Abstract
This review presents an introduction to the synthesis of metallic nanoparticles by radiation-induced method, especially gamma irradiation. This method offers some benefits over the conventional methods because it provides fully reduced and highly pure nanoparticles free from by-products or chemical reducing agents, and is capable of controlling the particle size and structure. The nucleation and growth mechanism of metallic nanoparticles are also discussed. The competition between nucleation and growth process in the formation of nanoparticles can determine the size of nanoparticles which is influenced by certain parameters such as the choice of solvents and stabilizer, the precursor to stabilizer ratio, pH during synthesis, and absorbed dose.
Keywords: Radiation-induced method, Metallic nanoparticles, Nucleation, Growth, Redox potential
Review
Introduction and background
In the past few decades, revolutionary developments of science and engineering have moved at a very fast pace towards synthesis of materials in the nanosize region in order to achieve unique properties that are significantly different from those of the individual atoms and their bulk counterparts [1-3]. When the dimension of a particle decreases below 100 nm, it exhibits many intriguing properties that arise mainly from two physical effects. First, the quantization of electronic states becomes apparent leading to very sensitive size-dependent effects such as optical and magnetic properties [4,5]. Second, the high surface-to-volume ratio alters the thermal, mechanical, and chemical properties of materials [6]. Various nanoparticle synthesis approaches are available, which can be broadly classified into top-down and bottom-up approaches [7]. In the former category, nanoparticles can be obtained by techniques such as milling or lithography which generates small particles from the corresponding bulk materials [8,9]. However, in the latter approach, nanoparticles can be formed atom-by-atom in the gas phase, solid phase, or liquid phase [10]. In the liquid phase, nanoparticles are chemically synthesized in a colloidal solution containing precursors, a reducing agent, a particle capping agent, and a solvent [11,12]. Although colloidal synthesis has the potential to produce large quantity of nanoparticles with good control of size, shape, crystallinity, morphology, composition, and surface chemistry at reasonably low cost.
Colloidal Metallic Nanoparticles
Colloids are composed of suspensions of one phase, either solid or liquid, in a second liquid phase [13]. They are very attractive because of their huge surface-to-volume ratio and their high specific surface area. This insures contact of a large part of the particle atoms with the surrounding liquid, to form almost as soluble macromolecules, which leads to larger interactions or faster reactions [14]. The colloids, which we are concerned with in this review, are particles of metallic elements with respect to their surrounding phase.
Most of the preparation techniques of the metal colloids are based on reduction of precursor metal ions in solution (aqueous or otherwise) in the presence of a stabilizing agent. The most widely used techniques are thermolysis [15], chemical reduction [16], sonochemical route [17,18], and irradiation methods [19,20]. One of the great advantages of the radiolytic synthesis in comparison with the other available methods lies in the fact that the experiment can be carried out at very mild conditions, such as ambient pressure and room temperature with high reproducibility [21]. Another important advantage of this method is that the main reducing agent in the absence of oxygen is the hydrated electron which has a very negative redox potential. This enables any metal ions to be reduced to zero-valent metal atoms without using chemical reducing agents. Thus, the generation of primary atoms occurs as an independent event and at the origin; the atoms are separated and homogeneously distributed as were the ionic precursors [14,22,23]. In other words, two main factors which lead to formation of uniformly dispersed and highly stable nanoparticles without unwanted by-products of the reductants are homogeneous formation of nuclei and elimination of excessive chemical reducing agents. The choice of the absorbed dose is crucial in order to control the cluster size and crystal structure by precise tuning of nucleation and growth steps especially for multi-metallic clusters [24]. Therefore, the radiation technique has proven to be an environmentally benign and low-cost method for preparation of a large quantity of size and structure controllable metal nanoparticles [24-26].
In this review, a few examples among recent works were selected in which colloidal metal particles were synthesized by radiolytic reduction method and used either as a part of elaborate structures.
Experimental process
Radiolytic reduction method
The radiolytic reduction has been proven to be a powerful tool to produce monosized and highly dispersed metallic clusters [25]. The normal ionization radiations which are used for synthesis of nanoparticles are electron beam, X-ray, gamma-ray, and UV light. The metallic nanoparticles can be prepared in an aqueous solution in the presence of a stabilizer without using chemical reducing agents, namely by using of radiolytic method [26-29].
Large number of hydrated electrons and H• atoms are produced during radiolysis of aqueous solutions by irradiation (Equation 1). They are strong reducing agents with redox potentials of and E0 (H+/H•) = -2.3 VNHE, respectively [30]. Therefore, they can reduce metal ions into zero-valent metal particles (Equations 2 and 3).
| (1) |
| (2) |
| (3) |
This mechanism avoids the use of additional reducing agents and the following side reactions. Moreover, by varying the dose of the irradiation, the amount of zero-valent nuclei can be controlled.
On the other hand, hydroxyl radicals (OH•), induced in radiolysis of water, are also strong reducing agents with E0 = (OH•/H2O) = +2.8 VNHE, which could oxidize the ions or the atoms into a higher oxidation state. An OH• radical scavenger, such as primary or secondary alcohols or formate ions, is therefore added into the precursor solutions before irradiation. For example, isopropanol can scavenge OH• and H• radicals and at the same time changes into the secondary radicals, which eventually reduce metal ions (M+) into zero-valent atoms (M0) as shown in the following reactions [24]:
| (4) |
| (5) |
| (6) |
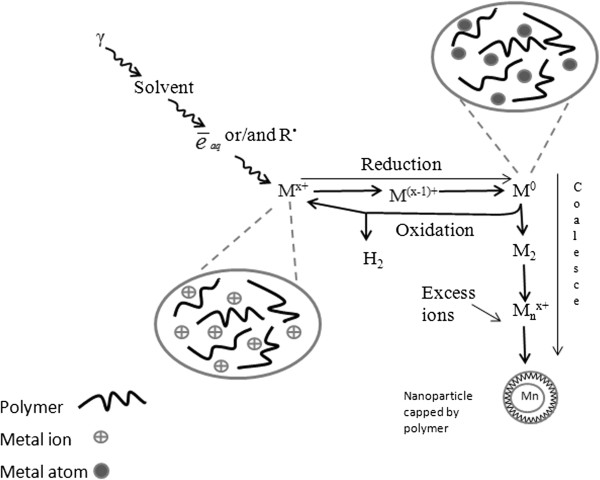
Multivalent ions are also reduced up to the atoms, by multi-step processes possibly including disproportion of lower valence states. These processes are illustrated by a schematic diagram in Figure 1.
Figure 1.
Scheme of metal ion reduction in solution by ionizing radiation in the presence of stabilizer. The isolated atoms M0 coalesce into clusters. They are stabilized by ligands, polymers, or supports [24].
Nucleation and growth under irradiation
The hydrated electrons arising from the radiolysis of water can easily reduce all metal ions up to the zero-valent atoms (M0). Also, the multivalent metal ions could be reduced by multi-step reductions including intermediate valencies. The atoms, which are formed via radiolytic method, are distributed homogeneously throughout the solution. This is as a result of the reducing agents generated by radiation which can deeply penetrate into the sample and randomly reduce the metal ions in the solution. These newly formed atoms act as individual centre of nucleation and further coalescence. The binding energy between two metal atoms or atoms with unreduced ions is stronger than the atom-solvent or atom-ligand bond energy [24]. Therefore, the atoms dimerize when encountering or being associated with the excess metal ions:
| (7) |
| (8) |
The charged dimer clusters M2+ may further be reduced to form a centre of cluster nucleation. The competition between the reduction of free metal ions and absorbed ones could be controlled by the rate of reducing agent formation [31].
Reduction of ions which are fixed on the clusters favours to cluster growth rather than formation of new isolated atoms. The bonding between clusters with unreduced ions or two charged clusters is also strong and these association processes are fast:
| (9) |
| (10) |
| (11) |
where m, n and p represent the nuclearities, and x, y and z, symbolize the number of associated ions. The control of the final size depends on the limitation applied to the coalescence beyond certain nuclearity. For free clusters such as nanocolloids in solution, the coalescence may be limited by a polymeric molecule acting as a cluster stabilizer.
Stabilization
All nanostructured materials possess a huge surface energy due to the large surface area; thus, they are thermodynamically unstable or metastable. Overcoming the large surface energy to prevent the nanostructures from growing is one of the great challenges in the synthesis of nanomaterials [32]. Nanoparticles, exclusively colloidal particles, in a short distance, are attracted to each other by the van der Waals force. If there is no counteracting force, the particles will aggregate and the colloidal system will be destabilized. The stability is achieved when the repulsion forces balance the attraction forces by electrostatic stabilization and/or steric stabilization.
There are several types of colloidal metal stabilizers which depend on the type of metal, method of preparation, and the application of the resultant metallic nanoparticles. For example, polymers having functional groups such as -NH2, -COOH, and -OH have high affinity for metal atoms; however, the use of stabilizers is not desirable for some applications such as catalysis. For example, activities of supported metal nanoparticle catalysts by coordination capture method are higher than those of polyvinyl-pyrrolidone (PVP)-stabilized metal colloidal catalysts [33,34]. Due to functional groups namely C = O and N, and long polymer chains, PVP can associate with the metal nanoparticles [35,36]. The functional groups containing lone pairs of electrons help in the stabilization of metal nanoparticles at their surfaces by covalent interaction, whereas the polymer chain restricts aggregation of metal nanoparticles by steric hindrance. For example, the long chains of PVP stretch out around nickel atom on the surface of the crystal, causing a steric hindrance effect and thus prevent particle growth effectively [37]. Apart from this, PVP is a biocompatible polymer. Hence, nanoparticles synthesized in PVP can be used in biological applications.
There are several reports about using poly(vinyl alcohol) (PVA) as a colloidal stabilizer for the synthesis of metallic nanoparticles by ionizing radiation [38-40]. The PVA chain plays a significant role in avoiding the formation of metal hydroxide clusters by hydrolysis of metal ions, thus preventing them from aggregation. Several active -OH groups in PVA are capable of absorbing metal ions through secondary bonds and steric entrapment [41]. A reaction of metal ions (M+) with PVA that leads to their associations can be expressed as:
| (12) |
where R-OH represents a PVA monomer.
In the absence of a radical scavenger, the radiation crosslinking of PVA molecules is known to be induced mainly by OH radicals in aqueous medium as shown in Equations 13 and 14 [42]:
| (13) |
| (14) |
The hydroxyl radicals almost exclusively react with PVA and the reduction of metal ions can take place both by hydrated electrons and the polymeric radicals PVA•.
The interactions between the surface of Ag colloids prepared by γ-irradiation and organic molecules containing ethanol and C12H25NaSO4 were discussed by Wang and his group [43]. It was observed that these molecules can restrain the growth of Ag particles and produce a dendrite pattern. The interaction of metallic surfaces with the solvent makes the surfaces become homogeneous; thus, Ag particles lost the anisotropy which played an important role in the formation of dendritic patterns.
Another kind of stabilizer for metallic nanoparticles is inorganic compounds such as metal oxides. They were originally used as catalyst supports. The catalysts are generally transition noble metals (Pt, Re, Rh, etc.) supported on various oxides. For example, Al2O3 supported Ni nanocluster was synthesized via γ-irradiation by Keghouche and his co-workers [44]. The solution of Ni(HCOO)2 · 7H2O, Al2O3, isopropanol, and ammonium hydroxide was γ-irradiated at a total dose of 100 kGy. Since alumina has an amphoteric character, it can play an important role in the fixation of metal ions.
Bimetallic Nanoparticles
When a mixed solution of two metal ionic precursors M+ and M'+ is irradiated, three main types of structures can be identified: intermetallic or alloyed structures, core/shell, and heterostructure [45,46]. The reduction process of ionic solution is controlled by the respective redox potential of metallic ions which is the key factor to determine the structure of resultant particles.
Alloy, core/shell, and heterostructured nanoparticles
Nanoparticles with alloy structure form when initial reduction reactions follow by mix coalescence and association of atoms and clusters with unreacted ions. These alternate associations and then reduction reactions progressively build bimetallic alloyed clusters [24].
The mechanism of alloyed structure formation by radiolysis has been studied in detail, for example for Al3+ and Ni2+ ionic solution under gamma irradiation by Abedini and her co-workers [47]. Nickel ions can be reduced easier than aluminium ions, and as a result, when the precursor ion solution is irradiated, reduction occurs by successive steps. The unreacted ions are absorbed on the surface of the newly formed clusters to form a charged cluster. These ions then get reduced in situ by hydrated electrons to form alloyed structure.
Different stoichiometries of Ag-Ni alloy nanoparticles were prepared from an aqueous solution containing AgClO4, NiSO4, sodium citrate, and methanol, in presence of PVA using the radiolytic method by Nenoff and her co-workers [48]. Figure 2 shows a zero-loss image of Ag-Ni nanoparticles, Ni, and Ag maps by energy-filtered transmission electron microscopy (EFTEM). The shape of bright spots from both Ni and Ag maps is the same which indicates that both Ag and Ni are present in particles with alloyed structure.
Figure 2.
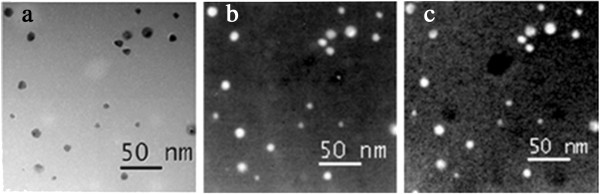
EFTEM maps of Ag0.9-Ni0.1NPs. (a) Zero-loss image, (b) Ni map, and (c) Ag map [48].
If the ionic precursors are multivalent and both metals have some probabilities to be reduced by hydrated electrons and radiolytic radicals, the less noble metal ions (M'+) will act as electron donors to the more noble metal ions (M+). Thus, at the first step, monometallic clusters of noble metal (Mn) will be formed. Then, when concentration of M+ ions decreases, M'+ ions are reduced afterwards at the surface of Mn. The final result is a core-shell cluster where the more noble metal M is coated by the other one M' [24]. For example, the Cu(core)/Al2O3(shell) nanoparticles were formed when mixed CuCl2 and AlCl3 solution in the presence of PVP was gamma-irradiated [49]. Copper ions have a higher possibility to be reduced (higher redox potential, E0(V) = +0.34) than aluminum ions ( E0(V) = -1.66), so the rate of reaction of hydrated electrons in the solution with Cu ions was higher than with Al ions. Thus, when bivalent Cu ions were irradiated, the reduction occurred until Cu zero-valent content increased. Then in a further step, when Cu2+ ions were depleted, the reduction of Al3+ increased which occurred exclusively at the surface of the Cu particles to form core-shell structure. The core/shell structure of the clusters, as analysed by transmission electron microscopy (TEM; Figure 3), electron diffraction, and XRD, was clearly confirmed [49]. The boundary between the core and shell was not sharp, since the shells are CuAlO2 and Al2O3 instead of pure Al.
Figure 3.
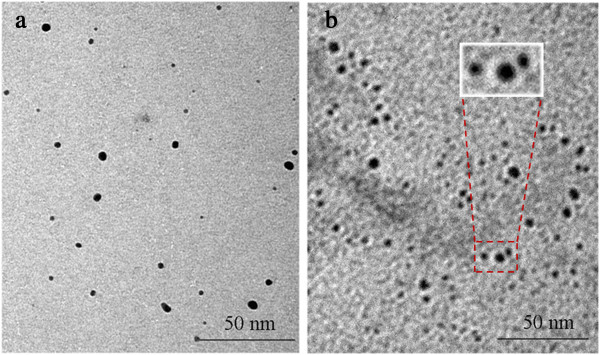
TEM images of Cu and Cu@CuAlO2-Al2O3nanoparticles. (a) pure Cu nanoparticles and (b) Cu@CuAlO2-Al2O3 nanoparticles in core-shell structure [49].
Under proper conditions, individual nucleation and growth of two kinds of metal atoms can occur separately to form heterostructure. For example, when FePt nanoparticles reacted with AuCl-(PPh3) in the presence of 1,2-dichlorobenzene containing 1-hexadecylamine, the successive growth of Au on to the FePt seeds was observed which resulted in the formation of heterodimers of FePt-Au (Figure 4) [50].
Figure 4.
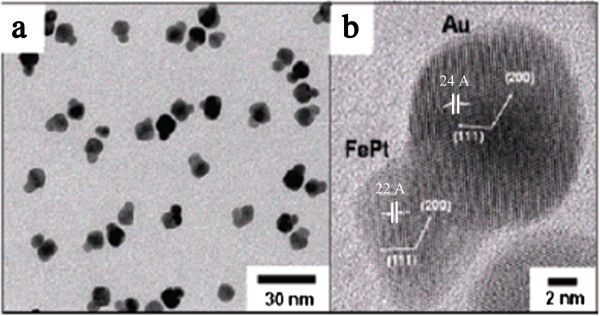
TEM and HRTEM images of FePt-Au heterostructured nanoparticles. (a) TEM image, and (b) HRTEM image of FePt-Au heterodimer nanoparticles reported by Choi et al. [50].
Effects of synthesis parameters
The synthesis of metallic nanoparticles by irradiation is governed by a number of experimental parameters such as the choice of solvent and stabilizer, the precursor to stabilizer ratio, pH value during synthesis, and absorbed dose. All of these parameters determine the final ordering, particle size and distribution, and surface area of resultant nanoparticles. A preliminary study should be done in order to determine the best conditions for an efficient dispersion, and to prepare the further homogeneous fixation of the metal nanoparticles on the support.
Effect of the solvent type
It has been suggested that the reduction rate under irradiation can be modified by using the appropriate solvent. The reducing agents are the key parameters that can affect the speed of reduction and therefore the particle size and distribution. The hydrated electrons (E0 = -2.9 VNHE), produced by water radiolysis, are stronger reducing agents than 2-propyl radicals. The existence of different reducing agents in the media varies the speed of reduction that makes a broad size distribution.
Misra and his co-workers [36] have synthesized the Au nanoparticles with narrow size distribution by gamma radiolysis method. They used acetone and 2-propyl alcohol in aqueous media as solvent. Acetone is known to scavenge aqueous electron to give 2-propyl radical (E0 = -1.8 VNHE) by the following reaction:
| (15) |
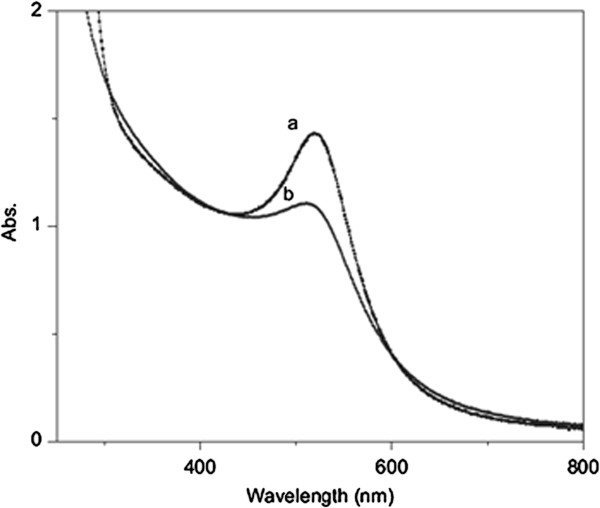
The only reducing agent in the system is the 2-propyl radical [51]. Reduction by this radical is slower than that by hydrated electron which is suitable for achieving narrower size distribution. It could be clearly observed from Figure 5 that FWHM of absorption peak, which shows size distribution of the particles in a solution, decreases by adding acetone. Also, in the synthesis of Ag nanoparticles by gamma irradiation reported by Mukherjee et al. [52], it has been investigated that as the mole fraction of ethylene glycol in aqueous media increased, the amount of reduced particle increased. The results show the participation of organic radicals in the reduction of silver ions adsorbed over the surface of silver particles.
Figure 5.
Absorption spectra of aqueous Au nanoparticle solution. Absorption spectra obtained (a) with acetone and (b) without acetone for absorbed dose of 1.7 kGy [36].
Effect of pH of the medium
The optimized pH corresponds to three issues namely, a compromise between the valence state and the charge of ionic precursor in relation with the electrostatic surface charge of the support, preventing reoxidation and minimizing the corrosion of the metallic nanoparticles, and preventing the preparation of unpleasant precipitation. For example, LIU et al. [53] have founded that Cu2+ ions in aqueous solution could be oxidized easily when the solution pH was lower than 9.
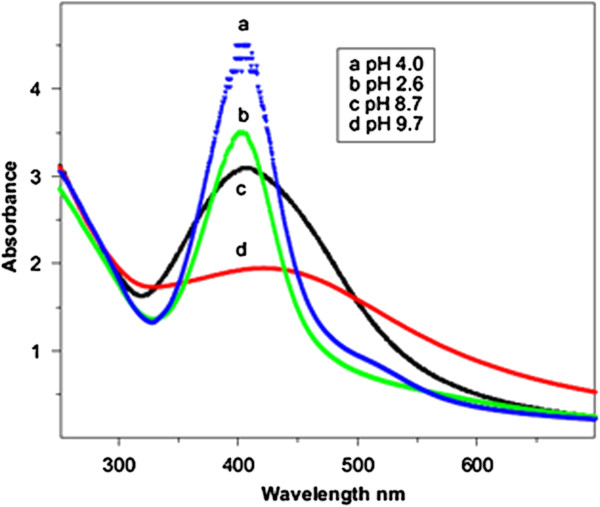
Silver nano-clusters on SiO2 support have been synthesized in aqueous solution using gamma radiation by Ramnani and co-workers [54]. They observed that, the surface plasmon resonance band, recorded after irradiation, shifts to the red side of the visible spectrum with enhanced broadness when pH was increased (Figure 6). In alkaline media, Ag clusters that formed on the surface of silica were not stable and probably underwent agglomeration. With increasing pH of the irradiated solution, the solubility of SiO2 increased and therefore affected stabilization of Ag clusters which resulted in their agglomeration.
Figure 6.
Radiation-induced formation of Ag nanoparticles on SiO 2 at various pH’s: (a) 4.0, (b) 2.6, (c) 8.7 and (d) 9.7; Radiation dose = 0.6 kGy [54].
Influence of radiation dose
Nucleation and aggregation processes in the formation of bimetallic nanoparticles could be affected by varying the absorbed dose. The rates of growth could be determined by probabilities of the collisions between several atoms, between one atom and a nucleus, and between two or more nuclei [55]. At low radiation doses, the concentration of unreduced metal ions is higher than the nucleus concentration because of low reduction rate. Thus, the unreduced ions can ionize bimetallic nanoparticles to form large bimetallic ions before they undergo reduction and aggregation processes to form even larger bimetallic nanoparticles. However, at higher doses, most of the metal ions are consumed during the nucleation process; therefore, the nucleus concentration is higher than the concentration of unreduced metal ions. As a result, the bimetallic nanoparticles are smaller in size at higher radiation doses [47].
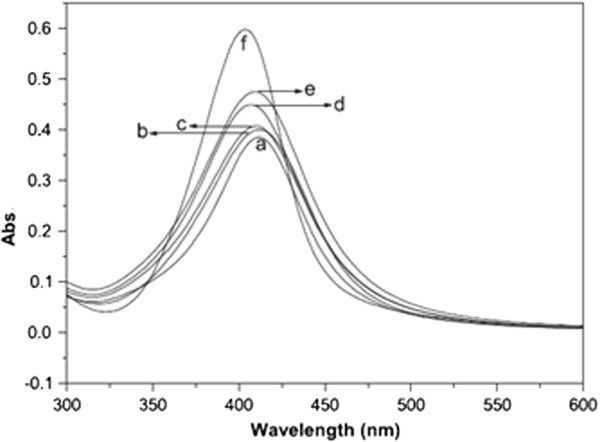
On the other hand, there is a possibility of inter- and intra-molecular crosslinking within the polymer molecules via radical interaction mechanism as secondary step in gamma-ray reduction. At higher doses, the polymer becomes a more complex matrix due to the occurrence of inter- and intra-molecular hydrogen bonding as well as radical linkage initiated by gamma irradiation between the cyclic structure constituents of the polymer molecules [56]. Therefore, it inhibits the aggregation of colloidal nanoparticles resulting in the formation of smaller nanoparticles. For example, Rau et al. [31], in the synthesis of silver nanoparticles by gamma radiation in the presence of gum acacia, have found that as the irradiation dose increases the corresponding optical absorption intensity increases with concomitant blue shifts. An increase in the intensity of optical absorption spectra indicates the increase of number of silver nanoparticles. In addition, the peak shift may be attributed to the change in particle size (Figure 7).
Figure 7.
Optical absorption spectra of silver nanoparticles. Optical absorption of samples when irradiated at (a) 1.0 kGy, (b) 2.0 kGy, (c) 4.5 kGy, (d) 12.0 kGy, (e) 18.0 kGy and (f) 24 kGy [31].
It was reported that the radiation crosslinking of gum acacia molecules can directly affect the growth process of silver nanoparticles [31]. It is important to mention here that we cannot generalize this for all kinds of polymers, for example in contrast with gum acacia, chitosan cannot facilitate the formation of Ag nanoparticles at higher doses and black precipitation was observed at a dose >20 kGy [57]. However, for binary Al-Ni nanoparticles prepared by gamma radiation method the average size of particles decreased from 32.7 nm at 60 kGy dose to 4.4 nm at 100 kGy dose (Figure 8) [47].
Figure 8.
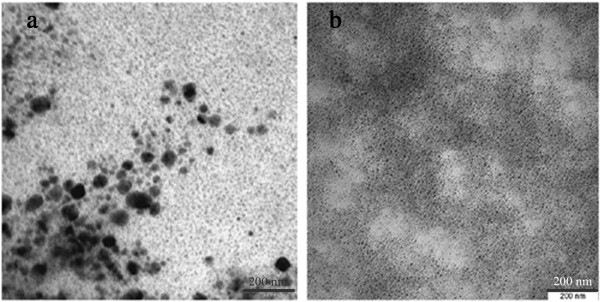
TEM images of colloidal Al-Ni nanoparticles. TEM images of Al-Ni nanoparticles at doses of (a) 60 kGy and (b) 100 kGy [47].
A similar trend has been reported for PVP-capped Cu@CuAlO2-Al2O3 nanoparticles synthesized by gamma radiation in aqueous solution at various radiation doses [49]. The average size of Cu@CuAlO2-Al2O3 nanoparticles decreased from 12 nm at 80 kGy to 4.5 nm at 120 kGy. Variation in the particle size could be referred to the difference in the rate of nucleation and growth processes.
Effect of precursor's concentration
By increasing the initial ion concentration, final size of metal nanoparticles increase [49]. There are three main reasons for the results. Firstly, the rate of ion association that forms larger particles increases by increasing the concentration of metal ions. Secondly, particle aggregation occurs by collision of small particle in solution. The viscosity of the aqueous solution and subsequently the speed of particles movement can be changed by varying the ratio of polymer to ions. Increasing the concentration increases the number of ions and collision probability. Finally, the surface energy and further agglomeration of nanoparticles can be reduced by the adsorption of polymer molecules on the surface of metal nanoparticles [58,59]. Therefore, increasing ion concentration reduces the polymer capping performance on the surface of nanoparticles which leads to the formation of larger particles.
Li et al. [60] have synthesized silver and gold nanoparticles from aqueous solution of AgNO3 and HAuCl4 in the presence of 2-propanol and PVP by gamma irradiation method. TEM results showed the average size of Au nanoparticles increased from 7 nm at the lowest ion concentration (2 × 10-4 M) to 15 nm at the highest (2 × 10-3 M) (Figure 9).
Figure 9.
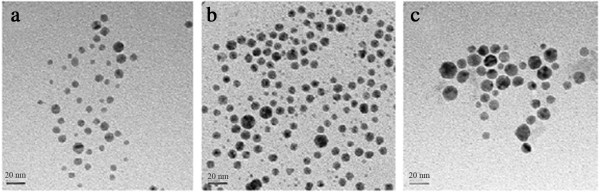
TEM images of gold nanoparticles. TEM images of gold nanoparticles prepared by γ-irradiation at various concentration of HAuCl4: (a) 2 × 10-4, (b) 1 × 10-3, and (c) 2 × 10-3 M [60].
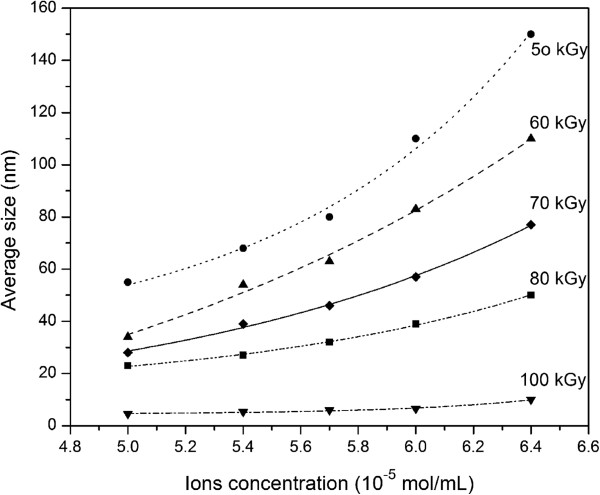
The size of silver and gold nanoparticles increased with the increase in concentration of starting AgNO3 and HAuCl4 solutions [60]. It indicated that when the number of nuclei remained constant or increased at a slower rate than that of the total ions, the particle size would become larger with the increase of ion concentration. From the data of the UV–vis spectra the irradiation-induced silver colloids from the lowest AgNO3 concentration of 2.0 × 10-4 M had a light yellow colour with maximum plasmon band at 416 nm. As the concentration of the precursor salt solution increased up to 1.0 × 10-2 M, the colour of the silver colloidal solution changed to dark yellow and the absorbance accordingly increased, indicating an increase in the density of resultant Ag nanoparticles formed under irradiation [60]. We could anticipate that the same thing happens to most kinds of bimetallic nanoparticles synthesized by gamma irradiation. Effect of ion concentration on growth process of Al-Ni and Al-Cu bimetallic under gamma irradiation has also been reported [47,49], where the average particle size increased with increasing ion concentration and with decreasing dose (Figure 10).
Figure 10.
Exponential trend of changing average size of colloidal Al-Ni nanoparticles versus ion concentration for several doses [47].
Conclusion
In this review, we have surveyed the radiation-induced synthesis and the characterization studies of metallic nanoparticles especially prepared by gamma irradiation. It has been illustrated that the type of solvent, solution pH, precursors' concentration, and the absorbed dose do influence the composition, crystalline structure, particle size, size distribution, and optical properties of the final products. These effects are due to the variation in the nucleation, growth, and aggregation processes in the formation of colloidal metallic nanoparticles. This information could be useful in describing underlying principles in controlling the size of metal nanoparticles by analyzing different combinations of physical factors in monometallic and bimetallic nanoparticle formation.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AA collected and reviewed the data and drafted the manuscript. ARD and MAAH modified the draft in first version and after revision. NKO participated in the discussion. ES participated in analysis and interpretation of data. All authors read and approved the final manuscript.
Contributor Information
Alam Abedini, Email: alamabedini@gmail.com.
Abdul Razak Daud, Email: ard@ukm.my.
Muhammad Azmi Abdul Hamid, Email: azmi@ukm.my.
Norinsan Kamil Othman, Email: insan@ukm.my.
Elias Saion, Email: elias@science.upm.edu.my.
Acknowledgements
The financial support from the Universiti Kebangsaan Malaysia (UKM) with project code DIP-2012-14 is acknowledged.
References
- Petit C, Taleb A, Pileni M. Cobalt nanosized particles organized in a 2D superlattice: synthesis, characterization, and magnetic properties. J Phys Chem B. 1999;8:1805–1810. doi: 10.1021/jp982755m. [DOI] [Google Scholar]
- Wang L, Zhang Z, Han X. In situ experimental mechanics of nanomaterials at the atomic scale. NPG Asia Mater. 2013;8:e40. doi: 10.1038/am.2012.70. [DOI] [Google Scholar]
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;8:MR17–MR71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- Turton R. The quantum dot: A journey into the future of microelectronics. New York, NY, USA: Oxford University Press, Inc; 1995. [Google Scholar]
- Chen S, Sommers JM. Alkanethiolate-protected copper nanoparticles: spectroscopy, electrochemistry, and solid-state morphological evolution. J Phys Chem B. 2001;8:8816–8820. doi: 10.1021/jp011280n. [DOI] [Google Scholar]
- Burda C, Chen X, Narayanan R, El-Sayed MA. Chemistry and properties of nanocrystals of different shapes. Chem Rev. 2005;8:1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- Toshima N, Yonezawa T. Bimetallic nanoparticles—novel materials for chemical and physical applications. New J Chem. 1998;8:1179–1201. doi: 10.1039/a805753b. [DOI] [Google Scholar]
- Haynes CL, Haes AJ, Van Duyne RP. Materials Research Society Symposium Proceedings. 635. Cambridge: Cambridge Univ Press; 2001. Nanosphere lithography: synthesis and application of nanoparticles with inherently anisotropic structures and surface chemistry; pp. C631–C636. [Google Scholar]
- Marques-Hueso J, Abargues R, Canet-Ferrer J, Valdes J, Martinez-Pastor J. Resist-based silver nanocomposites synthesized by lithographic methods. Microelectron Eng. 2010;8:1147–1149. doi: 10.1016/j.mee.2009.10.043. [DOI] [Google Scholar]
- Madou MJ. Fundamentals of Microfabrication and Nanotechnology: From MEMS to Bio-MEMS and Bio-Nems: manufacturing techniques and applications. Boca Raton, FL: CRC PressInc; 2011. [Google Scholar]
- Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J Chem Soc Chem Commun. 1994;8:801–802. [Google Scholar]
- Rodriguez A, Amiens C, Chaudret B, Casanove M-J, Lecante P, Bradley JS. Synthesis and isolation of cuboctahedral and icosahedral platinum nanoparticles. ligand-dependent structures. Chem Mater. 1996;8:1978–1986. doi: 10.1021/cm960338l. [DOI] [Google Scholar]
- Seifert G. Clusters and Colloids. From Theory to Applications. Z Kristallogr. 1995;8:816–816. [Google Scholar]
- Belloni J. Metal nanocolloids. Curr Opin Colloid. Interface Sci. 1996;8:184–196. [Google Scholar]
- Cushing BL, Kolesnichenko VL, O'Connor CJ. Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem Rev-Columbus. 2004;8:3893–3946. doi: 10.1021/cr030027b. [DOI] [PubMed] [Google Scholar]
- Long NN, Kiem CD, Doanh SC, Nguyet CT, Hang PT, Thien ND, Quynh LM. Synthesis and optical properties of colloidal gold nanoparticles. J Phys Conference Series. 2009;8:012026. [Google Scholar]
- Chen W, Cai W, Zhang L, Wang G, Zhang L. Sonochemical processes and formation of gold nanoparticles within pores of mesoporous silica. J Colloid Interface Sci. 2001;8:291–295. doi: 10.1006/jcis.2001.7525. [DOI] [PubMed] [Google Scholar]
- Darroudi M, Khorsand Zak A, Muhamad M, Huang N, Hakimi M. Green synthesis of colloidal silver nanoparticles by sonochemical method. Mater Lett. 2012;8:117–120. doi: 10.1016/j.matlet.2011.08.016. [DOI] [Google Scholar]
- Scaiano JC, Billone P, Gonzalez CM, Marett L, Marin ML, McGilvray KL, Yuan N. Photochemical routes to silver and gold nanoparticles. Pure Appl Chem. 2009;8:635–647. doi: 10.1351/PAC-CON-08-09-11. [DOI] [Google Scholar]
- Akhavan A, Kalhor H, Kassaee M, Sheikh N, Hassanlou M. Radiation synthesis and characterization of protein stabilized gold nanoparticles. Chem Eng J. 2010;8:230–235. doi: 10.1016/j.cej.2010.02.010. [DOI] [Google Scholar]
- Kharisov BI, Kharissova OV, Méndez UO. Radiation Synthesis of Materials and Compounds. Boca Raton, FL: CRC Press; 2013. [Google Scholar]
- Henglein A. Physicochemical properties of small metal particles in solution: “microelectrode” reactions, chemisorption, composite metal particles, and the atom-to-metal transition. The J Phys Chem. 1993;8:5457–5471. doi: 10.1021/j100123a004. [DOI] [Google Scholar]
- Henglein A. Electronics of colloidal nanometer particles. Berichte der Bunsen-Gesellschaft. 1995;8:903–913. [Google Scholar]
- Belloni J. Nucleation, growth and properties of nanoclusters studied by radiation chemistry: application to catalysis. Catal Today. 2006;8:141–156. doi: 10.1016/j.cattod.2005.11.082. [DOI] [Google Scholar]
- Marignier J, Belloni J, Delcourt M, Chevalier J. New microaggregates of non noble metals and alloys prepared by radiation induced reduction. Nature. 1985;8:344–345. doi: 10.1038/317344a0. [DOI] [Google Scholar]
- Lee K-P, Gopalan AI, Santhosh P, Lee SH, Nho YC. Gamma radiation induced distribution of gold nanoparticles into carbon nanotube-polyaniline composite. Compos Sci Technol. 2007;8:811–816. doi: 10.1016/j.compscitech.2005.12.030. [DOI] [Google Scholar]
- Seino S, Kinoshita T, Nakagawa T, Kojima T, Taniguci R, Okuda S, Yamamoto TA. Radiation induced synthesis of gold/iron-oxide composite nanoparticles using high-energy electron beam. J Nanopart Res. 2008;8:1071–1076. doi: 10.1007/s11051-007-9334-3. [DOI] [Google Scholar]
- Karim MR, Lim KT, Lee CJ, Bhuiyan MTI, Kim HJ, Park LS, Lee MS. Synthesis of core‒shell silver–polyaniline nanocomposites by gamma radiolysis method. J Polym Sci Part A: Polym Chem. 2007;8:5741–5747. doi: 10.1002/pola.22323. [DOI] [Google Scholar]
- Tang X-F, Yang Z-G, Wang W-J. A simple way of preparing high-concentration and high-purity nano copper colloid for conductive ink in inkjet printing technology. Colloids Surf A: Physicochemical and Engineering Aspects. 2010;8:99–104. doi: 10.1016/j.colsurfa.2010.02.011. [DOI] [Google Scholar]
- Rojas J, Castano C. Production of palladium nanoparticles supported on multiwalled carbon nanotubes by gamma irradiation. Radiat Phys Chem. 2012;8:16–21. doi: 10.1016/j.radphyschem.2011.08.010. [DOI] [Google Scholar]
- Rao Y, Banerjee D, Datta A, Das S, Guin R, Saha A. Gamma irradiation route to synthesis of highly re-dispersible natural polymer capped silver nanoparticles. Radiat Phys Chem. 2010;8:1240–1246. doi: 10.1016/j.radphyschem.2010.07.004. [DOI] [Google Scholar]
- Cao G. Nanostructures & nanomaterials: synthesis, properties & applications. London: Imperial College Pr; 2004. [Google Scholar]
- Zuo X, Liu H, Guo D, Yang X. Enantioselective hydrogenation of pyruvates over polymer-stabilized and supported platinum nanoclusters. Tetrahedron. 1999;8:7787–7804. doi: 10.1016/S0040-4020(99)00415-9. [DOI] [Google Scholar]
- Tu W-x, Zuo X-b, Liu H-f. Study on the interaction between polyvinylpyrrolidone and platinum metals during the formation of the colloidal metal nanoparticles. Chin J Polym Sci. 2008;8:23–29. doi: 10.1142/S0256767908002625. [DOI] [Google Scholar]
- Choi S-H, Zhang Y-P, Gopalan A, Lee K-P, Kang H-D. Preparation of catalytically efficient precious metallic colloids by γ-irradiation and characterization. Colloids Surf A: Physicochemical and Engineering Aspects. 2005;8:165–170. doi: 10.1016/j.colsurfa.2004.07.022. [DOI] [Google Scholar]
- Misra N, Biswal J, Gupta A, Sainis J, Sabharwal S. Gamma radiation induced synthesis of gold nanoparticles in aqueous polyvinyl pyrrolidone solution and its application for hydrogen peroxide estimation. Radiat Phys Chem. 2012;8:195–200. doi: 10.1016/j.radphyschem.2011.10.014. [DOI] [Google Scholar]
- Haque K, Hussain M. Synthesis of Nano-sized Nickel Particles by a Bottom-up Approach in the Presence of an Anionic Surfactant and a Cationic Polymer. J Sci Res. 2010;8:313–321. [Google Scholar]
- Torigoe K, Remita H, Beaunier P, Belloni J. Radiation-induced reduction of mixed silver and rhodium ionic aqueous solution. Radiat Phys Chem. 2002;8:215–222. doi: 10.1016/S0969-806X(01)00453-4. [DOI] [Google Scholar]
- Doudna CM, Bertino MF, Blum FD, Tokuhiro AT, Lahiri-Dey D, Chattopadhyay S, Terry J. Radiolytic synthesis of bimetallic Ag-Pt nanoparticles with a high aspect ratio. J Phys Chem B. 2003;8:2966–2970. doi: 10.1021/jp0273124. [DOI] [Google Scholar]
- Seino S, Kinoshita T, Otome Y, Maki T, Nakagawa T, Okitsu K, Mizukoshi Y, Nakayama T, Sekino T, Niihara K. γ-ray synthesis of composite nanoparticles of noble metals and magnetic iron oxides. Scripta Mater. 2004;8:467–472. doi: 10.1016/j.scriptamat.2004.06.003. [DOI] [Google Scholar]
- Gautam A, Tripathy P, Ram S. Microstructure, topology and X-ray diffraction in Ag-metal reinforced polymer of polyvinyl alcohol of thin laminates. J mater Sci. 2006;8:3007–3016. doi: 10.1007/s10853-006-6768-4. [DOI] [Google Scholar]
- Ulanski P, Bothe E, Rosiak JM, von Sonntag C. OH radical induced crosslinking and strand breakage of poly (vinyl alcohol) in aqueous solution in the absence and presence of oxygen. A pulse radiolysis and product study. Macromol Chem Phys. 1994;8:1443–1461. doi: 10.1002/macp.1994.021950427. [DOI] [Google Scholar]
- Wang S, Xin H. Fractal and dendritic growth of metallic Ag aggregated from different kinds of γ-irradiated solutions. J Phys Chem B. 2000;8:5681–5685. doi: 10.1021/jp000225w. [DOI] [Google Scholar]
- Keghouche N, Chettibi S, Latrèche F, Bettahar M, Belloni J, Marignier J. Radiation-induced synthesis of α-Al2O3 supported nickel clusters: Characterization and catalytic properties. Radiat Phys Chem. 2005;8:185–200. doi: 10.1016/j.radphyschem.2005.04.021. [DOI] [Google Scholar]
- Liz-Marzan LM, Kamat PV. Nanoscale materials. Netherlands: Springer Netherlands; 2003. [Google Scholar]
- Ferrando R, Jellinek J, Johnston RL. Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem Rev. 2008;8:845–910. doi: 10.1021/cr040090g. [DOI] [PubMed] [Google Scholar]
- Abedini A, Larki F, Saion E, Zakaria A, Zobir Hussein M. Influence of dose and ion concentration on formation of binary Al-Ni alloy nanoclusters. Radiat Phys Chem. 2012;8:1653–1658. doi: 10.1016/j.radphyschem.2012.05.015. [DOI] [Google Scholar]
- Nenoff TM, Zhang Z, Leung K, Stumpf R, Huang J, Lu P, Berry DT, Provencio PP, Hanson D, Robinson D. Room Temperature Synthesis of Ni-Based Alloy Nanoparticles by Radiolysis. Livermore: Sandia National Laboratories; 2009. Room temperature synthesis of Ni-based alloy nanoparticles by radiolysis. [Google Scholar]
- Abedini A, Saion E, Larki F, Zakaria A, Noroozi M, Soltani N. Room temperature radiolytic synthesized Cu@ CuAlO2-Al2O3nanoparticles. Int J Mol Sci. 2012;8:11941–11953. doi: 10.3390/ijms130911941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J-s C, Y-w J, Yeon S-I, Kim HC, Shin J-S, Cheon J. Biocompatible heterostructured nanoparticles for multimodal biological detection. J Am Chem Soc. 2006;8:15982–15983. doi: 10.1021/ja066547g. [DOI] [PubMed] [Google Scholar]
- Biswal J, Ramnani S, Shirolikar S, Sabharwal S. Seedless synthesis of gold nanorods employing isopropyl radicals generated using gamma radiolysis technique. Int J Nanotechnol. 2010;8:907–918. doi: 10.1504/IJNT.2010.034697. [DOI] [Google Scholar]
- Mukherjee T. Synthesis and characterization of silver nanoparticles in viscous solvents: A γ-radiolytic study. Int J Chem. 2012;8:10–15. [Google Scholar]
- Liu Q-m, Yasunami T, Kuruda K, Okido M. Preparation of Cu nanoparticles with ascorbic acid by aqueous solution reduction method. Trans Nonferrous Met Soc China. 2012;8:2198–2203. doi: 10.1016/S1003-6326(11)61449-0. [DOI] [Google Scholar]
- Ramnani S, Biswal J, Sabharwal S. Synthesis of silver nanoparticles supported on silica aerogel using gamma radiolysis. Radiat Phys Chem. 2007;8:1290–1294. doi: 10.1016/j.radphyschem.2007.02.074. [DOI] [Google Scholar]
- Wu M-L, Chen D-H, Huang T-C. Synthesis of Au/Pd bimetallic nanoparticles in reverse micelles. Langmuir. 2001;8:3877–3883. doi: 10.1021/la010060y. [DOI] [Google Scholar]
- Kassaee M, Akhavan A, Sheikh N, Beteshobabrud R. γ-Ray synthesis of starch-stabilized silver nanoparticles with antibacterial activities. Radiat Phys Chem. 2008;8:1074–1078. doi: 10.1016/j.radphyschem.2008.06.010. [DOI] [Google Scholar]
- Long D, Wu G, Chen S. Preparation of oligochitosan stabilized silver nanoparticles by gamma irradiation. Radiat Phys Chem. 2007;8:1126–1131. doi: 10.1016/j.radphyschem.2006.11.001. [DOI] [Google Scholar]
- Zhou F, Zhou R, Hao X, Wu X, Rao W, Chen Y, Gao D. Influences of surfactant (PVA) concentration and pH on the preparation of copper nanoparticles by electron beam irradiation. Radiat Phys Chem. 2008;8:169–173. doi: 10.1016/j.radphyschem.2007.05.007. [DOI] [Google Scholar]
- Linfeng ZXZRHE, Lihui R. Influence of PVA and PEG on Fe3O4nano-particles prepared by EB irradiation. J Radiat Res Radiat Proces. 2005;8:325–328. [Google Scholar]
- Li T, Park HG, Choi S-H. γ-Irradiation-induced preparation of Ag and Au nanoparticles and their characterizations. Mater Chem Phys. 2007;8:325–330. doi: 10.1016/j.matchemphys.2007.04.069. [DOI] [Google Scholar]