Abstract
Context
Sexual function is the health-related quality of life (HRQOL) domain most commonly impaired after prostate cancer treatment; however, validated tools to enable personalized prediction of erectile dysfunction after prostate cancer treatment are lacking.
Objective
To predict long-term erectile function following prostate cancer treatment based on individual patient and treatment characteristics.
Design
Pretreatment patient characteristics, sexual HRQOL, and treatment details measured in a longitudinal academic multicenter cohort (Prostate Cancer Outcomes and Satisfaction With Treatment Quality Assessment; enrolled from 2003 through 2006), were used to develop models predicting erectile function 2 years after treatment. A community-based cohort (community-based Cancer of the Prostate Strategic Urologic Research Endeavor [CaPSURE]; enrolled 1995 through 2007) externally validated model performance. Patients in US academic and community-based practices whose HRQOL was measured pretreatment (N = 1201) underwent follow-up after prostatectomy, external radiotherapy, or brachytherapy for prostate cancer. Sexual outcomes among men completing 2 years’ follow-up (n = 1027) were used to develop models predicting erectile function that were externally validated among 1913 patients in a community-based cohort.
Main Outcome Measures
Patient-reported functional erections suitable for intercourse 2 years following prostate cancer treatment.
Results
Two years after prostate cancer treatment, 368 (37% [95% CI, 34%–40%]) of all patients and 335 (48% [95% CI, 45%–52%]) of those with functional erections prior to treatment reported functional erections; 531 (53% [95% CI, 50%–56%]) of patients without penile prostheses reported use of medications or other devices for erectile dysfunction. Pretreatment sexual HRQOL score, age, serum prostate-specific antigen level, race/ethnicity, body mass index, and intended treatment details were associated with functional erections 2 years after treatment. Multivariable logistic regression models predicting erectile function estimated 2-year function probabilities from as low as 10% or less to as high as 70% or greater depending on the individual’s pretreatment patient characteristics and treatment details. The models performed well in predicting erections in external validation among CaPSURE cohort patients (areas under the receiver operating characteristic curve, 0.77 [95% CI, 0.74–0.80] for prostatectomy; 0.87 [95% CI, 0.80–0.94] for external radiotherapy; and 0.90 [95% CI, 0.85–0.95] for brachytherapy).
Conclusion
Stratification by pretreatment patient characteristics and treatment details enables prediction of erectile function 2 years after prostatectomy, external radiotherapy, or brachytherapy for prostate cancer.
Because most patients survive early-stage prostate cancer after treatment,1 health-related quality of life (HRQOL) outcomes have emerged as a major emphasis in treatment decisions. Erectile dysfunction is commonplace after prostate cancer treatment and has significant consequences for HRQOL. Among urinary, bowel, vitality, and sexual HRQOL domains—outcomes commonly impaired by prostate cancer treatment—sexual function in previously potent men is the most commonly impaired and is closely related to outcome satisfaction.2–8
Individual characteristics, such as pretreatment erectile function, that influence posttreatment sexual outcome are known to vary at diagnosis, yet tools to predict posttreatment erectile dysfunction based on pretreatment sexual HRQOL at baseline have been limited. Treatment refinements, such as nerve-sparing techniques, can mitigate erectile dysfunction consequences of prostate cancer treatment, while other treatment variations, such as use of neoadjuvant hormone therapy,9 can adversely affect sexual outcome. Although associations of these and other factors with patient-reported sexual outcome have been studied,3,10–13 information regarding how the combination of pretreatment patient characteristics and treatment factors relate to individualized sexual outcome remains limited.14,15
We sought to determine whether an individual man’s sexual outcomes after the most common treatments for early-stage prostate cancer (radical prostatectomy, external radiotherapy, or brachytherapy)16 can be predicted accurately based on baseline characteristics and treatment planning details.
METHODS
Study Patients and Measures
The Prostate Cancer Outcomes and Satisfaction With Treatment Quality Assessment (PROSTQA) is a prospective, longitudinal, multicenter cohort comprising men with previously untreated clinical stage T1 to T2 prostate cancer who had elected prostatectomy, external beam radiotherapy, or brachytherapy as primary treatment and were enrolled from 2003 to 2006 at 9 US university-affiliated hospitals into an institutional review board–approved protocol after providing written informed consent.3
Patient demographic, race/ethnicity, and clinical data were collected (because such factors have known associations with prostate cancer aggressiveness) by research coordinators via direct patient contact, eg, clinical visits supplemented by medical record review. Details of treatment, such as plan for nerve sparing during prostatectomy or neoadjuvant hormone therapy with radiation, were collected prior to treatment to enable predictive models based on pretreatment information. Patient-reported outcome measures, including the Expanded Prostate Cancer Index Composite (EPIC-26)17 and information regarding use of medications or devices for erectile dysfunction,18 were collected by third-party telephone interview before treatment and at 2, 6, 12, and 24 months after treatment; men who completed a pretreatment evaluation (1201/1371 eligible patients who had agreed to be contacted) comprised the PROSTQA cohort.
Among the 1201 men registered for follow-up, 1027 (86%) completed the 24-month interview and are the focus of this study. Their primary treatment included either prostatectomy (n = 524), external beam radiotherapy (n = 241), or brachytherapy (n = 262).
Statistical Analysis
Having functional erections suitable for intercourse was defined as the patient selecting the response option of “firm enough for intercourse” to the EPIC-26 question, “How would you describe the usual quality of your erections during the last 4 weeks?” (other responses indicated erectile dysfunction). Erectile function 2 years after treatment was modeled separately, according to planned treatment, using logistic regression. The pretreatment patient and disease characteristics as well as planned treatment details considered are summarized in eTable1, available at http://www.jama-com.
Multivariable model development used a backward elimination selection procedure with 2-sided α = .05. Model selection was internally validated using bootstrap resampling (500 resamples), and bootstrap estimates of parameter estimates, standard errors, pointwise 95% confidence intervals for model-predicted probabilities, and area under the receiver operating characteristic curve (AUC) were also obtained for each final model. Individual predicted probabilities of functional erections at 2 years were calculated using the inverse logistic function {exp[X′ β]/[1 + exp(X′ β)]}, where X′ β is the sum with X representing individual characteristics observed and β representing the associated log odds ratios for the individual characteristics estimated from the model.
The omission of 2-year nonrespondents from model development assumes data are missing completely at random. A sensitivity analysis assessed the effect of this assumption by refitting each final model using a model weighted for inverse probability of response; the probability of response was estimated using multivariable logistic regression including factors associated with nonresponse (education level, number of comorbid conditions, race, and pretreatment sexual functioning [eTable 1]). This approach had little effect on the estimates, and results were not reported.
Use of medications or devices to assist erection function as measured by patient self-report at 2 years was summarized overall and in detail among the subset of 694 men who were potent (ie, reported functional erections) before treatment, excluding patients with implanted erectile aid devices.
All analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, North Carolina).
External Validation
The community-based Cancer of the Prostate Strategic Urologic Research Endeavor the CaPSURE cohort registry served as an external validation cohort for the developed models. Men in the CaPSURE cohort reported HRQOL at baseline and every 6 months in follow-up; sexual function and bother (severity and impact of patient-reported erectile dysfunction) were determined from the UCLA Prostate Cancer Index (UCLA-PCI), the instrument from which the EPIC-26 was previously derived and from which it retained 6 items.19 Characteristics of the CaPSURE cohort have been previously described.20,21
Of the 1913 CaPSURE patients who completed pretreatment and 2 years posttreatment evaluation of sexual HRQOL using the UCLA-PCI, 1655 had data for all available model covariates available for validation. The PROSTQA model–predicted probability of 2-year erectile function was computed for each CaPSURE patient (from the inverse logistic function of the final model equations) and compared with his reported (actual) 2-year erectile function (the definition of erectile function used in the CaPSURE validation was the same as that in the PROSTQA cohort). Validation was assessed by AUC from fitting univariable logistic regression of reported 2-year erectile function on model-predicted probability, and calibration was assessed by examining the average model-predicted probability vs observed proportion of men reporting functional erections at 2 years, which is summarized overall and according to quintiles of the distribution of model-predicted probabilities.
RESULTS
Pretreatment patient characteristics and planned treatment details for the 1027 PROSTQA cohort patients who completed HRQOL interviews at 2 years’ follow-up are summarized in eTable 1. In the PROSTQA cohort used for predictive model development and excluding men with unknown erection quality, erectile dysfunction was reported by 274 of 983 (28% [95% CI, 25%–31%]) men prior to treatment (86/510 [17% {95% CI, 14%–20%}] in the prostatectomy group, 107/228 [47% {95% CI, 40%–54%}] in the external radiotherapy group, 81/245 [33% {95% CI, 27%–39%}] in the brachytherapy group) (eTable 2). At 2 years after treatment, erectile dysfunction was reported by 619 of 987 (63% [95% CI, 60%–66%]) men (334/511 [65% {95% CI, 61%–69%}] in the prostatectomy group, 145/229 [63% {95% CI, 57%–70%}] in the external radiotherapy group, and 140/247 [57% {95% CI, 50%–63%}] in the brachytherapy group) (eTable 2) and in 358 of 693 (52% [95% CI, 48%–55%]) men who were potent prior to treatment (248/414 [60% {95% CI, 55%–65%}] in the prostatectomy group, 51/121 [42% {95% CI, 33%–51%}] in the external radiotherapy group, 59/158 [37% {95% CI, 30%–45%}] in the brachytherapy group).
Probability of Functional Erections After Prostatectomy
The ability to attain functional erections suitable for intercourse at 2 years after treatment was reported among 177 of 511 (35% [95% CI, 30%–39%]) men who underwent prostatectomy. In univariable analyses, younger age, fewer comorbid conditions, lower prostate-specific antigen (PSA) level, lower cancer severity/risk category, pretreatment potency, better (higher) pretreatment EPIC-26 sexual HRQOL score, better (lower) pretreatment American Urological Association Symptom Index, and plan for nerve-sparing surgical technique were associated with greater probability of attaining functional erections at 2 years (each P < .05) (eTable 3); association of prostate size with sexual outcome was not statistically significant (P=.07).
In multivariable analysis, younger age, lower PSA level, better pretreatment sexual functioning score, and nerve-sparing surgery were associated with increased log-odds of functional erections (each P < .05; AUC, 0.77 [95% CI, 0.72–0.82]) (Table 1). The log-odds of erectile function increased approximately linearly with decreasing age and with increasing pretreatment sexual functioning score.
Table 1.
Multivariable Logistic Regression Models Predicting Functional Erections Suitable for Intercourse at 2 Years After Treatment, According to Planned Primary Prostate Cancer Treatment in the PROSTQA Cohorta
| Treatment, Variable | Parameter Estimate (SE) |
OR (95% CI) | Wald χ2 P Value |
Bootstrap Parameter Estimate (SE) |
||
|---|---|---|---|---|---|---|
| Prostatectomy | ||||||
| Intercept | −2.96 (1.38) | −3.00 (1.42) | ||||
| Pretreatment sexual HRQOL score (per 10 points) | 0.45 (0.07) | 1.6 (1.4–1.8) | <.001 | 0.45 (0.07) | ||
| Age (per 10 y) | −0.56 (0.16) | 0.6 (0.4–0.8) | <.001 | −0.58 (0.16) | ||
| Nerve-sparing | 1.29 (0.52) | 3.6 (1.3–10.1) | .01 | 1.39 (0.57) | ||
| PSA ≤10 ng/mL | 0.85 (0.36) | 2.3 (1.2–4.7) | .02 | 0.88 (0.37) | ||
| External radiotherapy | ||||||
| Intercept | −5.22 (0.76) | −5.37 (0.79) | ||||
| Pretreatment sexual HRQOL score (per 10 points) | 0.54 (0.08) | 1.7 (1.4–2.0) | <.001 | 0.55 (0.09) | ||
| No neoadjuvant hormone therapy | 1.18 (0.39) | 3.3 (1.5–7.0) | .003 | 1.24 (0.41) | ||
| PSA <4 ng/mL | 1.17 (0.46) | 3.2 (1.3–8.0) | .01 | 1.24 (0.48) | ||
| Brachytherapy | ||||||
| Intercept | −3.13 (2.21) | −3.40 (2.34) | ||||
| Pretreatment sexual HRQOL score (per 10 points) | 0.72 (0.11) | 2.1 (1.7–2.5) | <.001 | 0.75 (0.11) | ||
| Age (per 10 y) | −0.63 (0.28) | 0.5 (0.3–0.9) | .03 | −0.64 (0.30) | ||
| African American race/ethnicity | 1.13 (0.60) | 3.1 (0.9–10.0) | .06 | 1.18 (0.64) | ||
| BMIb | ||||||
| <25 | 2.22 (0.86) | 9.2 (1.7–50.0) | .01 | 2.30 (0.94) | ||
| 25–34.9 | 1.40 (0.77) | 4.0 (0.9–18.4) | .07 | 1.45 (0.85) | ||
| ≥35 | 0 | 1 [Reference] | ||||
Abbreviations: BMI, body mass index; CI, confidence interval; HRQOL, health-related quality of life; OR, odds ratio; PROSTQA, Prostate Cancer Outcomes and Satisfaction With Treatment Quality Assessment; PSA, prostate-specific antigen.
Model areas under the receiver operating characteristic curve were 0.77 (95% CI, 0.72–0.82) for prostatectomy, 0.83 (95% CI, 0.78–0.88) for external radiotherapy, and 0.89 (95% CI, 0.85–0.94) for brachytherapy. Individual predicted probabilities of functional erections suitable for intercourse at 2 years can be calculated using the inverse logistic function {exp[X′ β]/[1 + exp(X′ β)]}, where X′ β is the sum with X representing individual characteristics observed and β representing the associated model parameter estimates for the individual characteristics.
Calculated as weight in kilograms divided by height in meters squared.
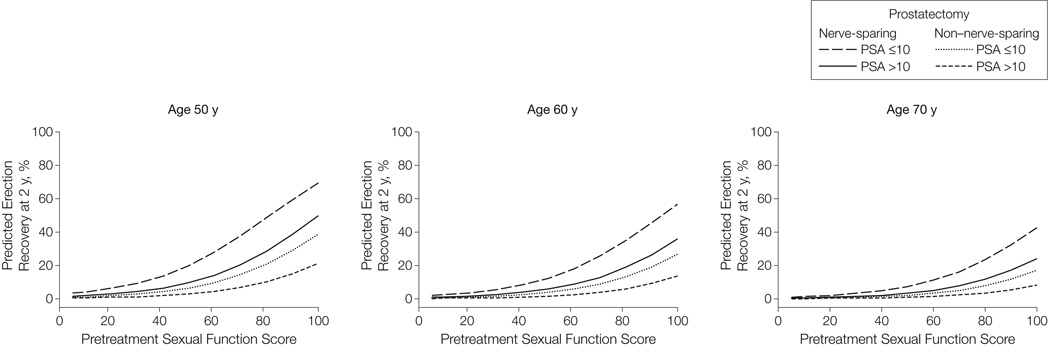
Model-predicted probabilities of functional erections after prostatectomy based on selected pretreatment sexual HRQOL scores are summarized in Figure 1 and Table 2. For example, a 50-year-old man’s prospects for having functional erections after prostatectomy vary, as tabulated, from 6% (95% CI, 2%–18%) to 70% (95% CI, 61%–77%) depending on pretreatment sexual HRQOL score, plan for nerve-sparing technique, and pretreatment serum PSA level (Table 2).
Figure 1.
Model-Predicted Probability of Functional Erections Suitable for Intercourse 2 Years After Radical Prostatectomy
Model-predicted probabilities based on pretreatment Expanded Prostate Cancer Index Composite sexual function score stratified by age, pretreatment prostate-specific antigen (PSA) level, and planned nerve sparing. Higher sexual function score denotes better sexual function. N=524 (66 [13%] with PSA level >10 ng/mL and 43 [8%] undergoing non–nerve-sparing surgery); median age, 60 years.
Table 2.
PROSTQA Model–Predicted Probabilities of Men Having Functional Erections Suitable for Intercourse 2 Years After Radical Prostatectomy
| Planned Surgical Technique |
Patient Age, y |
Pretreatment PSA Level, ng/mL |
Predicted Functional Erections After Treatment, % (95% CI) (by Pretreatment Sexual HRQOL Score)a,b |
||
|---|---|---|---|---|---|
| 67 | 83 | 100 | |||
| Nerve-sparing | 50 | ≤10 | 35 (25–45) | 52 (43–61) | 70 (61–77) |
| >10 | 18 (9–34) | 32 (18–49) | 50 (32–68) | ||
| 60 | ≤10 | 23 (18–30) | 38 (33–44) | 57 (49–64) | |
| >10 | 11 (0–21) | 21 (12–34) | 36 (22–53) | ||
| 70 | ≤10 | 15 (10–22) | 26 (19–35) | 43 (32–55) | |
| >10 | 7 (3–14) | 13 (7–24) | 24 (13–41) | ||
| Non–nerve-sparing | 50 | ≤10 | 13 (5–30) | 23 (9–46) | 39 (18–64) |
| >10 | 6 (2–18) | 11 (4–31) | 21 (7–48) | ||
| 60 | ≤10 | 8 (3–19) | 14 (6–32) | 27 (12–50) | |
| >10 | 3 (1–11) | 7 (2–19) | 13 (4–34) | ||
| 70 | ≤10 | 5 (2–12) | 9 (3–22) | 17 (7–37) | |
| >10 | 2 (1–7) | 4 (1–12) | 8 (3–23) | ||
Abbreviations: HRQOL, health-related quality of life; PROSTQA, Prostate Cancer Outcomes and Satisfaction With Treatment Quality Assessment; PSA, prostate-specific antigen.
Specific values of pretreatment sexual functioning (Expanded Prostate Cancer Index Composite scores of 67, 83, and 100; range of 0–100, with a higher score representing better function) are tabulated to represent cohort distribution (25th, 50th, and 75th percentiles of scores among prostatectomy-treated patients) as well as to reflect a clinically relevant set of possible pretreatment sexual functioning. Across all treatment groups, these percentiles correspond to 358 patients (36%) with pretreatment sexual HRQOL scores of 67 or less, 441 (45%) with scores of 68–99, and 186 (19%) with scores of 100.
Individual model-predicted probabilities were calculated using the inverse logistic function {exp[X′ β]/[1 + exp(X′ β)]}, where X′ β is the sum with X representing individual characteristics observed and β representing the associated model parameter estimates for the individual characteristics in Table 1.
Probability of Functional Erections After External Radiotherapy
The ability to attain functional erections suitable for intercourse at 2 years after treatment was reported among 84 of 229 (37% [95% CI, 30%–43%]) men who opted for external radiotherapy as their primary therapy. In univariable analyses, younger age, lower PSA level, lower risk category, better pretreatment sexual functioning score, better pretreatment American Urological Association Symptom Index, and no use of neoadjuvant hormone therapy were associated with greater probability of functional erections 2 years after treatment (each P < .05) (eTable 3).
In multivariable analysis, lower PSA level, better pretreatment sexual functioning score, and no use of neoadjuvant hormone therapy were associated with increased log-odds of functional erections after treatment (each P < .05; AUC, 0.83 [95% CI, 0.78–0.88]) (Table 1). The log-odds of functional erections increased approximately linearly with increasing pretreatment sexual HRQOL score.
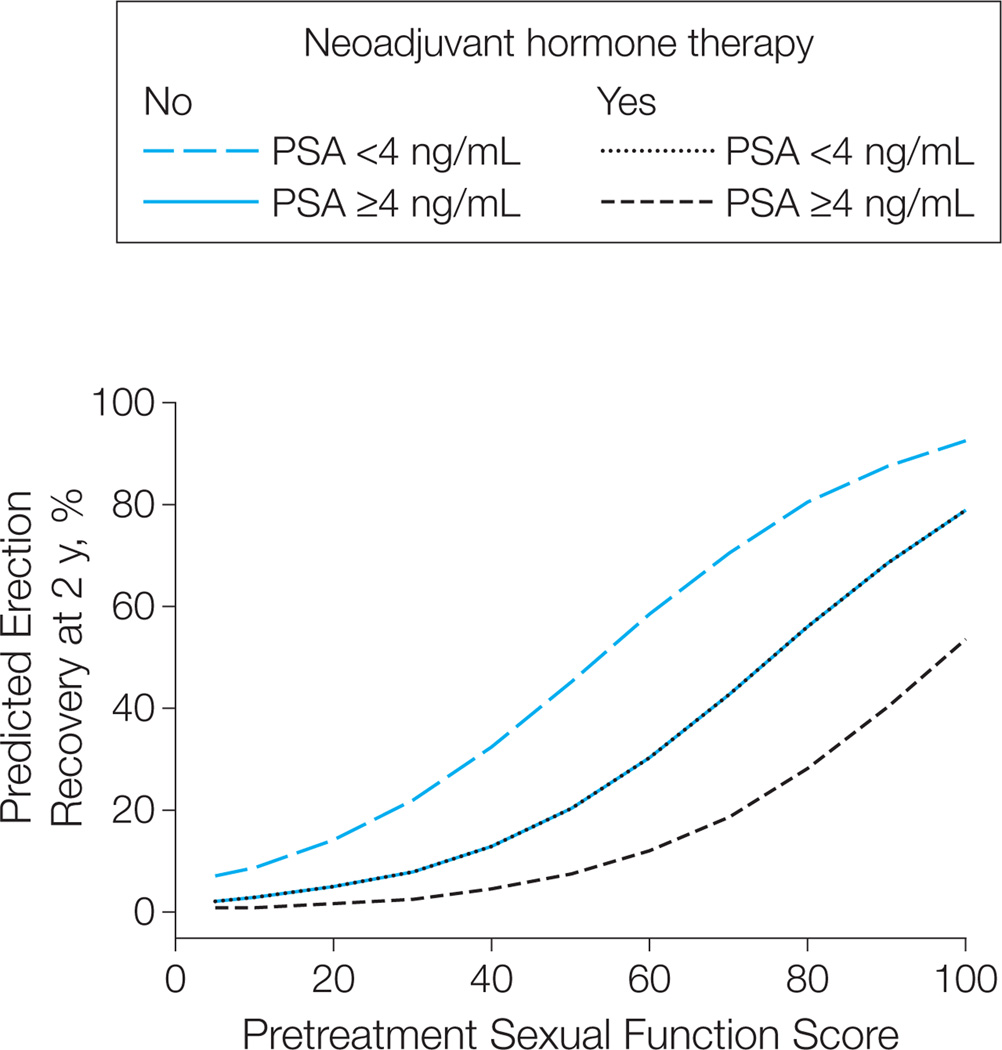
Model-predicted probabilities of functional erections after external radiotherapy at selected pretreatment sexual HRQOL scores are summarized in Figure 2 and Table 3. A man’s predicted probability of having functional erections after external radiotherapy varies from 16% (95% CI, 9%–28%) to 92% (95% CI, 81%–97%) depending on pretreatment sexual HRQOL score, use of neoadjuvant hormone therapy, and pretreatment serum PSA level (Table 3).
Figure 2.
Model-Predicted Probability of Functional Erections Suitable for Intercourse 2 Years After External Radiotherapy for Prostate Cancera
Model-predicted probabilities based on pretreatment Expanded Prostate Cancer Index Composite sexual function score stratified by pretreatment prostate-specific antigen (PSA) level and planned use of neoadjuvant hormone therapy. Higher sexual function score denotes better sexual function. N=241 (39 [16%] with PSA level <4 ng/mL and 74 [31%] receiving neoadjuvant hormone therapy.
aNote that curves for no use of neoadjuvant hormone therapy/PSA ≥4 ng/mL and use of neoadjuvant hormone therapy/PSA <4 ng/mL overlap.
Table 3.
PROSTQA Model–Predicted Probabilities of Men Having Functional Erections Suitable for Intercourse 2 Years After External Radiotherapy for Prostate Cancer
| Planned Neoadjuvant Hormone Therapy |
Pretreatment PSA Level, ng/mL |
Predicted Functional Erections After Treatment, % (95% CI) (by Pretreatment Sexual HRQOL Score)a,b |
||
|---|---|---|---|---|
| 67 | 83 | 100 | ||
| No | <4 | 67 (47–83) | 83 (66–92) | 92 (81–97) |
| ≥4 | 39 (30–49) | 60 (49–70) | 79 (67–87) | |
| Yes | <4 | 39 (19–63) | 60 (35–80) | 79 (55–92) |
| ≥4 | 16 (9–28) | 31 (19–48) | 53 (35–71) | |
Abbreviations: HRQOL, health-related quality of life; PROSTQA, Prostate Cancer Outcomes and Satisfaction With Treatment Quality Assessment; PSA, prostate-specific antigen.
Specific values of pretreatment sexual functioning (Expanded Prostate Cancer Index Composite scores of 67, 83, and 100; range of 0–100, with a higher score representing better function) are tabulated to represent cohort distribution (25th, 50th, and 75th percentiles of scores among prostatectomy-treated patients) as well as to reflect a clinically relevant set of possible pretreatment sexual functioning. These values correspond to 50th, 80th, and 93rd percentiles of the distribution among patients treated with external radiotherapy. Across all treatment groups, these percentiles correspond to 358 patients (36%) with pretreatment sexual HRQOL scores of 67 or less, 441 (45%) with scores of 68–99, and 186 (19%) with scores of 100.
Individual model-predicted probabilities were calculated using the inverse logistic function {exp[X′ β]/[1 + exp(X′ β)]}, where X′ β is the sum with X representing individual characteristics observed and β representing the associated model parameter estimates for the individual characteristics in Table 1.
Probability of Functional Erections After Brachytherapy
The ability to attain functional erections suitable for intercourse at 2 years was reported among 107 of 247 (43% [95% CI, 37%–50%]) men who opted for brachytherapy as primary treatment. In univariable analyses, younger age, college graduate, fewer comorbid conditions, and better pretreatment sexual HRQOL score were associated with greater probability of functional erections 2 years after treatment (each P < .05) (eTable 3).
In multivariable analysis, better pretreatment sexual HRQOL score, younger age, African American race/ethnicity, and lower body mass index were associated with increased log-odds of better erectile function (each P < .05; AUC = 0.89 [95% CI, 0.85–0.94]) (Table 1). The log-odds of erectile function 2 years after treatment increased approximately linearly with increasing pretreatment sexual HRQOL score and decreased approximately linearly with increasing age.
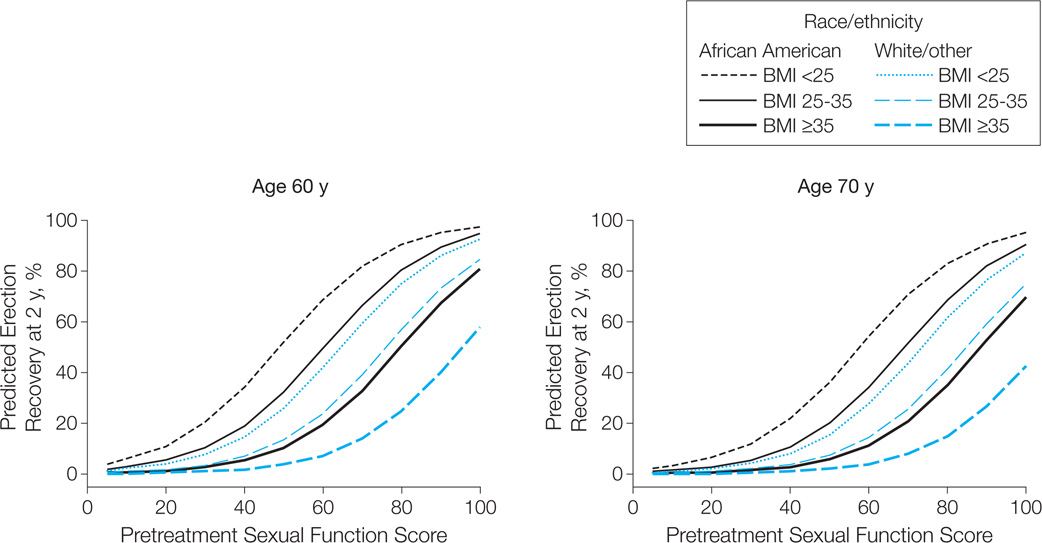
Model-predicted probabilities of functional erections after brachytherapy at selected pretreatment sexual HRQOL scores are summarized in Figure 3 and Table 4. Consequently, a 60-year-old man’s probability for having functional erections varies from 11% (95% CI, 3%–37%) to 98% (95% CI, 89%–99%) depending on pretreatment sexual HRQOL score, age, race/ethnicity, and BMI (Table 4).
Figure 3.
Model-Predicted Probability of Functional Erections Suitable for Intercourse 2 Years After Brachytherapy for Prostate Cancer
Model-predicted probabilities based on pretreatment Expanded Prostate Cancer Index Composite sexual function score stratified by age, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), and race. Higher sexual function score denotes better sexual function. N = 262 (28 [11%] African American; 57 [22%] with BMI <25, 187 [71%] with BMI 25–35, and 18 [7%] with BMI ≥35); median age, 66 years.
Table 4.
PROSTQA Model–Predicted Probabilities of Men Having Functional Erections Suitable for Intercourse 2 Years After Brachytherapy for Prostate Cancer
| Age, y | Race/Ethnicitya | BMIb | Predicted Functional Erections After Treatment, % (95% CI) (by Pretreatment Sexual HRQOL Score)c,d |
||
|---|---|---|---|---|---|
| 67 | 83 | 100 | |||
| 60 | African American | <25 | 78 (48–94) | 92 (73–98) | 98 (89–99) |
| 25–<35 | 61 (35–83) | 83 (62–94) | 94 (83–98) | ||
| ≥35 | 28 (7–69) | 56 (18–88) | 81 (41–96) | ||
| White/other | <25 | 54 (33–74) | 79 (61–90) | 93 (83–97) | |
| 25–<35 | 34 (22–48) | 62 (50–73) | 85 (75–91) | ||
| ≥35 | 11 (3–37) | 29 (9–63) | 58 (24–86) | ||
| 65 | African American | <25 | 73 (41–91) | 89 (67–97) | 97 (86–99) |
| 25–<35 | 54 (28–77) | 79 (54–92) | 93 (77–98) | ||
| ≥35 | 22 (5–62) | 48 (14–84) | 76 (33–95) | ||
| White/other | <25 | 46 (28–66) | 73 (55–86) | 90 (79–96) | |
| 25–<35 | 27 (18–39) | 54 (44–65) | 80 (69–88) | ||
| ≥35 | 9 (2–30) | 23 (6–56) | 50 (18–82) | ||
| 70 | African American | <25 | 66 (34–88) | 86 (60–96) | 95 (81–99) |
| 25–<35 | 46 (21–73) | 73 (45–90) | 90 (70–97) | ||
| ≥35 | 17 (3–56) | 40 (10–80) | 69 (25–94) | ||
| White/other | <25 | 39 (22–58) | 67 (47–82) | 87 (72–95) | |
| 25–<35 | 22 (13–33) | 47 (34–60) | 75 (60–86) | ||
| ≥35 | 6 (1–25) | 18 (4–50) | 42 (13–78) | ||
Abbreviations: BMI, body mass index; HRQOL, health-related quality of life; PROSTQA, Prostate Cancer Outcomes and Satisfaction With Treatment Quality Assessment.
White race/ethnicity included 3% of patients of other (non–African American) or unknown races/ethnicities.
Calculated as weight in kilograms divided by height in meters squared.
Specific values of pretreatment sexual functioning (Expanded Prostate Cancer Index Composite scores of 67, 83, and 100; range of 0–100, with a higher score representing better function) are tabulated to represent cohort distribution (25th, 50th, and 75th percentiles of scores among prostatectomy-treated patients) as well as to reflect a clinically relevant set of possible pretreatment sexual functioning. These values correspond to the 45th, 60th, and 85th percentiles among patients treated with brachytherapy. Across all treatment groups, these percentiles correspond to 358 patients (36%) with pretreatment sexual HRQOL scores of 67 or less, 441 (45%) with scores of 68–99, and 186 (19%) with scores of 100.
Individual model-predicted probabilities were calculated using the inverse logistic function {exp[X′ β]/[1 + exp(X′ β)]}, where X′ β is the sum with X representing individual characteristics observed and β representing the associated model parameter estimates for the individual characteristics in Table 1.
Validation of the Predictive Models in a Community-Based Cohort
To assess the generalizability of these models for predicting erectile function after primary prostate cancer treatment, we evaluated the performance of these models in a separate cohort of 1913 men who underwent prostatectomy, external radiotherapy, or brachytherapy in the community setting and whose HRQOL had been measured via their participation in the CaPSURE registry (eTable 4).9,20,21 The CaPSURE cohort reported higher pretreatment and posttreatment erectile dysfunction (42% [95% CI, 40%–44%] and 78% [95% CI, 76%–80%] of men, respectively).
The PROSTQA models performed well in predicting functional erections suitable for intercourse 2 years after treatment, with AUCs of 0.77 (95% CI, 0.74–0.80) for men undergoing prostatectomy, 0.87 (95%CI, 0.80–0.94) for those undergoing external radiotherapy, and 0.90 (95% CI, 0.85–0.95) for those undergoing brachytherapy. Calibration showed that model-predicted probabilities of functional erections corresponded to the observed outcome in the CaPSURE cohort (Table 5).
Table 5.
Calibration of PROSTQA Models in the CaPSURE Cohort: Comparison of Model-Predicted Probabilities of Functional Erections Suitable for Intercourse to Observed Proportions of Men Reporting Functional Erections 2 Years After Treatmenta
| Quintiles (Ranges) of PROSTQA Model–Predicted Probabilities |
No. | Functional Erections 2 y After Treatmentb |
||
|---|---|---|---|---|
| Mean PROSTQA Model–Predicted Probabilities |
Observed Proportion |
|||
| Prostatectomy | ||||
| 1st (0.001–0.030) | 216 | 0.01 | 0.04 | |
| 2nd (0.031–0.114) | 215 | 0.07 | 0.08 | |
| 3rd (0.115–0.246) | 215 | 0.18 | 0.20 | |
| 4th (0.247–0.403) | 219 | 0.32 | 0.36 | |
| 5th (0.404–0.760) | 212 | 0.52 | 0.48 | |
| All | 1077 | 0.22 | 0.23 | |
| External radiotherapy | ||||
| 1st (0.005–0.013) | 47 | 0.01 | 0.04 | |
| 2nd (0.014–0.031) | 50 | 0.02 | 0.00 | |
| 3rd (0.032–0.089) | 44 | 0.05 | 0.02 | |
| 4th (0.090–0.251) | 47 | 0.16 | 0.13 | |
| 5th (0.252–0.789) | 47 | 0.43 | 0.45 | |
| All | 235 | 0.13 | 0.15 | |
| Brachytherapy | ||||
| 1st (0.005–0.006) | 69 | 0.003 | 0.014 | |
| 2nd (0.007–0.035) | 68 | 0.02 | 0.04 | |
| 3rd (0.036–0.148) | 68 | 0.09 | 0.18 | |
| 4th (0.149–0.469) | 69 | 0.30 | 0.36 | |
| 5th (0.470–0.950) | 69 | 0.68 | 0.78 | |
| All | 343 | 0.22 | 0.27 | |
Abbreviations: CaPSURE, Cancer of the Prostate Strategic Urologic Research Endeavor; PROSTQA, Prostate Cancer Outcomes and Satisfaction With Treatment Quality Assessment.
Characteristics that differed between CaPSURE respondents and nonrespondents included race/ethnicity, relationship status, education, Gleason score at biopsy, and nerve sparing. Calibration was assessed within quintiles (fifths) of the distribution of model-predicted probabilities.
Individual model-predicted probabilities were calculated using the inverse logistic function {exp[X′ β]/[1 + exp(X′ β)]}, where X′ β is the sum with X representing individual characteristics observed and β representing the associated model parameter estimates for the individual characteristics in Table 1.
Use of Medications or Devices for Erectile Dysfunction
Prior to treatment, 6 men reported having a penile prosthesis (5 prostatectomy, 1 brachytherapy); among all other men, 269 of 1014 (27% [95% CI, 24%–29%]) reported having used medications or devices for erectile dysfunction (26% [95% CI, 22%–30%] in the prostatectomy group, 23% [95% CI, 18%–29%] in the external radiotherapy group, 31% [95% CI, 25%–37%] in the brachytherapy group). At 2 years, 14 men (9 prostatectomy, 1 external radiotherapy, 4 brachytherapy) reported having a penile prosthesis, and 53% (95% CI, 50%–56%) of all other men reported having used medications or devices for erectile dysfunction (66% [95% CI, 61%–70%] prostatectomy, 32% [95% CI, 26%–38%] external radiotherapy, 47% [95% CI, 41%–53%] brachytherapy), and 61% (95% CI, 57%–64%) of men who were potent prior to treatment reported having used any such aids at 2 years (69% [95% CI, 65%–74%] prostatectomy, 40% [95% CI, 31%–49%] external radiotherapy, 54% [95% CI, 46%–62%] brachytherapy) (Table 6).
Table 6.
Use and Reported Effectiveness of Medications and Devices for Erectile Dysfunction 2 Years After Primary Prostate Cancer Treatment Among 694 Men Who Had Reported Functional Erections Suitable for Intercourse Pretreatment in the PROSTQA Cohorta
| Medication or Device | Patient-Reported Effectiveness, No. (%)b | |||||
|---|---|---|---|---|---|---|
| Have Not Tried It |
Ineffective (Tried It But It Was Not Helpful) |
Effective | ||||
| I Use It Always |
I Use It Sometimes |
It Helped, But I Am Not Using It Now |
||||
| PDE-5 inhibitors | ||||||
| Prostatectomy (n = 412) | 131 (32) | 111 (27) | 51 (12) | 86 (21) | 33 (8) | |
| External radiotherapy (n = 120) | 72 (60) | 10 (8) | 11 (9) | 22 (18) | 5 (4) | |
| Brachytherapy (n = 162) | 75 (46) | 13 (8) | 29 (18) | 34 (21) | 11 (7) | |
| Overall (of 416 trying) | 134 (32) | 91 (22) | 142 (34) | 49 (12) | ||
| Intraurethral alprostadil | ||||||
| Prostatectomy (n = 412) | 351 (85) | 31 (8) | 5 (1) | 13 (3) | 12 (3) | |
| External radiotherapy (n = 120) | 115 (96) | 1 (1) | 1 (1) | 2 (2) | 0 | |
| Brachytherapy (n = 162) | 159 (98) | 1 (1) | 0 | 0 | 1 (1) | |
| Overall (of 67 trying) | 33 (49) | 6 (0) | 15 (22) | 13 (19) | ||
| Penile injections | ||||||
| Prostatectomy (n = 412) | 352 (86) | 16 (4) | 12 (3) | 10 (2) | 21 (5) | |
| External radiotherapy (n = 120) | 116 (97) | 1 (1) | 0 | 2 (2) | 1 (1) | |
| Brachytherapy (n = 162) | 159 (98) | 0 | 0 | 0 | 3 (2) | |
| Overall (of 66 trying) | 17 (26) | 12 (18) | 12 (18) | 25 (38) | ||
| Vacuum erection device | ||||||
| Prostatectomy (n = 412) | 332 (81) | 27 (7) | 12 (3) | 21 (5) | 20 (5) | |
| External radiotherapy (n = 120) | 115 (97) | 0 | 1 (1) | 1 (1) | 1 (1) | |
| Brachytherapy (n = 162) | 161 (99) | 0 | 0 | 0 | 1 (1) | |
| Overall (of 84 trying) | 27 (32) | 13 (15) | 22 (26) | 22 (26) | ||
Abbreviations: PDE-5, phosphodiesterase 5; PROSTQA, Prostate Cancer Outcomes and Satisfaction With Treatment Quality Assessment.
Use of medications or devices was measured by a previously described standard question set for use with the Expanded Prostate Cancer Index Composite.18 From among 694 men across all 3 treatment groups who reported erections suitable for intercourse at baseline, 328 reported erections suitable for intercourse at 2 years after treatment; 199 (61% [95% CI, 55%–66%]) of these 328 reported use of aids or medications for erectile dysfunction. Of the remaining 352 who reported erections not suitable for intercourse at 2 years, 218 (62% [95% CI, 57%–67%]) reported use of aids or medications for erectile dysfunction.
Values may not sum to total number of patients because of occasional missing values. Medications and devices considered effective if response was “I use it always,” “I use it sometimes,” or “It helped, but I am not using it now”; denominator for response rates is limited to patients reporting having tried the medication/device.
Among men who were potent prior to treatment, phosphodiesterase 5 inhibitors were tried most commonly (60% [95% CI, 56%–64%]), followed by vacuum erection devices (12% [95% CI, 10%–15%]); penile injections and intraurethral alprostadil were least commonly used (10% [95% CI, 7%–12%]) (Table 4). Of men trying each treatment, penile injections were most often reported as effective (74% [95% CI, 62%–84%] of men who tried them), followed by phosphodiesterase 5 inhibitors and vacuum erection devices (each effective among 68% [95% CI, 63%–72%] and 68% [95% CI, 57%–78%], respectively) and intraurethral alprostadil (effective among 51% [95% CI, 38%–63%]).
COMMENT
Erectile dysfunction is a well-recognized consequence of primary prostate cancer treatment. Accurate prediction of this adverse sexual HRQOL outcome is pivotal to set appropriate expectations and facilitate medical decision-making. However, the ability to inform individual patients how likely they are to develop erectile dysfunction based on their personal baseline sexual function, cancer severity, individual clinical characteristics, and treatment plan has been elusive. Our findings address this need by providing a validated, broadly applicable framework to predict the probability of long-term, posttreatment erectile dysfunction for individual patients.
Stratifying posttreatment outcome by pretreatment sexual HRQOL has been limited to a few single-institution studies.22,23 Our findings extend to the multicenter setting the observed relationship of pretreatment baseline sexual HRQOL with posttreatment outcome. We had initially described the PROSTQA cohort in a report that characterized the time course of mean HRQOL score changes across multiple HRQOL domains and identified factors broadly associated with such changes from pretreatment baseline to early follow-up.3 In the current study, we now expand on the prior report by developing models that predict erectile dysfunction based on individual factors; by extending the follow-up to focus on sexual outcome at a minimum of 2 years after treatment; by validating these predictive models in the external, community-based CaPSURE cohort to ascertain their generalizability; and by evaluating use of medications and devices by patients for erectile dysfunction. Moreover (and unlike in our prior report), the predictive models reported herein focus on a practical and clinically relevant dichotomous primary outcome—patient-reported ability of achieve erections firm enough for intercourse—based on response to question 26 on the EPIC-26 questionnaire, rather than evaluating total HRQOL score as the end point. Our approach herein does use sexual HRQOL scores to quantify baseline status, but we focus on the end point of erections “firm enough for intercourse” to provide a concrete metric having practical relevance to routine clinical care.17,24
The use and effectiveness of medications or devices for improving erections was previously limited to a few single-institution studies and a claims-based report.25–27 Medications and devices to assist with erectile function were used by slightly more than one-half of men in our study and were used more commonly among patients after prostatectomy, as was also noted in 2 single-institution studies.18,27 Phosphodiesterase 5 inhibitors were the most commonly used treatment for erectile dysfunction. Intracorporal penile injections were the most effective (helpful in 74% of those who tried them) but were the least used, perhaps owing to inconvenience or discomfort. The sparse use of mechanical devices (eg, vacuum erection device), particularly after external radiotherapy, suggests an underused approach to mitigating erectile dysfunction among prostate cancer survivors.
Our observation that baseline PSA level is associated with erectile function outcome after prostatectomy or external radiotherapy has not been previously described. Patients with higher PSA levels may have more extensive primary cancers or larger prostates that can affect surgical approach, even among those undergoing nerve-sparing surgery; 1 single-institution study showed a trend for inverse association of PSA level and erectile function following radical prostatectomy (univariable P=.06) and a significant association of lower PSA level with greater nerve preservation (P < .001).12 Prior studies have linked larger prostate size with worse postoperative erectile function, and larger prostate size is associated with higher serum PSA levels; we did observe a marginal association of prostate size with sexual outcome.28–31 Higher PSA levels can reflect greater cancer severity that may temper the extent of nerve sparing during prostatectomy or lead to broader distribution of higher radiotherapy doses during treatment planning. The concurrent association of larger prostate size as well as of greater cancer severity with higher serum PSA level may be the basis for the association that we observed between pretreatment PSA levels and posttreatment sexual outcome.
Although our study revealed poorer recovery of erectile function with increasing number of comorbid conditions in univariable analyses, these were not significant on multivariate analysis. Other researchers have found diabetes and peripheral vascular disease to be associated with worse posttreatment sexual outcome; however, those studies did not adjust for differences in pretreatment sexual function.32 The lack of significance of comorbid conditions in our multivariable analyses may be attributable, in part, to comorbid conditions influencing the sexual HRQOL score at baseline (comorbidity in the PROSTQA cohort was significantly correlated with pretreatment sexual HRQOL score; Spearman r=−0.31), whereby baseline sexual HRQOL score effect may supercede concurrent effects of comorbidity on posttreatment sexual outcome.
Consistent with prior reports, expected benefits of nerve sparing during prostatectomy10,13,33,34 and detriments associated with use of adjuvant androgen-suppressive therapy during radiation35 were observed in our study, wherein the nerve-sparing benefit was extended to an intent-to-treat analysis. Our predictive models further extend the characterization of these treatment modifications by indicating how their effects can be mitigated by other factors, such as poor sexual functioning at baseline (as reflected by lower EPIC-26 sexual HRQOL score) or high pretreatment PSA level. Some features of the predictive models have broad confidence intervals because of relatively small numbers, but our internal assessment of model overfitting confirmed robustness of the contribution of the factors, and consideration of clinical relevance (eg, nerve-sparing vs non– nerve-sparing surgical techniques) maintained the factors in the models.
Of interest, the models were more accurate in predicting erections following external radiotherapy than those following prostatectomy (AUCs of 0.77 [95% CI, 0.74–0.80] for prostatectomy, 0.87 [95% CI, 0.80–0.94] for external radiotherapy, and 0.90 [95% CI, 0.85–0.95] for brachytherapy). In an exploratory analysis, we did not detect any association between prostatectomy volume at individual centers and sexual HRQOL outcome (correlation r = 0.04, P=.17), suggesting that treatment proficiency is not attributable simply to individual center treatment volume. Whether factors such as surgeon proficiency or unmeasured factors (eg, variations in specific surgical techniques) contribute to the broader range of outcomes we observed after prostatectomy warrants further study.
Limitations of our study are related to its observational design, introducing the possibility of selection bias by treatment. Therefore, our predictions of erectile function are best suited to guide outcome expectations within treatment groups based on individual patient characteristics and do not provide conclusive evidence of treatment superiority. Our focus on a time of 2 years after treatment for these analyses does not discern effects of baseline factors on the dynamics of erection recovery (eg, after prostatectomy) or deterioration (eg, after radiotherapy); however, we selected this point as a focal point of long-term outcomes based on prospective studies suggesting relative stabilization of sexual HRQOL changes at 2 to 3 years following treatment, although there may be some potential for continued improvement and deterioration of sexual function following prostatectomy and radiotherapy, respectively, even after 2 years.36,37
The HRQOL instruments used to measure baseline sexual function in the development cohort (EPIC-26) and validation cohort (UCLA-PCI) were not identical; nevertheless, both instruments contain the question regarding whether erections are firm enough for intercourse, which is the principal end point of the predictive models, and correlation of sexual function scores between the EPIC-26 and the UCLAPCI have been shown to be highly correlated when both of these questionnaires were administered to the same patients in other studies.38,39 Measuring sexual HRQOL at the point of care with the EPIC-26 may be cumbersome and could impede use of our findings in routine practice. To address this barrier (separately from this study), we have developed the EPIC for Clinical Practice, a one-page HRQOL questionnaire that can be completed in 5 minutes and allows HRQOL scores to be easily calculated by clinicians at the point of care.40
Last, our study did not evaluate the usage or possible effects of erectile rehabilitation regimens, nor did the models control for use of medications or devices (that we instead reported as a concurrent consequence of erectile dysfunction). Nevertheless, our model validation in the external PROSTQA cohort indicates that this predictive model is generalizable despite these limitations.
We have developed clinically applicable models to predict recovery of erectile function following prostatectomy, external radiotherapy, or brachytherapy for early-stage prostate cancer based on pretreatment sexual function, patient characteristics, and specific plan of treatment. External validation of this predictive model in a community-based cohort suggests that these findings are generalizable and may help physicians and patients to set personalized expectations regarding prospects for erectile function in the years following primary treatment for prostate cancer.
Acknowledgments
Funding/Support: The study was supported by NIH grants R01 CA 95662 and 1RC1CA146596
Role of Sponsor: The NIH had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Additional Contributions: We acknowledge the help of Jill Hardy, MS (Michigan State University), Beth Doiron, BA (Beth Israel Deaconess Medical Center), and Catrina Crociani, MPH (Beth Israel Deaconess Medical Center), for project management and of Alan Paciorek, BS (University of California, San Francisco), for analytic support. The individuals and the coordinators at each clinical site participated as research study staff but were not compensated specifically for this manuscript.
Footnotes
Author Contributions: Dr Sanda had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Alemozaffar and Regan contributed equally as first authors. Drs Cooperberg and Wei contributed equally as second authors. Drs Carroll and Sanda shared senior authorship.
Study concept and design: Alemozaffar, Regan, Wei, Michalski, Hembroff, Saigal, Litwin, Klein, Wood, Carroll, Sanda.
Acquisition of data: Cooperberg, Wei, Michalski, Sandler, Hembroff, Saigal, Litwin, Klein, Kibel, Hamstra, Pisters, Kuban, Kaplan, Wood, Ciezki, Carroll, Sanda.
Analysis and interpretation of data: Regan, Cooperberg, Michalski, Sandler, Sadetsky, Saigal, Litwin, Klein, Pisters, Wood, Dunn, Carroll, Sanda.
Drafting of the manuscript: Alemozaffar, Regan, Cooperberg, Hamstra, Pisters, Kaplan, Sanda.
Critical revision of the manuscript for important intellectual content: Regan, Cooperberg, Wei, Michalski, Sandler, Hembroff, Sadetsky, Saigal, Litwin, Klein, Kibel, Hamstra, Pisters, Kuban, Kaplan, Wood, Ciezki, Dunn, Carroll, Sanda.
Statistical analysis: Alemozaffar, Regan, Sadetsky, Dunn.
Obtained funding: Saigal, Kibel, Carroll, Sanda.
Administrative, technical, or material support: Wei, Michalski, Hembroff, Saigal, Litwin, Klein, Kibel, Hamstra, Pisters, Wood, Ciezki, Carroll, Sanda.
Study supervision: Hembroff, Kuban, Carroll, Sanda.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. None of the coauthors have been compensated specifically for work related to this manuscript other than having received research support from the National Institutes of Health (NIH) to cover costs of conducting the study. Dr Cooperberg reported serving as a consultant for Denreon and Ortho-Centocor and receiving payment for lectures from Takeda, Abbott, and Amgen. Dr Wei reported serving as a consultant for Envisioneering Inc and sanofi-aventis; serving as an expert witness for Genprobe; serving as a proctor for American Medical Systems; and receiving research funding from sanofiaventis. Dr Michaliski reported making donations to charity in lieu of honoraria for his role as a board member for Elekta Inc, Augmenix Inc, and Viewray. Dr Sandler reported serving as a consultant for Varian, Calypso Medical, Millennium-Takeda, and Centocor-Ortho Biotech. Dr Kibel reported serving as a consultant for Myraid, Caris, Dendreon, sanofi-aventis, Cougar, and Ferring; receiving payment for lectures from Dendreon; and holding stock in Myriad. Dr Kuban serving as a consultant for Ferring Pharmaceuticals and bioTheranostics and as a lecturer supported by the Radiation Oncology Institute. Dr Wood reported serving as a consultant for Intuitive Surgical and holding stock in that company. Dr Carroll reported receiving an institutional research grant from Abbott. Dr Sanda reported receiving a lecture honnorarium from Eli Lily. No other authors reported financial disclosures.
Online Only Material: eTables 1–4 and Author Interview are available at http://www.jama.com.
REFERENCES
- 1.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman RM, Hunt WC, Gilliland FD, Stephenson RA, Potosky AL. Patient satisfaction with treatment decisions for clinically localized prostate carcinoma: results from the Prostate Cancer Outcomes Study. Cancer. 2003;97(7):1653–1662. doi: 10.1002/cncr.11233. [DOI] [PubMed] [Google Scholar]
- 3.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 4.Ferrer M, Suárez JF, Guedea F, et al. Multicentric Spanish Group of Clinically Localized Prostate Cancer. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72(2):421–432. doi: 10.1016/j.ijrobp.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Madalinska JB, Essink-Bot ML, de Koning HJ, Kirkels WJ, van der Maas PJ, Schröder FH. Health-related quality-of-life effects of radical prostatectomy and primary radiotherapy for screen-detected or clinically diagnosed localized prostate cancer. J Clin Oncol. 2001;19(6):1619–1628. doi: 10.1200/JCO.2001.19.6.1619. [DOI] [PubMed] [Google Scholar]
- 6.Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92(19):1582–1592. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 7.Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273(2):129–135. doi: 10.1001/jama.273.2.129. [DOI] [PubMed] [Google Scholar]
- 8.Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ. 2009;339:b4817. doi: 10.1136/bmj.b4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu AK, Cooperberg MR, Sadetsky N, Carroll PR. Health related quality of life in patients treated with multimodal therapy for prostate cancer. J Urol. 2008;180(6):2415–2422. doi: 10.1016/j.juro.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Quinlan DM, Epstein JI, Carter BS, Walsh PC. Sexual function following radical prostatectomy: influence of preservation of neurovascular bundles. J Urol. 1991;145(5):998–1002. doi: 10.1016/s0022-5347(17)38512-9. [DOI] [PubMed] [Google Scholar]
- 11.Briganti A, Capitanio U, Chun FK, et al. Prediction of sexual function after radical prostatectomy. Cancer. 2009;115(13) suppl:3150–3159. doi: 10.1002/cncr.24349. [DOI] [PubMed] [Google Scholar]
- 12.Rabbani F, Stapleton AM, Kattan MW, Wheeler TM, Scardino PT. Factors predicting recovery of erections after radical prostatectomy. J Urol. 2000;164(6):1929–1934. [PubMed] [Google Scholar]
- 13.Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283(3):354–360. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 14.Nelson CJ, Choi JM, Mulhall JP, Roth AJ. Determinants of sexual satisfaction in men with prostate cancer. J Sex Med. 2007;4(5):1422–1427. doi: 10.1111/j.1743-6109.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 15.Talcott JA, Manola J, Clark JA, et al. Time course and predictors of symptoms after primary prostate cancer therapy. J Clin Oncol. 2003;21(21):3979–3986. doi: 10.1200/JCO.2003.01.199. [DOI] [PubMed] [Google Scholar]
- 16.Miller DC, Gruber SB, Hollenbeck BK, Montie JE, Wei JT. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst. 2006;98(16):1134–1141. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 17.Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology. 2010;76(5):1245–1250. doi: 10.1016/j.urology.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller DC, Wei JT, Dunn RL, et al. Use of medications or devices for erectile dysfunction among long-term prostate cancer treatment survivors: potential influence of sexual motivation and/or indifference. Urology. 2006;68(1):166–171. doi: 10.1016/j.urology.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 19.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36(7):1002–1012. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Lubeck DP, Litwin MS, Henning JM. CaPSURE Research Panel. The CaPSURE database: a methodology for clinical practice and research in prostate cancer. Urology. 1996;48(5):773–777. doi: 10.1016/s0090-4295(96)00226-9. [DOI] [PubMed] [Google Scholar]
- 21.Cooperberg MR, Broering JM, Litwin MS. CaPSURE Investigators. The contemporary management of prostate cancer in the United States: lessons from the Cancer of the prostate Strategic Urologic Research Endeavor (CapSURE), a national disease registry. J Urol. 2004;171(4):1393–1401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 22.Giberti C, Chiono L, Gallo F, Schenone M, Gastaldi E. Radical retropubic prostatectomy versus brachytherapy for low-risk prostatic cancer: a prospective study. World J Urol. 2009;27(5):607–612. doi: 10.1007/s00345-009-0418-9. [DOI] [PubMed] [Google Scholar]
- 23.Chen RC, Clark JA, Talcott JA. Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J Clin Oncol. 2009;27(24):3916–3922. doi: 10.1200/JCO.2008.18.6486. [DOI] [PubMed] [Google Scholar]
- 24.Krupski TL, Saigal CS, Litwin MS. Variation in continence and potency by definition. J Urol. 2003;170(4, pt 1):1291–1294. doi: 10.1097/01.ju.0000085341.63407.46. [DOI] [PubMed] [Google Scholar]
- 25.Schover LR, Fouladi RT, Warneke CL, et al. The use of treatments for erectile dysfunction among survivors of prostate carcinoma. Cancer. 2002;95(11):2397–2407. doi: 10.1002/cncr.10970. [DOI] [PubMed] [Google Scholar]
- 26.Prasad MM, Prasad SM, Hevelone ND, et al. Utilization of pharmacotherapy for erectile dysfunction following treatment for prostate cancer. J Sex Med. 2010;7(3):1062–1073. doi: 10.1111/j.1743-6109.2009.01644.x. [DOI] [PubMed] [Google Scholar]
- 27.Bergman J, Gore JL, Penson DF, Kwan L, Litwin MS. Erectile aid use by men treated for localized prostate cancer. J Urol. 2009;182(2):649–654. doi: 10.1016/j.juro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Hu JC, Elkin EP, Pasta DJ, et al. Predicting quality of life after radical prostatectomy: results from CaPSURE. J Urol. 2004;171(2, pt 1):703–708. doi: 10.1097/01.ju.0000107964.61300.f6. [DOI] [PubMed] [Google Scholar]
- 29.Hollenbeck BK, Dunn RL, Wei JT, Montie JE, Sanda MG. Determinants of long-term sexual health outcome after radical prostatectomy measured by a validated instrument. J Urol. 2003;169(4):1453–1457. doi: 10.1097/01.ju.0000056737.40872.56. [DOI] [PubMed] [Google Scholar]
- 30.Pinkawa M, Fischedick K, Asadpour B, et al. Toxicity profile with a large prostate volume after external beam radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):83–89. doi: 10.1016/j.ijrobp.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 31.Ahlering TE, Kaplan AG, Yee DS, Skarecky DW. Prostate weight and early potency in robot-assisted radical prostatectomy. Urology. 2008;72(6):1263–1268. doi: 10.1016/j.urology.2008.05.055. [DOI] [PubMed] [Google Scholar]
- 32.Karakiewicz PI, Bhojani N, Neugut A, et al. The effect of comorbidity and socioeconomic status on sexual and urinary function and on general health-related quality of life in men treated with radical prostatectomy for localized prostate cancer. J Sex Med. 2008;5(4):919–927. doi: 10.1111/j.1743-6109.2007.00741.x. [DOI] [PubMed] [Google Scholar]
- 33.Ayyathurai R, Manoharan M, Nieder AM, Kava B, Soloway MS. Factors affecting erectile function after radical retropubic prostatectomy: results from 1620 consecutive patients. BJU Int. 2008;101(7):833–836. doi: 10.1111/j.1464-410X.2007.07409.x. [DOI] [PubMed] [Google Scholar]
- 34.Michl UH, Friedrich MG, Graefen M, Haese A, Heinzer H, Huland H. Prediction of postoperative sexual function after nerve sparing radical retropubic prostatectomy. J Urol. 2006;176(1):227–231. doi: 10.1016/S0022-5347(06)00632-X. [DOI] [PubMed] [Google Scholar]
- 35.Bolla M, de Reijke TM, Van Tienhoven G. EORTC Radiation Oncology Group and Genito-Urinary Tract Cancer Group. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 36.Penson DF, McLerran D, Feng Z, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol. 2005;173(5):1701–1705. doi: 10.1097/01.ju.0000154637.38262.3a. [DOI] [PubMed] [Google Scholar]
- 37.Gore JL, Kwan L, Lee SP, Reiter RE, Litwin MS. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009;101(12):888–892. doi: 10.1093/jnci/djp114. [DOI] [PubMed] [Google Scholar]
- 38.Schroeck FR, Donatucci CF, Smathers EC, et al. Defining potency: a comparison of the International Index of Erectile Function short version and the Expanded Prostate Cancer Index Composite. Cancer. 2008;113(10):2687–2694. doi: 10.1002/cncr.23887. [DOI] [PubMed] [Google Scholar]
- 39.Levinson AW, Ward NT, Sanda MG, et al. Comparison of validated instruments measuring sexual function in men. Urology. 2010;76(2):380–386. doi: 10.1016/j.urology.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 40.Chang P, Szymanski KM, Dunn RL, et al. Expanded Prostate Cancer Index Composite for clinical practice: development and validation of a practical health related quality of life instrument for use in the routine clinical care of patients with prostate cancer. J Urol. doi: 10.1016/j.juro.2011.04.085. [published online ahead of print July 23, 2011]. [DOI] [PMC free article] [PubMed] [Google Scholar]