Summary
High expression of the transcriptional activator ComK occurs in 10–20% of the cells in stationary phase cultures of Bacillus subtilis strain 168. ComK drives the expression of more than 100 genes constituting the semidormant K-state, distinct from sporulation and vegetative growth. Among the genes so activated are those that permit competence for genetic transformation. We have addressed the origin of bistability in expression of ComK. We show that bistability requires positive autoregulation at the promoter of comK, but not a potential toggle switch, in which ComK represses the promoter of rok and Rok represses the promoter of comK. We further address the source of the noise that results in the stochastic selection of cells that will express comK. A revised model for the regulation of comK expression is proposed that partially explains bistability.
Introduction
Phenotypic population heterogeneity and, in some cases, cell fate may be viewed as noise-driven processes (reviewed in Rao et al., 2002). The soil bacterium Bacillus subtilis responds to environmental stress with an arsenal of survival strategies, some of which are expressed heterogeneously and are likely to be probabilistically invoked. B. subtilis can become motile, secrete degradative enzymes or antibiotics, produce spores or become competent for genetic transformation. The heterogeneity during competence development will be considered in this report.
In B. subtilis, competence is part of a physiological state, the K-state (Berka et al., 2002), distinct from vegetative growth and sporulation, that develops during late exponential growth under specific nutritional conditions. Remarkably, competence is expressed in only 10–20% of the cells in laboratory strains (Nester and Stocker, 1963; Hadden and Nester, 1968; Haseltine-Cahn and Fox, 1968). Transcriptional profiling has revealed that well over 100 genes in this distinct subpopulation, many of which have no obvious role in transformation, are induced concomitantly with the development of transformability (Berka et al., 2002; Hamoen et al., 2002; Ogura et al., 2002). It has been proposed that the competent cells have embarked on a survival strategy, of which transformability is only one feature. This competence-associated survival mode has been called the K-state (Berka et al., 2002), because it depends on the synthesis of the master regulator, ComK (van Sinderen et al., 1995). As there are two clearly distinguishable cell types, competent and non-competent, we find it useful to describe this system as exhibiting bistability. This is not a mathematically rigorous usage, in part because the expression of competence occurs during the transition to stationary phase when the system is not at steady state. However, the term ‘bistability’ is a useful approximation, leading to the consideration of mechanisms that have been invoked elsewhere to explain true bistability.
Theoretical studies have described two such regulatory arrangements that can exhibit bistability (reviewed in Ferrell, 2002). In the first, cell-signalling systems containing positive feedback loops together with a cooperative (nonlinear) response to an activator can exhibit noise-driven bistability. Only those random cells with higher than average concentrations of the activator initiate the loop. In the second arrangement, a system containing two mutually repressing negative feedback loops can lead to population heterogeneity. In this case, random cells with a high concentration of the first repressor, will have a decreased amount of the second one, leading to the expression of a set of target genes. Artificial bistable systems of these two types have been engineered in Escherichia coli and Saccharomyces cerevisiae and their properties have been explored (Gardner et al., 2000; Becskei et al., 2001). In fact, a naturally occurring bistable system involving a positive feedback loop was studied nearly 50 years ago in E. coli (Novick and Weiner, 1957).
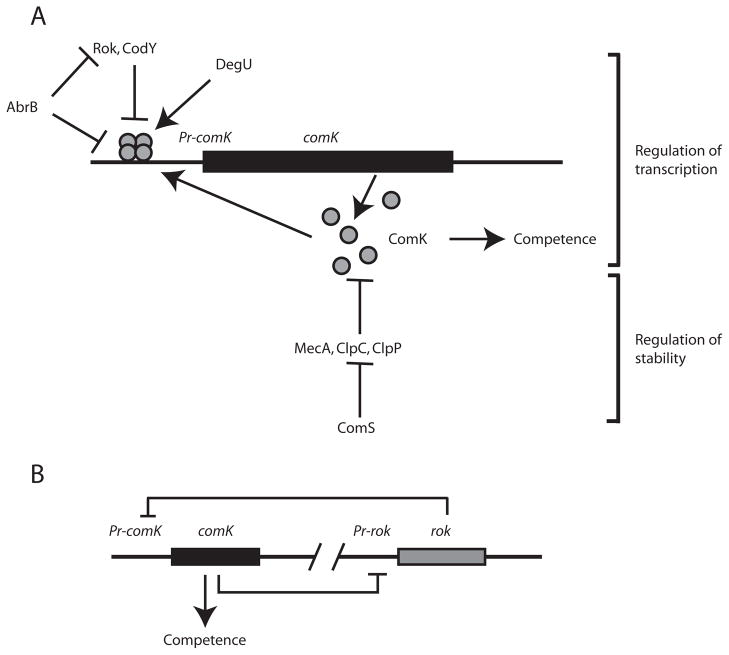
In B. subtilis, genes encoding proteins required for the binding and uptake of DNA are transcribed in the presence of the positively acting transcription factor ComK (van Sinderen et al., 1995), which is synthesized only in the competent (K-state) fraction of cells (van Sinderen and Venema, 1994; Haijema et al., 2001). ComK synthesis, in turn, is regulated by a complex series of reactions that involve quorumsensing, as well as controls at the transcriptional and post-transcriptional levels (summarized in Fig. 1A, reviewed in Hamoen et al., 2003a). ComK is required for the transcription of its own gene and binds to the promoter of comK (PcomK) as a dimer of dimers (Hamoen et al., 1998). The system therefore contains positive feedback and involves a non-linear response at the promoter of comK.
Fig. 1.
Regulation of competence.
A. Summary of competence regulation. Arrows and perpendiculars represent positive and negative regulation respectively. ComK binds to its promoter as a dimer of dimers.
B. Illustration of the putative toggle switch, in which ComK represses the transcription of rok and Rok represses the transcription of comK.
Additional proteins bind to the promoter of comK and act as activators or repressors. DegU increases the affinity of ComK for PcomK (Hamoen et al., 2000), whereas AbrB, CodY and Rok bind to PcomK and act as repressors (Serror and Sonenshein, 1996; Hoa et al., 2002; Hamoen et al., 2003b). AbrB must be maintained within a narrow window of concentrations (Hahn et al., 1995a), as it acts to repress both the promoters of comK and rok (Hoa et al., 2002). In addition to this transcriptional regulation, the stability of ComK is precisely controlled (Turgay et al., 1997; Turgay et al., 1998). Prior to the onset of stationary phase, ComK is bound by the adapter protein MecA, which targets it for degradation by the ClpC-ClpP protease. As cell density increases during growth and the cells approach stationary phase, a process initiated by quorum-sensing (Solomon et al., 1995) leads to the synthesis of the small protein ComS, which binds to MecA, preventing the targeting of ComK for degradation.
As noted in this brief summary, a positive feedback loop exhibiting a non-linear response to the cellular concentration of ComK is central to competence regulation. The essential features of a bistable system of the first type are therefore present. However, the features required for a toggle switch mechanism are also apparently present. It has been shown that Rok represses PcomK and that ComK represses Prok (Hoa et al., 2002). This arrangement hints at the possible existence of a bistable system of the second type (Fig. 1B). In this model, cells with higher concentrations of ComK would repress rok expression and therefore produce even more ComK. When a threshold is passed, this situation would become ‘irreversible’ and the cells would enter the competent state. Conversely, cells with less ComK would express more rok and remain in the non-competent state.
Although these qualitative features are suggestive, their function in the cell depends on the relevant binding and rate constants, on the concentrations of active proteins and on the cell-to-cell variations (noise) in these concentrations. In other words, the mere presence of reciprocally acting repressors or of a positive feedback loop with nonlinearity does not prove that these features are responsible for bistability, and an experimental approach to decide this question is required.
Here we show that ComK autoregulation is necessary for bistability. We also show that the mutually repressing Rok–ComK interactions are probably not major factors in establishing bistability. Finally, we discuss the possible sources of the noise that presumably determines which cells express the K-state. A similar study has independently concluded that the ComK autoregulatory loop is needed for bistability (Smits et al., 2005).
Results
Bistability in competence development
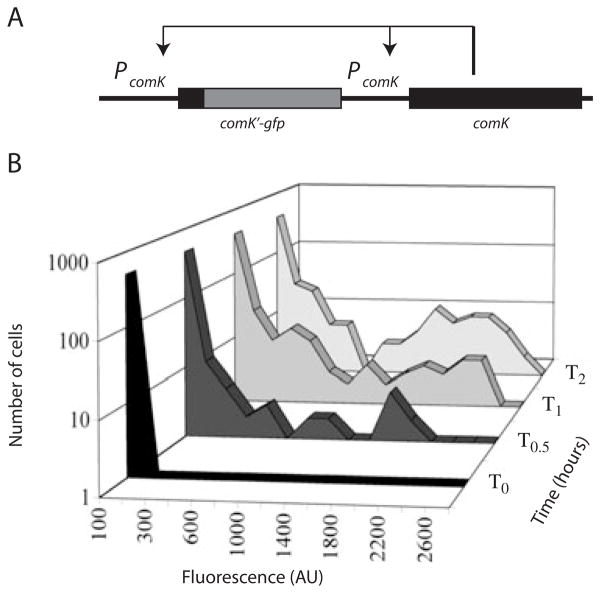
To study the heterogeneity of cultures expressing the K-state, we utilized an in frame fusion of the comK promoter, ribosomal binding site and first seven amino acid residues to the green fluorescent protein (GFP). Transcription from this promoter is completely dependent on the binding of ComK itself (van Sinderen and Venema, 1994). Hence, the fluorescence signals exhibited by cells containing this fusion provide a readout for the level of active ComK and of the K-state, as this transcription factor is both necessary and sufficient for induction of K-state genes (Hahn et al., 1996). We have shown elsewhere that the cells expressing comK-gfp are the ones expressing the competence protein comEA (Haijema et al., 2001) and are also the cells binding and internalizing DNA (J. Hahn, B. Maier and D. Dubnau, unpubl.). For the experiment shown in Fig. 2, which illustrates bistability, the comK-gfp fusion was placed in the comK locus by Campbell-like recombination, placing both the fusion and an intact copy of comK under control of the native promoter (Fig. 2A). In this strain (BD2711, Haijema et al., 2001), all the other relevant regulatory genes remain intact.
Fig. 2.
Bistability during the development of competence.
A. The construction (BD2711) used to demonstrate bistability. The arrows represent positive regulation.
B. The frequency distributions of fluorescence intensities in cells carrying comK-gfp (strain BD2711) are plotted at various times during the development of competence. T0 refers to the time of departure from exponential growth. At each time point, the fluorescent intensities of 500 cells were evaluated.
At T0, the time of transition to stationary phase, nearly all of the cells in the culture exhibited near background levels of fluorescence (Fig. 2B), characteristic of strains lacking a GFP reporter. At 30 and 60 min following the end of exponential growth, a number of cells with intermediate levels of fluorescence were evident (Fig. 2B), although the high-expressing class is also clearly represented. Many of these are likely to be cells that are approaching the maximal expression level. Later, at T2, the cellular distribution of fluorescent intensities was clearly bimodal, with 11% of the cells in a high-expressing subpopulation. In several independent experiments using this strain, the total high-expressing class consisted of 10–20% of the total population. Within the lower intensity class of cells at T2, a fraction (about 7.5% of the total) exhibited fluorescence higher than the background (Fig. 2B). This fraction may consist of cells that will go on to enter the high-expressing class, which would then comprise about 18.5% of the total population. It is also possible that these cells represent a distinct subgroup and that the system is more complex than a two-state model would assume.
This experiment demonstrates that the expression of comK, and hence of the K-state, is bistable and that ComK-GFP fluorescence provides a suitable readout for the study of bistability.
Positive autoregulation of comK is essential for bistability
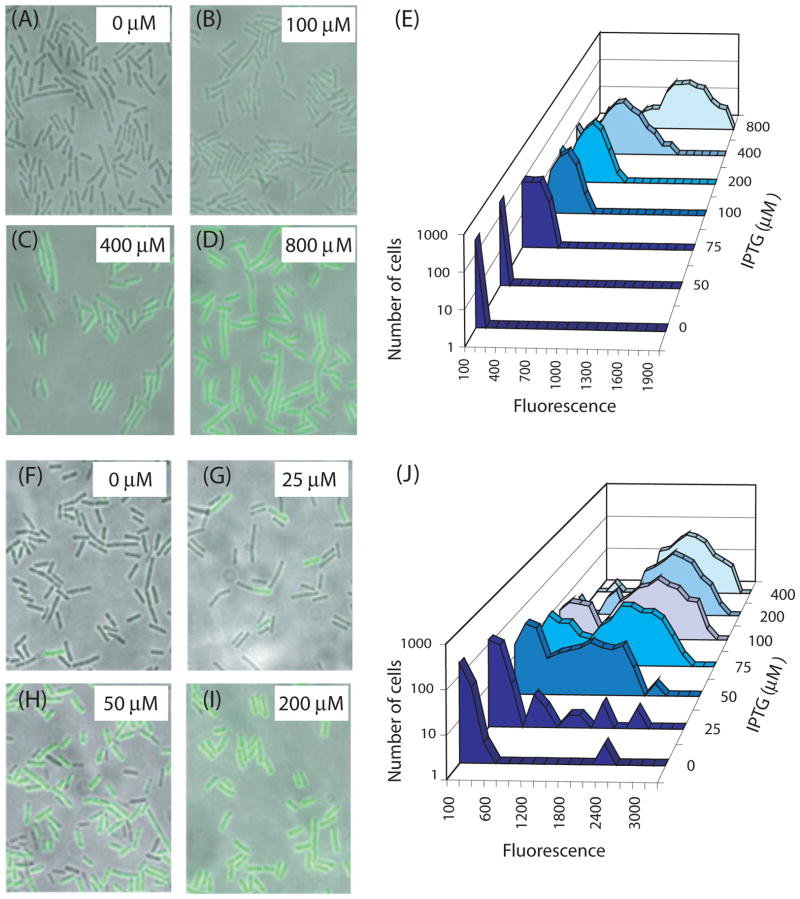
We next asked if the positive autoregulatory loop is essential for bistability. For this, we placed a copy of comK under control of the Phyperspank (Phs) promoter, at the ectopic amyE locus. This promoter (kindly provided by Dr David Rudner, Harvard Medical School, Boston, MA), is inducible by isopropyl-β-D-thiogalactoside (IPTG) and exhibits stronger induction and less leakiness than previous versions of IPTG-inducible promoters for use in B. subtilis. At the comK locus, we placed two different constructs. In the first (Fig. 3A), an intact copy of comK driven by its native promoter was present, together with the same comK-gfp fusion used for BD2711 (Fig. 2A). This strain retains a positive feedback loop, because expression of the comK copy at the native locus activates its own transcription. The second strain (Fig. 3B) was isogenic with this one, but the copy of comK at the native site was inactivated by insertion of a kanamycin-resistance cassette. This strain does not exhibit positive auto-regulation as it lacks an intact copy of comK driven by the promoter of comK. As a copy of comK under Phs control is present in both strains, it is possible to prime the system by the addition of the inducer, IPTG. Most important, the use of this inducible promoter provided active ComK in the absence of autoregulation. In the strain that retains the positive feedback loop, growth to T2 in the absence of IPTG permits the expression of GFP fluorescence in 16–18% of the cells, demonstrating that the normal regulatory machinery remains intact and functional (not shown).
Fig. 3.
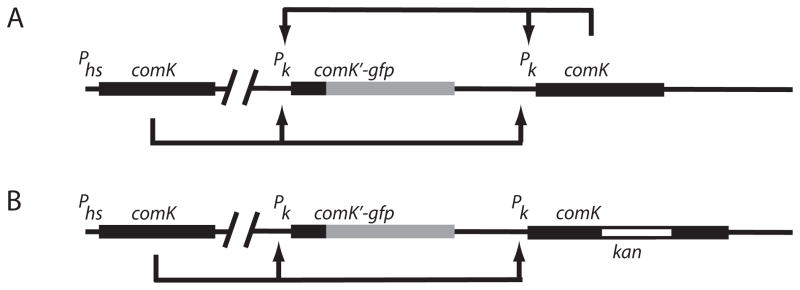
Constructs used to test the requirement for the autoregulatory loop for bistability. The arrowheads indicate positive regulation.
A. Construct containing the loop (strain BD4010).
B. Construct lacking the loop (strain BD4011).
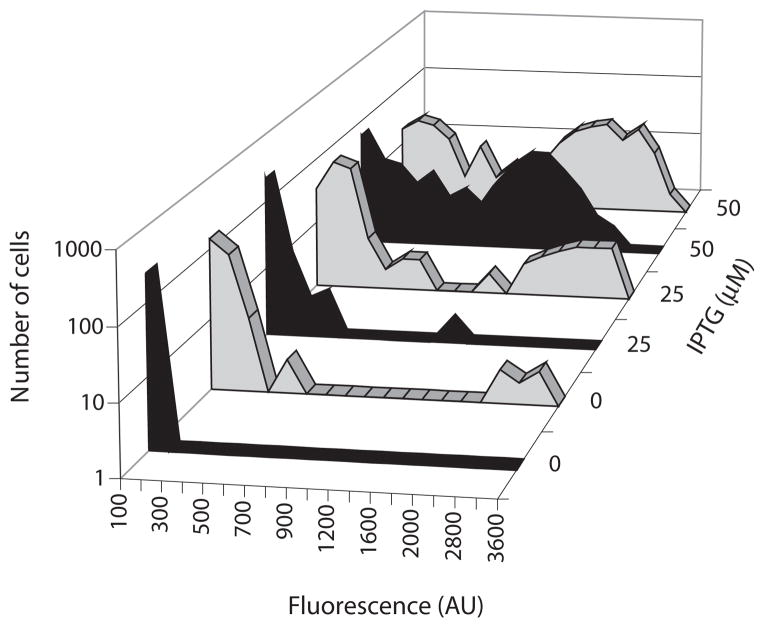
Figure 4 presents the results of a typical experiment in which the two strains were induced during logarithmic growth with varying concentrations of IPTG and harvested for microscopy 30 min later, prior to T0. At this time, in the wild-type strain (BD2711), no fluorescent cells were detectable (Fig. 2B), demonstrating that the fluorescent signals presented in Fig. 4 reflect the presence of the Phs–comK construct. The upper panels (Fig. 4A–D) show superimposed fluorescent and bright field images of cells from the control strain that lacks the positive loop, as well as frequency distributions of fluorescent intensities in the population (Fig. 4E). At intermediate IPTG concentrations (Figs 4B and C), nearly all of the cells were fluorescent, with intensities that were intermediate between those of the uninduced cells and those induced with higher IPTG concentrations. In other words, as the concentration of IPTG was increased, the cells responded in a graded manner, exhibiting a unimodal population response.
Fig. 4.
Distributions of ComK-GFP in cells without (A–E, strain BD4011) and with (F–J, strain BD4010) the ComK autoregulatory loop. The representative micrographs consist of fluorescent images overlaid with bright field images. All of the micrographs were recorded just before T0. In panel F, the arrow points to a rare cell expressing comK-gfp. The concentrations of IPTG used for each panel are indicated. For each IPTG concentration, the fluorescent intensities of 250 cells were determined.
When the autoregulatory loop was intact however, a strikingly different pattern was evident. Most notably, the cells responded in a distinctly bimodal fashion, qualitatively similar to the wild-type response shown in Fig. 2B. It is also evident that this strain exhibited a hypersensitive response to IPTG, compared to the control lacking the autoregulatory loop. In the absence of IPTG, occasional cells with near maximal fluorescence signals were observed and these were not seen in the control strain without the loop. These fluorescent cells may be seen both in the representative micrograph (Fig. 4F) as well as in the frequency distribution (Fig. 4J). In fact, 1.2% of the cells exhibited a near maximal level of fluorescence in the absence of IPTG. This expression may be as a result of the slight leakiness of the Phs promoter. We conclude from these observations that K-state bistability requires feedback regulation of comK. A similar conclusion, regarding the need for auto-stimulation at the promoter of comK, has been reached independently (Smits et al., 2005). We cannot conclude, however, that the positive autoregulatory loop rather than a toggle switch is at work, because the control construct depicted in Fig. 3B also eliminates the toggle switch configuration shown in Fig. 1B. This is because Rok presumably cannot repress at Phs.
Influence of regulatory factor mutations on K-state development. Is the toggle switch required?
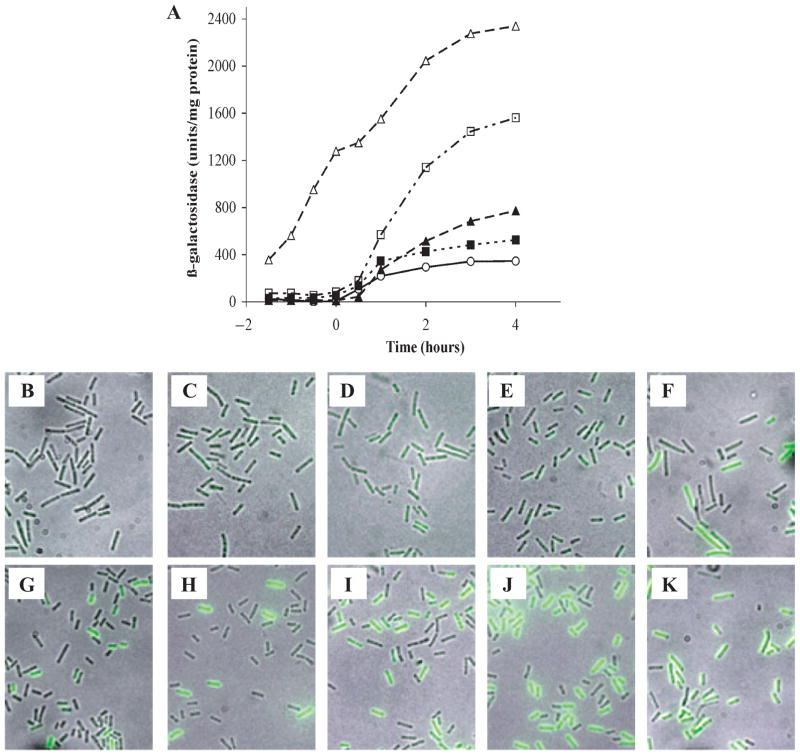
To gain further insight into the roles of various regulatory genes in the regulation of comK, and to test whether the toggle switch plays a role in bistability, we have introduced knockout mutations in regulatory genes and determined their effects on comK expression. For measurements in bulk cultures, we used a comK–lacZ reporter, inserted ectopically at amyE. To determine the effect on bistability, we utilized the comK–gfp fusion described above, in the strain BD2711 background (Fig. 2A). Figure 5A presents the results of β-galactosidase measurements in samples withdrawn during growth. Inactivation of the repressor genes (codY or rok) had little or no effect prior to T0, but increased the final level of β-galactosidase in the culture. Even a double codY rok mutant did not result in the premature expression of comK–lacZ, although it further increased the plateau level of β-galactosidase expression (T.T. Hoa and D. Dubnau, unpubl.). Overexpression of degU on a multicopy plasmid had little or no effect on the plateau level, again without any detectable effect before T0. These results were entirely confirmed using the comK–gfp fusion; representative microscopic fields are shown in Fig. 5B–E and G–J. At T2, in a typical experiment, the fractions of cells expressing ComK-GFP in the wild-type, rok, codY and degU overexpressing strains were 10 ± 2%, 60± 11%, 41 ± 18% and 10 ± 4% respectively.
Fig. 5.
Effect of competence regulatory mutations on the expression of comK-lacZ (A) and of comK-gfp (B–K). In A, the following strains are shown: wild-type (○; strain BD1991), multicopy degU (■; strain BD4017), codY (▲; strain BD2607), rok (□; strain BD4016) and multicopy comS (△; strain BD4018). B–F exhibit cells taken at T0 and G–K exhibit cells taken at T2. The strains used were wild-type for regulatory genes (B, G; strain BD2711), carried a multicopy plasmid with degU (C, H; strain BD4013), were codY (D, I; strain BD4014), rok (E, J; strain BD4012) or carried a multicopy plasmid with comS (F, K; strain BD4015).
A markedly different result was obtained when comS was overexpressed on a multicopy plasmid (Fig. 5A and Hahn et al., 1995b). In this case the timing of expression was dramatically altered, with substantial β-galactosidase activity and frequent fluorescent cells detected before T0 (Fig. 5F). At T−1, T0 and T2, the percentage fluorescent cells were 10%, 28% and 72% respectively. Similar results have been obtained with inactivation of mecA and clpC, two other genes on the pathway that regulates ComK stability (not shown and Hahn et al., 1995b; Hoa et al., 2002).
We conclude that CodY, Rok and DegU do not affect the timing of competence development, but that the levels of CodY and Rok in the wild-type strain act negatively to limit the final fraction of the population that expresses ComK. The fact that rok and codY mutants still exhibit bimodal distributions in comK-gfp expression, shows that these genes are individually not required for bistability and in the case of rok, provides strong evidence that the putative toggle switch is not needed.
The putative toggle switch is not required for bistability in strain BD4010
To further test whether the toggle switch contributes to bistability, we introduced a knockout of rok into the strain described in Fig. 3A that retains the positive autoregulatory loop (BD4010). Figure 6 demonstrates that in this strain, the bimodal response is retained in the absence of Rok. Furthermore, the response to IPTG is hypersensitive compared to the results seen with BD4010, because of the absence of Rok-repression at the promoter of comK. This experiment shows that Rok is not required for bistability, in this synthetic construct. This result supports the observations reported in Fig. 5 and further suggests that the ComK autoregulatory loop rather than the postulated toggle switch mechanism is essential for bistability.
Fig. 6.
Effect of rok knockout mutation on bistability in the strain BD4010 background (Fig. 3A). The distributions for the rok (BD4019, shaded) and rok+ (BD4010, black) strains are shown. The fluorescent intensities of 250 cells were evaluated for each IPTG concentration.
We have also asked whether Rok and CodY might somehow function redundantly to effect a toggle switch-like mechanism, although this seems unlikely, as there is no evidence that ComK can act at the promoter of codY. To test this possibility, we have examined the fluorescence of a rok codY comK-gfp strain (BD4042) grown to T2. In four experiments with this strain, an average of 78 ± 11% of the cells were fluorescent, demonstrating that even in this double knockout strain, comK-gfp is expressed heterogeneously.
Discussion
It has been known for many years (Nester and Stocker, 1963; Hadden and Nester, 1968; Haseltine-Cahn and Fox, 1968) that competence in the domesticated laboratory strains of B. subtilis, is expressed in 10–20% of the cells in a given culture (Fig. 2B). In natural isolates of B. subtilis, the fraction of cells expressing competence is markedly lower than this, presumably because these strains have not been artificially selected for high transformability. In one such isolate, only about 1% of the cells express a comK–gfp fusion, but in these rare cells, expression is at a high level (J. Hahn, H. Maamar and D. Dubnau, unpubl.) This dramatic example of population heterogeneity may have evolved so that few cells in a clone will commit to a particular fitness-enhancing strategy. As the prolonged semidormancy that accompanies the K-state (Haijema et al., 2001) poses a potential challenge to survival, this strategy serves to minimize risks to the genotype. If, on the other hand, the few cells expressing the K-state happen to enjoy an advantage, the chances that the genotype will survive will be enhanced. Presumably the heterogeneity mechanism has evolved to maximize the benefit-to-risk ratio. There may be many examples of population heterogeneity selected by evolution in single celled organisms (see for instance Balaban et al., 2004), and an understanding of the mechanisms that regulate this heterogeneity would be of general interest.
It has been shown, both experimentally and from modelling studies, that biological systems can achieve bistability using at least two distinct regulatory circuits (Gardner et al., 2000; Becskei et al., 2001; Ferrell, 2002). The first employs a positive feedback loop and the second uses reciprocally active repressors. Although the comK regulatory system contains all of these elements, we have shown in two different genetic backgrounds that the Rok repressor and the putative toggle switch are not needed for bistability and that instead, the first of these mechanisms plays a central role in generating the bimodal expression pattern of competence and the K-state.
Figure 5 suggests that in wild-type cells, the MecA mediated degradation pathway will eliminate any ComK that is synthesized prior to T0. The degradative machinery has the capacity to suppress the effect of fluctuations in the level of CodY and Rok, because even the total absence of each of these proteins (Fig. 5) or of both of them simultaneously (T.T. Hoa and D. Dubnau, unpubl.) has little or no effect on expression before T0. The same appears to be true of cells that express an enhanced level of DegU. As cells approach T0, enough ComS is synthesized in at least 10–20% of the cells to protect ComK from degradation. In contrast to the situation before T0, elimination of rok or codY by mutation, causes an increase in the final fraction of ComK-expressing cells, showing that these factors normally limit activation of the positive auto-regulatory loop.
What selects cells for the K-state? The choice of K-state-expressing cells must be stochastic, because the cultures used for these experiments are well shaken (exposed to uniform environments) as well as clonal (genetically uniform). We propose that those cells exceeding a threshold concentration of ComK because of noise in the synthesis or stability of this protein are the ones that express the K-state. Once a cell has achieved a certain level of comK expression, the positive autoregulatory loop will be activated and the switch will be thrown. In other words, the basal amount of ComK itself ultimately selects a cell for high-level expression. In accordance with this notion, we have observed that any mutation enhancing ComK synthesis, including the overexpression of comS, or the inactivation of mecA, clpC, rok or codY, increases the percentage of cells that express the K-state (this study and Hahn et al., 1995b; Hoa et al., 2002). In the cultures of BD2711 represented by Fig. 2B, rare cells could be detected that express high levels of GFP fluorescence, even during exponential growth (not shown), consistent with the occasional noise-driven activation of the ComK loop.
Noise can originate in the random nature of the initiation of transcription or translation (intrinsic noise) or may be as a result of variations in the activities of cellular components that influence gene expression (extrinsic noise) (Elowitz et al., 2002; Swain et al., 2002). In the case of comK, five transcription factors are known to bind to the promoter (CodY, Rok, DegU, AbrB and ComK) and it is possible that fluctuations in one or more of these determine which cells express comK. In addition, the post-translational mechanism that regulates the stability of the ComK protein may be a source of extrinsic noise in the expression of ComK, as the levels of MecA, ClpC, ClpP and ComS may vary from cell to cell. Such variations would influence the basal concentration of ComK at T0, which would then alter the rate of transcription at PcomK. At one extreme, it is conceivable that the variation in comK expression that selects cells for the K-state is dominated by noise in a single one of these many factors. At the other extreme, the variation may be determined by the joint probability that all of these factors vary to raise the expression of comK above a threshold value in 10–20% of the cells in a culture.
It is instructive to consider three models for the source of the noise that determines the K-state. These models are heuristic, and clearly all three may be partially valid. In the first model, the intrinsic noise in expression of comK selects cells for the K-state. A cell that randomly fires PcomK more often may achieve a critical level of ComK, thereby activating the loop. The role of ComS synthesis may be to simply permit the survival of ComK after T0.
In the second model, like the first, all the cells achieve a high enough level of ComS after T0 to protect ComK from degradation, but variations in the proteins that act at PcomK select cells for expression. The use of GFP fusions to CodY and Rok shows that these proteins are expressed in all the cells (not shown), but it is possible that only those cells at the low end of the CodY and/or Rok distribution activate the ComK loop. Another protein, AbrB, represses at PcomK (Hamoen et al., 2003b), but it is unlikely that variation in AbrB helps select cells for comK expression. As cells approach T0, the concentration of AbrB decreases, because of repression by the phosphorylated form of the master regulator Spo0A (Strauch et al., 1990). However, AbrB also represses at Prok, thereby exerting a positive effect as a repressor of a repressor (Hoa et al., 2002). As a result, the effect on comK expression of variations in the concentration of AbrB should be buffered by opposing effects. This buffering has been observed experimentally (Hahn et al., 1995a). Consequently, we are left with CodY and Rok as candidates for the second hypothesis.
The third model proposes that noise in the level of ComS, or another component of the degradation mechanism, selects cells for the K-state. It is known that transcription at the comS promoter takes place in all the cells (Hahn et al., 1994) and that a ComS-YFP fusion protein is actually synthesized in all the cells in a culture (J. Hahn, H. Maamar and D. Dubnau, unpubl.). However, there is undoubtedly noise in the expression of comS and this may provide the variability that selects cells for stabilization of ComK. Although this model seems attractive, because of the important effects of ComS over-production (Fig. 5) or of mecA and clpC inactivation before T0, it is actually no more compelling than the first two models.
We may summarize our current understanding of the regulation of the K-state as follows. As cells grow and divide exponentially, the expression of comK is limited by redundant mechanisms. CodY, Rok and AbrB repress expression at PcomK, and any ComK synthesized despite this repression will be targeted to the ClpC/ClpP proteosome and rapidly degraded. The concentration of ComK in the cell will be low under these conditions, and the positive feedback loop that acts at PcomK will not be activated. As a culture approaches T0, the concentration of pheromone in the medium rises, and consequently ComS is produced. ComS can act to prevent the degradation of ComK, by releasing it from binding to MecA. We propose that at this point, the system is delicately balanced, because at least some ComS is produced in all the cells. Any variation that further increases the concentration of ComK (high ComS, low MecA, ClpC, CodY or Rok or a stochastic increase in the transcription or translation of comK) may potentially activate the positive feedback loop. The expression of comK will then rapidly increase, favoured by the non-linear response of PcomK to ComK.
This model formulated above does not explain why all the cells do not eventually enter the K-state. At least two factors potentially act temporally to limit the expression of comK. As noted above, comK is only expressed within a narrow range of AbrB concentration (Hahn et al., 1995a). As cells enter stationary phase, the amount of AbrB drops continually (Strauch et al., 1990), thus eventually mitigating comK expression. During this period, the activity of SinR also decreases (Shafikhani et al., 2002), potentially activating rok transcription and further repressing comK expression (Hoa et al., 2002). Thus, the K-state may develop only within a temporal window of opportunity, restricted stochastically to the cells that ‘get there first’.
A major unanswered question is whether the noise in a single extrinsic factor or the intrinsic noise in comK expression dominates the selective process, or if the selection results from multiple variations.
Experimental procedures
General methods and materials
The B. subtilis strains used are all derivatives of strain BD630 and are described in Table 1. Bacillus strains were grown either in liquid competence medium (Albano et al., 1987) supplemented with glucose (0.5%), L-histidine, L-leucine and L-methionine (50 μg ml−1) or on tryptose blood agar base (TBAB, Difco) supplemented with chloramphenicol, erythromycin or kanamycin (5 μg ml−1) or spectinomycin (100 μg ml−1). B. subtilis competent cells were prepared as described previously (Albano et al., 1987). E. coli XL1 blue (Stratagene) was used for cloning comK into the Phs vector, and transformants were selected on Luria–Bertani (LB) plates containing ampicillin (100 μg ml−1). DNA manipulation and other molecular biological procedures were performed using standard protocols.
Table 1.
B. subtilis strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| BD630 | his leu met | – |
| BD1833 | his leu met multicopy degU (kana) | Hahn et al. (1996) |
| BD1991 | his leu met amyE:: comK-lacZ (cata) | Hahn et al. (1994) |
| BD2121 | his leu met comK::kana | D. Van Sinderren |
| BD2589 | his leu met codY::erma | L. Sonenshein and P. Serror |
| BD2711 | his leu met comK-gfp (CBLb, cata) | Haijema et al. (2001) |
| BD3196 | his leu met rok::kana | I. Chen |
| BD3836 | his leu met amyE:: Phs–comK (spca) | This study |
| BD4010 | his leu met amyE:: Phs–comK (spca) comK-gfp (CBLb, cata) | This study |
| BD4011 | his leu met amyE:: Phs–comK (spca) comK-gfp (CBLb, cata) comK::kana | This study |
| BD4012 | his leu met comK-gfp (CBLb, cata) rok::kana | This study |
| BD4013 | his leu met comK-gfp (CBLb, cata) multicopy degU (kana) | This study |
| BD4014 | his leu met comK-gfp (CBLb, cata) codY::erma | This study |
| BD4015 | his leu met comK-gfp (CBLb, cata) multicopy comS (kana) | Hahn et al. (1996) |
| BD4016 | his leu met amyE:: comK-lacZ (cata) rok::kana | This study |
| BD4017 | his leu met amyE:: comK-lacZ (cata) multicopy degU (kana) | This study |
| BD2607 | his leu met amyE:: comK-lacZ (cata) codY::erma | J. Hahn |
| BD4018 | his leu met amyE:: comK-lacZ (cata) multicopy comS (kana) | This study |
| BD4019 | his leu met amyE:: Phs–comK (spca) comK-gfp (CBLb, cata) rok::kana | This study |
| BD4042 | his leu met comK-gfp (CBLb, cat) rok::kana, codY::erma | This study |
kan, cat, erm and spc stand for resistance to kanamycin, chloramphenicol, erythromycin and spectinomycin respectively.
Inserted by Campbell like integration.
Construction of Phs-comK
The comK gene including the open reading frame and ribosome binding site was synthesized by polymerase chain reaction (PCR) using the primers, comKFor 5′-CCCAAGCTTAACAGATGATAGATTATTAGTA-3′ and comKRev 5′-ACATGCATGCATTGACATCTCAGGTATATGG-3′. The product was cut with HindIII and SphI (sites are underlined above) ligated to pdr111 hyper-SPANK, previously digested with the same enzymes and transformed into E. coli XL1 blue, with selection for ampicillin resistance. This plasmid, a generous gift from D. Rudner (Harvard Medical School), is derived from pDR66, and therefore carries front and back sequences of amyE. It also contains the hyper-SPANK promoter (Phs), inducible by IPTG, and is not capable of replication in B. subtilis. The recombinant plasmid was transferred to BD630 with selection for spectinomycin resistance and the transformants were screened for the absence of amylase activity.
Microscopy
The strains with Phs-comK at amyE were grown in competence medium to mid-log phase. IPTG was added to the indicated concentrations, and samples were taken thereafter for microscopy. For non-inducible strains, samples were taken throughout growth as indicated. Cells were permitted to attach to polylysine-coated slides. Cross walls were visualized by staining RNA and DNA with propidium iodide (PI). Samples were mounted in Slow Fade (Molecular Probes). Microscopy was performed with a Zeiss Axiovert 135 M microscope equipped with an Orca Digital Camera (Hamamatsu), and a Zeiss 1.3 NA Plan Neo-Fluor 100 X oil immersion objective. Openlab software (Improvision) and Adobe Photoshop were used for image acquisition and processing. Three images were captured for each field of cells: phase contrast and fluorescence images for GFP and PI. Omega Optical filter sets XF23 and XF34 were used for GFP and PI respectively.
To calculate the fluorescence intensity of single cells, the average pixel value of non-GFP-containing cells (BD630) was subtracted from the measured pixel value of each gfp-containing cell. When negative values were obtained, these were set to zero. The Improvision lasso selection tool was used to delimit cell boundaries in the propidium iodide fields and the average pixel intensities were recorded in the corresponding GFP fields.
Acknowledgments
We thank Wiep Klaas Smits, Leendert Hamoen, David Wah and Jeanie Dubnau, as well as all the members of our lab for useful discussions. We thank David Rudner for the kind gift of the Phs promoter before publication. This work was supported by NIH grant GM 57720.
References
- Albano M, Hahn J, Dubnau D. Expression of competence genes in Bacillus subtilis. J Bacteriol. 1987;169:3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science. 2004;305:1622–1625. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- Becskei A, Seraphin B, Serrano L. Positive feedback in eukaryotic gene networks: cell differentiation by graded to binary response conversion. EMBO J. 2001;20:2528–2535. doi: 10.1093/emboj/20.10.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berka RM, Hahn J, Albano M, Draskovic I, Persuh M, Cui X, et al. Microarray analysis of the Bacillus subtilis K-state: genome-wide expression changes dependent on ComK. Mol Microbiol. 2002;43:1331–1345. doi: 10.1046/j.1365-2958.2002.02833.x. [DOI] [PubMed] [Google Scholar]
- Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- Ferrell JE., Jr Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403:339–342. doi: 10.1038/35002131. [DOI] [PubMed] [Google Scholar]
- Hadden C, Nester EW. Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol. 1968;95:876–885. doi: 10.1128/jb.95.3.876-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Kong L, Dubnau D. The regulation of competence transcription factor synthesis constitutes a critical control point in the regulation of competence in Bacillus subtilis. J Bacteriol. 1994;176:5753–5761. doi: 10.1128/jb.176.18.5753-5761.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Roggiani M, Dubnau D. The major role of Spo0A in genetic competence is to downregulate abrB, an essential competence gene. J Bacteriol. 1995a;177:3601–3605. doi: 10.1128/jb.177.12.3601-3605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Bylund J, Haines M, Higgins M, Dubnau D. Inactivation of mecA prevents recovery from the competent state and interferes with cell division and the partitioning of nucleoids in Bacillus subtilis. Mol Microbiol. 1995b;18:755–767. doi: 10.1111/j.1365-2958.1995.mmi_18040755.x. [DOI] [PubMed] [Google Scholar]
- Hahn J, Luttinger A, Dubnau D. Regulatory inputs for the synthesis of ComK, the competence transcription factor of Bacillus subtilis. Mol Microbiol. 1996;21:763–775. doi: 10.1046/j.1365-2958.1996.371407.x. [DOI] [PubMed] [Google Scholar]
- Haijema BJ, Hahn J, Haynes J, Dubnau D. A ComGA-dependent checkpoint limits growth during the escape from competence. Mol Microbiol. 2001;40:52–64. doi: 10.1046/j.1365-2958.2001.02363.x. [DOI] [PubMed] [Google Scholar]
- Hamoen LW, Van Werkhoven AF, Bijlsma JJE, Dubnau D, Venema G. The competence transcription factor of Bacillus subtilis recognizes short A/T-rich sequences arranged in a unique, flexible pattern along the DNA helix. Genes Dev. 1998;12:1539–1550. doi: 10.1101/gad.12.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamoen LW, Van Werkhoven AF, Venema G, Dubnau D. The pleiotropic response regulator DegU functions as a priming protein in competence development in bacillus subtilis. Proc Natl Acad Sci USA. 2000;97:9246–9251. doi: 10.1073/pnas.160010597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamoen LW, Smits WK, de Jong A, Holsappel S, Kuipers OP. Improving the predictive value of the competence transcription factor (ComK) binding site in Bacillus subtilis using a genomic approach. Nucleic Acids Res. 2002;30:5517–5528. doi: 10.1093/nar/gkf698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamoen LW, Venema G, Kuipers OP. Controlling competence in Bacillus subtilis: shared use of regulators. Microbiology. 2003a;149:9–17. doi: 10.1099/mic.0.26003-0. [DOI] [PubMed] [Google Scholar]
- Hamoen LW, Kausche D, Marahiel MA, Sinderen DV, Venema G, Serror P. The Bacillus subtilis transition state regulator AbrB binds to the -35 promoter region of comK. FEMS Microbiol Lett. 2003b;218:299–304. doi: 10.1111/j.1574-6968.2003.tb11532.x. [DOI] [PubMed] [Google Scholar]
- Haseltine-Cahn F, Fox MS. Fractionation of transformable bacteria from competent cultures of Bacillus subtilis on renografin gradients. J Bacteriol. 1968;95:867–875. doi: 10.1128/jb.95.3.867-875.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoa TT, Tortosa P, Albano M, Dubnau D. Rok (YkuW) regulates genetic competence in Bacillus subtilis by directly repressing comK. Mol Microbiol. 2002;43:15–26. doi: 10.1046/j.1365-2958.2002.02727.x. [DOI] [PubMed] [Google Scholar]
- Nester EW, Stocker BAD. Biosynthetic latency in early stages of deoxyribonucleic acid transformation in Bacillus subtilis. J Bacteriol. 1963;86:785–796. doi: 10.1128/jb.86.4.785-796.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick A, Weiner M. Enzyme induction as an all-or-none phenomenon. Proc Natl Acad Sci USA. 1957;43:553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura M, Yamaguchi H, Kobayashi K, Ogasawara N, Fujita Y, Tanaka T. Whole-genome analysis of genes regulated by the Bacillus subtilis competence transcription factor ComK. J Bacteriol. 2002;184:2344–2351. doi: 10.1128/JB.184.9.2344-2351.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao CV, Wolf DM, Arkin AP. Control, exploitation and tolerance of intracellular noise. Nature. 2002;420:231–237. doi: 10.1038/nature01258. [DOI] [PubMed] [Google Scholar]
- Serror P, Sonenshein AL. CodY is required for nutritional repression of Bacillus subtilis genetic competence. J Bacteriol. 1996;178:5910–5915. doi: 10.1128/jb.178.20.5910-5915.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafikhani SH, Mandic-Mulec I, Strauch MA, Smith I, Leighton T. Postexponential regulation of sin operon expression in Bacillus subtilis. J Bacteriol. 2002;184:564–571. doi: 10.1128/JB.184.2.564-571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sinderen D, Venema G. comK acts as an autoregulatory control switch in the signal transduction route to competence in Bacillus subtilis. J Bacteriol. 1994;176:5762–5770. doi: 10.1128/jb.176.18.5762-5770.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sinderen D, Luttinger A, Kong L, Dubnau D, Venema G, Hamoen L. comK encodes the competence transcription factor, the key regulatory protein for competence development in Bacillus subtilis. Mol Microbiol. 1995;15:455–462. doi: 10.1111/j.1365-2958.1995.tb02259.x. [DOI] [PubMed] [Google Scholar]
- Smits WK, Eschevins CC, Susanna KA, Bron S, Kuipers OP, Hamoen LW. Stripping Bacillus: ComK auto-stimulation is responsible for the bistable response in competence development. Mol Microbiol. 2005 doi: 10.1111/j.1365-2958.2004.04488.x. [DOI] [PubMed] [Google Scholar]
- Solomon J, Magnuson R, Srivastava A, Grossman AD. Convergent sensing pathways mediate response to two extracellular competence factors in Bacillus subtilis. Genes Dev. 1995;9:547–558. doi: 10.1101/gad.9.5.547. [DOI] [PubMed] [Google Scholar]
- Strauch M, Webb V, Spiegelman G, Hoch JA. The SpoOA protein of Bacillus subtilis is a repressor of the abrB gene. Proc Natl Acad Sci USA. 1990;87:1801–1805. doi: 10.1073/pnas.87.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain PS, Elowitz MB, Siggia ED. Intrinsic and extrinsic contributions to stochasticity in gene expression. Proc Natl Acad Sci USA. 2002;99:12795–12800. doi: 10.1073/pnas.162041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgay K, Hamoen LW, Venema G, Dubnau D. Biochemical characterization of a molecular switch involving the heat shock protein ClpC, which controls the activity of ComK, the competence transcription factor of Bacillus subtilis. Genes Dev. 1997;11:119–128. doi: 10.1101/gad.11.1.119. [DOI] [PubMed] [Google Scholar]
- Turgay K, Hahn J, Burghoorn J, Dubnau D. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 1998;17:6730–6738. doi: 10.1093/emboj/17.22.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]