Abstract
Geobacter sulfurreducens, previously classified as a strict anaerobe, tolerated exposure to atmospheric oxygen for at least 24 h and grew with oxygen as the sole electron acceptor at concentrations of 10% or less in the headspace. These results help explain how Geobacter species may survive in oxic subsurface environments, being poised to rapidly take advantage of the development of anoxic conditions.
Geobacter species have previously been considered strict anaerobes (4, 18, 19). However, when organic electron donors are introduced into previously oxic subsurface sediments in order to stimulate anaerobic dissimilatory metal reduction, Geobacter species rapidly become the predominant Fe(III)-reducing microorganisms in a diversity of subsurface sediments (2, 13, 29). Thus, it appears that there is a reservoir of Geobacter species in oxic aquifers.
The ability of strict anaerobes to survive under oxic conditions is not unprecedented. Many anaerobes contain enzymes, such as superoxide reductase (14), superoxide dismutase (23), and NADH oxidase (32), which permit them to tolerate oxygen exposure. Some organisms generally regarded as anaerobes also have the ability to lower the oxygen concentration in their surroundings. For example, homoacetogenic bacteria isolated from termite guts oxidized hydrogen with the reduction of oxygen and thereby established favorable conditions for growth (3). Desulfovibrio species, which, like Geobacter species, are δ-Proteobacteria, can survive oxygen exposure (1, 11) and express oxidative-stress enzymes such as superoxide dismutases (10, 12), superoxide reductases (28), and ruberythrin or rubredoxin oxidoreductase (21). Desulfovibrio species also contain enzymes that can reduce oxygen (5, 16, 23) and, in some cases, conserve energy from oxygen reduction (8, 9). However, no examples of sustained growth by Desulfovibrio species with oxygen as the electron acceptor have been documented.
The sequence of the Geobacter sulfurreducens genome has revealed many previously unsuspected metabolic capabilities of this organism (6, 17, 22). Genes predicted to code for proteins involved in response to oxidative stress, such as rubredoxins, A-type flavodoxins, catalase, superoxide dismutase, and superoxide reductase, are readily apparent (22) and perhaps not unexpected. More surprising is the presence of genes appearing to encode a terminal cytochrome c oxidase, suggesting that G. sulfurreducens might have the ability to utilize oxygen as a terminal electron acceptor (22).
Survival in atmospheric oxygen.
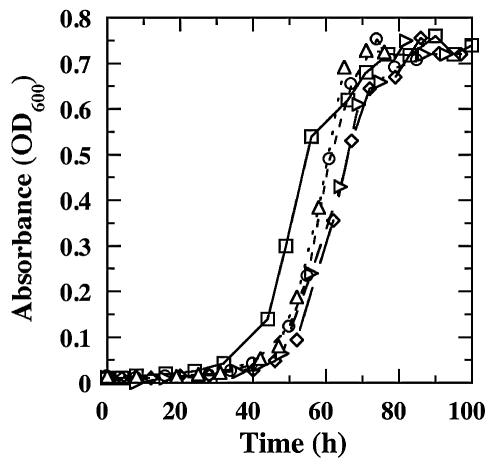
In order to determine the ability of G. sulfurreducens to tolerate oxygen exposure, mid-log-phase cultures were grown at 30°C under N2 in a 3-(N-morpholino)propanesulfonic acid (MOPS)-buffered (20 mM, pH 7.0) variation of the previously described acetate-fumarate medium (7). The cells were then diluted 10-fold into the aerobically prepared MOPS-acetate-fumarate medium and shaken under air. Following exposure to air for various periods of time, the cells were diluted another 10-fold into an anoxic MOPS-acetate-fumarate medium that was supplemented with 1 mM cysteine and 0.5% yeast extract and were incubated at 30°C. Exposure to atmospheric oxygen resulted in only a slight increase in the lag phase prior to the onset of anaerobic growth (Fig. 1). Furthermore, the length of the lag phase was not significantly affected by the duration of the exposure to air. These results demonstrate that G. sulfurreducens is aerotolerant.
FIG. 1.
Anaerobic growth of G. sulfurreducens following exposure to atmospheric oxygen for 0 h (□), 4 h (⋄), 8 h (▹), 16 h (○), and 24 h (▵). Data shown are the means of results from three replicate cultures. OD600, optical density at 600 nm.
Growth with oxygen as the sole electron acceptor.
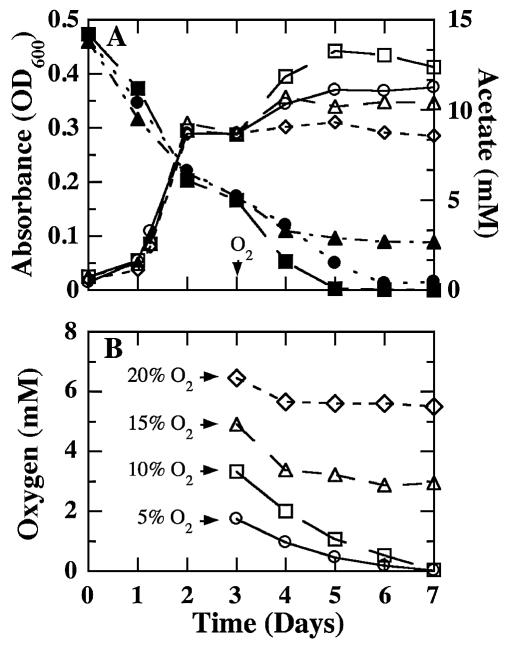
In order to determine whether G. sulfurreducens is able to grow with oxygen as the sole electron acceptor, G. sulfurreducens was grown in a modification of the previously described anoxic, bicarbonate-buffered medium (7) with excess acetate (15 mM) as the electron donor and limiting fumarate (20 mM) as the electron acceptor. Cysteine and resazurin were omitted from the medium. The atmosphere overlying the medium was He-CO2 (80:20). Cultures were grown in 1.1-liter bottles in a manner similar to the method described by Dilling and Cypionka (9), with 200 ml of medium at 30°C with shaking at 45 rpm. Acetate and fumarate concentrations were measured with a liquid chromatograph (model SPD-10A; Shimadzu, Kyoto, Japan) equipped with a 100- by 7.8-mm Aminex Fast Acid column (Bio-Rad, Hercules, Calif.) with an 8 mM H2SO4 mobile phase at a flow rate of 0.5 ml/min. Oxygen concentration in the headspace was measured with a Hewlett-Packard series HP6890 gas chromatograph equipped with a thermal conductivity detector (Agilent Technologies, Inc., Albany, N.Y.) and a Carboxen 1010 PLOT capillary column (Supelco, Bellefonte, Pa.). The temperatures of the detector, injector, and column were 230, 230, and 40°C, respectively. Cell number was determined with acridine orange staining and epifluorescence microscopy, as previously described (20). When a 2% inoculum of mid-log-phase cells grown in anoxic acetate-fumarate medium was added to fresh anoxic medium, the cells consumed acetate and grew to an absorbance of ca. 0.3 within 2 days (Fig. 2A). At this point, all of the fumarate in the medium had been consumed, and no further growth was observed unless the medium was amended with additional fumarate (data not shown). When oxygen was added to the headspace at an initial concentration of 5 or 10%, G. sulfurreducens grew, as was indicated by an increase in absorbance (Fig. 2A) and a net increase of 1.93 × 108 and 2.82 × 108 cells/ml, respectively. Cell growth was accompanied by the concurrent consumption of acetate and oxygen (Fig. 2). If the concentration of oxygen was increased to 15 or 20%, oxygen consumption stopped within a day and cell growth was less than that with 5% oxygen, suggesting that aerobic growth could not be sustained at these higher oxygen levels.
FIG. 2.
Oxygen-dependent growth of G. sulfurreducens. (A) Growth (open symbols) and acetate consumption (filled symbols) following the introduction of 5% (○), 10% (□), 15% (▵), or 20% (⋄) oxygen into the headspace of cultures pregrown on 20 mM fumarate; (B) oxygen consumption, with symbols designating the same oxygen concentrations as described for panel A. The time oxygen was added is indicated by the downward-pointing arrowhead. Data shown are the means of results from two replicate cultures. OD600, optical density at 600 nm.
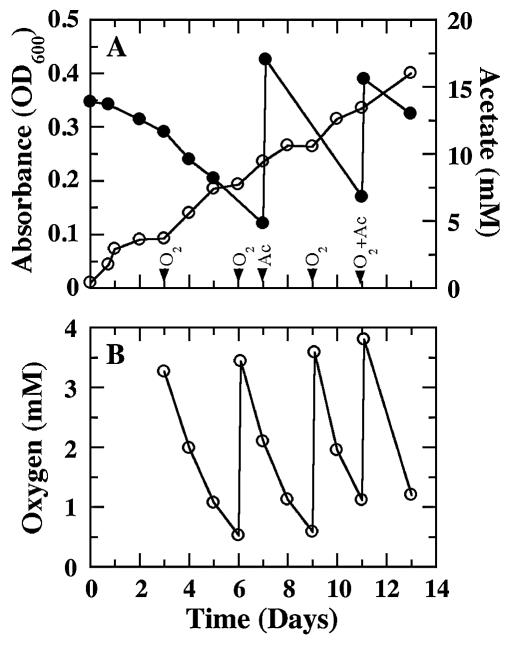
In order to determine if sustained growth on acetate and oxygen was possible, cells were initially grown on acetate with 5 mM fumarate as the electron acceptor; this was followed by oxygen addition after fumarate was depleted. Growth on acetate and oxygen continued for 10 days with multiple additions of 5% oxygen and 15 mM acetate (Fig. 3). When 10% oxygen was added, growth stopped after the second addition. However, growth could be sustained after an initial addition of 10% oxygen if 5% oxygen was used for subsequent additions (data not shown).
FIG. 3.
Sustained growth of G. sulfurreducens on oxygen. (A) Growth (open symbols) and acetate consumption (filled symbols) following the introduction of 5% oxygen into the headspace of cultures pregrown on 5 mM fumarate; (B) oxygen consumption by G. sulfurreducens. The times at which 5% oxygen (O2) and/or 15 mM acetate (Ac) was added are indicated by downward-pointing arrowheads. Data shown are the means of results from two replicate cultures.
Growth on oxygen required that the cells be pregrown on fumarate. There was no growth when either anaerobically grown cells or cells that had been grown with 5 or 10% oxygen were inoculated directly into fresh medium with 5%, 10%, or atmospheric oxygen, even when the medium was supplemented with organic acids that were present in the fumarate-grown cultures, such as succinate and malate.
The stoichiometry of oxygen to acetate consumption was 2.4 ± 0.8 (mean ± standard deviation from four replicate experiments, as determined from the results shown in Fig. 2 and 3) for the cultures grown with an initial oxygen concentration of 5%, and there was no growth on oxygen in acetate-depleted cultures. These results suggest that acetate was oxidized to carbon dioxide, with oxygen serving as the sole electron acceptor according to the reaction CH3COOH + 2O2 → 2CO2 + 2H2O. In comparison, the stoichiometry of fumarate-to-acetate consumption for the initial growth on fumarate was 3.8 ± 0.6, which also corresponded well with the predicted reaction, where acetate is oxidized to CO2, with fumarate serving as the sole electron acceptor: 4COOHCHCHCOOH + CH3COOH + 2H2O → 4COOHCH2CH2COOH + 2CO2.The levels of growth (measured as the increase in cell number per millimole of acetate consumed) were similar in the presence of oxygen and in the presence of fumarate. Growth on fumarate alone yielded 5.7 × 107 cells per mmol of acetate, while growth on 5 and 10% oxygen yielded 5.9 × 107 and 6.3 × 107 cells per mmol of acetate, respectively.
Implications.
The finding that G. sulfurreducens can survive exposure to atmospheric oxygen and readily grows at low oxygen concentrations has important implications for the growth and survival of Geobacter species in subsurface environments. In water-saturated soils and aquatic sediments, Fe(III) is likely to be most abundant near the oxic-anoxic interface, where the oxidation of Fe(II) can regenerate Fe(III) produced from microbial Fe(III) reduction (30). Thus, oxygen intrusions into the zone of Fe(III) reduction may be common, especially in sediments with substantial bioturbation or other physical disturbances. In shallow aquifers, an environment where Fe(III) reduction is often an important terminal electron-accepting process, periods of high surface recharge can result in the introduction of dissolved oxygen into previously anoxic zones (31). Microorganisms carrying out anaerobic respiration in the interior anoxic zone of soil aggregates (24, 25, 26, 27) in otherwise oxic soils are also likely to face periodic oxygen exposure. The ability to tolerate exposure to high levels of oxygen and to grow at low oxygen levels may permit G. sulfurreducens to thrive in these environments. It is well documented that once oxygen is consumed in subsurface environments, Geobacter species can rapidly become the predominant Fe(III)-reducing microorganisms (2, 13, 29).
In summary, the results presented here demonstrate that G. sulfurreducens should no longer be classified as a strict anaerobe. Given the potential importance of the survival and growth of Geobacter species in oxic environments, further investigations into the mechanisms by which G. sulfurreducens survives oxidative stress and grows on oxygen are warranted.
Acknowledgments
This research was supported by the Microbial Genome, Biological and Environmental Research, Office of Science, Department of Energy (DE-FG02-01ER63145), and Genomes to Life (DE-FC02-02ER63446) programs.
We thank Evgenya Shelobolina for her valuable assistance.
REFERENCES
- 1.Abdollahi, H., and J. W. T. Wimpenny. 1990. Effects of oxygen on the growth of Desulfovibrio desulfuricans. J. Gen. Microbiol. 136:1025-1030. [Google Scholar]
- 2.Anderston, R. T., H. A. Vrionis, I. Ortiz-Bernad, C. T. Resch, P. E. Long, R. Dayvault, K. Karp, S. Marutzky, D. R. Metzler, A. Peacock, D. C. White, M. Lowe, and D. R. Lovley. 2003. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69:5884-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boga, H. I., and A. Brune. 2003. Hydrogen-dependent oxygen reduction by homoacetogenic bacteria isolated from termite guts. Appl. Environ. Microbiol. 69:779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caccavo, F., Jr., D. J. Lonergan, D. R. Lovley, M. Davis, J. F. Stolz, and M. J. McInerney. 1994. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microoganism. Appl. Environ. Microbiol. 60:3752-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, L., M.-Y. Liu, J. LeGall, P. Fareleira, H. Santos, and A. V. Xavier. 1993. Rubredoxin oxidase, a new flavo-heme-protein, is the site of oxygen reduction to water by the ′strict anaerobe' Desulfovibrio gigas. Biochem. Biophys. Res. Commun. 193:100-105. [DOI] [PubMed] [Google Scholar]
- 6.Childers, S. E., S. Ciufo, and D. R. Lovley. 2002. Geobacter metallireducens accesses insoluble Fe(III) oxide by chemotaxis. Nature 416:767-769. [DOI] [PubMed] [Google Scholar]
- 7.Coppi, M. V., C. Leang, S. J. Sandler, and D. R. Lovley. 2001. Development of a genetic system for Geobacter sulfurreducens. Appl. Environ. Microbiol. 67:3180-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cypionka, J. 2000. Oxygen respiration by Desulfovibrio species. Annu. Rev. Microbiol. 54:827-848. [DOI] [PubMed] [Google Scholar]
- 9.Dilling, W., and J. Cypionka. 1990. Aerobic respiration in sulfate-reducing bacteria. FEMS Microbiol. Lett. 71:123-128. [Google Scholar]
- 10.Dos Santos, W. G., I. Pacheco, M.-Y. Liu, M. Teixeira, A. V. Xavier, and J. LeGall. 2000. Purification and characterization of an iron superoxide dismutase and a catalase from the sulfate-reducing bacterium Desulfovibrio gigas. J. Bacteriol. 182:796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fareleira, P., B. S. Santos, C. Antonio, P. Moradas-Ferreira, J. LeGall, A. V. Xavier, and H. Santos. 2003. Response of a strict anaerobe to oxygen: survival strategies in Desulfovibrio gigas. Microbiology (Reading) 149:1513-1532. [DOI] [PubMed] [Google Scholar]
- 12.Hatchikian, E. C., and Y. A. Henry. 1977. An iron-containing superoxide dismutase from the strict anaerobe Desulfovibrio desulfuricans (Norway 4). Biochimie 59:153-161. [DOI] [PubMed] [Google Scholar]
- 13.Holmes, D. E., K. T. Finneran, R. A. O'Neil, and D. R. Lovley. 2002. Enrichment of members of the family Geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. Appl. Environ. Microbiol. 68:2300-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenney, F. E., Jr., M. F. Verhagen, X. Y. Cui, and M. W. W. Adams. 1999. Anaerobic microbes: oxygen detoxification without superoxide dismutase. Science 286:306-309. [DOI] [PubMed] [Google Scholar]
- 15.LeGall, J., and A. V. Xavier. 1996. Anaerobes response to oxygen: the sulfate-reducing bacteria. Anaerobe 2:1-9. [DOI] [PubMed] [Google Scholar]
- 16.Lemos, R. S., C. M. Gomes, M. Santana, J. LeGall, A. V. Xavier, and M. Texeira. 2001. The “strict” anaerobe Desulfovibrio gigas contains a membrane-bound oxygen-reducing respiratory chain. FEBS Lett. 496:40-43. [DOI] [PubMed] [Google Scholar]
- 17.Lovley, D. R. 2003. Cleaning up with genomics: applying molecular biology to bioremediation. Nat. Microbiol. Rev. 1:35-44. [DOI] [PubMed] [Google Scholar]
- 18.Lovley, D. R. 2000. Dissimilatory Fe(III)- and Mn(IV)-reducing prokaryotes. In E. Stackebrandt (ed.), The prokaryotes. [Online.] Springer-Verlag, Inc., New York, N.Y. http://141.150.157.117:8080/prokPUB/chaphtm/279/COMPLETE.htm.
- 19.Lovley, D. R., S. J. Giovannoni, D. C. White, J. E. Champine, E. J. P. Phillips, Y. A. Gorby, and S. Goodwin. 1993. Geobacter metallireducens gen. nov. sp. nov., a microoganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch. Microbiol. 159:336-344. [DOI] [PubMed] [Google Scholar]
- 20.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumppio, H. L., N. V. Shevni, A. O. Summers, G. Voordouw, and D. M. Kurtz, Jr. 2001. Rubrerythrin and rubredoxin oxidoreductase in Desulfovibrio vulgaris: a novel oxidative stress protection system. J. Bacteriol. 183:101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Methe, B. A., K. E. Nelson, J. A. Eisen, et al. 2003. Genome of Geobacter sulfurreducens: metal reduction in subsurface environments. Science 302:1967-1969. [DOI] [PubMed] [Google Scholar]
- 23.Nakanishi, T., H. Inoue, and M. Ketamura. 2003. Cloning and expression of the superoxide dismutase gene from the obligate anaerobe bacterium Desulfovibrio vulgaris (Miyazaki F). J. Biochem. 133:387-393. [DOI] [PubMed] [Google Scholar]
- 24.Philippot, L., P. Renault, J. Sierra, C. Henault, A. Clays-Josserand, C. Chenu, R. Chaussod, and R. Lensi. 1996. Dissimilatory nitrate-reductase provides a competitive advantage to Pseudomonas sp. RTCO1 to colonize the centre of soil aggregates. FEMS Microbiol. Ecol. 21:175-185. [Google Scholar]
- 25.Ranjard, L., and A. Fichaume. 2001. Quantitative and qualitative microscale distribution of bacteria in soil. Res. Microbiol. 152:707-716. [DOI] [PubMed] [Google Scholar]
- 26.Renault, P., and J. Sierra. 1994. Modelling oxygen diffusion in aggregated soil. 2. Anaerobiosis in topsoil layers. Soil Sci. Soc. Am. J. 58:1023-1030. [Google Scholar]
- 27.Sierra, J., P. Renault, and V. Valles. 1995. Anaerobiosis in saturated soil aggregates: modeling and experiment. Eur. J. Soil Sci. 46:4413-4420. [Google Scholar]
- 28.Silva, G., J. LeGall, A. V. Xavier, M. Teixeira, and C. Rodrigues-Pousada. 2001. Molecular characterization of Desulfovibrio gigas neelaredoxin, a protein involved in oxygen detoxification in anaerobes. J. Bacteriol. 183:4413-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snoeyenbos-West, O. L., K. P. Nevin, R. T. Anderston, and D. R. Lovley. 2000. Enrichment of Geobacter species in response to stimulation of Fe(III) reduction in sandy aquifer sediments. Microb. Ecol. 39:153-167. [DOI] [PubMed] [Google Scholar]
- 30.Thamdrup, B. 2000. Bacterial manganese and iron reduction in aquatic sediments. Adv. Microb. Ecol. 16:41-84. [Google Scholar]
- 31.Vroblesky, D. A., and F. H. Chapelle. 1994. Temporal and spatial changes of terminal electron accepting processes in a petroleum hydrocarbon contaminated aquifer and the significance for contaminant biodegradation. Water Resour. Res. 30:1561-1570. [Google Scholar]
- 32.Ward, D. E., C. J. Donnelly, M. E. Mullendore, J. van der Oost, W. M. de Vos, and E. J. Crane III. 2001. The NADH oxidase from Pyrococcus furiosus: implications for the protection of anaerobic hyperthermophiles against oxidative stress. Eur. J. Biochem. 268:5816-5823. [DOI] [PubMed] [Google Scholar]