Abstract
Often viewed as a potential tool for preclinical diagnosis in early asymptomatic stages of Alzheimer’s disease (AD), the term “endophenotype” has acquired a recent popularity in the field. In this review, we analyze the construct of endophenotype—originally designed to discover genes, and examine the literature on potential endophenotypes for the late-onset form of AD (LOAD). We focus on the [18F]-fluoro-2-deoxyglucose (FDG) PET technique, which shows a characteristic pattern of hypo-metabolism in AD-related regions in asymptomatic carriers of the ApoE E4 allele and in children of AD mothers. We discuss the pathophysiological significance and the positive predictive accuracy of an FDG-endophenotype for LOAD in asymptomatic subjects, and discuss several applications of this endophenotype in the identification of both promoting and protective factors. Finally, we suggest that the term “endophenotype” should be reserved to the study of risk factors, and not to the preclinical diagnosis of LOAD.
Keywords: Alzheimer’s disease, FDG-PET, Endophenotype, Preclinical, Diagnosis, Genetics
Introduction
Alzheimer’s disease (AD) is the first cause of dementia and is characterized by progressive cognitive, functional and behavioral deficits. The number of patients worldwide is projected to almost quadruple by 2050, reaching 1 in 85 individuals [1]. While early neurofibrillary tangles and amyloid plaque pathology are estimated to occur decades before the symptoms [2, 3], a clinical diagnosis is not possible until a more advanced symptomatic stage of the disease, generally less than 10 years before death [4, 5]. Current research aims to detect the earliest manifestations of biological pathology, in the scope of a shift of paradigm from late intervention—where treatments are started after cognitive decline—to prevention, targeting at-risk asymptomatic patients with disease-modifying drugs. In this paradigm, in parallel with the development of biomarkers for AD, the term “endophenotype” has acquired a recent popularity [6–9].
The construct of endophenotype is designed for identifying genes involved in complex behaviors. The concept was first proposed to account for the geographical distribution of grasshoppers [10]. As opposed to exophenotypes, that are external and apparent features of a phenotype (signs and symptoms that can be assessed during a standard clinical evaluation), endophenotypes refer to internal or subclinical traits (signs that require neuropsychological, biochemical, electrophysiological and neuroanatomical measures) [11]. Gottesman and Gould [12] provide a practical definition of endophenotype:
It is associated with illness in the population.
It is heritable.
It co-segregates with the illness.
It is state-independent (i.e., it manifests in an individual whether or not illness is active).
It is found in non-affected family members at a higher rate than in the general population.
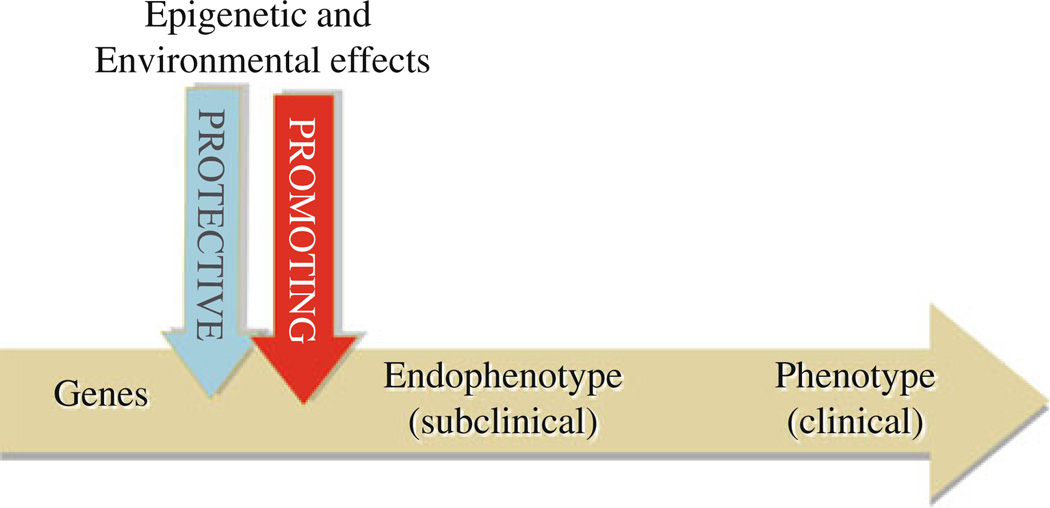
The construct of endophenotype is particularly useful in psychiatry, as it fills the gaps in the causal chain between genes and behavioral phenotypes. Identification of these “intermediate traits” can help decipher complex rules of etiology by measuring, closer to the genes than the full-blown syndrome, the effects of epigenetic factors and environment [13] (see Fig. 1). To date, several endophenotypes have been proposed and used in genetic studies of schizophrenic patients and their relatives: e.g., deficits in sensory motor gating [14–16], eye-tracking dysfunction [17, 18] that mapped to the loci on chromosome 15 and 6, respectively [19, 20]. Working memory deficits have been used for identifying loci on chromosome 1 [21] and led to a polymorphism in the gene coding for Catechol-O-methyl-transferase on chromosome 6 when associated to fMRI measurements [22].
Fig. 1.
The construct of endophenotype. Endophenotypes are defined as intermediate traits lying on the causal chain closer to the genes than the disease. They are designed to identify genes and measure the effect of epigenetic and environmental factors
In the field of AD research, the study of endophenotype could be particularly relevant in the late-onset form of AD (LOAD). As opposed to the rare “familial” early-onset form of the disease (EOAD), which is known to follow a Mendelian pattern of inheritance of highly penetrant mutations in three genes (APP, PSEN1 and PSEN2), the etiology of the more prevalent “sporadic” LOAD seems more complex and largely multifactorial, including a large number of genes with limited penetrance. Quantitative endophenotypes could, therefore, help capture and translate these small genetic effects into measurable scales.
Several studies have identified AD-related biological traits in both asymptomatic carriers of an apolipoprotein E (ApoE) E4 allele [23, 24] and in AD first-degree relatives [25], suggesting a number of possible endophenotypes for AD that include: neuropsychological tests [26], quantitative EEG [27], plasmatic and CSF Aβ42 [28, 29], volumetric MRI [30]. Positron emission tomography (PET) imaging of amyloid plaques measured by cortical binding of N-methyl-[11C]2-(4′-methylaminophenyl)-6-hydrox-ybenzothiazole, known as Pittsburgh Compound-B (PIB) [31–33] and PET glucose metabolism imaging with [18F]-fluoro-2-deoxyglucose (FDG) [34] are two techniques that could also identify endophenotypes of AD. We will concentrate this discussion on the use of FDG-PET.
FDG-PET provides qualitative and quantitative estimates of regional cerebral metabolic rates of glucose (CMRglc), considered as an index of synaptic activity [35] and density [36]. In AD, the most prominent and consistent CMRglc findings are decreased metabolism starting in the entorhinal cortex (EC) [37] and hippocampus (Hip) [38, 39], and spreading to posterior cingulate cortex (PCC), temporoparietal areas, precuneus and prefrontal cortex in more advanced stages [34, 40–42]. This pattern of regional hypometabolism appears to be strongly associated with AD, yielding sensitivity and specificity as high as 93% [40]. It also appears to have a higher accuracy than PIB-PET [43] (85 and 75%, respectively) in the distinction of normal versus mild cognitive impairment (MCI), a prodromal stage of dementia [44, 45] characterized by milder abnormalities in the same areas except for frontal cortex [46–53]. Last, in MCI and AD, it has been demonstrated that metabolism reductions exceed volume losses [38].
An FDG-PET endophenotype of AD has been described both in carriers of allele ApoE E4 and in subjects with a maternal family history of AD. This metabolic profile is similar and milder in cognitively normal than in symptomatic patients.
Although a precise definition of the concept is rarely given in the literature, the expression “endophenotype” is interchangeably used as “surrogate marker”, suggesting that manifestation of the endophenotype in asymptomatic subjects foreshadows clinical decline and could be used as a “surrogate endpoint” for assessing prevention treatment efficacy [54, 55]. In the event that all subjects who present the endophenotype decline to AD (positive predictive accuracy), preclinical metabolic abnormalities should be considered a diagnostic (or state) marker for the disease (after considering the negative predictive accuracy or specificity). This critical issue is discussed below.
The goals of this article are: (1) to review FDG-PET studies in populations potentially manifesting a heritable metabolic endophenotype of AD, i.e., normal subjects with family history (FH) of LOAD or with the known ApoE E4 genetic risk; (2) to characterize the metabolic pattern of an FDG-PET endophenotype of AD; (3) to weigh the rationale for considering the FDG-endophenotype a predictor of future decline to LOAD; (4) to discuss other applications of the FDG-endophenotype in AD.
The FDG-PET endophenotype
A large body of FDG-PET studies has been performed in AD and MCI patients; however, there is a scarcity of studies in asymptomatic subjects genetically at risk for LOAD. A total of ten FDG-PET studies have been published to date, eight in E4 carriers [56–63] and two in first-degree relatives of AD patients [64, 65]. These studies are summarized in Table 1.
Table 1.
FDG-PET studies in clinically and cognitively normal subjects
| Reference | Study design | Subjects | Method | Regions with CMRglc reductions | Interpretation | |
|---|---|---|---|---|---|---|
| Small et al. [63] | Cross-sectional study | 31 SCI and FH–subjects aged 44–67 years old: 12 E4+, 19E4− | ROI | Parietal (only ROI investigated) | N/A | |
| Reiman et al. [56] | Cross-sectional study | 33 normal subjects FH+ aged 50–62 years old: 11 E4+ homozygotes, 22 E4− | ROI | Frontal | Acceleration of certain aging processes that herald the onset of Alzheimer’s dementia | |
| Temporal | ||||||
| Parietal | ||||||
| Posterior cingulate cortex (effect is maximal) | ||||||
| Small et al. [57] | Longitudinal study over 2 years | At baseline, 54 normal subjects aged 57–75 years old: 27 E4+, 27E4− | SPM + ROI | Parietala | N/A | |
| Temporala | ||||||
| Posterior cingulate cortexa | ||||||
| At follow-up, 20 normal subjects: 10 E4+, 10 E4− | ||||||
| Reiman et al. [58] | Longitudinal study over 2 years | 25 normal FH+ aged 50–63 years old: 15 E4+ heterozygotes, 10 E4− | ROI | Frontal | Interaction between E4 allele and aging rather than a static trait Acceleration of aging processes, necessary but not sufficient for AD | |
| Temporal | ||||||
| Posterior cingulate cortex | ||||||
| Parahippocampal gyrus, | ||||||
| Hippocampal formation | ||||||
| Fusiform gyrus | ||||||
| OccipitoTemporal Cortex | ||||||
| Lingual gyrus | ||||||
| Basal forebrain | ||||||
| Thalamus | ||||||
| Midbrain | ||||||
| Cerebellum | ||||||
| Lentiform nucleus | ||||||
| Reiman et al. [59] | Cross-sectional study | 27 normal subjects aged 25–36 years old: 12 E4+ heterozygotes, 15E4− | ROI | Frontal | AD pathology Functional alterations provide a foothold (vulnerability) for subsequent onset of neuropathology Prenatal or early postnatal abnormal neurological development | |
| Temporal | ||||||
| Parietal | ||||||
| Posterior cingulate cortex | ||||||
| Reiman et al. [60] | Cross-sectional study | 160 normal subjects aged 47–68 years old: 82 E4+ (36 homozygotes, 46 heterozygotes), 78 E4− | SPM + ROI | Frontal | N/A | |
| Parietal, Precuneus | ||||||
| Temporal | ||||||
| Posterior cingulate cortex | ||||||
| Rimajova et al. [61] | Cross-sectional study | 30 E4+ normal or SCI subjects aged 55–78 (26 heterozygotes, 4 homozygotes, 23 healthy and 7 SCI), 30 healthy controls age-matched individuals from a database | VOI | Temporal | AD pathology | |
| Posterior cingulate cortex | ||||||
| Anterior cingulate cortex | ||||||
| All effects are higher in heterozygotes | ||||||
| (Japanese database of non demented elderly people aged 60–90 whose E4 status are unspecified) | ||||||
| Mosconi et al. [62] | Cross-sectional study | 28 normal or SCI subjects aged 45–70 years old: 13 E4+, 15 E4- | SPM | Frontal | N/A | |
| Parietal | ||||||
| Temporal | ||||||
| Fusiform gyrus | ||||||
| Middle occipital gyrus | ||||||
| Thalamus | ||||||
| Mosconi et al. [64] | Cross-sectional study | 49 FH+ normal or SCI subjects aged 46–80 years old: 16 FHm, 8 FHp, 25 FH- | SPM | FHm vs. FH−: | FHm vs. FHp: | Hypometabolism in FHm may be due to mitochondrial deficits, genetic imprinting, chromosome X-related mechanisms, or other causes |
| Frontal | – | |||||
| Parietal | Parietal | |||||
| Temporal | Temporal | |||||
| Posterior cingulate cortex | Posterior cingulate cortex | |||||
| Parahippocampal gyrus | Parahippocampal gyrus | |||||
| Hippocampus | Hippocampus | |||||
| Mosconi et al. [65] | Longitudinal 2-year study | 66 normal or SCI subjects aged 50–82 years old: 20 FHm, 9 FHp, 37 FH– | SPM | FHm vs. FH−: | FHm vs. FHp: | CMRglc decline may be due to a pathologic process that precedes cognitive deficit in healthy elderly |
| Frontal | Frontal | |||||
| Parietala | Parietal | |||||
| Temporala | Temporal | |||||
| Posterior cingulate cortexa | Posterior cingulate cortex | |||||
| Parahippocampal gyrus | Parahippocampal gyrus | |||||
| – | Hippocampus | |||||
Significantly higher rate of decline over 2 years
E4+ ApoE E4 carriers, E4− ApoE E4 non-carriers, FH+ family history of AD in at least one first-degree relative, FHm maternal FH, FHp paternal FH, CMRglc Cerebral metabolic rate of glucose, SCI subjective cognitive impairment, FH+ family history of AD in at least one first-degree relative, ROI region of interest, VOI volume of interest, SPM statistical parametric mapping, N/A not available
Studies in E4 carriers
FDG-PET studies in asymptomatic E4 carriers include six cross-sectional studies [56, 59–63] and two 2-year longitudinal studies measuring the rate of metabolic decline [57, 58]. Overall, the general common pattern is a CMRglc reduction in the temporal and parietal cortex, PCC and prefrontal cortex [55, 59–62]. Furthermore, longitudinal studies [57, 58] show that metabolism declines at a higher rate in temporoparietal cortices and PCC in E4 carriers compared to non-carriers, whereas metabolism in brain regions typically spared by AD pathology does not show ApoE E4 effects, indicating specificity to AD.
Studies in maternal family history subjects
Two FDG-PET studies have been published in asymptomatic individuals with parental family history (FH) of LOAD [64, 65]. The originality of these studies resides in the evaluation of parental gender effect, dividing individuals with a positive FH (FH+) into those with maternal (FHm) or paternal (FHp) FH of the disease. Compared to controls with a negative FH of dementia, the FHm group show hypometabolism in the temporoparietal, PCC, pre-frontal cortices, Hip and parahippocampal gyrus (PHG) compared to FHp and controls, whereas no regions showed metabolic differences between FHp and controls. Notably, these effects remain significant after controlling for ApoE status and other confounding factors such as age, gender, subjective cognitive impairment (SCI) or level of education. Longitudinally, over a 2-year follow-up interval, the FHm group shows significantly greater decline in temporal and parietal cortices and PCC compared to FHp and controls [65], indicating specificity to AD. As with the cross-sectional data, the FHp group shows no regions of increased metabolic loss over time relative to controls.
Characterization of the FDG-PET endophenotype of Alzheimer’s disease
Although comparability of patterns is limited due to the heterogeneity of methods between studies—region of interest (ROI) or voxel-based analysis with statistical parametric mapping (SPM), the CMRglc cross-sectional findings in E4 and FHm groups seem to be similar and for the most part overlapping, defining a pattern of hypometabolism in: prefrontal, temporal, parietal cortices and PCC. A large body of studies reports a similar pattern in AD patients [34, 40, 41] where the prefrontal cortex tends to be involved in more advanced patients [42]. Of interest, PHG, and Hip seem to be more consistently affected in FHm than in E4 carriers [63, 64], the latter region being reported to decline in most early stages of AD [37–39].
FDG-PET studies show a common pattern of hypometabolism in asymptomatic individuals at genetic risk in the same regions as reported in LOAD patients. The two populations found to manifest with this endophenotype being E4 carriers (homozygote or heterozygote) and children of AD mothers, should this trait reflect a genetic risk, it may not follow a simple Mendelian transmission. It is also found in these subjects and their relatives at a higher rate than in the general populations. Finally, the fourth criterion for an endophenotype is its state-independence, meaning that an endophenotype is not found only in subjects actually having the disease, but is found in the whole group of subjects at genetic risk. Although the concepts of endophenotype and predictive/diagnostic marker should remain distinct, the predictive value of an FDG-endophenotype with regards to AD has to be discussed.
Interpretation of the endophenotype
Discrepancy of the FDG pattern across ApoE4 and FHm studies: are there one or two endophenotypes?
FDG reports in ApoE4 carriers focused mainly on CMRglc changes in the neocortical regions typically affected by AD, such as the posterior cingulate, frontal and parieto-temporal cortices [55–58, 62] while studies in individuals with a maternal history of LOAD showed hypometabolism in AD-vulnerable neocortical regions as well as in medial temporal lobes (MTL). This additional finding is of interest as the MTL are early affected in AD. There are several possible explanations for the discrepancy across studies. First, previous studies focused specifically on AD-neocortical regions, while we also included the MTL as a region of interest in all analyses. Reiman and colleagues [58] listed the MTL as an affected region in ApoE-4 carriers, although this result was not emphasized as much as alterations in neocortex. Second, it is possible that the use of different image analysis software may have led to somewhat different results. For instance, we have demonstrated that Statistical Parametric Mapping, at least the 1999 version, underestimated hypometabolism in the MTL due to imprecise coregistration and spatial normalization in aging and dementia [42]. Another imaging software used in previous reports, Neurostat, does not allow one to specifically evaluate the MTL as results are assessed using cortical surface projection maps [58, 59]. Third, individuals with a maternal history of AD may be more prone to developing MTL damage than ApoE-4 carriers, although the biological substrates responsible for this effect are currently unknown. There may be more FDG-PET endophenotypes depending on the risk factor under study, or one AD-specific FDG-PET endophenotype (posterior cingulate, parieto-temporal and MTL hypometabolism) with some variability depending on the subjects’ ApoE genotype and family history, which may differentially affect AD-related regions.
Is the FDG-endophenotype a diagnostic marker of the disease?
FDG-PET data indicate that the E4 allele is associated with cortical CMRglc reductions that seem to be present in E4 carriers regardless of their cognitive status (asymptomatic, MCI or AD). This suggests a practical question: how much does the ApoE genotype impact on the detection of an abnormal pattern of hypometabolism? In other words, when examining a PET scan of an E4 carrier, if hypometabolism is present, is it to be considered a sign of AD or only a genetic consequence with no clinical implications?
The basis for viewing FDG-endophenotype as a marker of AD progression [58] in the case of ApoE4 is contextual. Historically, genetic linkage between allele ApoE E4 and AD preceded the characterization of an endophenotype [56]. In addition, ApoE E4 has been calculated to account for the majority of heritability (estimated to reach 80% [66]) for the “sporadic” LOAD [67], illustrated by a prevalence of E4 allele reaching 40% in AD, compared to 14% in non-AD population [24, 68]. Biochemical studies demonstrated the critical role of ApoE4 at several levels of the disease: in neuronal repair [69], Aβ fibrillization, plaque formation [70] and decreasing brain–blood clearance [71]. All these effects presumably accumulate and translate into an increased risk of the disease, threefold with one E4 copy, and 15-fold with two copies [69], and by reducing the age of onset by almost 10 years for each copy of the allele [23]. Furthermore, several studies corroborate the relationship between ApoE E4 and AD by demonstrating AD-related abnormalities in asymptomatic E4 carriers compared to non-carriers: cognitive performance of carriers is decreased as early as in adolescence [26], and fMRI studies reveal increased effort during memory tasks in carriers [72, 73]. Finally, CSF and PIB studies show evidence for higher levels of amyloid deposition [29, 74, 75] in E4 carriers compared to controls.
Although the contribution of other candidate genes for LOAD is probably minor [76], the prominence of ApoE E4 has now been nuanced by the observation of an FDG-endophenotype of AD [64, 65] in children of affected mothers controlled for ApoE E4. These preliminary FDG-PET observations are complemented and extended by an MRI study [30] that demonstrated significantly reduced gray matter volumes in AD-vulnerable regions (frontal cortex, precuneus and lingual gyrus) in FHm group compared to FHp and control groups. Additionally, a recent PIB-PET study showed that cognitively normal FHm have more severe and widespread fibrillar amyloid-beta deposits than demographically matched FHp group and controls [33]. These findings remained significant controlling for ApoE genotype. While specific genes remain to be identified, there is a growing body of evidence for a prevailing maternal transmission [76–78] in LOAD families. It was demonstrated in 1996 [77] that in AD patients with one affected parent, the ratio of AD mother:father is 3:1, and that it increases to 9:1 when patients have in addition two or more affected siblings. A review of the literature estimated that up to 30% of all LOAD cases are maternally inherited [76]. Several possible inheritance mechanisms may be involved in selective hypometabolism in FHm, including X-linked transmission, gender-dependent genomic imprinting, and mitochondrial inheritance. Among these, mitochondrial DNA (mtDNA) is inherited solely from the mother in humans and is transmitted equally to siblings. In the last 20 years, evidence has accumulated that AD is associated with mitochondrial dysfunction, oxidative stress and increased reactive oxygen species (ROS) production, as a consequence of reduced cytochrome oxidase (COX) activity [79, 80], which also may enhance Aβ toxicity [81]. A heritable deficient energy metabolism could by itself account for the functional and structural abnormalities found in children of AD mothers, which does not exclude the possibility of early subclinical consequences of AD pathology in some of these subjects [74].
“Trait marker” versus “state marker”: a conservative definition of “endophenotype”
In the recent years, a gradual shift has led to conflate the terms endophenotypes of AD with early markers of AD [27, 29]. We regard this as a conceptual fallacy contributing to the confusion between the concepts of “trait marker” and “state marker” of a disease [82], the former referring to an enduring and “state-independent” phenotype and the latter to the punctual expression of pathology. Such a simplification may be due to five main factors: (1) the high accuracy of FDG-PET in the diagnosis and prediction of both AD and MCI; (2) the almost mechanistic relationship between ApoE E4 and AD; (3) the concept of endophenotype itself—which allows being considered a vulnerability marker or measurement of risk for a disease [7, 8]; (4) its potential contribution to the study of disease mechanisms [6]; (5) and the fact that, for some authors, it can be age-normed [13], which places the endophenotype on a timeline between the gene and the disease (see Fig. 1).
From an epidemiological perspective, the relationship to AD for both ApoE E4 and maternal family history is limited; a large proportion of asymptomatic subjects carrying the risk factors do not develop AD, which suggests both partial penetrance and probable involvement of several other factors, genetic or not. Allele E4 and FHm status thus appear to act as risk modifiers rather than risk determinants for the disease. From an imaging standpoint, no longitudinal studies have yet been specifically conducted in subjects who are genetically at risk to monitor the evolution of the FDG pattern from the asymptomatic stage to MCI or AD. One longitudinal study followed 30 MCI patients over 16 months to evaluate the predictive value of ApoE status (n = 17 E4 carriers) and FDG-PET abnormalities for decline from MCI to AD [50]. Among the 17 E4 carriers a total of 12 declined to AD at study end. While FDG-PET and ApoE E4 genotype taken independently showed limited positive predictive values (PPV), the combination of the two risk factors (i.e., low metabolism in E4 carriers) increased PPV to 100%. This study highlights the pathological significance of reduced brain metabolism in a population predisposed to AD not only genetically but also due to their ongoing cognitive decline and a mean age of 70 years old. However, predictive value of hypometabolism in younger and asymptomatic subjects may be lower than in the elderly with MCI [57].
Three longitudinal FDG-PET studies [37, 39, 83] that followed individuals including some ApoE4 carriers from an asymptomatic stage to MCI or AD have been published, showing early involvement of the entorhinal cortex [37], Hip [39] and angular gyrus [83] to be highly predictive of future decline. Apart from the fact that these studies were conducted in elderly subjects (mean age = 67–72 years old), they were not designed to show differences in the pattern of metabolic decline between E4 carriers and noncarriers. In a clinico-pathologic series of cases with longitudinal FDG-PET exams, two cases were both carriers of the E4 allele and were followed from normal cognition to postmortem-confirmed AD [84]. On FDG-PET, both cases showed a progressive pattern of hypometabolism starting in Hip during the preclinical stage and spreading to cortical areas during progression to MCI and AD. These two E4 carriers did not present the typical AD pattern of cortical hypometabolism at the preclinical stage. Instead, hypometabolism was restricted to the medial temporal lobe (MTL) at the earliest stage of decline, and cortical hypometabolism reached statistical significance at the MCI stage of disease. These findings are consistent with previous studies showing early involvement of MTL in future decliners to AD, regardless of their genotype [37, 39]. No definitive conclusion can be drawn from these findings, as the sample size is too limited. Prevalence studies are warranted to evaluate individual variability in the expression of the potential FDG-endophenotype in E4 carriers and FHm subjects.
Until clinical outcome longitudinal studies are conducted, we caution that an FDG-endophenotype as defined above should be interpreted as an increased risk for developing AD dementia rather than a surrogate marker of the disease. In other words, the endophenotype could be seen as a “marker of risk” for AD, but evidence is still needed to consider it a marker of clinical outcome (“surrogate endpoint”).
Pathophysiology of the FDG-PET endophenotype
Age being established as the most important variable in AD processes and susceptibility, one can wonder whether findings of FDG-endophenotype in young adults may be interpreted as equivalent to hypometabolism occurring later in life. Reiman et al. [59] suggest that metabolic deficits in E4 carriers aged 25–36 years old may be due to a reduction in activity or density of terminal neuronal fields innervating the implicated regions. Pathological and imaging studies [85–87] suggest that FDG abnormalities in young adults, should the pathology have already started, may be due to functional rather than structural consequences of the disease. These early functional alterations could be a potential “foothold” for the subsequent onset of AD pathology in the same brain regions [58].
Other authors also suggested that metabolic changes may locally hasten AD progression. Buckner et al. [88] observed an important overlap between areas of preferential amyloid deposition and a pattern of regions known as the “default neural network” [89]—regions that are more active in passive states. Recently, findings of highly active regions (interconnecting nexuses) located in the same default network suggest that regions that are characterized by lifelong high levels of metabolism (i.e. active both during active tasks and at rest) may preferentially be vulnerable to AD pathology [88].
Future directions
In search for causative genes
It is believed that an important part of the genetically driven risk factors for LOAD remains to be unraveled [66]. The construct of endophenotype was originally designed for identifying genes. In this respect, mitochondrial genetic risk modifiers for LOAD have recently been suggested by evidence of findings of the FDG-endophenotype in children of AD mothers. Furthermore, although showing weaker association than ApoE E4, several other genes have demonstrated significant association with AD or its endophenotypes, e.g., episodic memory, hippocampal atrophy or plasma AB [90]. As reviewed by Glahn et al. [91], combining the fields of neuroimaging—based on a quantitative trait—and genetics presents several advantages and has proven more effective than an approach purely based on clinical diagnosis. Genome-wide association studies have recently shown strong evidence of novel AD genetic risk factors providing new insight into pathological pathways; for example: CLU, coding for clusterin (also known as ApoJ), implicated in fibril formation and Aβ clearance, and PICALM, involved in synaptic function and in the modulation of immune response [92]. Analysis of the genetic variants associated with AD would benefit from the use of endophenotypes. Using entorhinal cortex thickness as anendophenotype of AD, Saykin et al. [93] recently identified a single-nucleotide polymorphism of the gene PICALM associated with AD.
One limitation of classical case–control studies resides in the large size of sample required to detect gene effects. This is especially the case in LOAD, given the modest effect of new candidate genes, as compared to ApoE. Studies based on quantitative traits or continuous phenotypes have the advantage of both decreasing sample size requirements and increasing power. In the same way, as previously suggested by the authors [73], FDG-endophenotype could be used for investigating mtDNA or other genetic mechanisms in LOAD by pre-selecting homogeneous populations to increase the power of haplogroup and subhaplogroup-association studies.
In search for protective factors
In our analysis, we identify several factors contributing to the fallacy of interpreting an FDG-endophenotype as a marker for the disease. A second shortcut may reside in the assumption that a subject at risk, FHm or E4 carrier, systematically present with the FDG-endophenotype. Yet, as all available studies provide only group data, the prevalence of the AD pattern in these populations is unknown. Studies of the prevalence of the AD hypometabolic pattern in E4 carriers and FHm populations are thus warranted to provide useful information about individual variability as to the expression of metabolic effects.
A reversed application of endophenotype focusing on group of individuals genetically at risk (due to their ApoE status or to their maternal family history) but who do not express the endophenotype may help disentangle the causal relationship between endophenotype and AD pathology and lead to the identification of protective factors for AD— whether genetic or environmental. While aside from ApoE E2 [94, 95], no other genetic protective factor has yet been identified; a critical advance rests on the discovery of protective genes and preventive interventions and the exploration of their mechanism of action. Lack of metabolic deficits in cognitively normal FHp individuals as compared to FHm subjects also suggests protective mechanisms, although this remains to be investigated. This approach may eventually lead to the development of disease-modifying drugs.
Conclusion
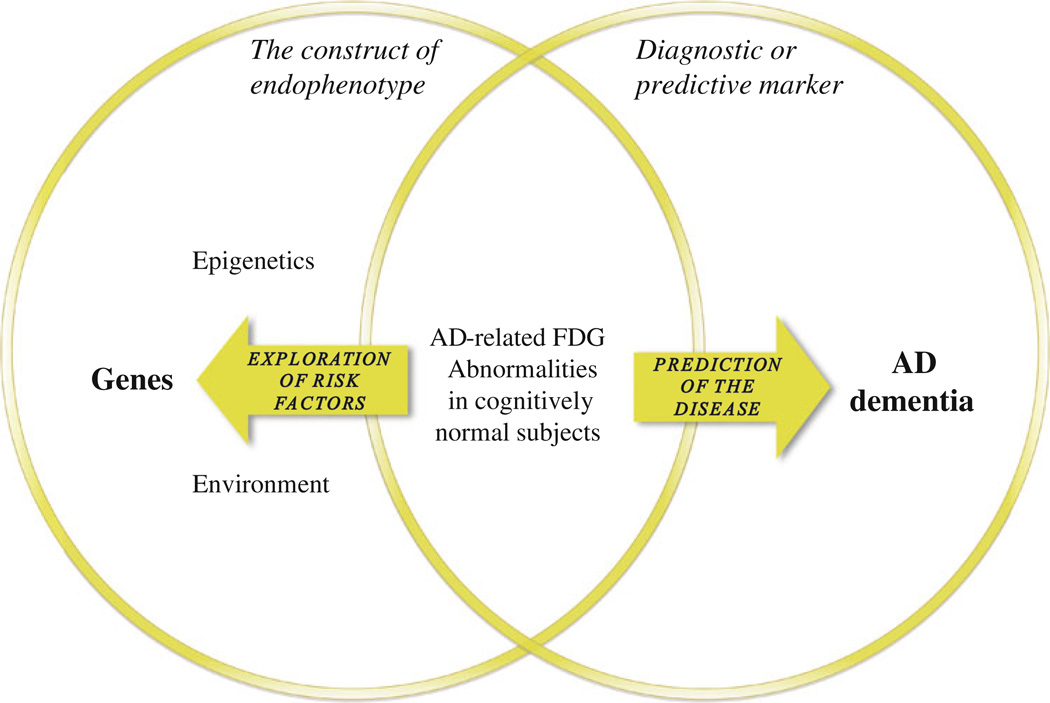
This review aimed to assess the validity of an FDG-endophenotype of LOAD and to discuss its application and significance with regards to the disease. We found with high consistency an FDG pattern of glucose hypometabolism in two subsets of populations: asymptomatic E4 carriers and subjects with a maternal family history of AD. This pattern of three regions—temporo-parietal cortices and PCC—that may be extended to MTL, seems to be heritable and specific to the disease, since it is otherwise only reported in patients diagnosed with AD or the prodromal stage of MCI. However, this metabolic finding meets the criteria for an endophenotype only when it is heritable or applied for investigating genetic risk factors (see Fig. 2). This criterion is essential for discerning two distinct frameworks: (1) the construct of endophenotype, where findings of AD-related metabolic abnormalities in asymptomatic subjects are considered in their relation to a genotype. This framework is directed toward the discovery of genetic or epigenetic risk factors, upstream to the FDG-endophenotype on the causal chain, oriented right to left; (2) the framework of preclinical diagnostic marker of the disease, where the same FDG finding is considered for its predictive value for AD. This framework is oriented left to right, downstream to what could rather be called an “intermediate trait”, and is applicable to any subjects regardless of their familial background, i.e., the population at large. The confusion may arise when one conceptual framework is taken for the other, illustrating that an AD-related FDG finding with preserved cognition may manifest—especially in advanced age—both a genetic risk and preclinical signs of pathology. To clarify communication in future research, we suggest that the term “endophenotype” only refer to the construct of endophenotype as defined above.
Fig. 2.
Two overlapping frameworks. The construct of endophenotype (left circle), where findings of AD-related metabolic abnormalities in asymptomatic subjects are considered in their relation to a genotype or familial background. This framework is directed toward the discovery of genetic and epigenetic risk factors, and is oriented right to left. The framework of preclinical diagnostic/predictive marker of the disease (right circle), where the same FDG-PET findings are considered in their predictive value for AD. This framework, oriented left to right, is applicable to any subject regardless of his familial background, i.e., the population at large
Finally, we suggest other applications of the FDG-endophenotype. First, as originally designed, an endophenotype represents a useful proxy for evaluating genetic risk for the disease, with several advantages including diminution of sample size requirements and increased power. Second, all reviewed FDG-PET studies compare group data, not individual data, and thus do not measure individual variability. A significant breakthrough could result from studying E4 carriers or FHm subsets of populations who do not manifest the endophenotype and/or do not decline to MCI/AD. This inversion of paradigm may allow for studying protective effects—whether genetic or environmental—in individuals who are otherwise genetically more expected to decline. Exploring these protective mechanisms could pave the way for the development of more efficient therapeutic interventions, whether secondary prevention with disease-modifying drugs or primary prevention hindering the first steps of the pathological cascade of AD.
Contributor Information
Emmanuel H. During, Center for Brain Health, Silberstein Alzheimer’s Institute, Center of Excellence on Brain Aging at NYU Langone Medical Center, NYU School of Medicine, 145 East 32nd Street, New York, NY 10016, USA, emmanuel.during@nyumc.org
R. S. Osorio, Center for Brain Health, Silberstein Alzheimer’s Institute, Center of Excellence on Brain Aging at NYU Langone Medical Center, NYU School of Medicine, 145 East 32nd Street, New York, NY 10016, USA Alzheimer’s Disease Research Unit, CIEN Foundation-Carlos III Institute of Health, Alzheimer Center Reina, Madrid, Spain.
F. M. Elahi, Department of Neurology, University of California, Los Angeles (UCLA), Los Angeles, CA, USA Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford, UK.
L. Mosconi, Center for Brain Health, Silberstein Alzheimer’s Institute, Center of Excellence on Brain Aging at NYU Langone Medical Center, NYU School of Medicine, 145 East 32nd Street, New York, NY 10016, USA
M. J. de Leon, Center for Brain Health, Silberstein Alzheimer’s Institute, Center of Excellence on Brain Aging at NYU Langone Medical Center, NYU School of Medicine, 145 East 32nd Street, New York, NY 10016, USA Nathan Kline Institute, Orangeburg, NY, USA.
References
- 1.Brookmeyer R, et al. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Ohm TG, et al. Close-meshed prevalence rates of different stages as a tool to uncover the rate of Alzheimer’s disease-related neurofibrillary changes. Neuroscience. 1995;64(1):209–217. doi: 10.1016/0306-4522(95)90397-p. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 4.Larson EB, et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140(7):501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 5.Mo¨lsa¨ PK, Marttila RJ, Rinne UK. Survival and cause of death in Alzheimer’s disease and multi-infarct dementia. Acta Neurol Scand. 1986;74(2):103–107. doi: 10.1111/j.1600-0404.1986.tb04634.x. [DOI] [PubMed] [Google Scholar]
- 6.Reiman EM. Linking brain imaging and genomics in the study of Alzheimer’s disease and aging. Ann N Y Acad Sci. 2007;1097:94–113. doi: 10.1196/annals.1379.011. [DOI] [PubMed] [Google Scholar]
- 7.Bearden CE, Freimer NB. Endophenotypes for psychiatric disorders: ready for primetime? Trends Genet. 2006;22(6):306–313. doi: 10.1016/j.tig.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Leboyer M, et al. Psychiatric genetics: search for pheno-types. Trends Neurosci. 1998;21(3):102–105. doi: 10.1016/s0166-2236(97)01187-9. [DOI] [PubMed] [Google Scholar]
- 9.Sunderland T, et al. Biomarkers in the diagnosis of Alzheimer’s disease: are we ready? J Geriatr Psychiatry Neurol. 2006;19(3):172–179. doi: 10.1177/0891988706291088. [DOI] [PubMed] [Google Scholar]
- 10.John B, Lewis KR. Chromosome variability and geographic distribution in insects. Science. 1966;152(3723):711–721. doi: 10.1126/science.152.3723.711. [DOI] [PubMed] [Google Scholar]
- 11.Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122(566):15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- 12.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 13.Hasler G, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Cadenhead KS, et al. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am J Psychiatry. 2000;157(10):1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- 15.Braff D, Geyer M, Swerdlow N. Human studies of pre-pulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 16.Braff D, Freedman R. Endophenotypes in studies of the genetics of schizophrenia. In: Davis K, et al., editors. Neuropsychopharamcology: the fifth generation of progress. 5th edn. Philadelphia: Lippincott Williams and Wilkins; 2002. pp. 703–716. [Google Scholar]
- 17.Diefendorf A, Dodge R. An experimental study of the ocular reactions of the insane from photographic records. Brain. 1908;31:451–489. [Google Scholar]
- 18.Lee K, Williams L. Eye movement dysfunction as a biological marker of risk for schizophrenia. Aust N Z J. 2000;34(Suppl):91–100. doi: 10.1080/000486700228. [DOI] [PubMed] [Google Scholar]
- 19.Freedman R, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arolt V, et al. Eye tracking dysfunction is a putative phenotypic susceptibility marker of schizophrenia and maps to a locus on chromosome 6p in families with multiple occurrence of the disease. Am J Med Genet. 1996;67(6):564–579. doi: 10.1002/(SICI)1096-8628(19961122)67:6<564::AID-AJMG10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Gasperoni TL, et al. Genetic linkage and association between chromosome 1q and working memory function in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2003;116B(1):8–16. doi: 10.1002/ajmg.b.10757. [DOI] [PubMed] [Google Scholar]
- 22.Callicott JH, et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003;160(4):709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- 23.Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 24.Poirier J, et al. Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet. 1993;342(8873):697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 25.Cupples LA, et al. Estimating risk curves for first-degree relatives of patients with Alzheimer’s disease: the REVEAL study. Genet Med. 2004;6(4):192–196. doi: 10.1097/01.gim.0000132679.92238.58. [DOI] [PubMed] [Google Scholar]
- 26.Bloss CS, et al. Decreased cognition in children with risk factors for Alzheimer’s disease. Biol Psychiatry. 2008;64(10):904–906. doi: 10.1016/j.biopsych.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponomareva NV, Korovaitseva GI, Rogaev EI. EEG alterations in non-demented individuals related to apolipoprotein E genotype and to risk of Alzheimer disease. Neurobiol Aging. 2008;29(6):819–827. doi: 10.1016/j.neurobiolaging.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Younkin SG, et al. Genetic elevation of plasma amyloid β protein in typical late onset Alzheimer’s disease. Abstr Soc Neurosci. 1998;24:263. [Google Scholar]
- 29.Sunderland T, et al. Cerebrospinal fluid beta-amyloid1–42 and tau in control subjects at risk for Alzheimer’s disease: the effect of APOE epsilon4 allele. Biol Psychiatry. 2004;56(9):670–676. doi: 10.1016/j.biopsych.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Honea RA, et al. Reduced gray matter volume in normal adults with a maternal family history of Alzheimer disease. Neurology. 2010;74(2):113–120. doi: 10.1212/WNL.0b013e3181c918cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klunk WE, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 32.Reiman EM, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106(16):6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosconi L, et al. Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer’s. Proc Natl Acad Sci USA. 2010;107(13):5949–5954. doi: 10.1073/pnas.0914141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Leon MJ, et al. Computed tomography and positron emission transaxial tomography evaluations of normal aging and Alzheimer’s disease. J Cereb Blood Flow Metab. 1983;3(3):391–394. doi: 10.1038/jcbfm.1983.57. [DOI] [PubMed] [Google Scholar]
- 35.Magistretti P, et al. Energy on demand. Science. 1999;283(5401):496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- 36.Rocher AB, et al. Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: a study in baboons. Neuroimage. 2003;20(3):1894–1898. doi: 10.1016/j.neuroimage.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 37.de Leon MJ, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET) Proc Natl Acad Sci USA. 2001;98(19):10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Santi S, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22(4):529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 39.Mosconi L, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29(5):676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herholz K, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17(1):302–316. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 41.Alexander GE, et al. Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer’s disease treatment studies. Am J Psychiatry. 2002;159(5):738–745. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- 42.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med. Mol Imaging. 2005;32(4):486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, et al. Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment, and Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2008;35(12):2169–2181. doi: 10.1007/s00259-008-0833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reisberg B, et al. Mild cognitive impairment (MCI): a historical perspective. Int Psychogeriatr. 2008;20(1):18–31. doi: 10.1017/S1041610207006394. [DOI] [PubMed] [Google Scholar]
- 45.Gauthier S, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 46.Nestor PJ, et al. Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment. Ann Neurol. 2003;54(3):343–351. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- 47.Mosconi L, et al. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology. 2005;64(11):1860–1867. doi: 10.1212/01.WNL.0000163856.13524.08. [DOI] [PubMed] [Google Scholar]
- 48.Mosconi L, et al. Visual rating of medial temporal lobe metabolism in mild cognitive impairment and Alzheimer’s disease using FDG-PET. Eur J Nucl Med Mol Imaging. 2006;33(2):210–221. doi: 10.1007/s00259-005-1956-z. [DOI] [PubMed] [Google Scholar]
- 49.Minoshima S, et al. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42(1):85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 50.Drzezga A, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30(8):1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 51.Mosconi L, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63(12):2332–2340. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- 52.Drzezga A, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med. 2005;46(10):1625–1632. [PubMed] [Google Scholar]
- 53.Chételat G, et al. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60(8):1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 54.Reiman EM, Langbaum JBS, Tariot PN. Alzheimer’s prevention initiative: a proposal to evaluate presymptomatic treatments as quickly as possible. Biomark Med. 2010;4(1):3–14. doi: 10.2217/bmm.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Small GW. Diagnostic issues in dementia: neuroimaging as a surrogate marker of disease. J Geriatr Psychiatry Neurol. 2006;19(3):180–185. doi: 10.1177/0891988706291089. [DOI] [PubMed] [Google Scholar]
- 56.Reiman EM, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 57.Small GW, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97(11):6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reiman EM, et al. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98(6):3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reiman EM, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reiman EM, et al. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci USA. 2005;102(23):8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rimajova M, et al. Fluoro-2-deoxy-D-glucose (FDG)-PET in APOEepsilon4 carriers in the Australian population. J Alzheimers Dis. 2008;13(2):137–146. doi: 10.3233/jad-2008-13203. [DOI] [PubMed] [Google Scholar]
- 62.Mosconi L, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008;63(6):609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Small GW, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273(12):942–947. [PubMed] [Google Scholar]
- 64.Mosconi L, et al. Maternal family history of Alzheimer’s disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci USA. 2007;104(48):9067–9072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosconi L, et al. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72(6):513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gatz M, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63(2):168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 67.Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25(5):641–650. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 68.Saunders AM, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 69.Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer’s disease. Trends Neurosci. 1994;17(12):525–530. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 70.Holtzman DM, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97(6):2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bell RD, et al. Transport pathways for clearance of human Alzheimer’s amyloid betapeptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu G, et al. The influence of parental history of Alzheimer’s disease and apolipoprotein E 4 on the BOLD signal during recognition memory. Brain. 2008;132(2):383–391. doi: 10.1093/brain/awn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bookheimer SY, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prince JA, et al. APOE epsilon4 allele is associated with reduced cerebrospinal fluid levels of Abeta42. Neurology. 2004;62(11):2116–2118. doi: 10.1212/01.wnl.0000128088.08695.05. [DOI] [PubMed] [Google Scholar]
- 75.Morris JC, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67(1):122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mosconi L, et al. Maternal transmission of Alzheimer’s disease: prodromal metabolic phenotype and the search for genes. Hum Genomics. 2010;4(3):170–193. doi: 10.1186/1479-7364-4-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Edland SD, et al. Increased risk of dementia in mothers of Alzheimer’s disease cases: evidence for maternal inheritance. Neurology. 1996;47(1):254–256. doi: 10.1212/wnl.47.1.254. [DOI] [PubMed] [Google Scholar]
- 78.Gómez-Tortosa E, et al. Variability of age at onset in siblings with familial Alzheimer disease. Arch Neurol. 2007;64(12):1743–1748. doi: 10.1001/archneur.64.12.1743. [DOI] [PubMed] [Google Scholar]
- 79.Parker WD, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40(8):1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 80.Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20(Suppl 2):S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cardoso SM, et al. Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Abeta toxicity. J Neurochem. 2004;89(6):1417–1426. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y, Bidwell LC, Norton D. Trait vs. state markers for schizophrenia: identification and characterization through visual processes. Curr Psychiatry Rev. 2006;2(4):431–438. doi: 10.2174/157340006778699729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jagust W, et al. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann Neurol. 2006;59(4):673–681. doi: 10.1002/ana.20799. [DOI] [PubMed] [Google Scholar]
- 84.Mosconi L, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36(5):811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gómez-Isla T, et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16(14):4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fjell AM, et al. Morphometric changes in the episodic memory network and tau pathologic features correlate with memory performance in patients with mild cognitive impairment. Am J Neuroradiol. 2008;29(6):1183–1189. doi: 10.3174/ajnr.A1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jack CR, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132(Pt 5):1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sleegers K, et al. The pursuit of susceptibility genes for Alzheimer’s disease: progress and prospects. Trends Genet. 2010;26(2):84–93. doi: 10.1016/j.tig.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 91.Glahn DC, Thompson PM, Blangero J. Neuroimaging endophenotypes: strategies for finding genes influencing brain structure and function. Hum Brain Mapp. 2007;28:488–501. doi: 10.1002/hbm.20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jun G, et al. Meta-analysis Confirms CR1, CLU, and PICALM as Alzheimer Disease Risk Loci and Reveals Interactions With APOE Genotypes. Arch Neurol (ahead of print) 2010 doi: 10.1001/archneurol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saykin AJ, et al. Alzheimer’s disease neuroimaging initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimer’s Dement. 2010;6(3):265–273. doi: 10.1016/j.jalz.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Corder EH, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7(2):180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 95.West HL, Rebeck GW, Hyman BT. Frequency of the apolipoprotein E [epsilon] 2 allele is diminished in sporadic Alzheimer disease. Neurosci Lett. 1994;175(1–2):46–48. doi: 10.1016/0304-3940(94)91074-x. [DOI] [PubMed] [Google Scholar]