Abstract
Owing to the worldwide increase in antibiotic resistance, researchers are investigating alternative anti-infective strategies to which it is supposed microorganisms will be unable to develop resistance. Prominent among these strategies, is a group of approaches which rely on light to deliver the killing blow. As is well known, ultraviolet light, particularly UVC (200–280nm), is germicidal, but it has not been much developed as an anti-infective approach until recently, when it was realized that the possible adverse effects to host tissue were relatively minor compared to its high activity in killing pathogens. Photodynamic therapy is the combination of non-toxic photosensitizing dyes with harmless visible light that together produce abundant destructive reactive oxygen species (ROS). Certain cationic dyes or photosensitizers have good specificity for binding to microbial cells while sparing host mammalian cells and can be used for treating many localized infections, both superficial and even deep-seated by using fiber optic delivered light. Many microbial cells are highly sensitive to killing by blue light (400–470 nm) due to accumulation of naturally occurring photosensitizers such as porphyrins and flavins. Near infrared light has also been shown to have antimicrobial effects against certain species. Clinical applications of these technologies include skin, dental, wound, stomach, nasal, toenail and other infections which are amenable to effective light delivery.
1. Introduction
The rising problem of antibiotic resistance has led to fears that medicine will return to the situation of a century ago when extensive wounds and surgery often led to death due to uncontrollable infection [1]. These fears have in turn spurred a major research effort to find alternative antimicrobial approaches which, it is hypothesized, will kill resistant micro-organisms while being unlikely to cause resistance to develop to themselves. At the present time many international research efforts to discovery new antimicrobials are underway. Recently, the emphasis is on how to take precautions against creating, and if possible eliminate, multidrug resistance in concert with exploring new methods to kill pathogenic microorganisms. Karen et al. recently pointed out that the investigation of novel non-antibiotic approaches, which can prevent and protect against infectious diseases should be encouraged, and should be looked upon as a high-priority for research and development projects [2].
Prominent among novel non-antibiotic approaches is likely to be the group of light-based technologies, including ultraviolet C (UVC) irradiation therapy, photodynamic therapy (PDT), blue light therapy and other light-based therapies. The most attractive advantages of light-based antimicrobial therapies lie in their ability to eradicate microbes regardless of antibiotic resistance, and the fundamental improbability of the microbes themselves developing resistance to these light based therapies due to the rather non-specific nature of the targets.
In this review, we will provide an overview of recent advances in exploration and understanding of light-based anti-infective therapies, including UVC, PDT, blue light and other promising light-based therapies. To our knowledge, this is the first time that there has been a review covering all these light-based therapies for infectious disease
2. Antimicrobial efficacy of UVC irradiation
2.1 Introduction
UV irradiation (wavelength: 100–400 nm) is electromagnetic irradiation, which is divided into four distinct spectral areas including: UVA (315–400 nm), UVB (280–315 nm), UVC (200–280 nm) and vacuum UV (100–200 nm) (Fig 1) [3]. Among these wavelength ranges, UVC has the best potential ability to inactivate microorganisms because the wavelength of 250–270nm, is strongly and mainly absorbed by the nucleic acids of microbial cells and, therefore is the most lethal range of wavelengths [4]. The bactericidal mechanism of UVC is to cause damage to their RNA and DNA, which often leads to the formation of dimers between pyrimidine residues in the nucleic acid strands. The consequence of this modification is that the production of cyclobutane pyrimidine dimers (CPDs) causes deformation of the DNA molecule, which might cause defects in cell replication and lead to cell death afterwards.
Figure 1.
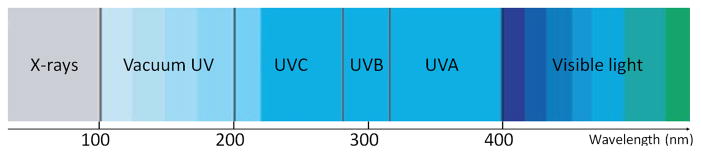
Spectrum of ultraviolet irradiation.
Because tissue penetration of light is limited by the extremely short wavelength and difficulties in the delivery approach, UVC for anti-infective diseases is likely to be applied exclusively to superficial infections. Studies that have investigated UVC inactivation of antibiotic-resistant bacteria have found them to be as equally sensitive as their naive counterparts [5].
2.2 UVC irradiation for bacterial/fungi infections in vivo
Reports have demonstrated that UVC efficiently inactivated methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecalis (VRE), antibiotic-susceptible strains of E. faecalis and S. aureus [5], Enterococci species, Streptococcus pyogenes [6], Escherichia coli and Pseudomonas aeruginosa in vitro [7]. UVC irradiation was able to disinfect catheters by destroying associated bacterial biofilms, including coagulase-negative Staphylococcus, Streptococcus, E. coli, E. faecalis, P. aeruginosa, Coryneforms and so on [8].
UVC also inactivated 3–5 log10 of dermatophyte suspensions in vitro, including Trichophyton mentagrophytes, Trichophyton rubrum, Microsporum canis and Epidermophyton floccosum [9]. When the inactivation efficacies of UVC were compared on pathogenic microorganisms, including bacteria (P. aeruginosa and Mycobacterium abscessus) and fungi (Candida albicans, Aspergillus fumigatus), in both mixed suspensions and single suspensions in vitro, it needed much higher fluences to kill fungi than bacteria [10]. The diverse structural features of the cell walls of bacteria and fungi are the main reason for the different killing rates. Different devices that deliver UVC with different power densities also induced different outcomes. More details about UVC germicidal efficacies are presented in Table 1.
Table 1.
Studies of UVC irradiation for inactivation of bacteria/fungi in vitro
| Light Source | Radiant Exposure | Bacterial/Fungi species/strains | Inactivation efficacy | Reference |
|---|---|---|---|---|
| 254nm UVC | 15.54 mW/cm2 | MRSA, VRE antibiotic-susceptible strains of S. aureus and E. faecalis | Illuminated 5 seconds, 99.9% MRSA and VRE inactivation; illuminated 9 seconds, 100% MRSA inactivation; illuminated 45 seconds, 100% VRE inactivation | 5 |
| 254nm UVC | 5mW/cm2 | MRSA, Streptococcus pyogenes | Illuminated 5 seconds, methicillin-resistant, coagulase-negative Staphylococcus and Streptococcus pyogenes inactivation; Illuminated 15 seconds, methicillin-susceptible S. aureus and Enterococci species inactivation | 6 |
| 265nm UVC | 1.93 mJ/cm2 | S. aureus, E. coli, Pseudomonas aeruginosa, S. pyogenes | Illuminated 1 seconds, 100% inhibition for all strains | 7 |
| 254nm UVC | 1500mJ/cm2 | catheter biofilms of E. coli, coagulase-negative Staphylococcus, E. faecalis, Streptococcus, P. aeruginosa, Coryneforms | Mean killing rates of the bacteria in catheter biofilms were 89.6% (11.8 mJ/cm2), 98% (47 mJ/cm2) and 99% (1500 mJ/cm2) | 8 |
| 254nm UVC | 120mJ/cm2 | Trichophyton rubrum, T. mentagrophytes, Epidermophyton floccosum, Microsporum canis. | 3–5 log10 of fungal inactivation | 9 |
| UVC | 15.54 mW/cm2 | bacteria (P. aeruginosa and Mycobacterium abscessus) and fungi (Candida albicans, Aspergillus fumigatus) | Illuminated 3–5 seconds, 99% bacteria inactivation; Illuminated 15–30 seconds, 99% fungi inactivation | 10 |
One of the key questions about the use of UVC as an anti-infective is the following [3]: Can UVC destroy microbial cells in tissue without causing irreversible and unacceptable severe damage to the host DNA and host cells? In an animal model infected by fungi with third-degree burn, C. albicans bioluminescence was showed a significant reduction by UV irradiation. The efficacy of fungicidal by UV was superior to using nystatin cream, one of topical antifungal drug [11]. Another animal study showed UVC irradiation (2.59 J/cm2 for burns and 3.24 J/cm2 for abrasions) significantly reduced Acinetobacter baumannii burden in UVC-treated wounds by approximately 10-fold compared with non-treated groups (p = 0.019 for burns, p = 0.004 for abrasions) [12].
2.3 Effects of UVC irradiation on host cells and tissues
It is well known that prolonged and repeated exposure to UV irradiation can damage host cells and be particularly hazardous to human skin. As to long-term UVC irradiation of human skin, it is also known to have potential carcinogenicity [13]. When UVC irradiation is applied to treat localized infections, one must consider the possible side-effects of UVC delivered at effective antimicrobial fluences on normal mammalian cells and tissue. The safety issue of UVC germicidal treatment requires that the pathogenic microbe is selectively eradicated while the normal tissue cells are spared.
Sosnin et al. convinced the UVC fluence that led to necrosis in Chinese hamster ovary cells, one type of fibroblasts, was more than ten-times higher than that used for killing of E. coli in vitro [14]. Dai et al. investigated UVC inactivated nearly all bacteria (99%) in suspensions over keratinocytes in confluent monolayer cultures, while it only reduced approximately 6% viability of keratinocytes [15]. Another study demonstrated that UVC selectively inactivated 2 logs (99%) of C. albicans, while only approximately 0.77 log10 (18.9%) of keratinocytes was killed at the same UVC fluence [11]. Dean et al. found that no significant adverse effects were induced in human primary corneal epithelial cells when the cells were exposed to 1.93mJ/cm2 UVC (265nm), which induced 100% inhibition of growth of all the bacterial species cultured on agar plates [7]. Mohr et al. used UVC to reduce pathogen contamination of platelet concentrates [16]. The results showed UVC inactivated more than 4log10 Gram-positive S. aureus, Bacillus cereus and S. epidermidis, and Gram-negative E. coli, P. aeruginosa and Klebsiella pneumoniae. However, UVC irradiation caused mild damage to platelet and leaded to more enhanced platelet metabolism, including enhancing glucose consumption, lactate accumulation and a stronger decrease in pH during storage.
In a study using a murine model, Dai et al. demonstrated that the mouse skin tolerated the UVC irradiation at the effective fungicidal fluence. Although UVC caused mild wrinkling of the skin at 24h after light exposure, it showed full recovery in the following days [11]. In order to evaluate the carcinogenicity of UVC, one animal study using hairless mice determined that UVC was less carcinogenic than UVB [17].
Most of the experimental results mentioned above suggest that UVC at appropriate fluences does not cause significant damages to host cells and tissues. However, UVC irradiation still has potential to induce nonspecific damage. Studies demonstrated that the DNA of mammalian cells could indeed be damaged by UVC at its effective antimicrobial fluences. Fortunately however, at the same time, the DNA repairing enzymes of the host cells could rapidly repair the damaged DNA [3]. In addition, to minimize the UVC-induced non-specific damage, the intact skin around the area to be treated could be shielded from UVC illumination. On the other hand, application of UVC is limited in some special locations due to its detrimental effects such as infections of the eyes [18].
2.4 The clinical application of UVC irradiation for infectious diseases
A few clinical studies have demonstrated the antimicrobial effect of UVC irradiation, including the disinfection of cutaneous wounds, promotion of wound healing in non-healing infected leg ulcers by increasing granulation tissue [19,20]. A study presented by Taylor et al., reported that the mean bacterial CFU in joint arthroplasty surgical wounds was reduced by 87% with 0.1 mW/cm2 (P < 0.001) and 92% with 0.3 mW/cm2 (P < 0.001) of UVC [21]. Thai et al. used UVC irradiation to treat cutaneous ulcers infected with MRSA [22]. In all three patients, UVC treatment reduced the bacterial burden in wounds and promoted wound healing. Two patients had complete wound closure following 1 week of UVC treatment. Another trial was carried out by the same investigators in 22 patients with chronic ulcers manifesting at least two signs of infection and critically colonized with bacteria. The patients received a single UVC treatment and demonstrated significantly reductions of the bacterial burden [23]. Boker et al. enrolled thirty patients with mild-to-moderate toenail onychomycosis to treat with UVC. Improvement by at least 1 measurement point was achieved in 60% of patient at 16-week follow-up compared with baseline. There were some unusual and slight side effects such as temporary mild eythema of the treated toe [24]. In addition to the inactivation of microbial cells in the cutaneous wound, UVC exposure is beneficial for wound healing by promoting the expression of basic fibroblast growth factor (bFGF) and transforming growth factor-β [25], although the exact mechanisms of UVC for wound healing is still unclear. Shimomura et al. investigated the prophylactic efficacies of UVC irradiation in 18 cases of catheter exit-site infections [26]. Although five cases remained unchanged, ten cases (55%) became culture negative and a further three cases showed a microbial decrease.
In summary, it has been known during the past one-hundred years that UVC irradiation is highly bactericidal; however, using UVC illumination for the prophylaxis and treatment of localized infections is still at very early stages of development. Most of the studies are limited to in vitro and ex vivo levels, while in vivo animal studies and clinical studies are much rarer. A major advantage of using UVC over antibiotics is that UVC can eradicate resistant and pathogenic microorganisms much more rapidly without any systemic side-effects. UVC may also be much more cost effective than the commonly used antibiotics.
3 Photodynamic therapy as an anti-infective
3.1 Introduction and mechanism of photodynamic therapy
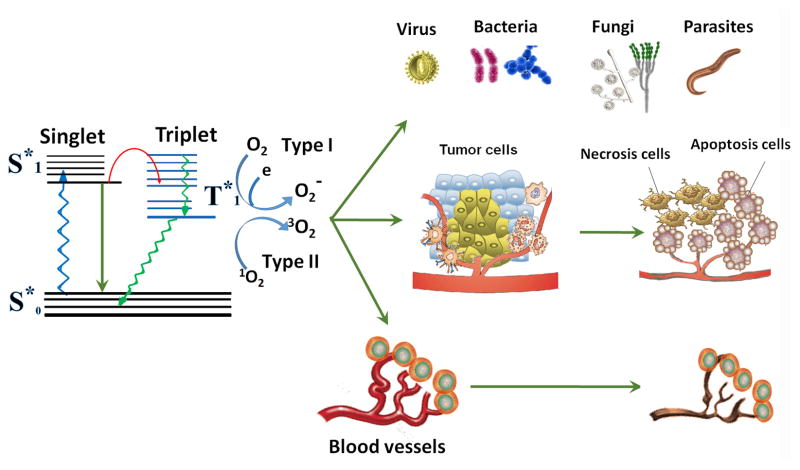
Another prominent member of the light-based therapies is known as antimicrobial photodynamic inactivation (PDI) or photodynamic therapy (PDT). PDT can direct injury to the target cells and tissues by producing reactive oxygen species (ROS) induced by the appropriate wavelength of visible light interacting with pre-applied photosensitizer (PS) drugs. The PS can be specifically activated by light of certain wavelength to its excited state. Interaction of the long-lived excited triplet state of the drug or dye with the ground state of molecular oxygen (also a triplet) results in development of ROS such as singlet oxygen (1O2) through energy transfer and hydroxyl radicals (HO•) through electron transfer. The formation of ROS and 1O2 can chemically attack a very wide range of biomolecules [27–30] (see Fig 2). The high selectivity of PDT for rapidly growing and hyperproliferating cells suggested it could be useful for selective inactivation of microorganisms which possess very fast growth rates [31]. Recently, PDT has been applied as a discovery and treatment alternative approach for localized infections, which represents an emerging new field [32]. This development has been motivated by the relentless growth in antibiotic resistance now found in all classes of microbial cells and in all countries of the world. The broad-spectrum and rapid killing effect of PDT is particularly appealing in this regard [33].
Figure 2.
Schematic illustration of photodynamic action. The PS initially absorbs a photon that excites it to the first excited singlet state and this can relax to the more long-lived triplet state. The triplet PS can interact with molecular oxygen in two pathways, Type I and Type II, leading to the formation of reactive oxygen species (ROS) and singlet oxygen 1O2, respectively. The formation of ROS and 1O2 can chemically attack a very wide range of biomolecules.
3.2 Antibacterial efficacy of photodynamic therapy
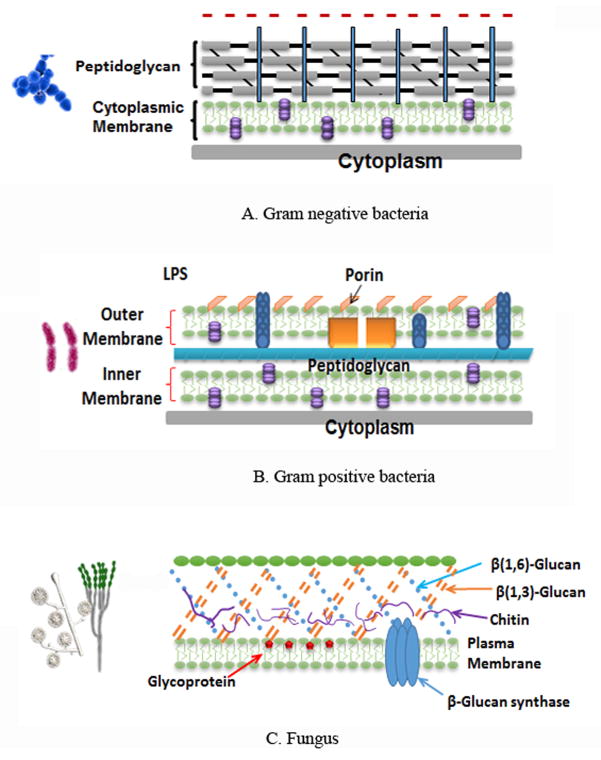
Bacterial cells can be divided into two groups called Gram-positive and Gram-negative bacteria, characterized by large differences in the cellular structure and organization (Fig 3A and B).
Figure 3.
Schematic illustration of cell wall structures of microbial pathogens. A: Gram-negative bacteria. B: Gram-positive bacteria. C: Fungal cells.
Gram-positive and Gram-negative bacteria have diversity in their three-dimensional architecture and outer cell wall. In Gram-positive bacteria the outer wall (15–80nm thick) demonstrates a relatively high degree of porosity which allows the most commonly used PSs to readily diffuse into the inner plasma membrane [34]. On the contrary, in Gram-negative bacteria, the outer wall possesses a highly organized compact structure which acts as a permeability barrier and prevents diffusion to the inner plasma membrane for most high molecular weight compounds [35]. The different permeability barriers between Gram-positive and Gram-negative bacteria are responsible for the observed sensitivity differences when treated with many antimicrobial agents. In principle, PDT has been shown to be more effective against Gram-positive than Gram-negative, especially when neutral or anionic PSs were used [31]. However, non-cationic PSs only bind to the outer membrane of Gram-negative bacteria cells, and do not inactivate them after illumination since the ROS is not produced in sensitive regions. The molecular structures of a range of PSs used for antimicrobial applications are shown in Fig 4.
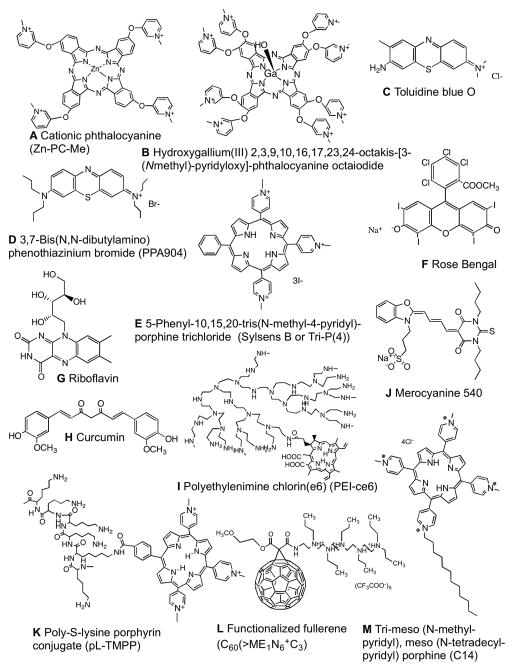
Figure 4.
Chemical structures of PSs described in this review. (A) Cationic phthalocyanine, Zn-PC-Me; (B) Hydroxygallium(III) 2,3,9,10,16,17,23,24-octakis-[3-(N-methyl) -pyridyloxy]-phthalocyanine octaiodide; (C) Toluidine blue O; (D) 3,7-Bis(N,N-dibutylamino) phenothiazinium bromide, PPA904; (E) 5-Phenyl-10,15,20-tris(N-methyl-4-pyridyl)-porphine trichloride, Sylsens B; (F) Rose Bengal; (G) Riboflavin; (H) Curcumin; (I) Polyethylenimine chlorin(e6), PEI-ce6; (J) Merocyanine 540; (K) Poly-S-lysine porphyrin conjugate, pL-TMPP; (L) Functionalized fullerene, C60(>ME1N6+C3); (M) Tri-meso (N-methyl-pyridyl), meso (N-tetradecyl-pyridyl) porphine, C14.
A number of PSs, including cationic substituted Zn(II)-phthalocyanines [36] (Fig 4A), poly-S-lysine-porphyrin conjugates [37] (Fig 4K), meso-tetrahydroporphyrin, tetrahydroporphyrin-tetratosylat (THPTS) [38,39] and cationic water-soluble gallium(III) phthalocyanines (GaPcs) [40] (Fig 4B), can substantially reduce MRSA populations (4–5 log10) after illumination. Moreover, it has been shown that a PS conjugate of polyethylenimine and chlorin(e6) (pEI-ce6) (Fig 4I) irradiated with red light is capable of reducing MRSA CFU by 2.7 log10 in a murine skin abrasion model [41]. Methylene blue (MB) mediated PDT inactivated VRE [42], and vancomycin-porphyrin conjugates were able to inactive in vitro vancomycin-sensitive and VRE [43]. Cationic PSs such as pL-ce6 and MB could destroy the protective effects of extracellular slime and stationary bacterial growth phase when PDI was used to inactive isogenic pairs of wild-type and mutant S. epidermidis and S. aureus [44].
Multidrug-resistant (MDR) and pandrug-resistant (PDR) Gram-negative bacteria are less prevalent than MRSA but bring about an equally grave threat of truly intractable infections [45,46]. In a model study, PDT also showed high efficiency to anti-infection of Gram-negative bacteria. Sixty MDR P. aeruginosa isolates were inactivated by toluidine blue O (TBO) (Fig 4C) mediated PDT with up to 6–7log10 reductions in CFU [47]. This study also demonstrated that antibiotic-resistant P. aeruginosa was just as sensitive to PDI as antibiotic-susceptible strains. Another report showed that MDR strains of Aeromonas hydrophila were inactivated by PDT mediated by cationic phthalocyanines [48]. Using 5-phenyl-10,15,20-tris(N-methyl-4pyridyl)-porphine trichloride (also known as Sylsens B or Tri-P(4)) (Fig 4E) as the PS, Trannoy et al. obtained a PDI reduction of Yersinia enterolitica CFU by 5 log10 [49].
MDR and extensively drug-resistant (XDR) strains of Mycobacterium tuberculosis (MDR-TB and XDR-TB) are a rising threat in the developing world which requires long term treatment. Infections have serious adverse effects, are difficult to cure and even fatal [50]. PDT studies on TB have been focused on the homologous system M. bovis Bacille Calmette Guerin (BCG) both in vitro applying phenothiazinium salts and in murine models of localized mycobacterial induced granulomatous formation [51,52]. In a similar fashion, the antimicrobial effects of PDT have been assessed on infections caused by M. marinum [53] and on rapidly growing nontuberculous mycobacteria keratitis [54].
Recently, some novel designed and synthesized PSs have been explored for anti-infective therapy with PDT such as the closed all-carbon cages called fullerenes. C60(>ME1N6 +C3) and C60(>ME3N6 +C3) (Fig 4L), which are two analogous pentacationic [60] fullerenyl monoadducts with variation of the methoxy-ethyleneglycol length, exhibited high activity for targeting and PDI Gram-positive and Gram-negative bacteria [55]. Polycationic chitosan-conjugated rose bengal (CSRB) as PS showed anti-biofilm ability on Enterococcus faecalis (Gram positive) using PDT [56]. Temoporfin liposomes conjugated with a specific lectin (wheat germ agglutinin, WGA) on the liposomal surface eradicated all MRSA by PDT compared with non-modified liposomes [57]. A new PS integrated a potent second generation photosensitizer (temoporfin) and a novel antimicrobial peptide (WLBU2) into liposomes that killed all MRSA upon illumination with 652 nm laser (1 W/cm2, 100 s, 100 J/cm2) [58]. Tsai et al. showed that 0.25 μM micellar hematoporphyrin (Hp) completely eradicated S. aureus under a light fluence of 50 J/cm2 [59]. The potentiating bactericidal effect of chitosan on Hp- or TBO-mediated PDI was also found with other Gram-positive bacteria (S. pyogenes and S. epidermidis) and two Gram-negative bacteria (A. baumannii and P. aeruginosa) while causing less damage to human normal tissues [60]. Tetracationic meso-arylsubstituted porphyrin (RM24) induced PDI can completely eradicate cultures of 108 CFU/mL P. aeruginosa PAO1 in stationary phase [61].
Moreover, PDT demonstrated significant killing effects on some bacteria which are very difficult to inactivate by antibiotics. For example, Hypocrellin A (HA)-mediated PDT significantly reduced the survival rate of Bacillus subtilis to less than 0.1% (P<0.05) [6]. Walther et al. reported that protochlorophyllide mediated PDT inactivated Listeria monocytogenes and Bacillus subtilis, while Yersinia pseudotuberculosis were found to be insensitive to protochlorophyllide treatment [62]. However, the two bacteria were showed susceptibility to eradication by protochlorophyllide (10 mg/L) combined with polymyxin B nonapeptide at 50 and 20 mg/L, respectively.
In addition to in vitro studies, several in vivo studies have been carried out to investigate and improve the efficacy of antimicrobial PDT in animal models that mimic clinical human infections. Different kinds of wounds such as burns, abrasions, soft tissue disruption, and excisional wounds have been investigated. These wounds are easy to infect and frequently involve Gram-negative as well as Gram-positive bacteria. Hamblin et al. first investigated the effect of PDT in vivo using mouse excisional wound models infected with P. aeruginosa and E. coli, respectively [63,64]. A light dose dependent response was observed in bacteria killing. Moreover, only the animals treated with PDT survived in P. aeruginosa infected wounds. PDT using TBO significantly inactivated Vibrio vulnificus that could otherwise lead to fatal sepsis. By using a mouse model of burn infected with Acinetobacter baumannii, Dai et al. found that, PEI-Ce6 mediated PDT produced 1.7–3 log10 reduction of bacteria while it did not inhibit the wound healing [65]. In a soft tissue model developed by intramuscular injection of bacterial suspension into the mouse thigh muscle, Berthiaume et al. studied the effect of PDT against P. aeruginosa using bacteria mixed in vitro with the tin (IV) chlorin e6—monoclonal antibody conjugate and shining the light after injection [66]. The significant decrease of bacteria was demonstrated. Hypocrellin B: lanthanum (HB:La+3) mediated PDT was able to reduce multidrug-resistant P. aeruginosa burden in burn wounds, inactive bacterial levels 2–3log in blood and delay bacteremia compared with untreated control group. In addition, Hashimoto et al. reported the in vitro reduction of P. aeruginosa [67]. Park and coworkers investigated chlorin(e6)-mediated PDT antimicrobial effects in a mouse model in which skin wounds were infected by bioluminescent S. aureus Xen29 [68]. MB-induced PDT produced maximal bacterial killing and minimized killing of host neutrophils in murine MRSA arthritis models [69]. PDT with Na-pheophorbide significantly prevented leg swelling, inhibited bone destruction on osteomyelitis models in rats infected by methicillin-sensitive S. aureus (MSSA) [70].
PDT has been widely applied in oral and dental infections where Gram-negative bacteria are really common and are thought to be responsible for the most damaging infections. The use of PDT in different models of peri-implantitis demonstrated a decrease of bacterial burden with different bacterial strains. The viabilities of A. actinomycetemcomitans, P. gingivalis, and Prevotella intermedia bacteria were reduced significantly after TBO mediated PDT [71]. PDT was able to reduce Fusobacterium sp and Prevotella sp counts [72,73]. Significant results were also obtained in non-surgical treatment of periodontitis in in vivo models. PDT using TBO caused a significant reduction of P. gingivalis CFU in a rat model [74]. The inactivation of P. gingivalis was achieved in infected sites using PDT with chlorin(e6) in a beagle dog model [75]. Bottura et al. used a model of immunosuppressed rats to demonstrate the effect of PDT on reducing bone loss resulted in experimental periodontitis [76]. Several more studies were in agreement with the previous literature, and further manifested the effectiveness of PDT in treatment of periodontal disease [77–79]. In order to evaluate the effect of PDT on inflammatory cells in periodontitis, Seguier et al. tested two different drug delivery systems [80]. The results demonstrated that PDT suppressed the gingival inflammatory reaction during chronic periodontitis and resulted in a specific decrease of antigen-presenting cell populations based on which drug delivery system was used.
3.3 Antifungal efficacy of photodynamic therapy
The clinical location and visible appearance of fungal infection varies widely, from superficial, subcutaneous to severe systemic infectious disease. Fungal pathogens are variable in their cell envelopes, possessing outer wall formed from mixtures of mannan, chitin polymers, and beta-glucans (Fig 3C). This feature causes them to be inherently more permeable to external substances when compared to Gram-negative bacteria.
The hypothesis that cationic PSs would be more efficient in PDI has been tested in Candida species, which are proposed to be the most common fungal pathogens, by several in vitro and in vivo studies [81–83]. C. albicans were inactivated by PDT using PSs including RB, Photofrin (Porfimer sodium) and Al (III)-tetrasulfonated phthalocyanine [84–86]. In another study, C. albicans suspensions were irradiated with helium–neon or gallium-aluminum-arsenide lasers using five different PSs including methylene blue (MB), thionin, crystal violet, toluidine blue O (TBO) and phthalocyanine. Except for phthalocyanine, all other PSs tested resulted in a significant reduction in C. albicans CFU following PDT [81]. PDT mediated by Photogem, a porphyrin PS, against four different species of Candida, C. tropicalis, C. albicans and C. dubliniensis were completely eliminated, except C. krusei [87]. MB-mediated PDT achieved a drug-dependent photo-killing of oral candidosis infected by C. albicans in a murine model [88]. Junqueira et al. demonstrated that the rats treated by MB-mediated PDT had less candidiasis lesions compared with the untreated control group [83]. On the other hand, Giroldo et al. confirmed that MB-induced PDT increased the permeability of cell membrane of C. albicans, which in turn could increase the sensitivity of this microorganism to other antifungal agents [89]. Effective killing of azole-resistant strains of C. albicans [88,90,91] and C. glabrata, [87,91] by PDT was also achieved with the use of TBO [88], MB [90], cationic porphyrin [91] and Photogem® [87] as PSs. However, investigators reported that PDT produced less substantial killing of azole-resistant strains in Candida [87,90]. Dovigo et al. investigated the PDI of oral candidiasis infected by C. albicans in a murine model using curcumin as the PS [92]. Results showed that exposure to curcumin (Fig 4H) combined with light-emitting diode (LED) light caused a significant reduction in C. albicans viability following PDT in a PS concentration dependent manner without harming the host tissue of mice. Mima et al. investigated that Photogem® illuminated by 455 or 630 nm LED light (305 J/cm2) inactivated C. albicans in a murine model of oral candidosis. The results showed a significant reduction in C. albicans recovered from the tongue (P < 0.001) compared control group [93]. Mima et al. did another in vitro study showed the effectiveness of Photogem® induced PDT for the inactivation of different five species of Candida on maxillary complete dentures, including C. albicans, C. tropicalis, C. glabrata, C. krusei and C. dubliniensis. The results showed PDT reduced 3.99 log10 CFU from dentures which were infected all species of Candida [94].
T. rubrum is a common pathogenic fungus genus of dermatophytes. PDT was suggested as a potential treatment modality to kill T. rubrum with the use of the broad band white light and PSs, including porphyrins deuteroporphyrin mono-methylester (DP-mme) and 5,10,15-tris(4-methylpyridinium)-20-phenyl-[21H,23H]-porphine trichloride (Sylsens B) [95]. PDT inactivated T. rubrum in both suspension culture and its isolated microconidia [96]. Red light showed the higher penetration was suggested to be more effective especially for nail infections by PDT. Results of the study demonstrated that Sylsens B was the better PS against both hyphae and the microconidia of T. rubrum which completely inactivated the fungal spores and destroyed the fungal hyphae.
Aspergillus fumigatus can cause opportunistic infections in the lung, sinuses and brain of patients with predisposing conditions [97]. Aspergillosis caused by this fungus is frequently fatal, increasing in frequency, and difficult to treat. The treatment is even more complicated by the increasing incidence of drug-resistant strains. Significant fungicidal effects of PDT on A. fumigatus was demonstrated in vitro, implying that PDT may be a treatment approach for certain localized fungal infections that form granulomas and can adapt themselves to invasive growth [98,99]. However, in a study performed by Gonzales et al. comparing the conidial phototoxicity of Metarhizium anisopliae and A. nidulans with TBO and MB under various incubation times and light doses, the investigators observed that even though conidial killing rates reached up to 99.7%, and germination of the surviving conidia was delayed, none of the treatments achieved a 100% fungicidal effect [100].
3.4 Anti-bacterial/fungi biofilm efficacy of photodynamic therapy
The formation of biofilm is most commonly associated with chronic infections which are typical of microorganisms adhering both to each other and/or to surfaces or interfaces and embedded in an extracellular polymeric matrix. Once biofilm is established, these communities form a protective niche that facilitates growth and survival of bacteria in a hostile environment [101]. The protected environment and the density of the film, as well as the significantly different cellular properties from those in free-floating bacteria of the same species, have been implicated in about 1,000-fold resistance to antiseptics, detergents and antibiotics [102]. Owing to the prevalence of biofilms as a cause of many diseases and the fact that they significantly increase the bacterial resistance to conventional antibiotic therapies, it is necessary to develop new modalities to treat microbial biofilms. PDI is a possible alternative to inactivate biofilms. Moreover, PDI is a simple non-mechanical therapy and it can be also used for the treatment of children, handicapped people and medically compromised individuals.
S. epidermidis and S. aureus are often the pathogens of persistent infections occurring with implanted medical devices that are hard to eradicate [103]. Sharma et al. studied the effects of TBO-induced PDT on the structure and the viability of biofilms of S. epidermidis and of a MRSA strain [104]. They found PDT significantly reduced staphylococcal biofilms, and the combination of tetrasodium EDTA with PDT enhanced the phototoxic efficacy in S. epidermidis biofilms, but not in S. aureus biofilms. Zanin et al. found that TBO (0.1 mg/ml) induced PDI reduced 2.10–3.11 log 10 CFU of S. mutans composed in biofilms treated in a dose-dependent manner [105,106]. The average reduction in CFU varied between 1.00 to 2.44 log 10 for the biofilms formed by two and three species, and it was verified that TBO-based PDT has a substantial effect on staphylococcal biofilms which can decrease 5 log10 CFU irradiated with red light, disrupt the architecture of biofilm and damage the membranes of bacterial cells. PDT also has an influence on S. mutans biofilms in different maturity stages (inactivated 4 log10 CFU), as well as mature S. sanguinis and S. sobrinus biofilms.
MB-PDT induced an average reduction of 2.81 log 10 of S. mutans biofilms, as well as an average CFU reduction of 3.29 log 10 of S. aureus biofilms [107]. Merocyanine 540 (Fig 4J) mediated PDT showed a significant inactivation effect on the survival of S. aureus biofilms. However, the light fluences required to inactivate biofilms were much higher than those used to photoinactivate planktonic cultures, due to hindrance of the penetration of light and/or PS within the biofilms [108]. PDI with the cationic porphyrin, tetra-substituted N-methyl-pyridylporphine (TMP) was effective in biofilm adhering to medical implant surfaces when combined with vancomycin and the host defenses [109]. Tri-meso (N-methyl-pyridyl), meso (N-tetradecyl-pyridyl) porphine (C14) (Fig 4M) presented a significantly greater PDI effect on the eradication of S. epidermidis biofilms when compared with the parental tetra-substituted N-methyl-pyridyl-porphine (C1) [110]. Erythrosine (ER) was found to be able to inactivate S. mutans biofilms better than protoporphyrin and MB with the inactivation effect enhanced by 2 log10 [111]. Erythrosine induced PDT was also more potent than MB, RB and TBO against Aggregatibacter actinomycetemcomitans biofilms [112]. PDI mediated by both 5-ALA and TMP at different concentrations could eliminate P. aeruginosa biofilms [113,114]. Recently, Hegge et al. investigated the phototoxicity of curcumin in S. epidermidis biofilm and suspension cells in two models in vitro [115]. The phototoxicity of curcumin towards planktonic S. epidermidis was dependent on the presence and type of nanocarrier (PEG 400, HPcCD and F 127). A different situation was observed in a biofilm, where the phototoxicity of curcumin was less sensitive to the selected nanocarrier. Therefore, curcumin should be optimally formulated to target both biofilms and planktonic bacteria in order to obtain maximum photoinactive effect.
Peri-implantitis involves the bacterial colonization, typically in the form of biofilms on the implant surfaces and may lead to system infection and destroy the implant surface. Approximately 20 % of the oral bacteria are streptococci, which are correlative of the dental plaque formation and the development of oral biofilms. Streptococcus mutans and Streptococcus sanguinis normally exist as regular members of the mature dental biofilm community, and causes caries and periodontal diseases. Pereira et al. examined the effect of PDT using ER and Rose Bengal (RB) as the PSs, respectively, and LED on S. mutans and S. sanguinis biofilms [116]. PDT induced significant decreases in the viability of both microorganisms, especially S. sanguinis.
Biofilm formation is also a key event in the development of fungal disease. Similar with the previously mentioned bacterial biofilms, fungal biofilms cause treatment resistance and high mortality, particularly in immunocompromised patients [117]. Candidiasis, caused most commonly by C. albicans and to a lesser extent by C. tropicalis, C. parapsilosis or C. glabrata, is often associated with biofilm formation on the surface of medical devices and tissues [118]. Candida biofilm is one of the most common causes of clinical complications found with implanted biomaterials such as intravascular catheters, prostheses, pacemakers, stents, urinary catheters, shunts and orthopedic implants and unfortunately treatment with current antifungal agents are challenging [119]. Candida biofilms resist most of the present antifungal agents, except echinocandins. However, an increase in resistance to echinocandins is now frequently reported [120].
It was revealed that C. albcans biofilms were susceptible to photofrin induced PDT [121], and C. albicans and C. dubliniensis were sensitive to erythrosine-mediated PDT, however the biofilms of both Candida spp. were more resistant than their planktonic counter parts [122]. Another study also showed the effects of photofrin (10μg/ml) mediated PDT against C. albicans biofilms and its germ tubes [121]. Biofilms treated with PDT showed significant reduction of metabolic activity compared to the biofilms treated with amphotericin B (10μg/ml). The same authors also investigated the effect of PDT against biofilms of C. albicans and C. dubliniensis using erythrosine (400 mM) illuminated by green LED light (532±10 nm, 237 mW/cm2, 42.63J/cm2) [123]. Scan electronic images demonstrated a decrease in the amounts of hyphae and conidia in the biofilm following PDT. However biofilms of both Candida species showed more resistance than their planktonic counterparts. The combination of erythrosine and RB-mediated PDT demonstrated some effect on biofilms, and was also effective in destroying and reducing the blastoconidia and hyphae of C. albicans [122]. MB-PDT combined with 660nm laser exhibited a modest reduction in biofilm C. albicans species [107]. Mentareva et al. demonstrated that the best phototoxic effects on C. albicans biofilms were obtained by Zn (II) and Ga (III) phtalocyanines, such as ZnPcMe (Fig 4A) and GaPc2 induced PDT, compared with other diverse PSs [40]. Coleman et al. demonstrated that through coupling of saponins with PSs (RB (Fig 4F) and chlorin[e6]), incubating with the yeasts for 30 min and irradiating with visible light, it was possible to inhibit the formation of C. albicans biofilm and dramatically potentiate PDI in vitro [124]. In one in vitro study, Donnelly et al. developed a mucoadhesive patch that contained TBO as a PS delivery system for PDT of oropharyngeal candidiasis [125]. This patch was able to resist dissolution when it interacted with artificial saliva and prevented staining of lips, teeth and buccal mucosa. When TBO was released directly into an aqueous sink upon illumination (635nm, 100mW/cm2, 200J/cm2), patches were capable of killing C. albicans biofilm. Recently, Ribeiro et al. evaluated that the effectiveness of chloroaluminum-phthalocyanine (ClAlPc) induced PDT in the treatment of C. albicans planktonic cultures and biofilm with different drug delivery system [126]. Cationic nanoemulsions –ClAlPc and free ClAlPc caused significant damage to the fungal cells membrane (P < 0.05); furthermore, cationic nanoemulsions –ClAlPc reduced significantly both cell metabolism and fungal colony counts and also showed better killing rates for Candida biofilms (P < 0.05).
PDT eradication of microbial biofilms could lead to a variety of applications in clinical conditions. For examples, PDT has been used to target to: 1) superficial infections, including elimination of dental plaque, periodonitis, gingivitis, endodontics and oral candidiasis; 2) subcutaneous infections including sinusitis, and osteomyetitis; 3) internal organs infection including endocarditis and the infection of cystic fibrosis; 4) various catheter infections and 5) some temporary or permanent indwelling devices such as joint prostheses, heart valves and other implants, and so on [32]
In conclusion, there is a wealth of literature describing PDT-based anti-biofilm strategies that focus mostly on the use of different PSs against a variety of microbial species. In contrast there are only a limited number of studies exploring the effects of PDT on phenotypic biofilm elements (e.g.,adhesins and extracellular polysaccharide ) [127,128]. Moreover, there is lack of standard reproducible models for assessing PDT efficacy against biofilms. The majority of published researches used methodologies where biofilms were grown in/on plastic or silicon microtiter plates and surfaces. These bioassays have been repeatedly criticized for lack of stability and clinical relevance and occasionally yield inconsistent results. For fungal biofilm, it involves a heterogeneous mixture that is composed of hyphae, pseudohyphae and blastoconidia embedded in extracellular polymeric substances which form pores and channels and display diverse phenotypic features compared to planktonic yeasts [129]. The extracellular matrix is composed of proteins, polysaccharides, hexosamine, uronic acid and DNA which promote biofilm formation and adhesion, protecting the cells from phagocytosis [129,130]. This in turn maintains and limits the diffusion of substances, such as antifungal agents, and also restricts penetration of PS and light during PDT [129,130]. Other reasons for increased resistance against both antifungal agents and PDT are the presence of cell wall thickening, up-regulation of drug-efflux pumps, and higher density and larger size of cells in yeast biofilms, decreased number of targets for singlet oxygen per unit of cell volume, existence of multiple species (bacteria and yeasts together), enzymatic alteration of active agent, presence of persisters which represent a subpopulation of cells spontaneously going into a dormant, non-dividing state and in turn remaining alive even after antimicrobial treatments [119,122,129,131,132]. Through modification of the treatment conditions such as incubation time, fluence rate, type of PS and PS’ concentration, the efficacy of photo-inactivation can be enhanced especially for resistant cases.
3.5 The efficacy of PDT on other pathogens
Several protozoa are quite dangerous and even deadly human pathogens and most of them are immune to the presently used antimicrobial therapeutic modalities. Typical examples are represented by Acanthamoeba, Leishmania spp, Entamoeba histolytica, Plasmodium spp and Giardia intestinalis.
Species of Acanthamoeba are facultative parasites, capable of living within the tissues of vertebrates and are the causative agents of granulomatous amoebic encephalitis and amoebic keratitis [133]. The life-cycle of Acanthamoeba species are divided into two stages: a dormant cyst and trophozoite, and both stages are infectious. The trophozoitic stage is delimited by a plasma membrane that mainly consists of phospholipids, while the mature cysts’ wall is composed of an inner (endocyst) and outer (exocyst) layer separated by a space with a tightly organized structure. The special osmotically inextensible wall, especially endocyst layer, makes the cyst highly resistant to various chemical agents [134]. Limited reports have shown that cationic porphyrins and phthalocyanines, excited by visible light, caused an efficient photosensitized killing of parasitic protozoa. More specifically, tetracationic phthalocyanine (RLP068)-mediated PDT showed the photo-toxicity effect on Acanthamoeba palestinensis which almost induced complete inhibition of the viability of A. palestinensis in the trophozoite stage [135]. Hypocrellins B (HB) induced PDT manifested effectiveness on Acanthamoeba cysts and trophozoites in vitro. The results showed HB-PDT induced a total inhibition of the trophozoites and cysts in a dose-dependent manner and a better phototoxic inactivation on the trophozoites than the cysts. However, HB-PDT also manifested phototoxicity on the corneal epithelial cells and stromal cells in the rabbit model [136]. Another study showed that MB-mediated PDT reduced the respiratory activity of Acanthamoeba castellanii (ATCC 50370) trophozoites in an MB-concentration dependent manner [137]. Recently, Siddiqui et al. used Sn (IV) porphyrin (a synthetic metalloporphyrin) as PSs illuminated by visible light to treat A. castellanii [138]. The results demonstrated that PDT suppressed A. castellanii growth, the ability of cytopathogenicity and decrease the adhesion to human corneal epithelial cells in a concentration-dependent manner. However, PDT had no effect on its viability.
Leishmania is an intracellular parasite that parasitizes the phagosomal compartment of macrophages in the vertebrate host and is transmitted to human body via the bite of infected sandflies. Leishmaniasis causes substantial disfigurement and sometimes mortality in the developing countries. The clinical manifestations of Leishmaniasis in humans include cutaneous, mucocutaneous and visceral forms with a wide range of geographic distribution [139]. Recent therapeutic modalities include systemic and topical treatments [140]. However, none of these treatments have been evaluated to be completely satisfactory, and only a few of the touted agents have been proved adequately in clinical trials. Up to now, multiple investigations performed have shown the effects of PDT on killing Leishmania in vivo and in vitro. Some PSs manifested phototoxicity to inactive Leishmania strains, including MB [141], (3,7-bis(N,N-dibutylamino) phenothiazinium bromide (PPA904) (Fig 4D), δ-aminolevulinic acid-derived protoporphyrin IX (ALA) [142–144], chloroaluminum phthalocyanine encapsulated ultra-deformable liposomes (UDL-ClAlPc) [145], dimethyl and diethyl carbaporphyrin ketals (CKOMe and CKOEt) [146]. PDT caused obvious inactivation on several Leishmania strains, such as L. panamensis, L. chagasi [145], L. tarentolae [146], regardless whether their life-stages were promastigotes, axenic or intracellular amastigotes. In some clinical studies, MB-, ALA- or MAL-mediated PDT showed effectiveness to treat cutaneous leishmaniasis. Cosmetic results were excellent and nearly no recurrence occurred after PDT [140,147–149]. Topical PDT showed safety as well as effectiveness, and can be applied as an alternative approach for cutaneous leishmaniasis.
Plasmodium spp are the main causative pathogens of mosquito-borne diseases transmitted from one individual to another resulting in transfusion-transmitted malaria (TTM). There are several infective members of the Plasmodium genus, including P. falciparum, P. ovale, P. vivax and P. malariae [150]. As a result of increased international travel, migration, and the spread of drug-resistant parasites, malaria is a growing issue in malaria-endemic areas and industrialized nations. Phthalocyanines (one of the most effective, HOSiPcOSi(CH,),(CH,),N(CH,), (Pc 4) ), illuminated by red light, manifested the photoinactivation of P. falciparum parasitized in red cells in a dose-dependent manner and could be used to make blood components safe [151]. Another study demonstrated that ALA-induced inactivation of plasmodial heme-cycle intermediates, by exposure to white light, reduced P. falciparum in culture to levels such that they were not detectable by light microscopy nor by lactate dehydrogenase assay [152].
Human African trypanosomiasis, caused by Trypanosoma brucei rhodesiense or Trypanosoma brucei gambiense is a disease contracted after biting by the tsetse fly, and is usually a disease involving the nervous system, also known as sleeping sickness. Conversely, American trypanosomiasis, or Chagas’ disease, is commonly produced by infection by T. cruzi [150]. None of the diseases have an obvious cutaneous appearance (except the puncture wound caused by the biting insect disease vector), but the protozoa exist in the bloodstream of infected persons. Recent studies showed silicon phthalocyanine Pc4, thiazole orange and gentian violet induced PDT manifested photoinaction for T. cruzi [153]. Especially, gentian violet -induced PDT has already been used to disinfect T. cruzi in donated blood, and this also offers a strong potential lead for conventional drug discovery against T. cruzi [154]. In one in vitro study, Do Campo et al. demonstrated the a 1% solution of MB or 0.001% solution of MB illuminated by white light (100W) immobilized T. brucei in citrated guinea pig blood without any subsequent disease when administered to mice [155]. Another study showed that the ultraviolet-absorbing compound Amotosalen (4′-aminomethyl-4,5′,8-trimethylpsoralen) when illuminated by UV completely inactivated the infective form of T. cruzi both in platelet concentrates and in fresh-frozen plasma [156]. Gottlieb et al. inactivated T. cruzi trypomastigote forms in the composition of blood by PDT with three phthalocyanine dyes, including HOSiPcOSi(CH3)2(CH2)3N(CH3)2(Pc4), HOSiPcOSi(CH3)2(CH2)3N+(CH3)3I-(Pc5) and aluminum tetrasulfophthalocyanine hydroxide (AlOHPcS4) [157]. The compound Pc 4 was shown to be highly effective in inactivating T. cruzi, Pc 5 was less effective and AlOHPcS4 was ineffective. Ultrastructural analysis showed the parasites’ mitochondria were a primary target of phthalocyanine dyes-induced PDT.
3.6 The efficacy of PDT on viruses
It has been reported that PDT can also be used to inactivate enveloped viruses. Pristine C60 fullerenes induced photo-inactivation of vesicular stomatitis virus (VSV) (Rhabdoviridae) or Semliki forest virus (Togaviridae), which produced up to 7-log10 of loss of infectivity [158,159]. Hirayama et al. demonstrated that methoxy-polyethylene glycol conjugated fullerene induced PDT, illuminated by white light (120 J/cm2), and damaged more than 5 log10 of plaque forming units of VSV [160]. Lin et al. compared two region-isomers of carboxyfullerene, illuminated with or without light, inactivation dengue-2 and other enveloped viruses [161]. The asymmetric isomer showed greater dark activity, albeit at much higher concentrations than required for its PDT effect. The authors concluded that this difference was attributed to the interaction with the lipid envelope of the virus.
In general, polyomaviruses are considered to be highly resistant to PDI. The degree of resistance is dependent upon the structure of the PS; TBO, acridine orange and MB are effective PSs for PDI of polyomaviruses. Higher doses of PS and light may be required in order to inactivate the oncogenic properties of these viruses than those required to inactivate infectivity [162]. Human papillomavirus (HPV) infection is very common in many populations and manifests several clinical features, including genital warts, verruca vulgaris, verruca plantaris, verruca plana and so on. The clinical applications of PDT have been well reviewed by Lee et al. in terms of efficacy, safety and cosmetic outcome [163]. In most clinical cases ALA and methyl aminolevulinic acid (MAL) are used as PSs, even if they are off-label treatments.
3.7 The efficacy of PDT on vectors
Insects act as the crucial roles to disseminate tropical disease via the blood of bitten individuals. Owing to deforestation, insect vectors can now invade cities and are thus considered responsible for developing new endemic regions of disease. Many of the affected countries spend enormous resources to control insect vectors, usually entailing the use of chemical insecticides. However, chemical insecticides are considered to possibly cause side effects on humans and are detrimental to the environment. Other strategies have been developed including biological control and photo-insecticides.
Photo-activated insecticides were demonstrated to be a novel compelling alternative to chemical insecticides. The ideal photo-activated insecticides possess the following characters. First of all they are non-toxic in the dark and second they are not permanently accumulated in the environment, because they are usually bleached and chemically degraded by light. The third is that usually, they employ light energy to produce their phototoxicity and are intrinsically less harmful to the environment because they are used at very low concentrations. The concept of using PDT inactivation of pest populations was realized as early as in the 1930’s using xanthene derivatives against Anopheles and Aedes larvae and the developments in this field have been reviewed by Ben Amor and Jori [164]. Among the limited reports in this area, porphyrins are particularly interesting as photo-insecticides because they exhibit several absorption bands in the UV/visible spectrum, being therefore efficiently excited by the solar spectrum [165]. Under this concept, Lucantoni et al. tested the phototoxicity of a synthetic meso-substituted porphyrin meso-tri(N-methylpyridyl), meso-mono(N-tetradecylpyridil) porphine (C14) [166]. The photo-activable porphyrin showed phototoxicity on the dengue vector Aedes aegypti in laboratory conditions. In artificial pool tests, greatest population reductions were achieved using dosages on the countryside of 20 and 40 g/ha. Shao et al. synthesized a series of tetraethynyl silanes (TETS) as photo-activated insecticides to test their killing effects on 4th-instar larvae of A. albopictus (Skuse) [167]. After 3h incubation in a dark room, A. albopictus were treated by ultraviolet irradiation (300–400 nm, maximum at 365 nm) for 1.5h at the fluence rate of 2.074 wW/cm2. Among them, the compound with four thiophene groups attached on the same silicon atom with the triple bond exhibited excellent photo-activated insecticidal activity. Relationship analysis between structure and activity showed that the presence of thiophene ring was critical for the overall activity [167].
3.8 Blood product disinfection by PDT
Transfusion-related infections and consequent fatalities continue to be reported in quite a few publications [168,169], and blood is currently not tested for many potentially dangerous known pathogens. In order to guarantee the safety of blood products from pathogenic microbes, PDT is an alternative approach with the main advantage of being potentially efficient against viruses, bacteria, fungi, and parasites. However, the main challenge in blood disinfection is to kill or inactivate the target microorganism without disruption of blood cells or serum proteins and moreover the PS should be without toxicity. MB is not an ideal PS for this application due to its ineffectiveness against intracellular viruses and tendency to cause collateral damage to blood components, typically to factor VIII and fibrinogen [170]. Thionin, the demethylated derivative of MB, mediating PDT has been shown to be efficient without damaging red blood cells and platelets [171]. Dimethylmethylene blue (DMMB), one type of phenothiazinium dye, illuminated by visible light showed good effects on the inactivation of DNA and RNA model viruses, including vesicular stomatitis virus and duck HBV (DHBV, a model for HBV; single-and double-stranded DNA virus), and leukocytes in RBCs. However, clinical studies have not yet been started with DMMB [172–174].
The naturally occurring vitamin B2, riboflavin (Fig 4G), illuminated by UVA or visible light, has also been developed as a nucleic acid-binding agent to be used for pathogen inactivation which results in photoinactivation of nucleic acid-containing pathogens in plasma, platelets, and RBCs. Preclinical studies have revealed the effectiveness of reduction in infectivity of some viruses, including HIV-1, bovine viral diarrhea virus (BVDV), a model for HCV; single-stranded RNA virus), and pseudorabies virus, as well as bacteria in blood products [175–178].
3.9 Aquaculture disinfection by PDT
Aquaculture comprises all types of culture of fish and other aquatic animals as well as culture of plants in fresh, brackish and marine environments. These enterprises are susceptible to water-borne disease caused by bacteria, viruses, helminths, protists and oomycetes, to a lesser extent, fungi. Among them, bacterial infection is one of the major concerns in the aquaculture industry [179]. Application of immobilized PS could mediate PDT to inactivate fish pathogenic microorganisms and if successful would manifests obvious advantage without low risk neither to the fishes nor to the environment. Only a few studies have been explored in this field, but preliminary results were gained at both laboratory level and at pilot stations. Porphyrin derivatives, as the PSs, have also a great potential for the disinfection of fish farming plant water, including Gram-positive bacteria (e.g. MRSA), Gram-negative bacteria (e.g. E. coli), fungi (e.g. C. albicans) and fungal-like pathogens (e.g. Saprolegnia spp.) and parasitic protozoa (e.g. Acanthamoeba palestinensis) [179]. Microbial population was decreased 5–6 log10 CFU after 10 min of irradiation with a low fluence rate (ca. 50 mW/cm2) in the presence of micromolar PS concentration [180]. Another study showed that a micromolar concentration of a porphyrinic PS eliminated the naturally or artificially induced infection of saprolegniosis in trout farming pools (inactivation of 6–7 log10) without collateral damage of the fish. The same low concentrations demonstrated also higher phototoxicity activity over more traditional pathogens MRSA and E. coli (up to 7 log10 decrease in bacterial CFU) [181]. The bactericidal effects of PDT were also exhibited for V. vulnificus that frequently infects fish farming water [182]. Similarly, cationic porphyrins-induced PDT inactivated up to 7 log10 of ten bacterial species (i.e. V. anguilarum, V. parahaemolyticus, Photobacterium damselae subsp. piscicida, Photobacterium damselae subsp. damselae, E. coli, Enterobacter, A. salmonicida, S. aureus, Pseudomonas sp, E. faecalis,) isolated from fish farming plant waters in vitro [179].
3.10 Waste water disinfection by PDT
At present, antimicrobial PDT is considered to have potential for use in the environmental area, including water disinfection. Effects were observed on the eradication of faecal bacteria [183–186], viruses [187] and helminth eggs [188] in environmental water. Bonnett et al. used a phthalocyanine immobilized on a polymeric membrane of chitosan in a model reactor of water disinfection [189]. Two mono-hydroxyl zinc-porphyrin derivatives with anionic and cationic net charge, respectively, were immobilized on poly(methyl methacrylate). The cationic derivative immobilized on poly(methyl methacrylate) polymer showed more effective inhibitory effect on the photo-inactivation of Deinococcus radiodurans [179].
3.11 Food disinfection by PDT
Numerous outbreaks of foodborne illness have been attributed to the contamination of produce due to inadequate sanitation of food contact surfaces [190]. Recent research has indicated that pathogens can acquire resistance to commonly used sanitizers or disinfectants and, as a result of such adaptation, cross-resistance of pathogens to antibiotics and production of multi-antibiotic resistant pathogens have been observed, which is a risk to animal health status and the safety of food products [191]. Brovko et al. investigated the photodynamic bactericidal effect of acriflavine neutral, phloxine B, RB and malachite green (oxalate salt) against two Gram-negative strains (E. coli LJH 128 and Salmonella Typhimurium C1058), two Gram-positive strains (Bacillus sp. C578 and Listeria monocytogenes LJH 375), and yeast (Saccharomyces cerevisiae C1172) [191]. The results showed that acriflavine neutral-induced PDT significantly reduced the viabilities of all pathogens tested. RB caused a significant reduction of bacterial viability incubated both in the dark and under illumination. Malachite green induced PDT was gave more effective inactivation of Gram-positive bacteria than Gram-negative bacteria and yeasts. Phloxine B demonstrated a significant photoinactivation on Gram-positive bacteria, whether in the dark or under illumination; but Gram-negative bacteria and yeasts were unaffected. Conjugation of RB and phloxine B with poly(vinylamine) resulted in an enhanced bactericidal effect during both dark and light incubation. No killing effect was observed for yeasts incubated with dye conjugates. The presented data suggest that a photodynamic approach for constructing “self-decontaminating” materials has potential in the food industry.
3.12 Effects of PDT on host cells and tissues
As mentioned above, PDT is an alternative approach to inactivate pathogenic microorganisms. The ideal outcome of antimicrobial PDT is that the target pathogenic microbes are selectively inactivated while host cells and tissues are preserved. Therefore, one of the criteria to evaluate the effectiveness of a PS is that it should demonstrate selectivity of microbial cells over host tissue and cells [192]. A recent study indicates that using tetracationic phthalocyanine (RLP068) as a PS leads to an about 5 log10 decrease in the CFU of microbial pathogens with no collateral damage at the same doses to typical human cells, such as fibroblasts and keratinocytes [36]. Soukos et al. found that poly-L-lysine (pL)-chlorin e6 (ce6) conjugates could efficiently target photo-destruction towards Gram-negative (P. gingivalis) and Gram-positive (Actinomyces viscosus) oral bacterial species while it spared the oral epithelial cell line (HCPC-1) [193]. Tegos et al. investigated the broad-spectrum antimicrobial photodynamic activities induced by two series of functionalized C60; one series had one, two, or three polar diserinol groups (BF1–3), another series had one, two, or three quarternary pyrrolidinium groups (BF4–6). The bis- and tris-cationic fullerenes showed highly germicidal effects on all tested microbes (4–6 log10), including Gram-positive bacteria, Gram-negative bacteria, and fungi, without any harm to mammalian cells (fibroblasts) [192]. One in vivo research, carried out by Trindade et al. evaluated the toxicity of Photogem® mediated PDT on rat palatal mucosa. Their data demonstrated Photogem®-induced PDT was not toxic to the rat palatal mucosa detected by macroscopic analysis and thermal mapping [194]. An in vitro study was carried out by Zeina et al. to investigate the effect of PDT on keratinocytes [195]. They found a therapeutic/dosing protocol whereby PDT inactivated microorganisms effectively without damaging adjacent keratinocytes. The researchers used a combination of MB and visible light previously used for microbial killing. The kill rates for keratinocytes were 200-fold lower than for bacteria and 18-fold lower than for Candida. Using different PSs, Tanaka et al. demonstrated the cytocidal effects of PDT on neutrophils [196]. They studied the morphological alteration and the viability of murine peripheral-blood neutrophils after PDT performed in vitro by using each PS at a concentration that achieved a maximum germicidal effect on MRSA. Most neutrophils survived (>80%) after PDT using TBO or MB, however, other PSs used for PDT (such as erythrosine B, RB, Photofrin, crystal violet, NMB and Laserphyrin) caused morphological change and viabilities reduction (<70%) to neutrophils. Shrestha et al. evaluated the potential of a PS-conjugated chitosan as a matrix-reinforcing agent on dentin collagen [197]. Rose Bengal-conjugated chitosan (CSRB) was synthesized by using a chemical cross-linking technique and it was characterized for its photobiological, photophysical, and cytotoxicity properties. Dentin collagen was applied for the CSRB cross-linking experiments and later on evaluated for mechanical properties, chemical changes, and resistance to enzymatic degradation. The CSRB-cross-linked dentin collagen demonstrated higher resistance to collagenase degradation and superior mechanical properties.
In conclusion, in vitro researches have shown that, under the same dosimetric parameters of the photodynamic process, host cells are less sensitive to PDI than bacteria, suggesting a safety margin between bacterial inactivation and host cell damage if PDT is used in vivo. This selectivity might be due to the protection that the plasma and nuclear membrane afford in mammalian cells that may act as an additional barrier for the PS or the PS high-energy reaction products (ROS) formed after light. Differences in sensitivity may also reflect differences in cell volume/size which increase the amount of ROS needed to kill the cell. Mammalian cells are approximately 25–50 times larger than the bacterial test species and may therefore they are likely harder to be killed [195]. The selectivity can also be explained by the selective uptake of cationic PSs by bacteria compared to that by mammalian cells [193].
3.13 Clinical applications of anti-infective PDT
Although PDT was discovered in the antimicrobial field over 100 years ago, the first clinical trials in humans did not take place until 1970s. The clinical applications of anti-infective PDT have been also evaluated and performed in vivo. Numerous studies indicate that PDT appears to be very efficient for the treatment of superficial and localized infections, including dental disease, cutaneous superficial and subcutaneous infection, gastric infections, and so on.
Qin et al. found an about 21% killing rate of microorganism in biofilms collected from patients with periodontitis, using 1 mg/ml TBO irradiated by 12 J/cm2 red laser [198]. Dörtbudak et al. reported that TBO-PDT successfully decontaminated implants with bacterial colonization of 15 patients, reducing about 2log10 bacterial CFU [199]. Soukos et al. used MB (25 μg/ml) PDT, illuminated by 665 nm red light (30 J/cm2), to fully inactivate all bacterial species except E. faecalis (53% killed) in infected root canals of extracted teeth [200]. A 97% elimination of bacteria biofilm infected by E. faecalis in root canals were achieved by PDT with the same concentration of MB and increased fluence (222 J/cm2) of red light. Fontana et al. reported that MB -PDT eliminated 63% subgingival species in the planktonic phase and 31% in the biofilm derived from patients’ dental plague with chronic periodontitis [201]. Another study, presented by William et al. showed that TBO-PDT could reduce 10-fold S. mutans which was present in a collagen matrix similar to mimicking carious dentin or appearing in decayed teeth [202]. Mima et al. used Photogem-PDT to treat five cases of denture stomatitis infected by Candida spp. The results showed four patients achieved clinical resolution and one patient demonstrated reduction in palatal inflammation. Two patients occurred recurrence of denture stomatitis during the follow-up period [203]. In one randomized clinical trial, Photogem®-induced PDT significantly reduced the CFU/ml of Candida species and showed clinical success rates 45% as effective as topical nystatin in the treatment of denture stomatitis [204].
Lee et al. used MAL-mediated PDT to treat six patients with refractory Malassezia folliculitis infected by Malassezia yeasts [205]. The results showed that a great improvement was achieved in four patients following continuous three sessions of PDT and a moderate improvement was achieved in one patient. Piracinini et al. treated a patient with recalcitrant onychomycosis infected by T. rubrum who did not respond to the topical and systemic antifungal agents’ treatment for 18 months. Following three sessions of ALA-PDT in a 15-days interval, remission of the infection was released and without recurrence during a 24-months’ follow-up period [206]. In another contrary study, thirty patients received ALA-PDT and achieved a 43.3% cure rate 12 months after treatment, however, cure rate dropped down to 36.6% after 18 months [207]. Nine patients with interdigital mycoses of the feet received 20% ALA-PDT treatment and achieved good therapeutic effects in most patients, however recurrences were seen quickly [208].
Another compelling application of PDT for superficial cutaneous infection is the treatment of acne vulgaris. Hongcharu et al. first revealed the efficacy of topical ALA-PDT to treat acne vulgaris on the back in a small but well administrated study in adults [209]. Another study was carried out by Itoh et al. using topical 20% ALA irradiated by lower fluence red light (13J/cm2) in Japanese patients. The acne lesions were improved and lasted at least 6 months [210,211]. Gold et al. evaluated the efficacy of ALA-PDT illuminated by intense pulsed light (IPL) and other light sources on moderate to severe inflammatory acne vulgaris in a clinical trial. Twenty patients were enrolled. Among them, fifteen completed the whole trial and twelve patients responded to the treatment. Among respondents, 50.1% patients reduced the active inflammatory acne lesions at the end of the four-week treatment, 68.5% was improved four weeks after the last treatment, and 71.8% was improved twelve weeks after the last treatment [212]. A large sample, randomized, split-face, placebo-controlled study was carried out by Yin et al. in China in 2010 [213]. These authors recruited one hundred and eighty patients and compared different topical ALA concentrations vs. placebo for PDT in patients with moderate to severe acne. Every patient was treated once at intervals of 10-days for four sessions. After 24 weeks, the sides treated by ALA-PDT demonstrated clinical improvement compared with the control sides treated only by red light (P < 0.01) (Fig 5). The beneficial outcomes were dose-dependent; however, the authors pointed out that the occurrence of side-effects also showed a dose-dependent increase.
Figure 5.
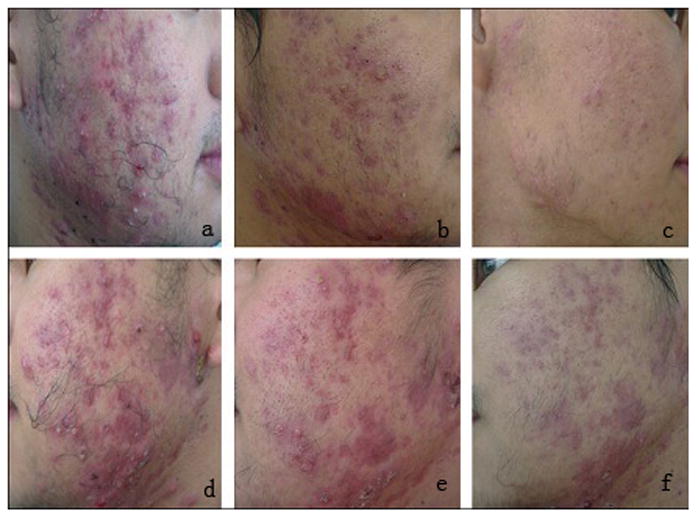
Improvements of severe inflammatory acne treated by 15% ALA-PDT (a c) and control (only red light alone) (d f). (a, d) before treatment; (b, e) 2 weeks after four consecutive treatments; (c, f) at follow-up visit after 24 weeks [213].
There have been further studies reporting beneficial effects of PDT on various bacterial infections, acne vulgaris, leishmaniasis, viral infections, gastric infections, etc. Table 2 summarizes the representative clinical applications of antimicrobial PDT. Although the overall clinical outcomes in all the studies were considered to be positive, discrepancies were found in the therapeutic efficacies in different trials. It was likely based on the use of different light sources, different PSs, concentration and incubation time between PS and infected tissue.
Table 2.
Summary of representative clinical applications of antimicrobial PDT
| Disease | Causative microorganism | Site of infection | Photosensitizer | No. of patients | Treatment outcome | Ref. |
|---|---|---|---|---|---|---|
| Localized bacterial infection | Gram (+)/Gram (−) bacteria | Leg ulcer, diabetic foot lesions | PPA904 | 10 | Up to 2-log reduction in bacterial load was achieved after a single PDT. | 270 |
| MRSA | Leg ulcer, diabetic foot lesions | PPA904 | 32 | Bacterial counts were significantly lower after PDT. No side effects were experienced. | 270,271 | |
| Gram (+)/Gram (−) bacteria | Leg ulcer, diabetic foot lesions | PPA904 | 48 | PDT was used once a week for 12 weeks. Interim data on 24 patients showed that 4 of 12 ulcers in the PDT group closed completely, compared with 0 of 12 ulcers in the placebo group. | 270,271 | |
| Leishmaniasis | L. major | Skin | ALA | 11 patients (32 lesions) | Completely healing was seen after one or two PDT sessions. | 140 |
| L. major | Skin | ALA | 20 patients (31 lesions) | 3 months after PDT, 29 lesions were completely healed. | 149 | |
| Acne | Acne vulgaris | Skin/sebaceous gland | Indole-3-acetic acid (IAA) | 25 | Numbers of both inflammatory and non-inflammatory acne lesions were significantly decreased. No adverse effects were observed except for transient pruritus in one patient. | 272 |
| Acne vulgaris | Skin/sebaceous gland | ALA | 78 | 22% of patients showed excellent improvement after one PDT session and another 34% showed excellent improvement after two PDT sessions. The rest (44%) required three PDT sessions to further reduce the number and size of residual lesions. Adverse effects were minimal. | 273 | |
| Viral infection | Herpes simplex virus | Skin | MB | 4 | No significant acute side effects were noted and the lesions healed rapidly. | 274 |
| Papillomavirus | Cervical condylomata | ALA | 56 | The overall complete remission rate of 1–4 sessions of PDT was 98.2% and the corresponding HPV clearance rate was 83.9%. Ten cases showed complete removal of cervical lesions and HPV infection after a single PDT. Adverse effects were minimal. | 275 | |
| Gastric infection | H. pylori | Stomach | ALA | 13 | A maximum eradication effect was achieved at 4 h post-irradiation when 85% of biopsies in the monochromatic and 66% in the white light exposed zones, and 58 and 33% in the respective control zones were HP-negative. | 276 |
| Dental infection | A. actinomycetemcomitans | Dental pockets and gingival tissue | Phenothiazine chloride | 10 | PDT was effective in reducing numbers of A. actinomycetemcomitans than scaling and root planning. | 277 |
| Onychomycosis | T. rubrum | Tonail | ALA | 30 | After one year, 13 patients (43.3%) were cured. The remaining patients had changes compatible with dermatophyte infection covering more than 10% of the nail plate. | 207 |
By and large, PDT has been investigated in the laboratory and the clinic, and was found to be able to inactivate numerous pathogenic microorganisms efficiently. However, the application of PDT as an anti-infective in the clinic is still at a relatively early stage and faces some limitations that need to be overcome before it can assume its future application as a therapeutic approach for deep infections. PDT will not replace antimicrobial chemotherapy, but may improve the treatment of localized infections, promote healing and reduce the cost of the treatment with low side-effects. It would be prudent to say that there is not any convincing evidence to support s that PDT has superior advantages over the traditional modalities at present. Furthermore, meta-analyses and randomized long term clinical studies are necessary to confirm the beneficial effect of antimicrobial PDT according to the tenets of evidence-based medicine. Further studies on the exploration of new PSs and more efficient light delivery systems are required to establish optimal treatment parameters. Antimicrobial PDT may hold promise as a substitute for currently available antibiotic therapy in the treatment of recalcitrant, recurrent, and multi-drug resistant bacterial and fungal infections especially in poorly vascularized tissues where antibiotics have difficulty reaching.
4 Blue light for infectious disease
4.1 Introduction
Another novel light-based approach, the use of blue light (400–500nm) as a mono-therapy, is gaining increasing attention owing to its potential antimicrobial effect without addition of exogenous PS. Moreover, it is accepted that blue light is much less harmful to mammalian cells than ultraviolet irradiation [214]. In addition to safety benefits, prolonged exposure of materials to 405-nm visible light would not induce the problematic levels of photodegradation that are associated with similar periods of material exposure to UV light, particularly in the UV-C region of around 254 to 260 nm. The biological response of blue light was firstly reported in 1881 by Darwin who reported phototropic response in plants to blue light. Up to now, the phototropic response of blue light has been identified in all three biological domains, including bacteria, eukaryotes, and archaea [215]. Recently, new exciting discoveries have been made concerning the genomes of many photosynthetic and chemotropic bacteria and fungi, which can encode photoreceptor proteins. For examples, blue light receptors have been studied in Neurospora crassa and other fungi, which can be divided into two general classes: the cryptochromes and the phototropins. The two major blue light photoreceptors are, (i) white collar (WC)-1 and (WC)-2, which control dark to light transition and (ii) VIVID, a protein important in the second light signaling system. Together, they control responses to daily changes in light intensity and also are involved in the modulation of the circadian clock [216,217]. Other photoreceptors have been studied in E.coli [218] and A. baumannii [219] and are functional coded for production of a BLUF protein (blue light sensing using flavin), encoded by the blue-light-sensing A (bslA) gene. The discoveries of those photoreceptors have triggered the new concept that not only phototrophic bacteria but also photosynthetic and chemotrophic microorganisms can sense light [220]. So far, it has been found that some bacteria and fungi can chose their lifestyle between the motile single-cellular planktonic state and the multicellular surface-attached community state (biofilms) by photo-regulated pathways, including the second messenger cyclic di-GMP system, and direct interactions of photoreceptors with transcription factors [221,222]. Blue light has been verified as a crucial factor that can affect all these physiological responses and decide the bacterial lifestyle [223,224]. Blue light not only can regulate bacterial motility, suppress biofilm formation, but also subsequently potentiate light inactivation of bacteria. On the other hand, the presence of blue light may also activate or increase bacterial virulence [219].
The lethality of blue light for bacteria has been demonstrated both in vitro and in vivo which can produce a broad-spectrum bactericidal effect on both Gram-negative and Gram-positive bacteria. While the wavelength range of 402–420 nm has been reported to be the most effective antimicrobial spectral range, both 455 nm and 470 nm wavelengths have also been found to be of antimicrobial potential for some bacterial species (e.g., S. aureus) [219]. The precise mechanism of the antimicrobial effect of blue light is not fully elaborated yet. The generally accepted hypothesis is that blue light excites endogenous intracellular porphyrins to behave as PSs, and this photon absorption sequentially leads to energy transfer and ultimately, the production of highly cytotoxic ROS – most notably 1O2 in a similar manner to PDT [225]. However, different bacteria demonstrate variable susceptibilities to blue light. Studies have reported that Gram-positive species, in general, were more sensitive to 405 nm light inactivation than Gram-negative species, which is generally consistent with the results obtained in a recent study [226,227]. It has also been theorized that the differences in inactivation kinetics may be due to organism-specific differences in porphyrin levels, individual porphyrin sub-types (i.e., porphyrin wavelength absorption maxima), or as a consequence of the relatively short distance that 1O2 molecules can diffuse (~20 nm in solution) within cell structures [33,226]. In addition, it has been speculated that less oxygen-tolerant bacterial species may be particularly susceptible to the effects of ROS as microorganisms, such as some microaerophilic species, have been found to possess fewer key antioxidant defenses than most aerobes [228,229]. For example, studies have demonstrated that blue light is able to inactivate the anaerobic oral pathogens Prevotella, Porphyromonas, and Fusobacterium as well as microaerophilic pathogens such as P. acnes and H. pylori [33,230]. However, inactivation results achieved with anaerobic/microaerophilic bacteria have not provided conclusive evidence as to whether oxygen-sensitive anaerobic bacteria are any more susceptible to blue light than aerobes as many of these bacteria are also known to accumulate high levels of porphyrins. Possible explanations for this difference in inactivation susceptibility could be that different characteristics of bacteria, the unusual cell envelope, confers some resistance to 405 nm light penetration and/or that bacterial cell envelopes provide greater innate resistance to ROS given that bacteria species are capable of evading phagocytic oxidative burst damage [227].
4.2 Blue light inactivation of bacteria and fungi
Blue light is lethal to many species of bacteria and fungi, even including filamentous fungi, although the effectiveness is dependent on the light wavelength, energy levels and microbial genus and species [216]. Blue non-coherent light sources, such as LED, the halogen lamp, and the plasma-arc curing (PAC) light, are often used in vivo and in vitro studies. The bactericidal efficacy in vitro of blue light for Escherichia coli, MRSA and P. aeruginosa, even Streptococcus mutans in biofilms has been reported [231].
Maclean et al. studied the bactericidal effect of 405-nm blue light emitted from a LED array on some bacterial species, including Gram-positive S. epidermidis, S. aureus, Enterococcus faecalis, Streptococcus pyogenes, Clostridium perfringens, Gram-negative E. coli, Acinetobacter baumannii, P. aeruginosa, Proteus vulgaris and K. pneumonia which are all associated with hospital acquired infection [226]. The results demonstrated that most of Gram-positive and Gram-negative species were successfully eliminated, except E. faecalis which was the least sensitive to inactivation by 405-nm blue light.
Murdoch et al. reported that bactericidal effect of high-intensity 405nm light, delivered by an array of LED, on taxonomically diverse bacterial pathogens from the genera Salmonella, Shigella, Escherichia, Listeria, and Mycobacterium [227]. L. monocytogenes was most readily inactivated in suspension, whereas S. enterica was most resistant. In surface exposure tests, L. monocytogenes was more susceptible than Gram-negative enteric bacteria to 405nm light when exposed on an agar surface but interestingly less susceptible than S. enterica after drying onto PVC and acrylic surfaces. Mycobacterium terrae, which was chosen as a safe, comparative, surrogate microorganism for the highly pathogenic M. tuberculosis, was inactivated by 4–5 log10 CFU/ml between a dose of 144 and 288 J/cm2 by 405nm light.
Blue light photoinactivation—as evaluated for other bacteria such as Helicobacter pylori, Propionibacterium acnes, Porphyromonas gingivalis and some black-pigmented bacteria [225,232–234] —has been attributed to the photo-excitation of endogenous intracellular porphyrins by blue light in the wavelength region from 400 nm to 500 nm and, more particularly, 400 nm to 420 nm in the cases of H. pylori and P. acnes [225,232]. The studies on the in vitro antimicrobial effect of blue light are summarized in Table 3
Table 3.
Studies on the in vitro antimicrobial effect of blue light
| Light Source | Radiant exposure | Bacterial species/strains | Inactivation efficacy | Ref |
|---|---|---|---|---|
| 405-nm diode laser | 20 J/cm2 | H. pylori | >99.9% | 225 |
| 405 nm light-emitting diode | 15 J/cm2 at lamp aperture | P. gingivalis | >75% | 233 |
| 380–520 nm broadband light | 4.2–42 J/cm2 | P. gingivalis, P. intermedia, P. nigrescens, P. elaninogenica, S. constellatus | P. intermedia and P. nigrescens: >5 log10 at 4.2 J/cm2; P. melaninogenica: >5 log10 at 21 J/cm2; P. gingivalis:1.83 log10 at 42 J/cm2 | 278 |
| 400–500 nm blue lamps | 260 and 1300 mW/cm2 for up to 3 min | P. gingivalis, F. nucleatum, S. mutans, E. faecalis | The minimal inhibitory dose for P. gingivalis and F. nucleatum was 16–62 J/cm2, for S. mutans and S. faecalis was 159–212 J/cm2 | 279 |
| 405 and 470 nm light | 15 J/cm2 | S. aureus, P. aeruginosa | S. aureus: 90% at 405 nm, 62% at 470 nm; P. aeruginosa: 95.1% at 405 nm, 96.5% at 470 nm | 280 |
| 407–420 nm | five P. acnes strains | decreased by 15.7% immediately and 24.4% at 60 min after the irradiation. | 231 | |
| 407–420 nm | 75J/cm2 | P. acnes | less than 2-log10 units (99%) illuminated once; decreased by 4-log10 units (99.99%) after two illuminations and by 5-log10 units (99.999%) after three illuminations | 281 |
However, the types and the amount of porphyrins can lead to different photo-inactivation rates in different bacteria. For example, Gram-positive S. epidermidis and S. aureus produced coproporphyrin as the predominant porphyrin species, and these species produce 2–3 times higher coproporphyrin than in Gram-negative strains (i.e. E. coli, Aeromonas strains and Acinetobacter) whereas there was no primary porphyrin produced in some Gram-negative bacteria [235]. Owing to fact that different bacteria produce different predominant porphyrins and their peak absorption wavelengths of these porphyrins may be at variance. As a consequence, different wavelengths may be required for their optimal photo-stimulation. All the aforementioned factors clearly induced the diversity of bactericidal efficacy by blue light. Other factor that must be taken into account are that the bacteria may produce different levels of porphyrins when cultured in laboratory cultivation conditions than they do in their natural physiological states, such as when they are infecting a mammalian host [236].
Fungi are able to sense light in a broad spectrum range, from UV to far-red light because they have blue-light receptors. Flavins or flavoproteins are considered to act as blue light receptors and can absorb blue light [237]. This observation makes them able to serve as a cue’ in the asexual development of fungal spores, and affecting growth, metabolism, pigment formation, and tropism [238,239]. This observation makes them able to serve as a cue’ in the asexual development of fungal spores, and affecting growth, metabolism, pigment formation, and tropism [237], or could photoexcite endogenous porphyrins in a similar manner to PDT [240]. However, there have been few reports demonstrating the fungicidal efficacy of blue light. Only recently, De Lucca et al. found that blue light (470nm) alone significantly reduced Fusarium graminearum (FG) germinating conidial viability by approximately 36, 42, and 47% respectively at fluences of 40, 80 and 100 J/cm2 [216]. However, no significant viability loss was observed for the FG non-germinated conidia treated with blue light (20–100 J/cm2).
4.3 Effects of blue light on host cells and tissues
In order to use blue light for the treatment of infectious diseases, it is very important to assess the possible harm that the blue light could cause against host cells and tissues so that they can avoid non-specific damage. However, only a few studies have been conducted so far in this area.
In one in vitro study, Hockberguer et al. showed that some cells, such as mouse 3T3 fibroblasts, foreskin keratinocytes from human, kidney epithelial cells from monkey were irradiated with blue light (450nm at 6.3 W/cm2) for 20 min respectively, produced hydrogen peroxide (H2O2), an important ROS which can lead cellular damage and could cause pathological changes associated with exposure to blue light [241].
Wataha et al. treated 3T3 mouse fibroblasts with three dental blue (400nm–500nm) light sources, and detected succinate dehydrogenase (SDH) activity of mitochondria after a range of fluences (1.3 to 60 J/cm2) [242]. Results showed that fluences ranging from 5 J/cm2 to 15 J/cm2 irreversibly completely suppressed SDH activity compared to dark controls up to 72 h post-exposure.
Godley et al. investigated the interaction of human primary retinal epithelial (PRE) cells with blue light (390–550 nm at 2.8 mW/cm2) and reported significant damage to the mitochondrial DNA after 3 h of light exposure. At the same time, release of ROS (1O2, O2−•, HO•) increased in isolated mitochondria after irradiation. Hence, it has been confirmed that blue light can damage cells via ROS release and subsequent DNA damage [243].
Shnitkind et al. studied the effects of blue light illumination on two immortalized keratinocyte cell lines, HaCaT and hTERT1. The cells were irradiated by 420nm blue light at fluences of 54 or 134 mJ/cm2. The results showed that blue light inhibited IL-1α release, which proved that blue light has anti-inflammatory potential on keratinocytes [244].
Conversely, a recent clinical research performed by Kleinpenning et al. did not demonstrate significant side effects on human skin [214]. Eight healthy volunteers were illuminated with a blue light source (390–460 nm) with a peak emission of 420 nm over 5 consecutive days at 20 J/cm2. To analyze the irradiation effects, skin biopsies were analyzed for p53 expression, vacuolization, sunburn cells, elastosis, matrix metalloproteinase-1 (MMP-1), and melan-A expression. As a result, neither inflammatory nor sunburn cells were observed before or after irradiation. No significant changes in p53 and MMP-1 expressions or sign of elastosis were observed after blue light exposure. Keratinocytes demonstrated a significant incremental perinuclear vacuolization during treatment (P=0.02) with a tendency towards significance after cessation of treatment (P=0.09). Irradiated skin demonstrated minor hyperpigmentation and a significant increase in melan-A-positive cells (P=0.03) histologically. Therefore, the investigators concluded that short-term use of blue light in cutaneous treatment could be considered safe.
In conclusion, blue light has been occasionally reported to be phototoxic to mammalian cells at certain spectrum ranges in a wavelength dependent manner. The photocytotoxic effect was assumed to be similar to that which occurred in bacteria, such as photo-excitation of intracellular chromophores and subsequent cytotoxic ROS formation. Thus, an ideal blue light therapy should choose an optimal wavelength to selectively excite the chromophores in pathogenic bacteria with minimized effect on chromophores in mammalian cells [219].
4.4 The clinical application of blue light for infectious diseases
One of the major application of blue light for infectious disease in the clinic is the treatment on acne vulgaris which is an important dermatologic disorder infected mainly by P. acnes. Several studies have reported the benefits of blue light on acne vulgaris. Table 4 summarizes the clinical studies of blue light therapy for acne vulgaris.
Table 4.
Studies on blue light therapy for acne vulgaris
| Light source | Clinical trial type | Acne type | Treatment regimen | No. of patients/acne location | Total follow-up time | outcome | Side-effects | Ref |
|---|---|---|---|---|---|---|---|---|
| 407–420 nm metal halide lamp | open clinical trial | Mild to moderate acne | twice a week up to 5 weeks | 30 | during the trial period (0, 1, 3 and 5 weeks) and at 1 month after the final treatment | Acne lesions were reduced by 64%. | Two patients experienced dryness. | 281 |
| Handle 412nm LED | prospective, single-center, open-label study | Mild-to-moderate, including inflammatory or non-inflammatory | 2J/cm2/day full-face treatment or 29J/cm2/day localized spot treatment, twice daily at home | 32/facial acne | eight weeks | 100 percent of subjects considered their overall appearance was improved, clarity (97%), radiance (73%), tone (80%), texture (80%), and smoothness (83%) | Three adverse events: minimal transient skin dryness and minimal transient hyperpigmentation | 282 |
| home use Blue-light LED | IRB approved randomized self-control study | inflammatory | Four Treatments were conducted twice daily in the clinic | 30/facial acne | followed-up till resolution | significant reduction in lesion size and lesion erythema as well as the improvement in the overall skin condition controlled with the placebo | NA | 283 |
| hand-held, Blue-light LED | open clinical trial | mild-to-mode rate inflammatory acne | a daily dose of ~29 J/cm2, twice daily | 33/facial acne | eight weeks | 90% improvements | NA | 284 |
| 407 to 420 nm LED | A prospective, randomized, open and comparative study | inflammatory acne lesions grades II or III | underwent 8 sessions of light therapy for 15 minutes each, twice a week, within minimum intervals of 48h | 60/facial acne | 4 weeks | The improvement achieved by the blue light was the same as the one with benzoyl peroxide | 23.3% patients with mild desquamation and/or dryness | 285 |
| 415nm LED | open-lab el study | 48 J/cm2, receiving two treatments of 20 minutes per week for a period of 4–8 weeks | 45 | 2, 4 and 8 weeks after treatment | mean improvement score was 3.14 at 4 weeks and 2.90 at 8 weeks | 286 | ||
| blue LED, peak wavelength | open-label study | mild to moderat | Over 4 weeks, patients | 30 | at weeks 5, 8 and 12 | An overall effect on inflammatory | 287 | |
| 409–419 nm | e acne | received eight 10- or 20-minute light treatments | counts was observed at week 5, and a statistically significant decrease in inflamed counts was detected at the week 8 assessments, which continued to week 12. |
Blue light also can inactivate H. pylori, which is the primary pathogen causing atrophic gastritis and peptic ulcers and is increasingly resistant to antibiotics [245]. Ganz et al. treated 10 patients with blue light (405 nm, 40.5J/cm2) via an optical fiber inserted in the endoscope channel that illuminated a 1-cm diameter area of the gastric mucosa [246]. Significant decreases (91%) in H. pylori colonies per gram of tissue in treated sites was observed by comparing biopsies from treated and adjacent untreated sites. Some patients showed reductions of bacterial burden approaching 99%. Another pilot trial was carried out by Lembo et al. using a blue (408 nm) laser source with multi-segmented balloon that allowed whole stomach illumination to treat 18 patients with H. pylori infection [247]. A generalized reduction in bacterial load was observed in the stomach. Significant reduction in bacterial colonization was found in the antrum (>97%), followed by the body (>95%) and the fundus (>86%) with no apparent dose-response correlation. However, repeated breath tests showed H. pylori repopulated in days following illumination.
In conclusion, blue light can treat inflammatory and non-inflammatory acne lesions caused by P. acnes and digestive disorders caused by H. pylori. Blue light therapy has been demonstrated in clinical studies to be feasible and safe, and may represent a novel alternative approach to the eradication of P. acnes and H. pylori, especially in patients who have failed standard antibiotic treatment. However further studies are necessary to establish appropriate protocols for each clinical condition.
4.5 Summary
In summary, blue light, at certain wavelengths (range 402–420 nm), has been found to be effective in the inactivation of pathogens in both laboratory and clinic. The overall outcomes were demonstrated to be positive in all the studies. This is a new compelling approach that can eliminate a wide range of medically important pathogenic bacteria, including MRSA, P. aeruginosa, A. baumannii and E. faecalis. The absence of a requirement for pretreatment with a PS increases its likelihood for widespread applications, although discrepancies have been seen in the antibacterial efficacy in different studies. It was likely that the differences were owing to the different light delivery regimen used in the studies, differences in the spectra of light sources, differences in light fluences, variable microbial genera, different patient populations, etc. In common, the clinical application of blue light for infectious diseases is still at the early stage of development. The sample sizes of all published clinical studies are still very limited and the target bacteria are mainly P. acnes and H. pylori. Further studies with larger sample sizes are warranted to optimize the parameters of blue light therapy, and to investigate the use of blue light for infections caused by a wider range of pathogens. Further development of blue light inactivation approaches could lead to potential applications as decontamination systems for air, contact surfaces, and medical instruments within the clinical environment [248,249]. Light with wavelengths centered at 405-nm has shown a significantly broad antibacterial spectrum which offer potential application in numerous fields including food sterilization, prevention of contamination, sewage disposal and other disinfection industries [236].
5. Other light-based anti-infectives
5.1. Anti-infective effect of femtosecond pulsed lasers
It has been proposed that femtosecond lasers, or lasers that have a pulse duration in the region 10−15s, are able to break down transparent or semitransparent biological tissues due to nonlinear absorption of laser energy with minimal thermal and mechanical effects [250]. As a result of the adverse thermal damage possible with other laser systems, femtosecond lasers have been hypothesized to be a new approach as anti-infectives. The basic idea is that the entire energy of a pulse (which could be quite small) is delivered in a very short time, so if the average power is 10 mW and the pulse duration is 100 fs then the peak power is 100 GW which is sufficient to produce efficient 2-photon absorption. Recently, a series of studies have demonstrated the efficacy of a visible femtosecond laser or a near-infrared subpicosecond fiber laser on elimination of several kinds of viral species, including M13 bacteriophage, tobacco mosaic virus, HPV, and human immunodeficiency virus (HIV) [251–258]
In 2007, Tsen et al. found that a very low power visible femtosecond laser (as low as 0.5 nJ/pulse, 425 nm wavelength, 100 fs pulse width, peak power density greater than or equal to 50 MW/cm2) irradiated M13 phages efficiently [254]. Their further research demonstrated that one kind of visible femtosecond diode-pumped continuous-wave (CW) mode-locked Ti-sapphire laser (λ = 425 nm) could inactivate M13 bacteriophage, encephalomyocarditis virus and Salmonella typhimurium. The laser emitted a continuous array of 60 fs pulses at a repetition rate of 80 MHz, average power of about 50 mW. It has a pulse width of full-width at half maximum (FWHM) 100fs. The laser inactivated nearly the entire aforementioned microorganism very efficiently, especial for Salmonella typhimurium [258]. Another recent study reported the inactivation of murine cytomegalovirus (MCMV), an envelope, double-stranded DNA virus, illuminated by visible femtosecond laser ( λ = 425 nm) which was reduced 5 log 10 titer and the virus demonstrated selective aggregation of viral capsid and tegument proteins [259]. However, the global structure of MCMV virions including membrane and capsid, was not changed significantly evaluated by electron microscopy; meanwhile the double-chain of MCMV genomic DNA did not show any break or crosslinking.
Manevitch and colleagues showed that the application of the femtosecond laser at a fluence of 7×1031 photons m−2 s−1 effectively and selectively inactivated T. rubrum from nail clippings obtained from infected toenails [260]. The laser wavelength used was 800nm which wavelength could effectively traverse the nail to eliminate dermatophytes at any level. Most importantly, it killed fungal cells without any collateral damage to the nail structure as confirmed by canning electron microscopy. This is the only study assessing the fungicidal efficacy of this laser system for onychomycosis in vitro at present. In fact, it is difficult to selectively delivery laser energy to microorganisms embedded in the nail plate without causing collateral damage to the nail structure overlying the fungi. Femtosecond infrared titanium sapphire lasers can theoretically circumvent this problem owing to the nonlinear interactions of femtosecond lasers with biological media and the ability to selectively deliver energy at the laser focus [261]. This study further demonstrated femtosecond infrared laser was a new alternative approach for the infection of fungus.
It has been proposed that use of a near-infrared (NIR) femtosecond laser via impulsive stimulated raman scattering (ISRS) to produce damage (e.g. to the protein coat of a virus) is a method for selectively inactivating microorganisms [254]. There were different mechanisms of inactivation of different microorganism by femtosecond laser. Inactivation of viruses involving the breaking of hydrogen/hydrophobic bonds or the separation of the weak protein links in the protein shell of a viral particle. On the contrary, inactivation of bacteria is related to the damage of their DNA due to 2-photon absorption of a visible femtosecond laser [258].
When a NIR femtosecond laser induced the inaction of virus and bacteria, its safety to the mammalian cells was considered. The relative research demonstrated that if the wavelength and pulse width of the femtosecond laser were appropriately selected, there was a window in power density that enabled them to achieve selective inactivation of target viruses and bacteria without causing cytotoxicity to mammalian cells. It was suggested that this strategy targeted the mechanical (vibrational) properties of micro-organisms, and thus its antimicrobial efficacy was likely to be affected by genetic differences in the micro-organisms [255].
Advantages of such a new laser technology over the presently prevailed disinfection methods have been proposed to be: 1. it is a noninvasive disinfection technology, because no extraneous materials are needed in the disinfection process; 2.it is a harmless environmental disinfection method since no chemicals are used in the pathogen inactivation process; 3. it is a general method for selective disinfection of pathogens with potential minimal side effects [258].
5.2. Anti-infective effect of dual wavelength (870/930 nm) laser
Recently by using the wavelengths that had previously demonstrated cellular light mediated damage effects in optical traps, Bornstein et al. developed a dual wavelength laser system (with the wavelengths of 870 nm and 930 nm) for the inactivation of bacteria and fungi [262]. During in vitro studies, by using 1.5cm diameter flat-top light spots (fluence rate of 5.66 W/cm2, at physiological temperatures), the investigators successfully inactivated S. aureus, E. coli, C. albicans and T. rubrum. Using non-lethal light fluence, the authors observed a decrease in transmembrane potentials and an increase in ROS generation in pathogenic microorganisms (MRSA and C. albicans) and human embryonic kidney cells. It was also postulated that the dual wavelengths caused an optically mediated —mechanotransduction of cellular redox pathways, decreased transmembrane potentials, and increased ROS generation. The cellular energetics of prokaryotic, fungal pathogens, as well as host cells, was affected in a similar manner when treated with the dual wavelengths at the fluence used. After thermal tolerance experiments in live porcine skin, human pilot studies were performed, examining the photo-inactivation of MRSA in the nose, and of fungi present in onychomycosis. The investigators did not observe non-specific damages either to the nares or the nail matrix, yet found successful photo-inactivation of pathogens.
Krespi et al. performed a prospective clinical study using a dual wavelength laser (870/930 nm) for nasal decolonization of S. aureus and MRSA [263]. Twenty-five patients colonized with S. aureus or MRSA were enrolled and non-randomly divided into 4 treatment groups: dual-wavelength laser with low-power alone (GR1); dual wavelength laser with low-power in combination with erythromycin (E-mycin) cream (GR2); dual wavelength laser with low-power in combination with peroxide irrigation (GR3); and single wavelength 940-nm laser with high-power alone (GR4). Laser illumination was delivered by using a fiber diffuser, with the fluences of 200 to 600 J/cm2 for each naris circumferentially. Nasal decolonization efficacy for the groups of GR1, GR2, GR3, and GR4 was respectively 1 out of 3, 13 out of 14, 2 out of 4, and 4 out of 4. No side effects or discomfort were observed. The authors concluded that dual wavelength laser therapy could eradicate S. aureus and MRSA. It also could potentially re-sensitize bacteria which was drug-resistant to the common antibiotic, erythromycin.
A possible mechanism of the resensitization of bacteria to antibiotics using dual wavelength laser was that, the laser illumination interacted with the respiratory processes of the bacterial plasma membranes, lowered the membrane potential, and subsequently inactivated the bacterial resistance mechanism, which depended on the membrane drug efflux pump [263].
6. Can pathogenic microorganisms develop resistance to light based anti-infectives?
Before light-based antimicrobials become widely used, one question that must be addressed is: can pathogenic microorganisms develop resistance to light-based anti-infectives? If this is indeed possible, the mechanism of resistance development is likely to be different for each light-based modality.
6.1 Can pathogenic microorganisms develop resistance to PDT?
An early study investigating the development of resistance by bacteria after PDI was carried out by Lauro et al. [264]. Peptostreptococcus micros and Actinobacillus actinomycetemcomitans were subject to 10 cycles of repeated sub-lethal PDI and regrowth using visible light combined with the PS consisting of porphycene-polylysine conjugates (2,7,12,17-tetrakis(2-methoxyethyl)-9-glutaramidoporphycene and 2,7,12,17-tetrakis(2-methoxyethyl)-9-p-carboxybenzyloxyporphycene). The investigators found that the repeated PDI of P. micros and A. actinomycetemcomitans by both PSs did not induce the development of resistance by bacteria to PDI in partially photo-inactivated bacteria.
Taveres et al. assessed the development of bacterial resistance to PDI mediated by white light and Tri-Py+-Me-PF (5,10,15-tris(1-Methylpyridinium-4-yl)-20-(pentafluorophenyl)-porphyrin triiodide) [265] Vibrio fischeri and recombinant E. coli were the studied bacteria. The results showed that after 10 consecutive cycles of sub-lethal PDI followed by regrowth, E. coli did not develop appreciable resistance to PDI. In another study, Giuliani et al. investigated the potential of P. aeruginosa, S. aureus and C. albicans strains to develop resistance to PDI after beingexposed to RLP068/Cl, a tetracationic Zn(II) phthalocyanine derivative [266]. The authors reported that 20 consecutive cycles of sub-lethal PDI with RLP068/Cl did not generate any resistant mutants.
6.2 Can pathogenic microorganisms develop resistance to UVC irradiation?
Dai et al. investigated the potential development of resistance to UVC (254 nm) inactivation by T. rubrum by performing five consecutive cycles of sub-lethal UVC inactivation followed by fungal regrowth in vitro [9]. The investigators did not observe significant difference in fungal inactivation rate among the five cycles of fungal inactivation and regrowth when 120 mJ/cm2 UVC energy was delivered. The investigators concluded that the development of resistance to UVC inactivation is not rapidly acquired by T. rubrum that are subject to repeated sub-lethal UVC inactivation.
Alcantara-Diaz et al. evaluated the divergent adaptation of E. coli to repeated cycles of sub-lethal UVC inactivation at 254 nm [267]. Five cultures of wild type E. coli PQ30 were exposed to 80 consecutive bacterial inactivation by UVC and regrowth. The initial fluence of UVC was 1mJ/cm2 for each cycle, and the fluence was increased 2-fold every 10 inactivation-regrowth cycles. Quantitative dose response curves were obtained by exposing the bacteria in exponentially growing phase to several UVC fluences. The investigators reported that all cultures were observed to develop different degrees resistance to UVC inactivation after 80 consecutive cycles of sub-lethal bacterial inactivation and regrowth. The adaptation of bacteria to cyclic UVC inactivation was believed to be a consequence of selecting advantageous mutations in those genes related to DNA repair and replication.
6.3 Can pathogenic microorganisms develop resistance to blue light?
To the best of our knowledge, this question has not yet been experimentally addressed. As the mechanism of blue light inactivation of microorganisms is suggested to be similar to that of PDI [219,225,268], it is expected that the potential of resistance development by pathogenic microorganisms to blue light inactivation is less than that of antibiotics. In a recent study carried out in our lab (data not published), we found that, after 10 consecutive cycles of sub-lethal bacterial (A. baumannii) inactivation by blue light at 415 nm and bacterial regrowth, there was a significant decrease (rather than an increase) in bacterial resistance to blue light inactivation between the first cycle and the 10th cycle (P <0.05). However, a contradictory finding was reported by Guffey et al. [261]. In that study, the investigators found that the inactivation of S. aureus by blue light (405 nm) was increasingly effective through the first 4 cycles of sub-lethal inactivation-regrowth (P<0.05). From the 5th cycle, the increase of bacterial resistance to blue light inactivation was observed (P<0.05).
6.4 Summary
There have been no reported cases of PDT induced resistance of microorganisms despite numerous attempts to induce resistance by repeated cycles of sub-lethal PDI and microbial re-growth. It is believed that PDT acts at multiple sites within microorganism cells [269], and, subsequently, offers less potential for the development of resistance by microorganisms to PDI than antibiotics, which usually act on a single-target. Development of resistance by microorganisms to UVC inactivation is possible after excessive repetition of UVC inactivation. This is because one primary function of UVC inactivation is to damage microbial DNA. However, for treatment of wound or other localized infections using UVC, only a limited number of repetitions of therapy are expected to be required. Variable results have been reported regarding the development of resistance by microorganisms to blue light inactivation. Further studies are warranted to elucidate the intrinsic mechanism of blue light inactivation of microorganisms.
7. Conclusion
Light based anti-infective therapies have become a rapidly growing area of interest in recent years. The reasons for this rise in interest are manifold; multi antibiotic resistance is the predominant growth-driving factor, but other relevant considerations may be the public distrust of big-pharmaceutical companies and their products, the desire to use —natural therapies and blue light is considered somewhat similar to sunlight; and the rapid microbial killing typically seen in phototherapy; so when the light is switched off the killing is done. Since many of the applications of phototherapy can in fact be performed by midday sunlight that has been suitable concentrated and/or filtered by cheap disposable devices, antimicrobial phototherapy may in future be transitioned into a therapy suitable for the developing world, where it is accepted that infectious diseases represent a much bigger burden to public health than they do in developed nations. As always in anti-infective therapies, selectivity towards microbial cells compared to host mammalian cells is also critical. Fortunately this appears to be the rule rather than exception for the phototherapies discussed above.
Highlights (for review).
Light-based anti-infectives work against biofilms and localized infections.
Ultraviolet C light will kill all pathogens without killing host mammalian cells.
Photodynamic inactivation produces reactive oxygen species that kill pathogens.
Blue light can kill many pathogens due to accumulation of porphyrins.
Femtosecond infrared laser pulses can selectively kill pathogens.
Acknowledgments
Research in the Hamblin laboratory is supported by US NIH grant R01AI050875. Rui Yin was supported in part by National Natural Science Foundation of China (No.81172495) in this work. Tianhong Dai was supported by an Airlift Research Foundation Extremity Trauma research grant (No. 109421). Ana Jorge and Wanessa de Melo were supported by CAPES (Coordination for the Improvement of Higher Level Personnel) from Brazil. Asheesh Gupta was supported by BOYSCAST Fellowship 2010–11, Department of Science and Technology, Government of India.
Abbreviations
- ALA
5-aminolevulinic acid methyl ester
- BCG
M. bovis Bacille Calmette Guerin
- bFGF
basic fibroblast growth factor
- BVDV
bovine viral diarrhea virus
- CFU
colony forming units
- ClAlPC
chloroaluminum-phthalocyanine
- CPD
cyclobutane pyrimidine dimer
- CSRB
chitosan-conjugated rose bengal
- DMMB
dimethylmethylene blue
- DP-mme
deuteroporphyrin mono-methylester
- EDTA
ethyelenediamine tetraacetic acid
- ER
erythrosine
- GaPc
cationic gallium phthalocyanine
- HA(B)
hypocrellin A(B)
- Hp
hematoporphyrin
- HPV
human papillomavirus
- LED
light-emitting diode
- MAL
5-aminolevulinic acid
- MB
methylene blue
- MDR
multi-drug resistant
- MRSA
methicillin resistant Staphylococcus aureus
- MSSA
methicillin sensitive Staphylococcus aureus
- PDI
photodynamic inactivation
- PDR
pan-drug resistant
- PDT
photodynamic therapy
- PEI-ce6
polyethylenimine chlorin(e6) conjugate
- PS
photosensitizer
- RB
rose Bengal
- RBC
red blood cell
- ROS
reactive oxygen species
- TB
Mycobacterium tuberculosis
- TBO
toluidine blue O
- THPTS
meso-tetrahydroporphyrin-tetratosylate
- TMP
tetra-substituted N-methyl-pyridylporphine
- Tri-P(4)
5-phenyl-10,15,20-tris(N-methyl-4pyridyl)-porphine trichloride
- UVC
ultraviolet c
- VRE
vancomycin resistant Enterococcus
- VSV
vesicular stomatitis virus
- WGA
wheat germ agglutinin
- XDR
extensively-drug resistant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bell SG. Antibiotic resistance: is the end of an era near? Neonatal Netw. 2003;22:47–54. doi: 10.1891/0730-0832.22.6.47. [DOI] [PubMed] [Google Scholar]
- 2**.Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, Jacoby GA, Kishony R, Kreiswirth BN, Kutter E, et al. Tackling antibiotic resistance. Nat Rev Microbiol. 2011;9:894–896. doi: 10.1038/nrmicro2693. An important review on the present state of antibiotic resistance and possible strategies to overcome the problem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai T, Vrahas MS, Murray CK, Hamblin MR. Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev Anti Infect Ther. 2012;10:185–195. doi: 10.1586/eri.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurzadyan GG, Gorner H, Schulte-Frohlinde D. Ultraviolet (193, 216 and 254 nm) photoinactivation of Escherichia coli strains with different repair deficiencies. Radiat Res. 1995;141:244–251. [PubMed] [Google Scholar]
- 5.Conner-Kerr TA, Sullivan PK, Gaillard J, Franklin ME, Jones RM. The effects of ultraviolet radiation on antibiotic-resistant bacteria in vitro. Ostomy Wound Manage. 1998;44:50–56. [PubMed] [Google Scholar]
- 6.Su Y, Sun J, Rao S, Cai Y, Yang Y. Photodynamic antimicrobial activity of hypocrellin A. J Photochem Photobiol B. 2011;103:29–34. doi: 10.1016/j.jphotobiol.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Dean SJ, Petty A, Swift S, McGhee JJ, Sharma A, Shah S, Craig JP. Efficacy and safety assessment of a novel ultraviolet C device for treating corneal bacterial infections. Clin Experiment Ophthalmol. 2011;39:156–163. doi: 10.1111/j.1442-9071.2010.02471.x. [DOI] [PubMed] [Google Scholar]
- 8.Bak J, Ladefoged SD, Tvede M, Begovic T, Gregersen A. Dose requirements for UVC disinfection of catheter biofilms. Biofouling. 2009;25:289–296. doi: 10.1080/08927010802716623. [DOI] [PubMed] [Google Scholar]
- 9.Dai T, Tegos GP, Rolz-Cruz G, Cumbie WE, Hamblin MR. Ultraviolet C inactivation of dermatophytes: implications for treatment of onychomycosis. Br J Dermatol. 2008;158:1239–1246. doi: 10.1111/j.1365-2133.2008.08549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan PK, Conner-Kerr TA. A comparative study of the effects of UVC irradiation on select procaryotic and eucaryotic wound pathogens. Ostomy Wound Manage. 2000;46:28–34. [PubMed] [Google Scholar]
- 11.Dai T, Kharkwal GB, Zhao J, St Denis TG, Wu Q, Xia Y, Huang L, Sharma SK, d’Enfert C, Hamblin MR. Ultraviolet-C light for treatment of Candida albicans burn infection in mice. Photochem Photobiol. 2011;87:342–349. doi: 10.1111/j.1751-1097.2011.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Dai T, Murray CK, Vrahas MS, Baer DG, Tegos GP, Hamblin MR. Ultraviolet C light for Acinetobacter baumannii wound infections in mice: potential use for battlefield wound decontamination? J Trauma Acute Care Surg. 2012;73:661–667. doi: 10.1097/TA.0b013e31825c149c. One of the few papers showing that UVC can have a beneficial in an animal model of localized bacterial infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trevisan A, Piovesan S, Leonardi A, Bertocco M, Nicolosi P, Pelizzo MG, Angelini A. Unusual high exposure to ultraviolet-C radiation. Photochem Photobiol. 2006;82:1077–1079. doi: 10.1562/2005-10-27-ra-728. [DOI] [PubMed] [Google Scholar]
- 14.Sosnin EASE, Erofeev MV, Kieft IE, Kunts SE. The effects of UV irradiation and gas plasma treatment on living mammalian cells and bacteria: a comparative approach. IEEE Tran Plasma Sci. 2004;32:1544–1550. [Google Scholar]
- 15.Dai T, Tegos GP, St Denis TG, Anderson D, Sinofsky E, Hamblin MR. Ultraviolet-C irradiation for prevention of central venous catheter-related infections: an in vitro study. Photochem Photobiol. 2011;87:250–255. doi: 10.1111/j.1751-1097.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohr H, Steil L, Gravemann U, Thiele T, Hammer E, Greinacher A, Muller TH, Volker U. A novel approach to pathogen reduction in platelet concentrates using short-wave ultraviolet light. Transfusion. 2009;49:2612–2624. doi: 10.1111/j.1537-2995.2009.02334.x. [DOI] [PubMed] [Google Scholar]
- 17.Sterenborg HJ, van der Putte SC, van der Leun JC. The dose-response relationship of tumorigenesis by ultraviolet radiation of 254 nm. Photochem Photobiol. 1988;47:245–253. doi: 10.1111/j.1751-1097.1988.tb02722.x. [DOI] [PubMed] [Google Scholar]
- 18.Young AR. Acute effects of UVR on human eyes and skin. Prog Biophys Mol Biol. 2006;92:80–85. doi: 10.1016/j.pbiomolbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Freytes HA, Fernandez B, Fleming WC. Ultraviolet Light in the Treatment of Indolent Ulcers. South Med J. 1965;58:223–226. doi: 10.1097/00007611-196502000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Nussbaum EL, Biemann I, Mustard B. Comparison of ultrasound/ultraviolet-C and laser for treatment of pressure ulcers in patients with spinal cord injury. Phys Ther. 1994;74:812–823. doi: 10.1093/ptj/74.9.812. discussion 824–815. [DOI] [PubMed] [Google Scholar]
- 21.Taylor GJ, Bannister GC, Leeming JP. Wound disinfection with ultraviolet radiation. J Hosp Infect. 1995;30:85–93. doi: 10.1016/0195-6701(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 22.Thai TP, Houghton PE, Campbell KE, Woodbury MG. Ultraviolet light C in the treatment of chronic wounds with MRSA: a case study. Ostomy Wound Manage. 2002;48:52–60. [PubMed] [Google Scholar]
- 23*.Thai TP, Keast DH, Campbell KE, Woodbury MG, Houghton PE. Effect of ultraviolet light C on bacterial colonization in chronic wounds. Ostomy Wound Manage. 2005;51:32–45. A clinical study of UVC in chronic MRSA wound infections. [PubMed] [Google Scholar]
- 24.Boker AR-CG, Cumbie B, Kimball AB. A single-center, prospective, open-label, pilot study of the safety, local tolerability, and efficacy of ultraviolet-C (UVC) phototherapy for the treatment of great toenail onychomycosis. J Am Acad Dermatol. 2007;58:AB82. [Google Scholar]
- 25*.Gupta A, Dai T, Avci P, Huang YY, Hamblin MR. Ultraviolet radiation in wound care: sterilization and stimulation. Adv Wound Care. 2013 doi: 10.1089/wound.2012.0366. in press. Review of the use of UV light in wound healing for either stimulating the tissue healing process or alternatively for killing microbial contamination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimomura A, Tahara D, Tominaga M, Uchigiri S, Yamaguchi Y, Ishioka H, Nakahata A. The effect of ultraviolet rays on the prevention of exit-site infections. Adv Perit Dial. 1995;11:152–156. [PubMed] [Google Scholar]
- 27.Foote CS. Definition of type I and type II photosensitized oxidation. Photochem Photobiol. 1991;54:659. doi: 10.1111/j.1751-1097.1991.tb02071.x. [DOI] [PubMed] [Google Scholar]
- 28.Tanielian C, Mechin R, Seghrouchni R, Schweitzer C. Mechanistic and kinetic aspects of photosensitization in the presence of oxygen. Photochem Photobiol. 2000;71:12–19. doi: 10.1562/0031-8655(2000)071<0012:makaop>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B. 1997;39:1–18. doi: 10.1016/s1011-1344(96)07428-3. [DOI] [PubMed] [Google Scholar]
- 30.Mitton D, Ackroyd R. A brief overview of photodynamic therapy in Europe. Photodiagnosis Photodyn Ther. 2008;5:103–111. doi: 10.1016/j.pdpdt.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Nitzan Y, Gutterman M, Malik Z, Ehrenberg B. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem Photobiol. 1992;55:89–96. doi: 10.1111/j.1751-1097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 32.St Denis TG, Dai T, Izikson L, Astrakas C, Anderson RR, Hamblin MR, Tegos GP. All you need is light: antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence. 2011;2:509–520. doi: 10.4161/viru.2.6.17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. A highly cited review covering antimicrobial photodynamic inactivation and its application to experimental and clinical infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med. 2006;38:468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 35.Malik Z, Ladan H, Nitzan Y. Photodynamic inactivation of Gram-negative bacteria: problems and possible solutions. J Photochem Photobiol B. 1992;14:262–266. doi: 10.1016/1011-1344(92)85104-3. [DOI] [PubMed] [Google Scholar]
- 36.Soncin M, Fabris C, Busetti A, Dei D, Nistri D, Roncucci G, Jori G. Approaches to selectivity in the Zn(II)-phthalocyanine-photosensitized inactivation of wild-type and antibiotic-resistant Staphylococcus aureus. Photochem Photobiol Sci. 2002;1:815–819. doi: 10.1039/b206554a. [DOI] [PubMed] [Google Scholar]
- 37.Tome JP, Neves MG, Tome AC, Cavaleiro JA, Soncin M, Magaraggia M, Ferro S, Jori G. Synthesis and antibacterial activity of new poly-S-lysine-porphyrin conjugates. J Med Chem. 2004;47:6649–6652. doi: 10.1021/jm040802v. [DOI] [PubMed] [Google Scholar]
- 38.Schastak S, Ziganshyna S, Gitter B, Wiedemann P, Claudepierre T. Efficient photodynamic therapy against gram-positive and gram-negative bacteria using THPTS, a cationic photosensitizer excited by infrared wavelength. PloS one. 2010;5:e11674. doi: 10.1371/journal.pone.0011674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schastak S, Gitter B, Handzel R, Hermann R, Wiedemann P. Improved photoinactivation of gram-negative and gram-positive methicillin-resistant bacterial strains using a new near-infrared absorbing meso-tetrahydroporphyrin: a comparative study with a chlorine e6 photosensitizer photolon. Methods Find Exp Clin Pharmacol. 2008;30:129–133. doi: 10.1358/mf.2008.30.2.1165448. [DOI] [PubMed] [Google Scholar]
- 40.Mantareva V, Kussovski V, Angelov I, Wohrle D, Dimitrov R, Popova E, Dimitrov S. Non-aggregated Ga(III)-phthalocyanines in the photodynamic inactivation of planktonic and biofilm cultures of pathogenic microorganisms. Photochem Photobiol Sci. 2011;10:91–102. doi: 10.1039/b9pp00154a. [DOI] [PubMed] [Google Scholar]
- 41.Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg Med. 2010;42:38–44. doi: 10.1002/lsm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wainwright M, Phoenix DA, Gaskell M, Marshall B. Photobactericidal activity of methylene blue derivatives against vancomycin-resistant Enterococcus spp. J Antimicrob Chemother. 1999;44:823–825. doi: 10.1093/jac/44.6.823. [DOI] [PubMed] [Google Scholar]
- 43.Xing B, Jiang T, Bi W, Yang Y, Li L, Ma M, Chang CK, Xu B, Yeow EK. Multifunctional divalent vancomycin: the fluorescent imaging and photodynamic antimicrobial properties for drug resistant bacteria. Chem Commun (Camb) 2011;47:1601–1603. doi: 10.1039/c0cc04434b. [DOI] [PubMed] [Google Scholar]
- 44.Gad F, Zahra T, Hasan T, Hamblin MR. Effects of growth phase and extracellular slime on photodynamic inactivation of gram-positive pathogenic bacteria. Antimicrob Agents Chemother. 2004;48:2173–2178. doi: 10.1128/AAC.48.6.2173-2178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordmann P, Naas T, Fortineau N, Poirel L. Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp and Pseudomonas aeruginosa in 2010. Curr Opin Microbiol. 2007;10:436–440. doi: 10.1016/j.mib.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 47.Tseng SP, Teng LJ, Chen CT, Lo TH, Hung WC, Chen HJ, Hsueh PR, Tsai JC. Toluidine blue O photodynamic inactivation on multidrug-resistant Pseudomonas aeruginosa. Lasers Surg Med. 2009;41:391–397. doi: 10.1002/lsm.20765. [DOI] [PubMed] [Google Scholar]
- 48.Kussovski V, Mantareva V, Angelov I, Orozova P, Wohrle D, Schnurpfeil G, Borisova E, Avramov L. Photodynamic inactivation of Aeromonas hydrophila by cationic phthalocyanines with different hydrophobicity. FEMS Microbiol Lett. 2009;294:133–140. doi: 10.1111/j.1574-6968.2009.01555.x. [DOI] [PubMed] [Google Scholar]
- 49.Trannoy LL, Terpstra FG, de Korte D, Lagerberg JW, Verhoeven AJ, Brand A, van Engelenburg FA. Differential sensitivities of pathogens in red cell concentrates to Tri-P(4)-photoinactivation. Vox sanguinis. 2006;91:111–118. doi: 10.1111/j.1423-0410.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 50.Jassal M, Bishai WR. Extensively drug-resistant tuberculosis. Lancet Infect Dis. 2009;9:19–30. doi: 10.1016/S1473-3099(08)70260-3. [DOI] [PubMed] [Google Scholar]
- 51.O’Riordan K, Sharlin DS, Gross J, Chang S, Errabelli D, Akilov OE, Kosaka S, Nau GJ, Hasan T. Photoinactivation of Mycobacteria in vitro and in a new murine model of localized Mycobacterium bovis BCG-induced granulomatous infection. Antimicrob Agents Chemother. 2006;50:1828–1834. doi: 10.1128/AAC.50.5.1828-1834.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Riordan K, Akilov OE, Chang SK, Foley JW, Hasan T. Real-time fluorescence monitoring of phenothiazinium photosensitizers and their anti-mycobacterial photodynamic activity against Mycobacterium bovis BCG in in vitro and in vivo models of localized infection. Photochem Photobiol Sci. 2007;6:1117–1123. doi: 10.1039/b707962a. [DOI] [PubMed] [Google Scholar]
- 53*.Wiegell SR, Kongshoj B, Wulf HC. Mycobacterium marinum infection cured by photodynamic therapy. Arch Dermatol. 2006;142:1241–1242. doi: 10.1001/archderm.142.9.1241. Use of PDT to cure an unusual clinical case of a mycobacterial inefction. [DOI] [PubMed] [Google Scholar]
- 54.Shih MH, Huang FC. Effects of photodynamic therapy on rapidly growing nontuberculous mycobacteria keratitis. Invest Ophthalmol Vis Sci. 2011;52:223–229. doi: 10.1167/iovs.10-5593. [DOI] [PubMed] [Google Scholar]
- 55.Thota S, Wang M, Jeon S, Maragani S, Hamblin MR, Chiang LY. Synthesis and characterization of positively charged pentacationic [60]fullerene monoadducts for antimicrobial photodynamic inactivation. Molecules. 2012;17:5225–5243. doi: 10.3390/molecules17055225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shrestha A, Kishen A. Polycationic chitosan-conjugated photosensitizer for antibacterial photodynamic therapy. Photochem Photobiol. 2012;88:577–583. doi: 10.1111/j.1751-1097.2011.01026.x. [DOI] [PubMed] [Google Scholar]
- 57.Yang K, Gitter B, Ruger R, Albrecht V, Wieland GD, Fahr A. Wheat germ agglutinin modified liposomes for the photodynamic inactivation of bacteria. Photochem Photobiol. 2012;88:548–556. doi: 10.1111/j.1751-1097.2011.00983.x. [DOI] [PubMed] [Google Scholar]
- 58.Yang K, Gitter B, Ruger R, Wieland GD, Chen M, Liu X, Albrecht V, Fahr A. Antimicrobial peptide-modified liposomes for bacteria targeted delivery of temoporfin in photodynamic antimicrobial chemotherapy. Photochem Photobiol Sci. 2011;10:1593–1601. doi: 10.1039/c1pp05100h. [DOI] [PubMed] [Google Scholar]
- 59.Tsai T, Yang YT, Wang TH, Chien HF, Chen CT. Improved photodynamic inactivation of gram-positive bacteria using hematoporphyrin encapsulated in liposomes and micelles. Lasers Surg Med. 2009;41:316–322. doi: 10.1002/lsm.20754. [DOI] [PubMed] [Google Scholar]
- 60.Tsai T, Chien HF, Wang TH, Huang CT, Ker YB, Chen CT. Chitosan augments photodynamic inactivation of gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2011;55:1883–1890. doi: 10.1128/AAC.00550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Orlandi VT, Caruso E, Banfi S, Barbieri P. Effect of organic matter on the in vitro photoeradication of Pseudomonas aeruginosa by means of a cationic tetraaryl-porphyrin. Photochem Photobiol. 2012;88:557–564. doi: 10.1111/j.1751-1097.2012.01122.x. [DOI] [PubMed] [Google Scholar]
- 62.Walther J, Brocker MJ, Watzlich D, Nimtz M, Rohde M, Jahn D, Moser J. Protochlorophyllide: a new photosensitizer for the photodynamic inactivation of Gram-positive and Gram-negative bacteria. FEMS microbiology letters. 2009;290:156–163. doi: 10.1111/j.1574-6968.2008.01413.x. [DOI] [PubMed] [Google Scholar]
- 63**.Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J Infect Dis. 2003;187:1717–1725. doi: 10.1086/375244. The first report to show that topical PDT could rescue mice with a localized infection from an otherwise certain death due to the infection spreading to systemic sepsis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64*.Hamblin MR, O’Donnell DA, Murthy N, Contag CH, Hasan T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol. 2002;75:51–57. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. First report of bioluminescent bacteria being used to monitor the efficcay of PDT in an animal model. [DOI] [PubMed] [Google Scholar]
- 65.Dai T, Tegos GP, Lu Z, Huang L, Zhiyentayev T, Franklin MJ, Baer DG, Hamblin MR. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother. 2009;53:3929–3934. doi: 10.1128/AAC.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66*.Berthiaume F, Reiken SR, Toner M, Tompkins RG, Yarmush ML. Antibody-targeted photolysis of bacteria in vivo. Biotechnology (N Y) 1994;12:703–706. doi: 10.1038/nbt0794-703. Impressive early study that used an antibody-conjugated photosensitizer to treat a mouse infection with PDT. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto MC, Prates RA, Kato IT, Nunez SC, Courrol LC, Ribeiro MS. Antimicrobial photodynamic therapy on drug-resistant Pseudomonas aeruginosa-induced infection. An in vivo study. Photochem Photobiol. 2012;88:590–595. doi: 10.1111/j.1751-1097.2012.01137.x. [DOI] [PubMed] [Google Scholar]
- 68.Park JH, Ahn MY, Kim YC, Kim SA, Moon YH, Ahn SG, Yoon JH. In vitro and in vivo antimicrobial effect of photodynamic therapy using a highly pure chlorin e6 against Staphylococcus aureus Xen29. Biol Pharm Bull. 2012;35:509–514. doi: 10.1248/bpb.35.509. [DOI] [PubMed] [Google Scholar]
- 69*.Tanaka M, Kinoshita M, Yoshihara Y, Shinomiya N, Seki S, Nemoto K, Hamblin MR, Morimoto Y. Photodynamic therapy using intra-articular Photofrin for murine MRSA arthritis: biphasic light dose response for neutrophil-mediated antibacterial effect. Lasers Surg Med. 2011;43:221–229. doi: 10.1002/lsm.21037. Shows that PDT can extert an antimicrobial effect by stimulating the host immune system rather than by the PDT directly killing the microbial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goto B, Iriuchishima T, Horaguchi T, Tokuhashi Y, Nagai Y, Harada T, Saito A, Aizawa S. Therapeutic effect of photodynamic therapy using Na-pheophorbide a on osteomyelitis models in rats. Photomed Laser Surg. 2011;29:183–189. doi: 10.1089/pho.2010.2803. [DOI] [PubMed] [Google Scholar]
- 71.Dortbudak O, Haas R, Bernhart T, Mailath-Pokorny G. Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin Oral Implants Res. 2001;12:104–108. doi: 10.1034/j.1600-0501.2001.012002104.x. [DOI] [PubMed] [Google Scholar]
- 72.Shibli JA, Martins MC, Theodoro LH, Lotufo RF, Garcia VG, Marcantonio EJ. Lethal photosensitization in microbiological treatment of ligature-induced peri-implantitis: a preliminary study in dogs. J Oral Sci. 2003;45:17–23. doi: 10.2334/josnusd.45.17. [DOI] [PubMed] [Google Scholar]
- 73*.Hayek RR, Araujo NS, Gioso MA, Ferreira J, Baptista-Sobrinho CA, Yamada AM, Ribeiro MS. Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature-induced peri-implantitis in dogs. J Periodontol. 2005;76:1275–1281. doi: 10.1902/jop.2005.76.8.1275. Study showing that PDT can be effective in a beagle dog model of peri-implantitis. [DOI] [PubMed] [Google Scholar]
- 74.Komerik N, Nakanishi H, MacRobert AJ, Henderson B, Speight P, Wilson M. In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother. 2003;47:932–940. doi: 10.1128/AAC.47.3.932-940.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sigusch BW, Pfitzner A, Albrecht V, Glockmann E. Efficacy of photodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog model. J Periodontol. 2005;76:1100–1105. doi: 10.1902/jop.2005.76.7.1100. [DOI] [PubMed] [Google Scholar]
- 76.Bottura PE, Milanezi J, Fernandes LA, Caldas HC, Abbud-Filho M, Garcia VG, Baptista MA. Nonsurgical periodontal therapy combined with laser and photodynamic therapies for periodontal disease in immunosuppressed rats. Transplant Proc. 2011;43:2009–2016. doi: 10.1016/j.transproceed.2011.03.083. [DOI] [PubMed] [Google Scholar]
- 77.Fernandes LA, de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Martins TM, Okamoto T, Garcia VG. Treatment of experimental periodontal disease by photodynamic therapy in immunosuppressed rats. J Clin Periodontol. 2009;36:219–228. doi: 10.1111/j.1600-051X.2008.01355.x. [DOI] [PubMed] [Google Scholar]
- 78.de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Bonfante S, Garcia VG. Treatment of experimental periodontal disease by photodynamic therapy in rats with diabetes. J Periodontol. 2008;79:2156–2165. doi: 10.1902/jop.2008.080103. [DOI] [PubMed] [Google Scholar]
- 79.de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Oshiiwa M, Garcia VG. Influence of photodynamic therapy on the development of ligature-induced periodontitis in rats. J Periodontol. 2007;78:566–575. doi: 10.1902/jop.2007.060214. [DOI] [PubMed] [Google Scholar]
- 80.Seguier S, Souza SL, Sverzut AC, Simioni AR, Primo FL, Bodineau A, Correa VM, Coulomb B, Tedesco AC. Impact of photodynamic therapy on inflammatory cells during human chronic periodontitis. J Photochem Photobiol B. 2010;101:348–354. doi: 10.1016/j.jphotobiol.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 81.Wilson M, Mia N. Sensitisation of Candida albicans to killing by low-power laser light. J Oral Pathol Med. 1993;22:354–357. doi: 10.1111/j.1600-0714.1993.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 82.de Souza SC, Junqueira JC, Balducci I, Koga-Ito CY, Munin E, Jorge AO. Photosensitization of different Candida species by low power laser light. J Photochem Photobiol B. 2006;83:34–38. doi: 10.1016/j.jphotobiol.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 83*.Junqueira JC, da Martins JS, Faria RL, Colombo CE, Jorge AO. Photodynamic therapy for the treatment of buccal candidiasis in rats. Lasers Med Sci. 2009;24:877–884. doi: 10.1007/s10103-009-0673-4. Shows that PDT can treat a rat model of oral candidiasis. [DOI] [PubMed] [Google Scholar]
- 84.Bliss JM, Bigelow CE, Foster TH, Haidaris CG. Susceptibility of Candida species to photodynamic effects of photofrin. Antimicrob Agents Chemother. 2004;48:2000–2006. doi: 10.1128/AAC.48.6.2000-2006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lazarova G. Effect of glutathione on rose bengal photosensitized yeast damage. Microbios. 1993;75:39–43. [PubMed] [Google Scholar]
- 86.Bertoloni G, Rossi F, Valduga G, Jori G, Ali H, van Lier JE. Photosensitizing activity of water- and lipid-soluble phthalocyanines on prokaryotic and eukaryotic microbial cells. Microbios. 1992;71:33–46. [PubMed] [Google Scholar]
- 87.Dovigo LN, Pavarina AC, Mima EG, Giampaolo ET, Vergani CE, Bagnato VS. Fungicidal effect of photodynamic therapy against fluconazole-resistant Candida albicans and Candida glabrata. Mycoses. 2011;54:123–130. doi: 10.1111/j.1439-0507.2009.01769.x. [DOI] [PubMed] [Google Scholar]
- 88.Teichert MC, Jones JW, Usacheva MN, Biel MA. Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:155–160. doi: 10.1067/moe.2002.120051. [DOI] [PubMed] [Google Scholar]
- 89.Giroldo LM, Felipe MP, de Oliveira MA, Munin E, Alves LP, Costa MS. Photodynamic antimicrobial chemotherapy (PACT) with methylene blue increases membrane permeability in Candida albicans. Lasers Med Sci. 2009;24:109–112. doi: 10.1007/s10103-007-0530-2. [DOI] [PubMed] [Google Scholar]
- 90.Jackson Z, Meghji S, MacRobert A, Henderson B, Wilson M. Killing the yeast and hyphal forms of candida albicans using a light-activated antimicrobial agent. Lasers Med Sci. 1999;14:150–157. doi: 10.1007/s101030050037. [DOI] [PubMed] [Google Scholar]
- 91*.Chabrier-Rosello Y, Foster TH, Mitra S, Haidaris CG. Respiratory deficiency enhances the sensitivity of the pathogenic fungus Candida to photodynamic treatment. Photochem Photobiol. 2008;84:1141–1148. doi: 10.1111/j.1751-1097.2007.00280.x. Interesting study using mutants of Candida to tease out the determinants of susceptibility to PDT. [DOI] [PubMed] [Google Scholar]
- 92.Dovigo LN, Carmello JC, de Souza Costa CA, Vergani CE, Brunetti IL, Bagnato VS, Pavarina AC. Curcumin-mediated photodynamic inactivation of Candida albicans in a murine model of oral candidiasis. Med Mycol. 2012 doi: 10.3109/13693786.2012.714081. [DOI] [PubMed] [Google Scholar]
- 93.Mima EG, Pavarina AC, Dovigo LN, Vergani CE, Costa CA, Kurachi C, Bagnato VS. Susceptibility of Candida albicans to photodynamic therapy in a murine model of oral candidosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:392–401. doi: 10.1016/j.tripleo.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 94.Mima EG, Pavarina AC, Ribeiro DG, Dovigo LN, Vergani CE, Bagnato VS. Effectiveness of photodynamic therapy for the inactivation of Candida spp. on dentures: in vitro study. Photomed Laser Surg. 2011;29:827–833. doi: 10.1089/pho.2011.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95*.Smijs TG, Schuitmaker HJ. Photodynamic inactivation of the dermatophyte Trichophyton rubrum. Photochem Photobiol. 2003;77:556–560. doi: 10.1562/0031-8655(2003)077<0556:PIOTDT>2.0.CO;2. Suggests that PDT may be effective against the causative agent of onychomycosis. [DOI] [PubMed] [Google Scholar]
- 96.Smijs TG, van der Haas RN, Lugtenburg J, Liu Y, de Jong RL, Schuitmaker HJ. Photodynamic treatment of the dermatophyte Trichophyton rubrum and its microconidia with porphyrin photosensitizers. Photochem Photobiol. 2004;80:197–202. doi: 10.1562/2004-04-22-RA-146. [DOI] [PubMed] [Google Scholar]
- 97.Denning DW. In: Aspergillus species. 5. Mandell GL, Benett JE, Dolin R, editors. Philadelphia: Churchill Livingstone; 2000. [Google Scholar]
- 98.Friedberg JS, Skema C, Baum ED, Burdick J, Vinogradov SA, Wilson DF, Horan AD, Nachamkin I. In vitro effects of photodynamic therapy on Aspergillus fumigatus. J Antimicrob Chemother. 2001;48:105–107. doi: 10.1093/jac/48.1.105. [DOI] [PubMed] [Google Scholar]
- 99.Jackson M, Flower CD, Shneerson JM. Treatment of symptomatic pulmonary aspergillomas with intracavitary instillation of amphotericin B through an indwelling catheter. Thorax. 1993;48:928–930. doi: 10.1136/thx.48.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gonzales FP, da Silva SH, Roberts DW, Braga GU. Photodynamic inactivation of conidia of the fungi Metarhizium anisopliae and Aspergillus nidulans with methylene blue and toluidine blue. Photochem Photobiol. 2010;86:653–661. doi: 10.1111/j.1751-1097.2009.00689.x. [DOI] [PubMed] [Google Scholar]
- 101**.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. Important review of biofilm resistance to antibiotics and other various antimicrobial techniques. [DOI] [PubMed] [Google Scholar]
- 102.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.O’Gara JP. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett. 2007;270:179–188. doi: 10.1111/j.1574-6968.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 104.Sharma M, Visai L, Bragheri F, Cristiani I, Gupta PK, Speziale P. Toluidine blue-mediated photodynamic effects on staphylococcal biofilms. Antimicrob Agents Chemother. 2008;52:299–305. doi: 10.1128/AAC.00988-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zanin IC, Goncalves RB, Junior AB, Hope CK, Pratten J. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study. J Antimicrob Chemother. 2005;56:324–330. doi: 10.1093/jac/dki232. [DOI] [PubMed] [Google Scholar]
- 106.Zanin IC, Lobo MM, Rodrigues LK, Pimenta LA, Hofling JF, Goncalves RB. Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode. Eur J Oral Sci. 2006;114:64–69. doi: 10.1111/j.1600-0722.2006.00263.x. [DOI] [PubMed] [Google Scholar]
- 107.Pereira CA, Romeiro RL, Costa AC, Machado AK, Junqueira JC, Jorge AO. Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: an in vitro study. Lasers Med Sci. 2011;26:341–348. doi: 10.1007/s10103-010-0852-3. [DOI] [PubMed] [Google Scholar]
- 108.Lin HY, Chen CT, Huang CT. Use of merocyanine 540 for photodynamic inactivation of Staphylococcus aureus planktonic and biofilm cells. Appl Environ Microbiol. 2004;70:6453–6458. doi: 10.1128/AEM.70.11.6453-6458.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Di Poto A, Sbarra MS, Provenza G, Visai L, Speziale P. The effect of photodynamic treatment combined with antibiotic action or host defence mechanisms on Staphylococcus aureus biofilms. Biomaterials. 2009;30:3158–3166. doi: 10.1016/j.biomaterials.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 110.Saino E, Sbarra MS, Arciola CR, Scavone M, Bloise N, Nikolov P, Ricchelli F, Visai L. Photodynamic action of Tri-meso (N-methyl-pyridyl), meso (N-tetradecyl-pyridyl) porphine on Staphylococcus epidermidis biofilms grown on Ti6Al4V alloy. Int J Artif Organs. 2010;33:636–645. doi: 10.1177/039139881003300909. [DOI] [PubMed] [Google Scholar]
- 111.Wood S, Metcalf D, Devine D, Robinson C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother. 2006;57:680–684. doi: 10.1093/jac/dkl021. [DOI] [PubMed] [Google Scholar]
- 112.de Goulart RC, Thedei G, Jr, Souza SL, Tedesco AC, Ciancaglini P. Comparative study of methylene blue and erythrosine dyes employed in photodynamic therapy for inactivation of planktonic and biofilm-cultivated Aggregatibacter actinomycetemcomitans. Photomed Laser Surg. 2010;28 (Suppl 1):S85–90. doi: 10.1089/pho.2009.2698. [DOI] [PubMed] [Google Scholar]
- 113.Lee CF, Lee CJ, Chen CT, Huang CT. delta-Aminolaevulinic acid mediated photodynamic antimicrobial chemotherapy on Pseudomonas aeruginosa planktonic and biofilm cultures. J Photochem Photobiol B. 2004;75:21–25. doi: 10.1016/j.jphotobiol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 114.Collins TL, Markus EA, Hassett DJ, Robinson JB. The effect of a cationic porphyrin on Pseudomonas aeruginosa biofilms. Curr Microbiol. 2010;61:411–416. doi: 10.1007/s00284-010-9629-y. [DOI] [PubMed] [Google Scholar]
- 115.Hegge AB, Bruzell E, Kristensen S, Tonnesen HH. Photoinactivation of Staphylococcus epidermidis biofilms and suspensions by the hydrophobic photosensitizer curcumin--effect of selected nanocarrier: studies on curcumin and curcuminoides XLVII. Eur J Pharm Sci. 2012;47:65–74. doi: 10.1016/j.ejps.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 116.Pereira CA, Costa AC, Carreira CM, Junqueira JC, Jorge AO. Photodynamic inactivation of Streptococcus mutans and Streptococcus sanguinis biofilms in vitro. Lasers Med Sci. 2013;28:859–864. doi: 10.1007/s10103-012-1175-3. [DOI] [PubMed] [Google Scholar]
- 117.Pereira Gonzales F, Maisch T. Photodynamic inactivation for controlling Candida albicans infections. Fungal Biol. 2012;116:1–10. doi: 10.1016/j.funbio.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 118.Ramage G, Martinez JP, Lopez-Ribot JL. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 2006;6:979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 119.Khan S, Alam F, Azam A, Khan AU. Gold nanoparticles enhance methylene blue-induced photodynamic therapy: a novel therapeutic approach to inhibit Candida albicans biofilm. Int J Nanomedicine. 2012;7:3245–3257. doi: 10.2147/IJN.S31219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat. 2010;13:180–195. doi: 10.1016/j.drup.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 121.Chabrier-Rosello Y, Foster TH, Perez-Nazario N, Mitra S, Haidaris CG. Sensitivity of Candida albicans germ tubes and biofilms to photofrin-mediated phototoxicity. Antimicrob Agents Chemother. 2005;49:4288–4295. doi: 10.1128/AAC.49.10.4288-4295.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Costa AC, Rasteiro VM, Pereira CA, Rossoni RD, Junqueira JC, Jorge AO. The effects of rose bengal- and erythrosine-mediated photodynamic therapy on Candida albicans. Mycoses. 2012;55:56–63. doi: 10.1111/j.1439-0507.2011.02042.x. [DOI] [PubMed] [Google Scholar]
- 123.Costa AC, de Campos Rasteiro VM, Pereira CA, da Silva Hashimoto ES, Beltrame M, Jr, Junqueira JC, Jorge AO. Susceptibility of Candida albicans and Candida dubliniensis to erythrosine- and LED-mediated photodynamic therapy. Arch Oral Biol. 2011;56:1299–1305. doi: 10.1016/j.archoralbio.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 124.Coleman JJ, Okoli I, Tegos GP, Holson EB, Wagner FF, Hamblin MR, Mylonakis E. Characterization of plant-derived saponin natural products against Candida albicans. ACS Chem Biol. 2010;5:321–332. doi: 10.1021/cb900243b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Donnelly RF, McCarron PA, Tunney MM, David Woolfson A. Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. J Photochem Photobiol B. 2007;86:59–69. doi: 10.1016/j.jphotobiol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 126.Ribeiro AP, Andrade MC, de da Silva JF, Jorge JH, Primo FL, Tedesco AC, Pavarina AC. Photodynamic inactivation of planktonic cultures and biofilms of Candida albicans mediated by aluminum-chloride-phthalocyanine entrapped in nanoemulsions. Photochem Photobiol. 2013;89:111–119. doi: 10.1111/j.1751-1097.2012.01198.x. [DOI] [PubMed] [Google Scholar]
- 127.Soares BM, da Silva DL, Sousa GR, Amorim JC, de Resende MA, Pinotti M, Cisalpino PS. In vitro photodynamic inactivation of Candida spp. growth and adhesion to buccal epithelial cells. J Photochem Photobiol B. 2009;94:65–70. doi: 10.1016/j.jphotobiol.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 128.Biel MA. Photodynamic therapy of bacterial and fungal biofilm infections. Methods Mol Biol. 2010;635:175–194. doi: 10.1007/978-1-60761-697-9_13. [DOI] [PubMed] [Google Scholar]
- 129.Seneviratne CJ, Jin L, Samaranayake LP. Biofilm lifestyle of Candida: a mini review. Oral Dis. 2008;14:582–590. doi: 10.1111/j.1601-0825.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- 130.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–36. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 131.Dai T, Fuchs BB, Coleman JJ, Prates RA, Astrakas C, St Denis TG, Ribeiro MS, Mylonakis E, Hamblin MR, Tegos GP. Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front Microbiol. 2012;3:120. doi: 10.3389/fmicb.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132*.Demidova TN, Hamblin MR. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob Agents Chemother. 2005;49:2329–2335. doi: 10.1128/AAC.49.6.2329-2335.2005. Study pointing out a fairly basic fact of in vitro antimicrobial PDT experiments that however is often ignored. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Illingworth CD, Cook SD. Acanthamoeba keratitis. Surv Ophthalmol. 1998;42:493–508. doi: 10.1016/s0039-6257(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 134.Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med. 2006;38:468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 135.Kassab K, Dei D, Roncucci G, Jori G, Coppellotti O. Phthalocyanine-photosensitized inactivation of a pathogenic protozoan, Acanthamoeba palestinensis. Photochem Photobiol Sci. 2003;2:668–672. doi: 10.1039/b300293d. [DOI] [PubMed] [Google Scholar]
- 136.Chen Z, Xuguang S, Zhiqun W, Ran L. In vitro amoebacidal activity of photodynamic therapy on Acanthamoeba. Br J Ophthalmol. 2008;92:1283–1286. doi: 10.1136/bjo.2007.134288. [DOI] [PubMed] [Google Scholar]
- 137.Mito T, Suzuki T, Kobayashi T, Zheng X, Hayashi Y, Shiraishi A, Ohashi Y. Effect of photodynamic therapy with methylene blue on Acanthamoeba in vitro. Invest Ophthalmol Vis Sci. 2012;53:6305–6313. doi: 10.1167/iovs.12-9828. [DOI] [PubMed] [Google Scholar]
- 138.Siddiqui R, Khan NA. Photochemotherapeutic strategies against Acanthamoeba keratitis. AMB Express. 2012;2:47. doi: 10.1186/2191-0855-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 140*.Enk CD, Fritsch C, Jonas F, Nasereddin A, Ingber A, Jaffe CL, Ruzicka T. Treatment of cutaneous leishmaniasis with photodynamic therapy. Arch Dermatol. 2003;139:432–434. doi: 10.1001/archderm.139.4.432. Clinical study of 5-amino-levulanic acid mediated PDT for treating Leishmaniasis. [DOI] [PubMed] [Google Scholar]
- 141.Song D, Lindoso JA, Oyafuso LK, Kanashiro EH, Cardoso JL, Uchoa AF, Tardivo JP, Baptista MS. Photodynamic therapy using methylene blue to treat cutaneous leishmaniasis. Photomed Laser Surg. 2011;29:711–715. doi: 10.1089/pho.2010.2915. [DOI] [PubMed] [Google Scholar]
- 142.Akilov OE, Kosaka S, O’Riordan K, Hasan T. Photodynamic therapy for cutaneous leishmaniasis: the effectiveness of topical phenothiaziniums in parasite eradication and Th1 immune response stimulation. Photochem Photobiol Sci. 2007;6:1067–1075. doi: 10.1039/b703521g. [DOI] [PubMed] [Google Scholar]
- 143*.Akilov OE, Kosaka S, O’Riordan K, Hasan T. Parasiticidal effect of delta-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis is indirect and mediated through the killing of the host cells. Exp Dermatol. 2007;16:651–660. doi: 10.1111/j.1600-0625.2007.00578.x. Study pointing out that the benefits of clinical PDT using 5-ALA must have been indirect as the parasite lacks the enzymes to synthesize porphyrins. [DOI] [PubMed] [Google Scholar]
- 144.Akilov OE, Yousaf W, Lukjan SX, Verma S, Hasan T. Optimization of topical photodynamic therapy with 3,7-bis(di-n-butylamino)phenothiazin-5-ium bromide for cutaneous leishmaniasis. Lasers Surg Med. 2009;41:358–365. doi: 10.1002/lsm.20775. [DOI] [PubMed] [Google Scholar]
- 145.Hernandez IP, Montanari J, Valdivieso W, Morilla MJ, Romero EL, Escobar P. In vitro phototoxicity of ultradeformable liposomes containing chloroaluminum phthalocyanine against New World Leishmania species. J Photochem Photobiol B. 2012;117C:157–163. doi: 10.1016/j.jphotobiol.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 146.Taylor VM, Cedeno DL, Munoz DL, Jones MA, Lash TD, Young AM, Constantino MH, Esposito N, Velez ID, Robledo SM. In vitro and in vivo studies of the utility of dimethyl and diethyl carbaporphyrin ketals in treatment of cutaneous leishmaniasis. Antimicrob Agents Chemother. 2011;55:4755–4764. doi: 10.1128/AAC.00671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gardlo K, Horska Z, Enk CD, Rauch L, Megahed M, Ruzicka T, Fritsch C. Treatment of cutaneous leishmaniasis by photodynamic therapy. J Am Acad Dermatol. 2003;48:893–896. doi: 10.1067/mjd.2003.218. [DOI] [PubMed] [Google Scholar]
- 148.Stojiljkovic I, Evavold BD, Kumar V. Antimicrobial properties of porphyrins. Expert Opin Investig Drugs. 2001;10:309–320. doi: 10.1517/13543784.10.2.309. [DOI] [PubMed] [Google Scholar]
- 149.Asilian A, Davami M. Comparison between the efficacy of photodynamic therapy and topical paromomycin in the treatment of Old World cutaneous leishmaniasis: a placebo-controlled, randomized clinical trial. Clin Exp Dermatol. 2006;31:634–637. doi: 10.1111/j.1365-2230.2006.02182.x. [DOI] [PubMed] [Google Scholar]
- 150.Baptista MS, Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) for the treatment of malaria, leishmaniasis and trypanosomiasis. Braz J Med Biol Res. 2011;44:1–10. doi: 10.1590/s0100-879x2010007500141. [DOI] [PubMed] [Google Scholar]
- 151.Lustigman S, Ben-Hur E. Photosensitized inactivation of Plasmodium falciparum in human red cells by phthalocyanines. Transfusion. 1996;36:543–546. doi: 10.1046/j.1537-2995.1996.36696269514.x. [DOI] [PubMed] [Google Scholar]
- 152.Smith TG, Kain KC. Inactivation of Plasmodium falciparum by photodynamic excitation of heme-cycle intermediates derived from delta-aminolevulinic acid. J Infect Dis. 2004;190:184–191. doi: 10.1086/421503. [DOI] [PubMed] [Google Scholar]
- 153.Wagner SJ, Skripchenko A, Salata J, O’Sullivan AM, Cardo LJ. Inactivation of Leishmania donovani infantum and Trypanosoma cruzi in red cell suspensions with thiazole orange. Transfusion. 2008;48:1363–1367. doi: 10.1111/j.1537-2995.2008.01712.x. [DOI] [PubMed] [Google Scholar]
- 154.Docampo R, Moreno SN, Gadelha FR, de Souza W, Cruz FS. Prevention of Chagas’ disease resulting from blood transfusion by treatment of blood: toxicity and mode of action of gentian violet. Biomed Environ Sci. 1988;1:406–413. [PubMed] [Google Scholar]
- 155.Docampo R, Moreno SN, Cruz FS. Enhancement of the cytotoxicity of crystal violet against Trypanosoma cruzi in the blood by ascorbate. Mol Biochem Parasitol. 1988;27:241–247. doi: 10.1016/0166-6851(88)90043-6. [DOI] [PubMed] [Google Scholar]
- 156*.Gottlieb P, Margolis-Nunno H, Robinson R, Shen LG, Chimezie E, Horowitz B, Ben-Hur E. Inactivation of Trypanosoma cruzi trypomastigote forms in blood components with a Psoralen and Ultraviolet A light. Photochem Photobiol. 1996;63:562–565. doi: 10.1111/j.1751-1097.1996.tb05656.x. Use of psoralens and UVA light for blood product sterilization. [DOI] [PubMed] [Google Scholar]
- 157.Gottlieb P, Shen LG, Chimezie E, Bahng S, Kenney ME, Horowitz B, Ben-Hur E. Inactivation of Trypanosoma cruzi trypomastigote forms in blood components by photodynamic treatment with phthalocyanines. Photochem Photobiol. 1995;62:869–874. doi: 10.1111/j.1751-1097.1995.tb09149.x. [DOI] [PubMed] [Google Scholar]
- 158.Kasermann F, Kempf C. Photodynamic inactivation of enveloped viruses by buckminsterfullerene. Antiviral Res. 1997;34:65–70. doi: 10.1016/s0166-3542(96)01207-7. [DOI] [PubMed] [Google Scholar]
- 159.Kasermann F, Kempf C. Buckminsterfullerene and photodynamic inactivation of viruses. Rev Med Virol. 1998;8:143–151. doi: 10.1002/(sici)1099-1654(199807/09)8:3<143::aid-rmv214>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 160.Hirayama J, Abe H, Kamo N, Shinbo T, Ohnishi-Yamada Y, Kurosawa S, Ikebuchi K, Sekiguchi S. Photoinactivation of vesicular stomatitis virus with fullerene conjugated with methoxy polyethylene glycol amine. Biol Pharm Bull. 1999;22:1106–1109. doi: 10.1248/bpb.22.1106. [DOI] [PubMed] [Google Scholar]
- 161.Lin YL, Lei HY, Wen YY, Luh TY, Chou CK, Liu HS. Light-independent inactivation of dengue-2 virus by carboxyfullerene C3 isomer. Virology. 2000;275:258–262. doi: 10.1006/viro.2000.0490. [DOI] [PubMed] [Google Scholar]
- 162.Nims RW, Plavsic M. Polyomavirus inactivation - a review. Biologicals. 2013;41:63–70. doi: 10.1016/j.biologicals.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 163.Lee Y, Baron ED. Photodynamic therapy: current evidence and applications in dermatology. Semin Cutan Med Surg. 2011;30:199–209. doi: 10.1016/j.sder.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 164*.Ben Amor T, Jori G. Sunlight-activated insecticides: historical background and mechanisms of phototoxic activity. Insect Biochem Mol Biol. 2000;30:915–925. doi: 10.1016/s0965-1748(00)00072-2. Review of photodynamic insecticides that can be activated by sunlight without polluting the environment. [DOI] [PubMed] [Google Scholar]
- 165.Ben Amor T, Bortolotto L, Jori G. Porphyrins and related compounds as photoactivatable insecticides. 3. Laboratory and field studies. Photochem Photobiol. 2000;71:124–128. doi: 10.1562/0031-8655(2000)071<0124:sippar>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 166.Lucantoni L, Magaraggia M, Lupidi G, Ouedraogo RK, Coppellotti O, Esposito F, Fabris C, Jori G, Habluetzel A. Novel, meso-substituted cationic porphyrin molecule for photo-mediated larval control of the dengue vector Aedes aegypti. PLoS Negl Trop Dis. 2011;5:e1434. doi: 10.1371/journal.pntd.0001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shao G, Jiang DX, Xu HH, Zeng W, Yu HJ, Tian YQ. Synthesis and photoactivated insecticidal activity of tetraethynylsilanes. J Photochem Photobiol B. 2010;98:52–56. doi: 10.1016/j.jphotobiol.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 168.Ling AE, Robbins KE, Brown TM, Dunmire V, Thoe SY, Wong SY, Leo YS, Teo D, Gallarda J, Phelps B, et al. Failure of routine HIV-1 tests in a case involving transmission with preseroconversion blood components during the infectious window period. JAMA. 2000;284:210–214. doi: 10.1001/jama.284.2.210. [DOI] [PubMed] [Google Scholar]
- 169.Al Shaer L, Abdulrahman M, John TJ, Alhashimi A. Trends in prevalence, incidence, and residual risk of major transfusion-transmissible viral infections in United Arab Emirates blood donors: impact of individual-donation nucleic acid testing, 2004 through 2009. Transfusion. 2012;52:2300–2309. doi: 10.1111/j.1537-2995.2012.03740.x. [DOI] [PubMed] [Google Scholar]
- 170.Aznar JA, Molina R, Montoro JM. Factor VIII/von Willebrand factor complex in methylene blue-treated fresh plasma. Transfusion. 1999;39:748–750. doi: 10.1046/j.1537-2995.1999.39070748.x. [DOI] [PubMed] [Google Scholar]
- 171.Wainwright M. Pathogen inactivation in blood products. Curr Med Chem. 2002;9:127–143. doi: 10.2174/0929867023371355. [DOI] [PubMed] [Google Scholar]
- 172.Wagner SJ, Skripchenko A, Robinette D, Mallory DA, Hirayama J, Cincotta L, Foley J. The use of dimethylmethylene blue for virus photoinactivation of red cell suspensions. Dev Biol (Basel) 2000;102:125–129. [PubMed] [Google Scholar]
- 173.Skripchenko AA, Wagner SJ. Inactivation of WBCs in RBC suspensions by photoactive phenothiazine dyes: comparison of dimethylmethylene blue and MB. Transfusion. 2000;40:968–975. doi: 10.1046/j.1537-2995.2000.40080968.x. [DOI] [PubMed] [Google Scholar]
- 174.Hirayama J, Wagner SJ, Abe H, Ikebuchi K, Ikeda H. Involvement of reactive oxygen species in hemoglobin oxidation and virus inactivation by 1,9-dimethylmethylene blue phototreatment. Biol Pharm Bull. 2001;24:418–421. doi: 10.1248/bpb.24.418. [DOI] [PubMed] [Google Scholar]
- 175.Schuyler R. Use of riboflavin for photoinactivation of pathogens in blood components. Transfus Apher Sci. 2001;25:189–190. doi: 10.1016/s1473-0502(01)00119-7. [DOI] [PubMed] [Google Scholar]
- 176.Sobral PM, Barros AE, Gomes AM, do Bonfim CV. Viral inactivation in hemotherapy: systematic review on inactivators with action on nucleic acids. Rev Bras Hematol Hemoter. 2012;34:231–235. doi: 10.5581/1516-8484.20120056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Antic A, Stanojkovic Z, Macukanovic-Golubovic L, Jelic M. Evaluation of coagulation factors in fresh frozen plasma treated with riboflavin and ultraviolet light. Vojnosanit Pregl. 2012;69:22–26. doi: 10.2298/vsp1201022a. [DOI] [PubMed] [Google Scholar]
- 178.Stanojkovic Z, Antic A, Stojanovic M. Effects of use of riboflavin and ultraviolet light for pathogen inactivation on quality of platelet concentrates. Vojnosanit Pregl. 2011;68:489–494. doi: 10.2298/vsp1106489s. [DOI] [PubMed] [Google Scholar]
- 179.Almeida A, Cunha A, Gomes NC, Alves E, Costa L, Faustino MA. Phage therapy and photodynamic therapy: low environmental impact approaches to inactivate microorganisms in fish farming plants. Mar Drugs. 2009;7:268–313. doi: 10.3390/md7030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jori GCO. Inactivation of pathogenic microorganisms by photodynamic techniques: mechanistic aspects and perspective applications. Anti-Infect Agents Med Chem. 2007;6:119–131. [Google Scholar]
- 181.Magaraggia M, Faccenda F, Gandolfi A, Jori G. Treatment of microbiologically polluted aquaculture waters by a novel photochemical technique of potentially low environmental impact. J Environ Monit. 2006;8:923–931. doi: 10.1039/b606975d. [DOI] [PubMed] [Google Scholar]
- 182.Wong PN, Mak SK, Lo MW, Lo KY, Tong GM, Wong Y, Wong AK. Vibrio vulnificus peritonitis after handling of seafood in a patient receiving CAPD. Am J Kidney Dis. 2005;46:e87–90. doi: 10.1053/j.ajkd.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 183.Jemli M, Alouini Z, Sabbahi S, Gueddari M. Destruction of fecal bacteria in wastewater by three photosensitizers. J Environ Monit. 2002;4:511–516. doi: 10.1039/b204637g. [DOI] [PubMed] [Google Scholar]
- 184.Carvalho CM, Gomes AT, Fernandes SC, Prata AC, Almeida MA, Cunha MA, Tome JP, Faustino MA, Neves MG, Tome AC, et al. Photoinactivation of bacteria in wastewater by porphyrins: bacterial beta-galactosidase activity and leucine-uptake as methods to monitor the process. J Photochem Photobiol B. 2007;88:112–118. doi: 10.1016/j.jphotobiol.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 185.Alves E, Carvalho CM, Tome JP, Faustino MA, Neves MG, Tome AC, Cavaleiro JA, Cunha A, Mendo S, Almeida A. Photodynamic inactivation of recombinant bioluminescent Escherichia coli by cationic porphyrins under artificial and solar irradiation. J Ind Microbiol Biotechnol. 2008;35:1447–1454. doi: 10.1007/s10295-008-0446-2. [DOI] [PubMed] [Google Scholar]
- 186.Alves E, Costa L, Carvalho CM, Tome JP, Faustino MA, Neves MG, Tome AC, Cavaleiro JA, Cunha A, Almeida A. Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiol. 2009;9:70. doi: 10.1186/1471-2180-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Costa L, Carvalho CM, Faustino MA, Neves MG, Tome JP, Tome AC, Cavaleiro JA, Cunha A, Almeida A. Sewage bacteriophage inactivation by cationic porphyrins: influence of light parameters. Photochem Photobiol Sci. 2010;9:1126–1133. doi: 10.1039/c0pp00051e. [DOI] [PubMed] [Google Scholar]
- 188.Alouini Z, Jemli M. Destruction of helminth eggs by photosensitized porphyrin. J Environ Monit. 2001;3:548–551. doi: 10.1039/b103471p. [DOI] [PubMed] [Google Scholar]
- 189.Bonnett R, Krysteva MA, Lalov IG, Artarsky SV. Water disinfection using photosensitizers immobilized on chitosan. Water Res. 2006;40:1269–1275. doi: 10.1016/j.watres.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 190.den Aantrekker ED, Boom RM, Zwietering MH, van Schothorst M. Quantifying recontamination through factory environments--a review. Int J Food Microbiol. 2003;80:117–130. doi: 10.1016/s0168-1605(02)00137-x. [DOI] [PubMed] [Google Scholar]
- 191.Brovko LY, Meyer A, Tiwana AS, Chen W, Liu H, Filipe CD, Griffiths MW. Photodynamic treatment: a novel method for sanitation of food handling and food processing surfaces. J Food Prot. 2009;72:1020–1024. doi: 10.4315/0362-028x-72.5.1020. [DOI] [PubMed] [Google Scholar]
- 192*.Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, Hamblin MR. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol. 2005;12:1127–1135. doi: 10.1016/j.chembiol.2005.08.014. One of the first studies to suggest that functionalized fullerenes may be effectivePD agents against infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother. 1998;42:2595–2601. doi: 10.1128/aac.42.10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Trindade FZ, Pavarina AC, Ribeiro AP, Bagnato VS, Vergani CE, Costa CA. Toxicity of photodynamic therapy with LED associated to Photogem(R): an in vivo study. Lasers Med Sci. 2012;27:403–411. doi: 10.1007/s10103-011-0909-y. [DOI] [PubMed] [Google Scholar]
- 195.Zeina B, Greenman J, Corry D, Purcell WM. Cytotoxic effects of antimicrobial photodynamic therapy on keratinocytes in vitro. Br J Dermatol. 2002;146:568–573. doi: 10.1046/j.1365-2133.2002.04623.x. [DOI] [PubMed] [Google Scholar]
- 196.Tanaka M, Kinoshita M, Yoshihara Y, Shinomiya N, Seki S, Nemoto K, Hirayama T, Dai T, Huang L, Hamblin MR, et al. Optimal photosensitizers for photodynamic therapy of infections should kill bacteria but spare neutrophils. Photochem Photobiol. 2012;88:227–232. doi: 10.1111/j.1751-1097.2011.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shrestha A, Hamblin MR, Kishen A. Characterization of a conjugate between Rose Bengal and chitosan for targeted antibiofilm and tissue stabilization effects as a potential treatment of infected dentin. Antimicrob Agents Chemother. 2012;56:4876–4884. doi: 10.1128/AAC.00810-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Qin Y, Luan X, Bi L, He G, Bai X, Zhou C, Zhang Z. Toluidine blue-mediated photoinactivation of periodontal pathogens from supragingival plaques. Lasers Med Sci. 2008;23:49–54. doi: 10.1007/s10103-007-0454-x. [DOI] [PubMed] [Google Scholar]
- 199.Dörtbudak O, Haas R, Bernhart T, Mailath-Pokorny G. Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin Oral Implants Res. 2001;12:104–108. doi: 10.1034/j.1600-0501.2001.012002104.x. [DOI] [PubMed] [Google Scholar]
- 200.Soukos NS, Chen PS, Morris JT, Ruggiero K, Abernethy AD, Som S, Foschi F, Doucette S, Bammann LL, Fontana CR, et al. Photodynamic therapy for endodontic disinfection. J Endod. 2006;32:979–984. doi: 10.1016/j.joen.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 201.Fontana CR, Abernethy AD, Som S, Ruggiero K, Doucette S, Marcantonio RC, Boussios CI, Kent R, Goodson JM, Tanner AC, et al. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J Periodontal Res. 2009;44:751–759. doi: 10.1111/j.1600-0765.2008.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Williams JA, Pearson GJ, Colles MJ, Wilson M. The photo-activated antibacterial action of toluidine blue O in a collagen matrix and in carious dentine. Caries Res. 2004;38:530–536. doi: 10.1159/000080582. [DOI] [PubMed] [Google Scholar]
- 203.Mima EG, Pavarina AC, Silva MM, Ribeiro DG, Vergani CE, Kurachi C, Bagnato VS. Denture stomatitis treated with photodynamic therapy: five cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:602–608. doi: 10.1016/j.tripleo.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 204.Mima EG, Vergani CE, Machado AL, Massucato EM, Colombo AL, Bagnato VS, Pavarina AC. Comparison of Photodynamic Therapy versus conventional antifungal therapy for the treatment of denture stomatitis: a randomized clinical trial. Clin Microbiol Infect. 2012;18:E380–388. doi: 10.1111/j.1469-0691.2012.03933.x. [DOI] [PubMed] [Google Scholar]
- 205.Lee JW, Kim BJ, Kim MN. Photodynamic therapy: new treatment for recalcitrant Malassezia folliculitis. Lasers Surg Med. 2010;42:192–196. doi: 10.1002/lsm.20857. [DOI] [PubMed] [Google Scholar]
- 206.Piraccini BM, Rech G, Tosti A. Photodynamic therapy of onychomycosis caused by Trichophyton rubrum. J Am Acad Dermatol. 2008;59:S75–76. doi: 10.1016/j.jaad.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 207.Sotiriou E, Koussidou-Eremonti T, Chaidemenos G, Apalla Z, Ioannides D. Photodynamic therapy for distal and lateral subungual toenail onychomycosis caused by Trichophyton rubrum: Preliminary results of a single-centre open trial. Acta Derm Venereol. 2010;90:216–217. doi: 10.2340/00015555-0811. [DOI] [PubMed] [Google Scholar]
- 208.Calzavara-Pinton PG, Venturini M, Capezzera R, Sala R, Zane C. Photodynamic therapy of interdigital mycoses of the feet with topical application of 5-aminolevulinic acid. Photodermatol Photoimmunol Photomed. 2004;20:144–147. doi: 10.1111/j.1600-0781.2004.00095.x. [DOI] [PubMed] [Google Scholar]
- 209.Hongcharu W, Taylor CR, Chang Y, Aghassi D, Suthamjariya K, Anderson RR. Topical ALA-photodynamic therapy for the treatment of acne vulgaris. J Invest Dermatol. 2000;115:183–192. doi: 10.1046/j.1523-1747.2000.00046.x. [DOI] [PubMed] [Google Scholar]
- 210.Itoh Y, Ninomiya Y, Tajima S, Ishibashi A. Photodynamic therapy for acne vulgaris with topical 5-aminolevulinic acid. Arch Dermatol. 2000;136:1093–1095. doi: 10.1001/archderm.136.9.1093. [DOI] [PubMed] [Google Scholar]
- 211.Itoh Y, Ninomiya Y, Tajima S, Ishibashi A. Photodynamic therapy of acne vulgaris with topical delta-aminolaevulinic acid and incoherent light in Japanese patients. Br J Dermatol. 2001;144:575–579. doi: 10.1046/j.1365-2133.2001.04086.x. [DOI] [PubMed] [Google Scholar]
- 212.Gold MH, Bradshaw VL, Boring MM, Bridges TM, Biron JA, Carter LN. The use of a novel intense pulsed light and heat source and ALA-PDT in the treatment of moderate to severe inflammatory acne vulgaris. J Drugs Dermatol. 2004;3:S15–19. [PubMed] [Google Scholar]
- 213.Yin R, Hao F, Deng J, Yang XC, Yan H. Investigation of optimal aminolaevulinic acid concentration applied in topical aminolaevulinic acid-photodynamic therapy for treatment of moderate to severe acne: a pilot study in Chinese subjects. Br J Dermatol. 2010;163:1064–1071. doi: 10.1111/j.1365-2133.2010.09860.x. [DOI] [PubMed] [Google Scholar]
- 214.Kleinpenning MM, Smits T, Frunt MH, van Erp PE, van de Kerkhof PC, Gerritsen RM. Clinical and histological effects of blue light on normal skin. Photodermatol Photoimmunol Photomed. 2010;26:16–21. doi: 10.1111/j.1600-0781.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- 215.Darwin C. The power of movement in plants. New York: Appleton and Company; 1881. [Google Scholar]
- 216.De Lucca AJ, Carter-Wientjes C, Williams KA, Bhatnagar D. Blue light (470 nm) effectively inhibits bacterial and fungal growth. Lett Appl Microbiol. 2012 doi: 10.1111/lam.12002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 217.Ohm RA, Aerts D, Wosten HA, Lugones LG. The blue light receptor complex WC-1/2 of Schizophyllum commune is involved in mushroom formation and protection against phototoxicity. Environ Microbiol. 2013;15:943–955. doi: 10.1111/j.1462-2920.2012.02878.x. [DOI] [PubMed] [Google Scholar]
- 218.Tschowri N, Lindenberg S, Hengge R. Molecular function and potential evolution of the biofilm-modulating blue light-signalling pathway of Escherichia coli. Mol Microbiol. 2012;85:893–906. doi: 10.1111/j.1365-2958.2012.08147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dai T, Gupta A, Murray CK, Vrahas MS, Tegos GP, Hamblin MR. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist Updat. 2012;15:223–236. doi: 10.1016/j.drup.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gomelsky M, Hoff WD. Light helps bacteria make important lifestyle decisions. Trends Microbiol. 2011;19:441–448. doi: 10.1016/j.tim.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 221.Purcell EB, Siegal-Gaskins D, Rawling DC, Fiebig A, Crosson S. A photosensory two-component system regulates bacterial cell attachment. Proc Natl Acad Sci USA. 2007;104:18241–18246. doi: 10.1073/pnas.0705887104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.van der Horst MA, Stalcup TP, Kaledhonkar S, Kumauchi M, Hara M, Xie A, Hellingwerf KJ, Hoff WD. Locked chromophore analogs reveal that photoactive yellow protein regulates biofilm formation in the deep sea bacterium Idiomarina loihiensis. J Am Chem Soc. 2009;131:17443–17451. doi: 10.1021/ja9057103. [DOI] [PubMed] [Google Scholar]
- 223.Ondrusch N, Kreft J. Blue and red light modulates SigB-dependent gene transcription, swimming motility and invasiveness in Listeria monocytogenes. PloS one. 2011;6:e16151. doi: 10.1371/journal.pone.0016151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Swartz TE, Tseng TS, Frederickson MA, Paris G, Comerci DJ, Rajashekara G, Kim JG, Mudgett MB, Splitter GA, Ugalde RA, et al. Blue-light-activated histidine kinases: two-component sensors in bacteria. Science. 2007;317:1090–1093. doi: 10.1126/science.1144306. [DOI] [PubMed] [Google Scholar]
- 225*.Ganz RAVJ, Ahmad A, Ahmadi A, Khalil A, Tolkoff MJ, Nishioka NS, Hamblin MR. Helicobacter pylori in patients can be killed by visible light. Lasers Surg Med. 2005;36:260–265. doi: 10.1002/lsm.20161. First clinical study suggesting that intragastric blue light could be effective against H. pylori in the human stomach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Maclean M, MacGregor SJ, Anderson JG, Woolsey G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl Environ Microbiol. 2009;75:1932–1937. doi: 10.1128/AEM.01892-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Murdoch LE, Maclean M, Endarko E, MacGregor SJ, Anderson JG. Bactericidal effects of 405 nm light exposure demonstrated by inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium species in liquid suspensions and on exposed surfaces. Sci World J. 2012;2012:137805. doi: 10.1100/2012/137805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jean D, Briolat V, Reysset G. Oxidative stress response in Clostridium perfringens. Microbiology. 2004;150:1649–1659. doi: 10.1099/mic.0.27017-0. [DOI] [PubMed] [Google Scholar]
- 229.Murphy C, Carroll C, Jordan KN. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J Appl Microbiol. 2006;100:623–632. doi: 10.1111/j.1365-2672.2006.02903.x. [DOI] [PubMed] [Google Scholar]
- 230.Feuerstein O, Ginsburg I, Dayan E, Veler D, Weiss EI. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem Photobiol. 2005;81:1186–1189. doi: 10.1562/2005-04-06-RA-477. [DOI] [PubMed] [Google Scholar]
- 231.Guffey JS, Wilborn J. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed Laser Surg. 2006;24:684–688. doi: 10.1089/pho.2006.24.684. [DOI] [PubMed] [Google Scholar]
- 232.Ashkenazi H, Malik Z, Harth Y, Nitzan Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol Med Microbiol. 2003;35:17–24. doi: 10.1111/j.1574-695X.2003.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 233.Fukui M, Yoshioka M, Satomura K, Nakanishi H, Nagayama M. Specific-wavelength visible light irradiation inhibits bacterial growth of Porphyromonas gingivalis. J Periodontal Res. 2008;43:174–178. doi: 10.1111/j.1600-0765.2007.01009.x. [DOI] [PubMed] [Google Scholar]
- 234.Soukos NS, Som S, Abernethy AD, Ruggiero K, Dunham J, Lee C, Doukas AG, Goodson JM. Phototargeting oral black-pigmented bacteria. Antimicrob Agents Chemother. 2005;49:1391–1396. doi: 10.1128/AAC.49.4.1391-1396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nitzan Y, Salmon-Divon M, Shporen E, Malik Z. ALA induced photodynamic effects on gram positive and negative bacteria. Photochem Photobiol Sci. 2004;3:430–435. doi: 10.1039/b315633h. [DOI] [PubMed] [Google Scholar]
- 236.Maclean M, MacGregor SJ, Anderson JG, Woolsey G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Applied and environmental microbiology. 2009;75:1932–1937. doi: 10.1128/AEM.01892-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Takahama U, Shimizu-Takahama M, Egashira T. Reduction of exogenous cytochrome c by Neurospora crassa conidia: effects of superoxide dismutase and blue light. J Bacteriol. 1982;152:151–156. doi: 10.1128/jb.152.1.151-156.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Casas-Flores S, Rios-Momberg M, Rosales-Saavedra T, Martinez-Hernandez P, Olmedo-Monfil V, Herrera-Estrella A. Cross talk between a fungal blue-light perception system and the cyclic AMP signaling pathway. Eukaryotic cell. 2006;5:499–506. doi: 10.1128/EC.5.3.499-506.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Purschwitz J, Muller S, Kastner C, Fischer R. Seeing the rainbow: light sensing in fungi. Curr Opin Microbiol. 2006;9:566–571. doi: 10.1016/j.mib.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 240.Oriel S, Nitzan Y. Photoinactivation of Candida albicans by its own endogenous porphyrins. Curr Microbiol. 2010;60:117–123. doi: 10.1007/s00284-009-9514-8. [DOI] [PubMed] [Google Scholar]
- 241.Hockberger PE, Skimina TA, Centonze VE, Lavin C, Chu S, Dadras S, Reddy JK, White JG. Activation of flavin-containing oxidases underlies light-induced production of H2O2 in mammalian cells. Proc Natl Acad Sci U S A. 1999;96:6255–6260. doi: 10.1073/pnas.96.11.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wataha JC, Lockwood PE, Lewis JB, Rueggeberg FA, Messer RL. Biological effects of blue light from dental curing units. Dent Mater. 2004;20:150–157. doi: 10.1016/s0109-5641(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 243.Godley BF, Shamsi FA, Liang FQ, Jarrett SG, Davies S, Boulton M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J Biol Chem. 2005;280:21061–21066. doi: 10.1074/jbc.M502194200. [DOI] [PubMed] [Google Scholar]
- 244.Shnitkind E, Yaping E, Geen S, Shalita AR, Lee WL. Anti-inflammatory properties of narrow-band blue light. J Drugs Dermatol. 2006;5:605–610. [PubMed] [Google Scholar]
- 245.Megraud F, Lamouliatte H. Review article: the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:1333–1343. doi: 10.1046/j.1365-2036.2003.01592.x. [DOI] [PubMed] [Google Scholar]
- 246.Ganz RA, Viveiros J, Ahmad A, Ahmadi A, Khalil A, Tolkoff MJ, Nishioka NS, Hamblin MR. Helicobacter pylori in patients can be killed by visible light. Lasers Surg Med. 2005;36:260–265. doi: 10.1002/lsm.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lembo AJ, Ganz RA, Sheth S, Cave D, Kelly C, Levin P, Kazlas PT, Baldwin PC, 3rd, Lindmark WR, McGrath JR, et al. Treatment of Helicobacter pylori infection with intra-gastric violet light phototherapy: a pilot clinical trial. Lasers Surg Med. 2009;41:337–344. doi: 10.1002/lsm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bache SE, Maclean M, MacGregor SJ, Anderson JG, Gettinby G, Coia JE, Taggart I. Clinical studies of the High-Intensity Narrow-Spectrum light Environmental Decontamination System (HINS-light EDS), for continuous disinfection in the burn unit inpatient and outpatient settings. Burns. 2012;38:69–76. doi: 10.1016/j.burns.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 249.Maclean M, Macgregor SJ, Anderson JG, Woolsey GA, Coia JE, Hamilton K, Taggart I, Watson SB, Thakker B, Gettinby G. Environmental decontamination of a hospital isolation room using high-intensity narrow-spectrum light. J Hosp Infect. 2010;76:247–251. doi: 10.1016/j.jhin.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 250.N S. Photodisruption in biological tissues using femtosecond laser pulses. Harvard University Press; Cambridge: 2003. [Google Scholar]
- 251.Tsen KT, TSW, Chang CL, Hung CF, Wu TC, Kiang JG. Inactivation of viruses with a very low power visible femtosecond laser. Virol J. 2007;4:50. doi: 10.1186/1743-422X-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tsen KT, TSW, Chang CL, Hung CF, Wu TC, Kiang JG. Inactivation of viruses by laser-driven coherent excitations via impulsive stimulated Raman scattering process. J Biomed Opt. 2007;12:064030. doi: 10.1117/1.2821713. [DOI] [PubMed] [Google Scholar]
- 253.Tsen KTTS, Fu Q, Lindsay SM, Kibler K, Jacobs B, Wu TC, Karanam B, Jagu S, Roden RB, et al. Photonic approach to the selective inactivation of viruses with a near-infrared subpicosecond fiber laser. J Biomed Opt. 2009;14:064042. doi: 10.1117/1.3275477. [DOI] [PubMed] [Google Scholar]
- 254.Tsen KT, TSWD, Chang CL, Hung CF, Wu TC, Kiang JG. Inactivation of viruses with a very low power visible femtosecond laser. J Phys: Condens Matter. 2007;19:322102. doi: 10.1186/1743-422X-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tsen KT, TSWD, Sankey OF, Kiang JG. Selective inactivation of microorganisms with near-infrared femtosecond laser pulses. J Phys: Condens Matter. 2007;19:472201. [Google Scholar]
- 256.Tsen KT, TSWD, Chang CL, Hung CF, Wu TC, Ramakrishna B, Mossman K, Kiang JG. Inactivation of viruses with a femtosecond laser via impulsive stimulated Raman scattering. Proc SPIE. 2008;6854:68540N. [Google Scholar]
- 257.Tsen SWD, TYSD, Tsen KT, Wu TC. Selective inactivation of viruses with femtosecond laser pulses and its potential use for in vitro therapy. J Healthcare Engineering. 2010;1:185–196. [Google Scholar]
- 258.Tsen KTTS, Fu Q, Lindsay SM, Li Z, Cope S, Vaiana S, Kiang JG. Studies of inactivation of encephalomyocarditis virus, M13 bacteriophage, and Salmonella typhimurium by using a visible femtosecond laser: insight into the possible inactivation mechanisms. J Biomed Opt. 2011;16:078003. doi: 10.1117/1.3600771. [DOI] [PubMed] [Google Scholar]
- 259.Tsen SWCT, Beatty W, Tsen KT, Yu D, Achilefu S. Inactivation of enveloped virus by laser-driven protein aggregation. J Biomed Opt. 2012;17:128002. doi: 10.1117/1.JBO.17.12.128002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Manevitch Z, Lev D, Hochberg M, Palhan M, Lewis A, Enk CD. Direct antifungal effect of femtosecond laser on Trichophyton rubrum onychomycosis. Photochem Photobiol. 2010;86:476–479. doi: 10.1111/j.1751-1097.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 261.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 262.Bornstein E, Hermans W, Gridley S, Manni J. Near-infrared photoinactivation of bacteria and fungi at physiologic temperatures. Photochem Photobiol. 2009;85:1364–1374. doi: 10.1111/j.1751-1097.2009.00615.x. [DOI] [PubMed] [Google Scholar]
- 263.Krespi YP, Kizhner V. Laser-assisted nasal decolonization of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus. Am J Otolaryngol. 2012;33:572–575. doi: 10.1016/j.amjoto.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 264.Lauro FM, Pretto P, Covolo L, Jori G, Bertoloni G. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene-polylysine conjugates. Photochem Photobiol Sci. 2002;1:468–470. doi: 10.1039/b200977c. [DOI] [PubMed] [Google Scholar]
- 265.Tavares A, Carvalho CM, Faustino MA, Neves MG, Tome JP, Tome AC, Cavaleiro JA, Cunha A, Gomes NC, Alves E, et al. Antimicrobial photodynamic therapy: study of bacterial recovery viability and potential development of resistance after treatment. Mar Drugs. 2010;8:91–105. doi: 10.3390/md8010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266*.Giuliani F, Martinelli M, Cocchi A, Arbia D, Fantetti L, Roncucci G. In vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob Agents Chemother. 2010;54:637–642. doi: 10.1128/AAC.00603-09. Showing that it apaers to be impossible to force bacteria to develop resistance to repeated rounds of PDT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Alcantara-Diaz D, Brena-Valle M, Serment-Guerrero J. Divergent adaptation of Escherichia coli to cyclic ultraviolet light exposures. Mutagenesis. 2004;19:349–354. doi: 10.1093/mutage/geh039. [DOI] [PubMed] [Google Scholar]
- 268.Maclean M, Macgregor SJ, Anderson JG, Woolsey GA. The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J Photochem Photobiol B. 2008;92:180–184. doi: 10.1016/j.jphotobiol.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 269.Wainwright M. ‘Safe’ photoantimicrobials for skin and soft-tissue infections. Int J Antimicrob Agents. 2010;36:14–18. doi: 10.1016/j.ijantimicag.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 270.Brown S. Clinical Antimicrobial Photodynamic Therapy: Phase II Studies in Chronic Wounds. J Natl Compr Canc Netw. 2012;10 (Suppl 2):S80–83. doi: 10.6004/jnccn.2012.0182. [DOI] [PubMed] [Google Scholar]
- 271**.Morley S, Griffiths J, Philips G, Moseley H, O’Grady C, Mellish K, Lankester CL, Faris B, Young RJ, Brown SB, et al. Phase IIa randomised, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonised, chronic leg ulcers and diabetic foot ulcers. A new approach to antimicrobial therapy. Br J Dermatol. 2012 doi: 10.1111/bjd.12098. Clinical study showing that topical PDT can have benefits in infected non-ealing leg ulcers. [DOI] [PubMed] [Google Scholar]
- 272.Huh SY, Na JI, Huh CH, Park KC. The effect of photodynamic therapy using indole-3-acetic Acid and green light on acne vulgaris. Ann Dermatol. 2012;24:56–60. doi: 10.5021/ad.2012.24.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wang XL, Wang HW, Zhang LL, Guo MX, Huang Z. Topical ALA PDT for the treatment of severe acne vulgaris. Photodiagnosis Photodyn Ther. 2010;7:33–38. doi: 10.1016/j.pdpdt.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 274.Marotti J, Aranha AC, de Eduardo CP, Ribeiro MS. Photodynamic therapy can be effective as a treatment for herpes simplex labialis. Photomed Laser Surg. 2009;27:357–363. doi: 10.1089/pho.2008.2268. [DOI] [PubMed] [Google Scholar]
- 275.Wang HW, Zhang LL, Miao F, Lv T, Wang XL, Huang Z. Treatment of HPV infection-associated cervical condylomata acuminata with 5-aminolevulinic acid-mediated photodynamic therapy. Photochem Photobiol. 2012;88:565–569. doi: 10.1111/j.1751-1097.2011.01060.x. [DOI] [PubMed] [Google Scholar]
- 276.Wilder-Smith CH, Wilder-Smith P, Grosjean P, van den Bergh H, Woodtli A, Monnier P, Dorta G, Meister F, Wagnieres G. Photoeradication of Helicobacter pylori using 5-aminolevulinic acid: preliminary human studies. Lasers Surg Med. 2002;31:18–22. doi: 10.1002/lsm.10066. [DOI] [PubMed] [Google Scholar]
- 277.Novaes AB, Jr, Schwartz-Filho HO, de Oliveira RR, Feres M, Sato S, Figueiredo LC. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: microbiological profile. Lasers Med Sci. 2012;27:389–395. doi: 10.1007/s10103-011-0901-6. [DOI] [PubMed] [Google Scholar]
- 278.Feuerstein O, Persman N, Weiss EI. Phototoxic effect of visible light on Porphyromonas gingivalis and Fusobacterium nucleatum: an in vitro study. Photochem Photobiol. 2004;80:412–415. doi: 10.1562/0031-8655(2004)080<0412:PEOVLO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 279*.Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK. Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med. 2008;40:734–737. doi: 10.1002/lsm.20724. Shows that MRSA is suceptible to blue light eradication. [DOI] [PubMed] [Google Scholar]
- 280.Enwemeka CS, Williams D, Enwemeka SK, Hollosi S, Yens D. Blue 470-nm light kills methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomed Laser Surg. 2009;27:221–226. doi: 10.1089/pho.2008.2413. [DOI] [PubMed] [Google Scholar]
- 281.Kawada A, Aragane Y, Kameyama H, Sangen Y, Tezuka T. Acne phototherapy with a high-intensity, enhanced, narrow-band, blue light source: an open study and in vitro investigation. J Dermatol Sci. 2002;30:129–135. doi: 10.1016/s0923-1811(02)00068-3. [DOI] [PubMed] [Google Scholar]
- 282.Wheeland RG, Koreck A. Safety and Effectiveness of a New Blue Light Device for the Self-treatment of Mild-to-moderate Acne. J Clin Aesthet Dermatol. 2012;5:25–31. [PMC free article] [PubMed] [Google Scholar]
- 283.Gold MH, Sensing W, Biron JA. Clinical efficacy of home-use blue-light therapy for mild-to moderate acne. J Cosmet Laser Ther. 2011;13:308–314. doi: 10.3109/14764172.2011.630081. [DOI] [PubMed] [Google Scholar]
- 284.Wheeland RG, Dhawan S. Evaluation of self-treatment of mild-to-moderate facial acne with a blue light treatment system. J Drugs Dermatol. 2011;10:596–602. [PubMed] [Google Scholar]
- 285.de Arruda LH, Kodani V, Bastos Filho A, Mazzaro CB. A prospective, randomized, open and comparative study to evaluate the safety and efficacy of blue light treatment versus a topical benzoyl peroxide 5% formulation in patients with acne grade II and III. Anais brasileiros de dermatologia. 2009;84:463–468. doi: 10.1590/s0365-05962009000500003. [DOI] [PubMed] [Google Scholar]
- 286.Tremblay JF, Sire DJ, Lowe NJ, Moy RL. Light-emitting diode 415 nm in the treatment of inflammatory acne: an open-label, multicentric, pilot investigation. J Cosmet Laser Ther. 2006;8:31–33. doi: 10.1080/14764170600607624. [DOI] [PubMed] [Google Scholar]
- 287.Morton CA, Scholefield RD, Whitehurst C, Birch J. An open study to determine the efficacy of blue light in the treatment of mild to moderate acne. J Dermatolog Treat. 2005;16:219–223. doi: 10.1080/09546630500283664. [DOI] [PubMed] [Google Scholar]