Abstract
Background/Aims
Mindfulness-based stress reduction (MBSR) has enhanced cognition, positive emotion, and immunity in younger and middle-aged samples; its benefits are less well known for older persons. Here we report on a randomized controlled trial of MBSR for older adults and its effects on executive function, left frontal asymmetry of the EEG alpha band, and antibody response.
Methods
Older adults (n = 201) were randomized to MBSR or waiting list control. The outcome measures were: the Trail Making Test part B/A (Trails B/A) ratio, a measure of executive function; changes in left frontal alpha asymmetry, an indicator of positive emotions or approach motivation; depression, mindfulness, and perceived stress scores, and the immunoglobulin G response to a protein antigen, a measure of adaptive immunity.
Results
MBSR participants had a lower Trails B/A ratio immediately after intervention (p <0.05); reduced shift to rightward frontal alpha activation after intervention (p = 0.03); higher baseline antibody levels after intervention (p <0.01), but lower antibody responses 24 weeks after antigen challenge (p <0.04), and improved mindfulness after intervention (p = 0.023) and at 21 weeks of follow-up (p = 0.006).
Conclusions
MBSR produced small but significant changes in executive function, mindfulness, and sustained left frontal alpha asymmetry. The antibody findings at follow-up were unexpected. Further study of the effects of MBSR on immune function should assess changes in antibody responses in comparison to T-cell-mediated effector functions, which decline as a function of age.
Keywords: Mindfulness-based stress reduction, Executive function, Frontal alpha asymmetry, Immune function, Older adults
Mindfulness-based stress reduction (MBSR) [1] is a brief, intensive psychoeducational program incorporating mindful meditation, yoga and other practices that is designed to train participants to cultivate moment-by-moment receptive attention to thoughts, emotions, and sensations. Numerous studies in clinical and nonclinical samples have shown beneficial effects of MBSR on psychological symptoms (see meta-analyses [2, 3]) as well as physical well-being (see Grossman et al. [2] for meta-analysis) in younger and middle-aged adults. In addition, a smaller but growing number of studies suggest that cognitive function and immune benefits may also accrue among younger adults trained in MBSR [4]. Research in older adult samples is largely lacking, although one recent small trial of MBSR for older adults showed promising results [5]. Cognitive and immune therapeutic targets are particularly important in older adults, as aging is associated with decrements in both immune [6-8] and cognitive function [9, 10]. In addition, older adults who practice MBSR may be able to capitalize on aging-related increases in motivational processes involved in maintaining positive affect via attentional control of emotional experience [2, 3, 9].
Another possible target of research in elderly persons is frontal alpha asymmetry in the brain. Relative increases in left frontal alpha activity are associated with tendencies toward appetitive, approach, or behavioral activation, whereas greater right frontal activity is related to avoidance or withdrawal, including depressive states [11]. Coan et al. [12] have advanced a capability model of frontal alpha asymmetry, based on a conceptualization of this phenomenon as ‘interactions between the emotional demands of specific situations and the emotion-regulatory abilities (that) individuals bring to those situations’ (p. 199).
In one study to date, MBSR training in younger and middle-aged adults resulted in functional changes in brain areas associated with positive emotion valence and emotional control [4] and a motivational state characterized by approach rather than avoidance of emotional challenge [13]. Davidson et al. [4] reported that MBSR training increased left relative to right frontal alpha activity, sugges ting that changes in functional patterns of one relevant brain region may index at least some of MBSR’s benefits. However, in a commentary following the appearance of the findings of Davidson et al. [4], Travis and Arenander [14] objected that these alpha asymmetry changes were obtained at central, rather than frontal, electrodes. Davidson [15] countered that these scalp sites are nevertheless sensitive to frontal activity. Thus, the assertion that improved control of emotional experience following MBSR-induced shifts toward left frontal alpha activity in younger and middle-aged adult samples is controversial; a 2006 review of EEG and meditation practices by Cahn and Polich [16] concluded that additional studies are warranted to determine whether MBSR is associated with such changes in left versus right prefrontal dominance.
Other studies of mindfulness have examined its cognitive benefits for attention [17-19], as well as learning and memory [17, 20]. Chambers et al. [17] observed a significant increase in working memory in subjects trained in mindfulness compared to control subjects; moreover, a significant decrease in overall reaction time was observed in the Internal Switching Task, an indicator of improved capacity for sustained attention. Jha et al. [19] also observed that mindfulness training was associated with an increase in working memory capacity, defined as the capacity to selectively sustain and manipulate task-relevant information without becoming distracted by irrelevant information. In an earlier study, Jha et al. [18] also observed that MBSR improved the ability to orient attention, and direct and focus attention to relevant inputs. A recent study by Bostanov et al. [21] demonstrated that mindful attention is associated with increased amplitude of an EEG evoked response potential to a neutral auditory test stimulus; the heightened late contingent negative variation response observed in subjects trained in mindfulness compared to control subjects was considered to be reflective of mobilization of attentional resources, whereas diminished late contingent negative variation amplitude is observed during inattention to a task.
In addition to enhanced cognitive function, some research suggests that MBSR may have benefits for immune function. MBSR studies among HIV-positive adults [22] and patients with breast or prostate cancer [23-26] have reported improvement in levels of the stress hormone cortisol [23, 26], increased natural killer cell function [22, 23], and improvements in circulating cytokine levels or in vitro cytokine production [23-25]. A recent small trial of MBSR with older adults showed that proinflammatory gene expression decreased in association with decreased loneliness in the MBSR group [5]. To our knowledge, only one study has examined the effects of MBSR on adaptive immune effector function; in the study by Davidson et al. [4] referenced above, young adults who completed MBSR training showed a modest but significant increase in antibody titer to influenza vaccination compared to control subjects. They also reported a correlation between this increase in immune function and the previously mentioned elevation of left frontal alpha asymmetry. Again, however, these findings have been a topic of debate [14, 15].
Based on suggestive prior findings for MBSR in a variety of younger and middle-aged samples, we conducted a randomized controlled trial of MBSR’s effects in persons 65 years of age and older. We compared the effects of MBSR to a waiting list control (WLC) group on objective measures of: (1) emotional approach orientation, as ostensibly assessed by EEG measurement of left prefrontal brain activity; (2) executive function, as assessed by a neuropsychological measure, the Trail Making Test [27]; (3) depression, perceived stress, and mindfulness, subjective responses that the intervention would be expected to affect, and (4) humoral, adaptive immunity, as assessed by the immunoglobulin G (IgG) antibody response to a challenge with the protein antigen keyhole limpet hemocyanin (KLH). We chose this antigen because we reasoned that it would be novel, and therefore, our subjects would be unlikely to have preexisting antibodies that might mask antibody responses to a subsequent challenge, as can be the case with influenza vaccines [28]. We hypothesized that MBSR participants would show greater enhancement in each of these measures compared to WLC subjects.
Methods
Design, Setting and Subjects
Between December 2006 and October 2009, women and men were recruited from the community via advertisements in local newspapers and from University of Rochester Strong Health-associated primary care offices using flyers. With the exception of 3 individuals (1 aged 63 years, 2 aged 64 years) who enrolled with spouses or partners, all participants were aged 65 years or older. Participants treated with antidepressant or anxiolytic medications were required to have had a stable medication regimen for 8 weeks prior to enrolling in the trial. Exceptions were made for individuals taking sedative-hypnotic medications for sleep, low-level psychotropic medications for pain management, or β-blockers for heart conditions. Given the didactic and self-directed nature of the MBSR program, individuals with major, uncorrected sensory impairments and cognitive deficits were excluded. Cognitive impairment was defined as a score of 24 or lower on the Mini-Mental State Examination [29]. Other exclusion criteria were: major depression with psychotic features; psychosis; life-time history of schizophrenia, bipolar disorder, organic brain syndrome, or mental retardation, and alcohol or substance abuse within the previous year, all based on the time 1 screening interview. Finally, individuals with allergies to shellfish were excluded from the study as a precaution because of immunization with KLH, which is purified from the mollusc Megathura crenulata.
A total of 228 subjects met inclusion criteria, gave written informed consent, and underwent initial assessment by 1 of 3 master’s level research assistants. Subjects were then randomized to an 8-week program of MBSR or a WLC condition. Randomization was conducted using an MS Excel spreadsheet function for generating random numbers. This function was used to determine the treatment arm (with equal chance for either arm) for each subject individually, except in cases where 2 individuals enrolled together. Analyses were conducted to discern the adequacy of the randomization and to determine whether treatment outcomes are moderated by demographic or clinical variables. Spouses, housemates, or close friends (with frequent, regular contact) were randomized to the same arm to prevent the contamination that could have occurred if a control group member observed or was told about MBSR techniques by a participant in the treatment arm. A total of 23 dyads were enrolled (12 to control, 11 to treatment), consisting of 20 pairs of spouses, 1 of housemates, and 2 pairs of close friends.
Data from both MBSR and WLC subjects were collected at 4 time points relative to the MBSR program: following randomization and before beginning of MBSR training (time 1); immediately following completion of the 8-week MBSR program (time 2); 3 weeks later (time 3), and 21 weeks following time 3 (time 4). At time 2, blood was drawn from study participants (preimmunization baseline), and they were then immunized with KLH. Blood was not obtained at study baseline (time 1) because in the absence of immunization with KLH, antibody titer would be expected to remain constant between time 1 and time 2. Further, we expected that KLH would be a novel antigen, and that antibody levels would therefore be low to undetectable prior to immunization at time 2. Blood was obtained again at times 3 and 4. At times 1, 2 and 4, resting EEG recordings were obtained. Assessments were conducted at the University of Rochester Medical Center or Monroe Community Hospital, Rochester, N.Y., USA. To examine the effectiveness of the randomization procedure with respect to potential confounds, participants completed a battery of self-report instruments, including the 20-item Center for the Epidemiologic Studies Depression Scale-Revised (CES-D-R) [30], the 10-item Perceived Stress Scale (PSS) [31] and the 15-item Mindful Attention Awareness Scale (MAAS) [32]. The protocol is depicted in table 1.
Table 1.
Study protocol
| Time 1: week 0 | 8 weeks | Time 2: week 8 | 3 weeks | Time 3: week 11 | 21 weeks | Time 4: week 32 |
|---|---|---|---|---|---|---|
| Randomization, psychosocial assessment, EEG, Trails B/A |
Treatment | 1st blood draw, KLH immunization, psychosocial assessment, EEG, Trails B/A |
Follow-up | 2nd blood draw, psychosocial assessment, Trails B/A |
Follow-up | 3rd blood draw, psychosocial assessment, EEG, Trails B/A |
Twenty subjects withdrew from the study prior to time 2 (3 randomized to MBSR, 8 randomized to WLC, and 9 prior to randomization). Data from all 4 study time points were collected on 208 participants, 105 in the MBSR arm and 103 WLC subjects. Of these, 201 who had complete data on all variables at all time points and 131 who participated in the EEG sessions comprise the present sample.
The study protocol was approved by the University of Rochester Institutional Review Board (RSRB00012363) and is registered with clinicaltrials.gov (NCT01027780).
Mindfulness-Based Stress Reduction
Standardized MBSR [1] was used. Subjects participated in a group-based (15-20 members) 8-week curriculum that included weekly 120-min sessions and one ‘all day intensive’ session of 7 h [1]. The active MBSR program was delivered in 6 cohorts during the spring, summer and fall of 2006-2008. Only 2 subjects in the active treatment group attended fewer than 5 MBSR sessions. All MBSR treatment groups were conducted by M.S.K., who received his training in teaching MBSR from Jon Kabat-Zinn, PhD, founder of the Center for Mindfulness, University of Massachusetts School of Me dicine, Worcester, Mass., USA and Saki Santorelli, EdD, Executive Director of the Center for Mindfulness. As part of the study design, videotapes of 25% of our active MBSR sessions were examined for fidelity by Florence Meyer, MS, MA, and Melissa Blacker, MA, Co-Directors, Professional Training Programs, Center for Mindfulness, University of Massachusetts School of Medicine, Worcester, Mass., USA, who reported full fidelity to the MBSR curriculum.
Four primary mindfulness practices were taught over the course of the program: sitting meditation, body scan meditation, mindful movement similar to Hatha Yoga, and walking meditation. In each, emphasis is placed on the deployment of mindful attention – the observation of experience as it is occurring in the present moment in a nonevaluative and nondiscursive way. MBSR practices are designed to enhance an ongoing and nonjudgmental awareness of moment-to-moment mind-body experience, including those of a cognitive, emotional, kinesthetic, and sensory nature. The reflection on one’s current thoughts and feelings, while refraining from judging them, is designed to promote acceptance of experiences rather than ongoing evaluations of experiences as ‘good’ or ‘bad’. The consequence of enhanced mindfulness is a shift from non-awareness and automatic, habitual behaviors and stress responses to conscious awareness and effective, deliberate action [33].
MBSR was structured to accommodate the mobility, sensory, and cognitive limitations of older adults who met the inclusion criteria. Accommodations were made on an individualized basis according to specific participant needs. For example, elderly people in a wheelchair completed mindful movement exercises with a variety of chair-based yoga postures and movements.
Outcome Variables
Anti-KLH Antibody Response
KLH [34-36] was injected intradermally by trained nurses into the deltoid muscle of the nondominant arm of participants in a volume of 0.1–0.2 ml of sterile saline following completion of MBSR (time 2) for both study groups. Sterile KLH (Intracel, Frederick, MD and biosyn Corporation, Carlsbad, Calif., USA) was prepared for injection by the staff of the University of Rochester Investigational Pharmacy. Embedded in this study was the hypothesis that group differences in antibody response would be a function of the dose of KLH received – such that maximum differences would be observed at ‘moderate’ doses, but that the potential effects of MBSR on antibody production would be masked at higher immunizing doses. Thus, subjects were immunized with a range of doses, from 8 to 1,000 μg of KLH. More subjects were assigned into groups receiving 100 and 200 μg of KLH (which, based on pilot data, were calculated to provide observable group differences); the final number of subjects randomized to receive 8, 40, 100, 200 and 1,000 μg of KLH were n = 3, 10, 57, 25 and 5 for the MBSR group and n = 2, 7, 65, 22, and 5 for the WLC group.
Immediately prior to immunization, and 3 and 24 weeks following immunization, 30 ml of venous blood was collected from subjects into heparinized vacutainer tubes (BD, Franklin Lakes, N.J., USA) by a nurse or a trained clinical coordinator/phlebotomist. Blood was centrifuged, and serum was collected and frozen at −80 °C.
Anti-KLH antibody levels in serum were determined by enzyme-linked immunoassay based on modifications of our published protocol in rodents [37-39]. Briefly, wells of 96-well microtiter plates were coated overnight with 10 μg/ml of KLH in a carbonate coating buffer (pH 9.6) and blocked for 1 h with phosphate-buffered saline (PBS) containing 1% bovine serum albumin at 37 °C. Serum samples were diluted 1: 400, 1: 800 and 1: 1,600 in PBS/Tween containing 1 M NaCl, and incubated in duplicate for 3 h at 37 °C. The plates were then incubated overnight at 4 °C with alkaline phosphatase-conjugated goat anti-human IgG antibodies (Zymed Laboratories, Inc., San Francisco, Calif., USA) diluted in PBS/Tween, followed by addition of the substrate, p-nitrophenyl phosphate (Sigma Chemical Co.) at 1 mg/ml in carbonate buffer (pH 9.6). Washes with PBS/Tween followed each step of the assays. Absorbance at 405 nm was measured using an Opsys MR Microplate Reader (Thermo Labsystems, Chantilly, Va., USA). Data are expressed as absorbance at 405 nm.
Trail Making Test
The Trail Making Test is a commonly used neuropsychological test of visual attention and task switching [27]. It consists of 2 parts: part A asks participants to connect a series of numbers in ascending order on a page, while part B requires participants to draw a line alternating between numbers and letters, both of which must be connected in ascending order. The first part places few cognitive demands on participants beyond counting, and thus primarily captures processing speed. The second part requires more complex attention shifting, planning, and concentration, and is considered a valid index of executive control [40]. The ratio of completion time of part B over part A (Trails B/A) thus reflects the participants’ attention and concentration skill, relative to or adjusting for their processing speed [27]. The Trails B/A ratio was used as an index of executive control throughout the trial, with lower scores indicating better performance.
Psychological Scales
Participants also completed the CES-D-R [30], the PSS [31], and the MAAS [32] at each measurement point. The CES-D asks about symptoms of depression within the past 2 weeks, the PSS asks about stress within the past week, and the MAAS assesses dispositional mindfulness.
Brain Electrical Activity
All EEG recordings were obtained for each participant at the same time of day ± 1 h in a quiet and low illumination environment. A trained experimenter monitored equipment and read instructions before each of eight 1-min baselines during which EEG was recorded while the subject sat quietly with eyes open and eyes closed in 1 of 2 counterbalanced sequences [41]. The start and end of each baseline were signified, respectively, by a single and a double 500-Hz tone. EEG from the left and right mastoids and 19 sites in the international 10/20 electrode system were recorded using a Lycra stretchable cap (Compumedics, Inc., El Paso, Tex., USA). This montage included 8 homologous pairs (FP1/2, F3/4, F7/8, C3/4, T7/8, P7/8, P3/4, O1/2) and 3 midline electrodes (Fz, Cz, Pz) referenced to the right mastoid. EEG recordings were re-referenced off-line to a digitally derived linked-mastoid reference. Cz-referenced recordings were also obtained, but we report only the linked-mastoid findings. Vertical (left supra- and infra-orbital) and horizontal (outer canthi) bipolar electrooculograms were also obtained to allow for detection and elimination of EEG data segments contaminated by eye movements.
Electrophysiological data were sampled at a rate of 1,000 Hz, amplified, and processed by Neuroscan NuAmp amplifiers and associated Neuroscan software (Compumedics, Inc.). EEG was recorded with a gain of 20,000 and electrooculograms with a gain of 10,000. Amplifier filters were set for high- and low-pass filter cutoffs of 0.10 and 200 Hz, respectively. Neuroscan software derived 1.024-second segments of artifact-free EEG, deleted those containing eye movement or other artifacts, overlapped them by 50%, and then processed them using a fast Fourier transform method to derive measures of spectral power density in the alpha band (8-13 Hz), which is inversely related to cerebral activation [42] and for which MBSR effects have been noted previously [4]. EEG asymmetric activation scores were calculated by averaging, for each electrode, alpha power densities over eyes open or closed trials for each session, weighted by the number of epochs with acceptable data, and subtracting log-transformed left-hemisphere alpha power densities from the comparable measures obtained from homologous right-hemisphere sites. On the basis of previous research associating asymmetric anterior activation with positive affect [42] and MBSR intervention [4], analyses of EEG data were focused on results from F3/4, but we performed exploratory analyses of additional anterior pairs of homologous sites: FP1/2, F3/4, F7/8, T3/4, and C3/4.
A total of 131 subjects participated in multiple EEG sessions, but the number of subjects with useable data was reduced to 110 because of equipment malfunction (n = 6); falling asleep (n = 9); unacceptable recordings at one or more electrodes (n = 5), and interruption by a fire alarm (n = 1). Change in alpha asymmetry from time 1 to either time 2 or 4 was not correlated with chronological age, gender, handedness, use of drugs affecting the CNS, prandial factors (recent meal, reporting hunger, etc.), or subjective rating of quality of sleep for the preceding night (all p values >0.05).
Data Analysis
The study was powered to detect significant differences in antibody rise between the MBSR versus WLC groups. Primary treatment effect estimation for IgG and Trails B/A, as well as the psychological scales, used generalized estimating equations (GEE), which accounted for the nesting of repeated measures within individuals. The multiple dilutions of IgG antibodies were also treated as repeated measures. GEE models used log links and gaussian distributions and an exchangeable working correlation matrix. Both the IgG and Trail Making Test distributions were not normal; therefore, it would have been difficult to fit a traditional repeated-measures linear model for them. We chose GEE because it can directly model continuous distributions that are nonnormal (i.e., gamma distributions). Additionally, GEE is a semi-parametric technique that does not make assumptions about the variance of the outcome distribution. The standard errors of model estimates will not be distorted by variances that do not match the selected distribution. For instance, gamma distributions assume that the variance increases with the square of each observed value, but in practice this is often not true and would lead to incorrect standard errors. We thus selected it as an accurate but cautious approach for repeated measures of these outcomes. Prior to modeling treatment effects, we screened for baseline factors on which the groups differed and which were at least marginally (p <0.10) correlated with outcomes [43].
Alpha asymmetry values were submitted to within-subject (3 visits) and between-subject (treatment group) analyses of variance for each scalp location; because asymmetry scores were relatively normal, a fully parametric model could be used to optimize power. These analyses were followed by analyses of baseline/time 2 and time 2/time 4; this approach took advantage of the larger number of subjects participating in the first 2 visits. We report results for only F3/4, the only site for which treatment differences were obtained, the most commonly used site in studies of frontal alpha asymmetry, as well as the location for which Davidson et al. [44] found a correlation between anterior alpha asymmetry and natural killer cell activity.
Results
The demographic characteristics of the two groups are shown in table 2. The sample was predominantly white (98%), and the MBSR and WLC groups did not differ in gender, age, and other demographic characteristics (including income, education, and marital status). Randomization failed to yield groups comparable on 3 factors: treatment participants scored higher on the PSS and the CES-D-R, but lower on the MAAS. To control for these baseline imbalances between groups, all models of treatment effects included baseline CES-D, PSS, and MAAS scores as covariates [43].
Table 2.
Baseline characteristics of MBSR and WLC groups
| MBSR group | WLC group | Test statistics (d.f.); p value | |
|---|---|---|---|
| Age (mean ± SD), years | 73.3±6.7 | 73.6±6.7 | t = 1.25 (d.f. = 198); p = 0.179 |
| Education (mean ± SD), years | 16.3±2.8 | 16.5±2.8 | t = 0.351 (d.f. = 198); p = 0.726 |
| Female, n | 62 (62%) | 62 (62%) | z = −0.217; p = 0.829 |
| Number of dyads, n | 12 (12%) | 11 (11%) | z = 0.221; p = 0.825 |
| PSS score (mean ± SD) | 13±6.3 | 11±6.7 | t = −2.55 (d.f. = 197); p = 0.012 |
| CES-D-R score (mean ± SD) | 5.9±6.8 | 4.1±6.3 | t = −2.09 (d.f. = 198); p = 0.038 |
| MAAS score (mean ± SD) | 5.1±0.8 | 5.3±0.7 | t = 3.36 (d.f. = 198); p < 0.001 |
| Trails B/A ratio (mean ± SD) | 2.0±1 | 1.9±0.9 | t = −0.094 (d.f. = 198); p = 0.372 |
Comparisons are independent-samples t tests for continuous variables, test of independent proportions. p values in italics are significant.
Trail Making Test
Table 3 shows the Trails B/A ratio means for each group across postintervention time points. Immediately after intervention (time 2), the treatment group showed a significantly lower ratio, indicating significantly better executive control compared to WLC subjects (p = 0.04). This difference was not sustained at times 3 and 4. No significant differences in Trails A or B individually were noted.
Table 3.
Effects of MBSR on Trails B/A ratio and IgG anti-KLH antibody response
| MBSR mean |
WLC mean |
p value | Effect size |
|
|---|---|---|---|---|
| MAAS scores | ||||
| Baseline | 5.08 | 5.30 | 0.017 | −0.29 |
| 8 weeks | 5.21 | 5.28 | 0.500 | −0.08 |
| 11 weeks | 5.53 | 5.39 | 0.153 | 0.18 |
| 32 weeks | 5.46 | 5.27 | 0.023 | 0.25 |
| Trails B/A ratio | ||||
| Baseline | 2.01 | 1.89 | 0.281 | −0.06 |
| 8 weeks | 1.75 | 2.01 | 0.04 | − 0.24 |
| 11 weeks | 2.37 | 2.43 | 0.666 | 0.05 |
| 32 weeks | 1.89 | 1.98 | 0.507 | 0.08 |
| IgG (absorbance) | ||||
| 8 weeks | 0.172 | 0.104 | 0.007 | 0.18 |
| 11 weeks | 0.203 | 0.256 | 0.293 | −0.14 |
| 32 weeks | 0.143 | 0.223 | 0.036 | − 0.22 |
Group differences with p < 0.05 are in italics. Week 8 = Immediately after intervention, at the time of KLH challenge. Effect size is Cohen's d and is the difference between treatment and control group at each time point, expressed in standard deviation units; analyses are adjusted for MAAS, CES-D, and PSS scores at baseline.
Brain Electrical Activity
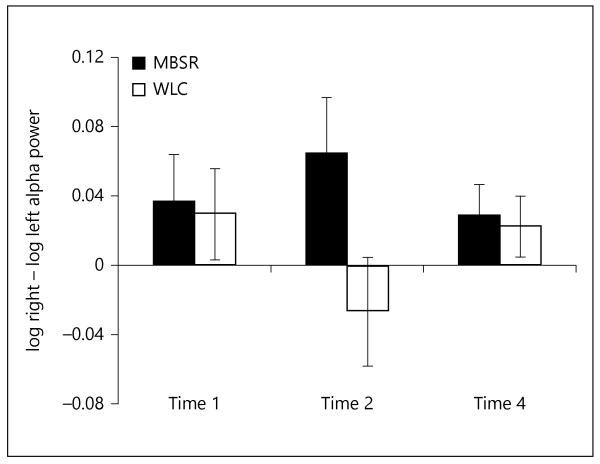
As previously mentioned, we focus on results for alpha asymmetry at the F3/4 (frontal) sites. Analysis of these data for time 1 (baseline) and time 2 (following intervention) showed a marginally significant treatment group × time interaction [F(1, 109) = 3.38, p = 0.07, η2 = 0.03]; this interaction reflected moderate differences between treatment groups at time 2 (fig. 1). At time 1 and time 4 (follow-up), there were no differences in F3/4 asymmetry between the MBSR and WLC groups. However, immedia tely following completion of the 8-week MBSR program (time 2), a significantly greater leftward alpha asymmetry was observed for MBSR subjects compared to WLC subjects [F(1, 129) = 4.66, p = 0.03, η2 = 0.04]. Specifically, between the baseline and the end of treatment, MBSR subjects maintained relatively constant alpha asymmetry [F(1, 63) <1, not significant, η2 <0.01], whereas WLC subjects exhibited a significant rightward shift in alpha asymmetry [F(1, 66) = 5.69, p = 0.02, η2 = 0.08].
Fig. 1.
Alpha asymmetry scores at F3/4 sites for MBSR and WLC at time 1 (baseline session), time 2, and time 4. Asymmetry was scored as the difference in log power of alpha band at the right frontal scalp site (F8) minus log power alpha at the homologous left site. Numerically larger values indicate greater activation of alpha on the left hemisphere for these two frontal sites.
Psychological Measures
Whereas no post-treatment or follow-up differences were noted in CES-D or PSS scores, the treatment group exhibited significantly higher MAAS scores after treatment (p = 0.023, d = 0.26) and at follow-up (p = 0.006, d = 0.33). As mentioned earlier, these analyses statistically controlled for initial group differences on all factors. CES-D and PSS scores tended to be low in both groups throughout the trial (i.e., coefficients of variation over 1 and long right-tailed distributions).
Antibody Response to KLH
The bottom portion of table 3 shows group IgG (absorbance) at each time point. Antigen concentration was not a significant factor in the analysis and thus, results are shown collapsed across concentration. The treatment group showed a slightly higher initial level compared to the WLC group immediately after intervention (time 2; p = 0.007), and slightly lower levels at long-term follow-up (time 4; p = 0.036). Antibody levels did not differ by group at time 3.
Discussion
Mindfulness and training in it are theorized to foster the adaptive regulation of attention, thought, and emotion [37, 45]. Consistent with this, MBSR practice in younger adult populations has previously been shown to be associated with increased attention and awareness in the present moment, resulting in increased functional attention in tests of cognitive performance [17, 19]. Moreover, Davidson et al. [46] demonstrated, following MBSR training of young adults, a relative increase in left brain activation, which is linked to positive emotional valence and an approach orientation to emotional challenge [13]. As noted earlier, Davidson’s group reported increased antibody responses to influenza vaccine [4]. Here we report on the first randomized controlled trial of MBSR for community-dwelling older adults (average age of MBSR subjects = 72.3 years), which included a measure of attention and executive control, examination of frontal brain asymmetry, stress, depression and mindfulness, and antibody response to the protein antigen KLH.
Immediately after intervention, the MBSR group had significantly better scores on the Trails B/A ratio than the WLC group, indicating that MBSR improved visual attention and executive control in older adult subjects, as has also been reported for younger to middle-aged adults [17-19]. Specifically, the intervention improved performance on a more complex executive function task (Trails B) relative to a simpler one (Trails A), rather than absolute speed on either. Since the Trails B/A ratio standardizes performance for individual processing speed, the cognitive gain would appear related to attentional control, planning or sequencing rather than processing speed itself.
Davidson et al. [4] reported a significant increase in left brain asymmetry following MBSR training. As mentioned earlier, left frontal activity has also been associated with approach motivation, and emotions related to it, including those typically understood as ‘positive’, as well as approach emotions such as anger [47]. Immediately following MBSR training, we found sustained left frontal (F3/4) brain activation relative to baseline in our older adult MBSR participants, as opposed to significant reductions in the WLC group. Our findings of stable, rather than increased, anterior alpha asymmetry after treatment resemble those of Barnhofer et al. [48] for remitted suicidal depressive patients. These results contrast with the sizeable increases in frontal left-sided alpha asymmetry reported by Moyer et al. [49] following meditation training, but their subjects were assessed while meditating; this procedure likely enhanced differences between groups. On the other hand, Keune et al. [50] reported comparable shifts toward right-dominant asymmetry for remitted patients with a history of chronic major depression following both mindfulness-based cognitive therapy and waiting list. In summary, when EEG was measured under resting conditions with populations other than healthy volunteers, MBSR training prevented a shift toward right-sided frontal alpha asymmetry in 2 out of 3 studies conducted after the report of Davidson et al. [4]. Actual increases in left-sided frontal asymmetry were found in a fourth study in which EEG was measured during meditation practice [49].
No improvements in perceived stress or depressive symptoms were observed as a result of the treatment examined here. However, other studies demonstrating such improvement have used depressed, rather than nondepressed or community samples. The distribution of depression and stress scores in our relatively healthy, older sample suggests that there was little room to improve on either variable; hence, it is not surprising that there was no observable effect. On the other hand, effect sizes in the ‘small’ range (i.e., Cohen’s d values around 0.3) were observed for mindfulness, and these treatment effects were maintained at follow-up. Thus, the intervention indeed appears to alter its target psychological factor.
Our follow-up over a period of 26 weeks after MBSR suggested that the better executive function during Trails B/A and stable left frontal alpha asymmetry activity were not sustained beyond the conclusion of treatment. In contrast to our findings, studies with younger adult populations have documented that some psychological and biological changes are sustained for at least 3 months [23] and some even up to 1 year [25] following training. We do not know to what extent our participants were still practicing mindfulness following the end of the intervention; it may be that ‘booster’ MBSR sessions could help to sustain improvements in these domains, perhaps particularly in older adult populations.
In contrast to the above findings, the antibody findings ran in the opposite of the expected direction, and we cannot definitively rule out an unanticipated methodological confound. We had expected baseline antibody levels to KLH, presumably a novel antigen, to be low to undetectable. This was not the case: significant preexisting antibody levels were present in both the MBSR and WLC groups at the time of challenge (time 2). In support of this finding, Smith et al. [36] documented good baseline proliferative responses to KLH in 7/21 students between 18 and 30 years of age. Further, the authors observed very considerable variability in the baseline antibody response to KLH. It seems likely, although it is not known, that by age 65, the percentage of individuals with preexisting antibody to KLH might be even greater than in young adults.
Significantly greater baseline levels (time 2) of anti-KLH antibody were observed in the MBSR group, which could explain the lower antibody at follow-up in that group versus the WLC group. Preexisting antibodies can lead to faster antigen clearance following immunization, and in turn to lower subsequent levels of antibody following challenge [28]. We cannot verify that such a process occurred here, but it is one plausible explanation for the lower KLH antibody levels in the MBSR group at time 4.
Our observations of antibody response contrast with the findings of Davidson et al. [4] of increased antibody to influenza vaccine in young adults (average age = 36 years). Future studies should test whether these differences in findings are due to differences in the choice of antigen challenge (flu vaccine vs. KLH) and the subsequent cell-mediated versus humoral immune responses. Studies with both younger and older subjects with panels of antigens and immune outcomes (including T helper 1 and T helper 2 effector functions), which are important for a balance between cell-mediated immunity and antibody responses [51, 52], could address these issues.
Our study has several limitations. First, our comparison group, a WLC group, was not matched for attention and time to our MBSR group. Future studies should include a study design carefully matching on these factors. Second, in our analyses, we did not consider the possible effects of various health behaviors, such as sleep and diet, on our outcome measures.
Third, our measure of executive function was the Trails B/A ratio; while accepted as a measure of executive function, a more comprehensive neurocognitive battery could better gauge the effects of mindfulness training in this population. However, findings from this easily administered test, part of a substantial battery of measures used in this study, suggest that executive function may be increased in older adults trained in mindfulness meditation, but the effects are transient. Fourth, a future study should be aimed at documenting whether subjects engage in sustained practice of MBSR following the 8-week training period, and should perhaps include booster sessions of mindfulness training.
A fifth limitation to our study was the observation of baseline differences in the PSS, the CES-D and the MAAS between our groups, such that the subjects randomized to MBSR reported more baseline stress and depressive symptoms, and lower mindfulness. Despite this issue, however, we did observe that reported mindfulness increased following MBSR, and was sustained at follow-up (time 4).
Finally, the unexpected antibody findings could have been better interpreted had we included a time 1 blood draw to compare preexisting antibody levels before and after intervention (but prior to antigen challenge). Although we would not expect antibody levels to change between time 1 and time 2 in the absence of immunization, we cannot definitively rule out that, for example, MBSR was associated with an overall increase in total serum IgG, resulting in higher time 2 anti-KLH antibody levels compared to the WLC subjects.
To our knowledge, this is one of the first reports of the use of MBSR for a relatively healthy older adult population. Predicted improvements in antibody response in favor of MBSR were not found, but this null effect could be explained by the unexpected presence of baseline antibody responses to an antigen that had been presumed to be novel. Our data do suggest that MBSR maintained initial levels of theoretically meaningful changes in left frontal brain alpha asymmetry, and increased executive control, consistent with theorized effects of mindfulness training [37, 45] and with some previous research with younger populations. Further study of the effects of MBSR and its constituent components on immunosenescence and cognitive aging are thus warranted.
Acknowledgments
We thank John J.B. Allen for generous advice on scoring of EEG measures. We also thank Jeffrey Swan and Iwona Juskiewicz for their assistance with the execution of the study. This research was supported by R24AG031089 (J.M.), R01AG25474 (J.M.), and K08AG31328 (B.C.).
Reference
- 1.Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. Delacorte; New York: 1990. [Google Scholar]
- 2.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78:169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Ur-banowski F, Harrington A, Bonus K, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- 5.Creswell JD, Irwin MR, Burklund LJ, Lieber-man MD, Arevalo JM, Ma J, Breen EC, Cole SW. Mindfulness-based stress reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun. 2012;26:1095–1101. doi: 10.1016/j.bbi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franceschi C, Bonafe M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000;18:1717–1720. doi: 10.1016/s0264-410x(99)00513-7. [DOI] [PubMed] [Google Scholar]
- 7.Ginaldi L, Loreto MF, Corsi MP, Modesti M, De Martinis M. Immunosenescence and infectious diseases. Microbes Infect. 2001;3:851–857. doi: 10.1016/s1286-4579(01)01443-5. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi C, Capri M, Monti D, Giunta S, Ol-ivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Aine CJ, Sanfratello L, Adair JC, Knoefel JE, Caprihan A, Stephen JM. Development and decline of memory functions in normal, pathological and healthy successful aging. Brain Topogr. 2011;24:323–339. doi: 10.1007/s10548-011-0178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson RJ. Emotion and affective style. Psychol Sci. 1992;3:39–43. [Google Scholar]
- 12.Coan JA, Allen JJ, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biol Psychol. 2006;72:198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauss IB, Robinson MD. Measures of emotion: a review. Cogn Emot. 2009;23:209–237. doi: 10.1080/02699930802204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Travis F, Arenander A. EEG asymmetry and mindfulness meditation. Psychosom Med ■■. 66:147–148. doi: 10.1097/00006842-200401000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Davidson RJ. Response. Psychosomatic Medicine. 2004;66:147–148. [Google Scholar]
- 16.Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- 17.Chambers R, Chuen Lee Yo B, Allen NB. The impact of intensive mindfulness training on attentional control, cognitive style, and affect. Cog Ther Res. 2008;32:303–322. [Google Scholar]
- 18.Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cogn Affect Behav Neurosci. 2007;7:109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- 19.Jha AP, Stanley EA, Kiyonaga A, Wong L, Gelfand L. Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion. 2010;10:54–64. doi: 10.1037/a0018438. [DOI] [PubMed] [Google Scholar]
- 20.Holzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, Lazar SW. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bostanov V, Keune M, Kotchoubey B, Hautz-inger M. Event-related brain potentials reflect increased concentration ability after mindfulness-based cognitive therapy for depression: a randomized clinical trial. Psychiatry Res. 2012;199:174–180. doi: 10.1016/j.psychres.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Robinson FP, Mathews HL, Witek-Janusek L. Psycho-endocrine-immune response to mindfulness-based stress reduction in individuals infected with the human immunodeficiency virus: a quasiexperimental study. J Altern Complement Med. 2003;9:683–694. doi: 10.1089/107555303322524535. [DOI] [PubMed] [Google Scholar]
- 23.Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22:969–981. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom Med. 2003;65:571–581. doi: 10.1097/01.psy.0000074003.35911.41. [DOI] [PubMed] [Google Scholar]
- 25.Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21:1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Matousek RH, Pruessner JC, Dobkin PL. Changes in the cortisol awakening response (CAR) following participation in mindfulness-based stress reduction in women who completed treatment for breast cancer. Complement Ther Clin Pract. 2011;17:65–70. doi: 10.1016/j.ctcp.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Lezak M, Howieson D, Loring D. Neuropsychological Assessment. ed 4 Oxford University Press; Oxford: 2004. [Google Scholar]
- 28.Sasaki S, He XS, Holmes TH, Dekker CL, Kemble GW, Arvin AM, Greenberg HB. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One. 2008;3:e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 31.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 32.Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84:822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- 33.Brown KW, Ryan RM, Creswell JD. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychol Inq. 2007;18:211–237. [Google Scholar]
- 34.Waldo FB, van den Wall Bake AW, Mestecky J, Husby S. Suppression of the immune response by nasal immunization. Clin Immunol Immunopathol. 1994;72:30–34. doi: 10.1006/clin.1994.1103. [DOI] [PubMed] [Google Scholar]
- 35.Valdez H, Smith KY, Landay A, Connick E, Kuritzkes DR, Kessler H, Fox L, Spritzler J, Roe J, Lederman MB, Lederman HM, Evans TG, Heath-Chiozzi M, Lederman MM, ACTG 375 team. AIDS Clin Trials Group Response to immunization with recall and neo-antigens after prolonged administration of an HIV-1 protease inhibitor-containing regimen. AIDS. 2000;14:11–21. doi: 10.1097/00002030-200001070-00002. [DOI] [PubMed] [Google Scholar]
- 36.Smith A, Vollmer-Conna U, Bennett B, Wakefield D, Hickie I, Lloyd A. The relationship between distress and the development of a primary immune response to a novel antigen. Brain Behav Immun. 2004;18:65–75. doi: 10.1016/s0889-1591(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 37.Moynihan J, Koota D, Brenner G, Cohen N, Ader R. Repeated intraperitoneal inject ions of saline attenuate the antibody res ponse to a subsequent intraperitoneal injection of antigen. Brain Behav Immun. 1989;3:90–96. doi: 10.1016/0889-1591(89)90008-1. [DOI] [PubMed] [Google Scholar]
- 38.Moynihan JA, Ader R, Grota LJ, Schachtman TR, Cohen N. The effects of stress on the development of immunological memory following low-dose antigen priming in mice. Brain Behav Immun. 1990;4:1–12. doi: 10.1016/0889-1591(90)90001-7. [DOI] [PubMed] [Google Scholar]
- 39.Karp JD, Moynihan JA, Ader R. Effects of differential housing on the primary and secondary antibody responses of male C57BL/6 and BALB/c mice. Brain Behav Immun. 1993;7:326–333. doi: 10.1006/brbi.1993.1032. [DOI] [PubMed] [Google Scholar]
- 40.Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol. 2000;22:518–528. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- 41.Sutton S, Davidson R. Prefrontal brain asymmetry: a biological substrate of the behavioral approach and inhibition systems. Psychol Sci. 1997;8:204–210. [Google Scholar]
- 42.Davidson RJ, Jackson D, Larson C. Human electroencephalography. In: Cacioppo JT, Tassinary L, Bernston GG, editors. Handbook of Psychophysiology. Cambridge University Press; New York: 2000. pp. 27–52. [Google Scholar]
- 43.Piantadosi S. Clinical Trials: A Methodologic Perspective. ed 3 Wiley; Hoboken: 2005. [Google Scholar]
- 44.Davidson RJ, Coe CC, Dolski I, Donzella B. Individual differences in prefrontal activation asymmetry predict natural killer cell activity at rest and in response to challenge. Brain Behav Immun. 1999;13:93–108. doi: 10.1006/brbi.1999.0557. [DOI] [PubMed] [Google Scholar]
- 45.Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Segal ZV, Abbey S, Speca M, Velting D, Devins G. Mindfulness: a proposed operational definition. Clin Psychol. 2004;11:230–241. [Google Scholar]
- 46.Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- 47.Harmon-Jones E. Contributions from research on anger and cognitive dissonance to understanding the motivational functions of asymmetrical frontal brain activity. Biol Psychol. 2004;67:51–76. doi: 10.1016/j.biopsycho.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Barnhofer T, Duggan D, Crane C, Hepburn S, Fennell MJ, Williams JM. Effects of meditation on frontal alpha-asymmetry in previously suicidal individuals. Neuroreport. 2007;18:709–712. doi: 10.1097/WNR.0b013e3280d943cd. [DOI] [PubMed] [Google Scholar]
- 49.Moyer CA, Donnelly MP, Anderson JC, Valek KC, Huckaby SJ, Wiederholt DA, Doty RL, Rehlinger AS, Rice BL. Frontal electroencephalographic asymmetry associated with positive emotion is produced by very brief meditation training. Psychol Sci. 2011;22:1277–1279. doi: 10.1177/0956797611418985. [DOI] [PubMed] [Google Scholar]
- 50.Keune PM, Bostanov V, Hautzinger M, Kotchoubey B. Mindfulness-based cognitive the rapy (MBCT), cognitive style, and the temporal dynamics of frontal EEG alpha asymmetry in recurrently depressed patients. Biol Psychol. 2011;88:243–252. doi: 10.1016/j.biopsycho.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Amsen D, Spilianakis CG, Flavell RA. How are T(H)1 and T(H)2 effector cells made? Curr Opin Immunol. 2009;21:153–160. doi: 10.1016/j.coi.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]