Abstract
The objective of this study was to evaluate the effects of inserting peptide nucleic acid (PNA) sequences into the protein-binding surface of an immobilized four-way junction (4WJ). Here we compare the classic immobile DNA junction, J1, with two PNA containing hybrid junctions (4WJ-PNA1 and 4WJ-PNA3). The protein interactions of each 4WJ were evaluated using recombinant high mobility group proteins from rat (HMGB1b and HMGB1b/R26A) and human histone H1. In vitro studies show that both HMG and H1 proteins display high binding affinity toward 4WJ's. A 4WJ can access different conformations depending on ionic environment, most simply interpreted by a two-state equilibrium between: (i) an open-x state favored by absence of Mg2+, low salt, and protein binding, and (ii) a compact stacked-x state favored by Mg2+. 4WJ-PNA3, like J1, shifts readily from an open to stacked conformation in the presence of Mg+2, while 4WJ-PNA1 does not. Circular dichroism spectra indicate that HMGB1b recognizes each of the hybrid junctions. H1, however, displays a strong preference for J1 relative to the hybrids. More extensive binding analysis revealed that HMGB1b binds J1 and 4WJ-PNA3 with nearly identical affinity (KDs) and 4WJ-PNA1 with two-fold lower affinity. Thus both the sequence/location of the PNA sequence and the protein determine the structural and protein recognition properties of 4WJs.
Keywords: four-way junction, cruciform DNA, peptide nucleic acid (PNA), high mobility group protein B 1 (HMGB1), histone H1, circular dichroism
Introduction
Peptide nucleic acids (PNAs) are nucleic acid analogues with standard purine and pyrimidine bases linked via amide bonds.1,2 The polyamide backbone of PNA permits more stable Watson–Crick pairing between PNA-DNA and PNA-RNA oligonucleotides than the corresponding DNA-DNA and DNA-RNA duplex/triplex structures.1,3 The polyamide backbone also confers resistance to endogenous nucleases.4 These favorable properties have spurred investigations of PNAs as novel reagents for biotechnology and antisense/antigene applications.5–12 Despite the growing number of biomolecular applications for PNAs, there have been a relatively small number of studies focused on their protein binding characteristics.13–15
In this study individual PNA strands were inserted into the immobilized four-way junction (4WJ), J1, to evaluate the influence of PNA on structure and protein recognition. Four-way junctions, or Holliday junctions, are central intermediates in both homologous genetic recombination and site-specific recombination.16–18 X-ray and NMR analysis have confirmed the global conformations of 4WJs as: (i) an open-x conformer, with unstacked arms and (ii) a stacked-x conformer, in which the helical arms are coaxially stacked.19–24 The conformation of 4WJs is sensitive to the presence of divalent metal ions. In the absence of metal ions, an open-x or unstacked conformer is the major structure present.25 The helical arms of the open structure tend to be extended to reduce electrostatic repulsion of the DNA phosphate backbones. In the presence of divalent metal ions (≥100 µM Mg+2), the helical arms of each duplex undergo pairwise coaxial stacking.25–28 Divalent ions effectively screen the phosphate backbone of each strand, allowing the arms to move closer together in the stacked-x conformer. A schematic of stacked-x and open-x conformations is displayed in Figure 1(A,B).
Figure 1.

Schematic of four-way junction structures in the open-x (A) and stacked-x conformation B).
The hybrid junctions investigated here are based on the model J1. J1 possesses a nonsymmetric sequence pattern that blocks branch migration.29–31 Junctions were assembled by hybridizing DNA strands (J1) and different combinations of DNA and PNA strands (4WJ-PNA1 and 4WJ-PNA3). The DNA strands are shown in black and each PNA strand is shown in red in Figure 1. The focus of this investigation centers on the replacement of strands 101 and 103. Future investigations will focus on the replacement of strands 102 and 104 with PNAs. In this study, the length of each PNA strand was reduced relative to the DNA strands (12 vs. 16) in order to minimize synthetic problems with longer PNAs. The resulting DNA overhangs are not expected to prohibit the formation of stable 4WJs because the nonbonded regions are not located at the branch point of the junction. Earlier results showed that truncated junctions, or DNA necks, located at the branch point of three-way junctions significantly reduced the stability of the resulting complex but reduction in the length of the arms did not change junction topology until only three base pairs per arm remain.32 In the case of PNA strands the additional stability may make it possible to reduce this number even further, although we have not explored this yet.
The protein recognition properties of each hybrid were evaluated using two “architectural” DNA-binding proteins: a recombinant High Mobility Group protein (HMGB1b) and histone H1. These proteins do not bind DNA in a sequence specific manner, but recognize branched or otherwise deformed DNA structures. Electrophoretic mobility shift assays (EMSAs) reveal that individual HMGB1 box subunits (i.e., HMGB1b) and H1 bind 4WJs with high affinity.33–38 We focus on HMGB1b because this box subunit of HMGB1 has been shown to be responsible for important extracellular functional roles of the protein.39–41 A schematic of the 3D structure of HMGB1b and the winged-helix region of the histone H1 variant H5 is displayed in Figure 2.42,43 Each protein possesses a helix-turn-helix (HTH) DNA binding motif.
Figure 2.
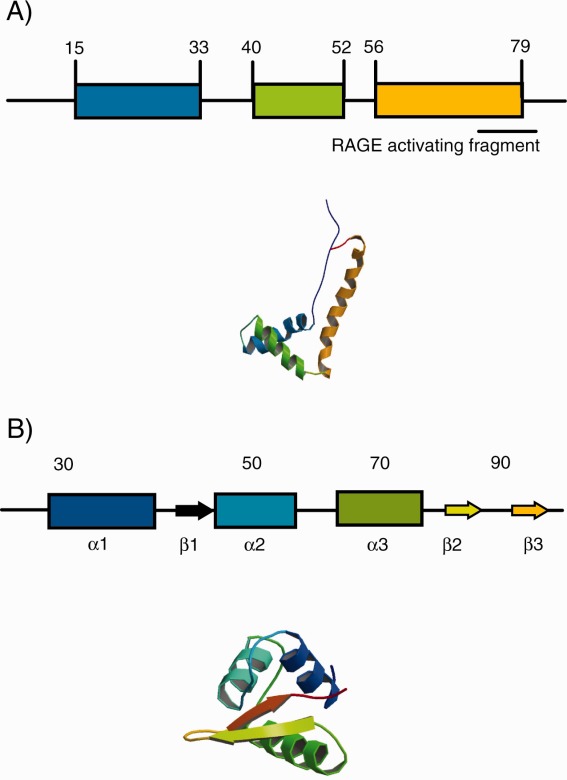
Schematic of HMGB1b and the Histone H1 variant H5. HMG1b and H5 structures correspond to PDB files 1HME and 1HST.
We show using circular dichroism (CD) spectroscopy that the secondary structure of hybrid 4WJs is strongly dependent upon the sequence and location of the PNA strand. In the case of 4WJ-PNA1, the global conformation of the junction does not undergo a clear shift between open-x and stacked-x conformations in the presence of Mg+2 in contrast to J1. 4WJ-PNA3, like J1, undergoes a clear shift between conformations in the presence of metals ions—this result is indicative of a more stable/discrete secondary structure. CD analysis of the structure of 4WJs in the presence of HMGB1b and H1 indicates that both proteins readily bind/recognize each junction to facilitate “unstacking” of the junctions. HMGB1b binds each junction in an analogous fashion. H1, however, displays stronger binding to J1 than to the hybrid 4WJs. Finally, EMSAs were used to define the binding properties of HMGB1b and 4WJ's in more detail. The EMSA data show that HMB1b binds 4WJ-PNA3 and J1 with nearly identical affinities (KDs), while HMGB1b binds 4WJ-PNA1 with significantly lower affinity (twofold). Based on these results, hybrid 4WJs that possess similar structural features to native 4WJs can retain protein-binding properties. However, the case of H1 shows that essential recognition surfaces are perturbed by the neutral PNA sequence.
It is now clear that the HMGB protein, HMGB1, performs dual roles as an architectural nuclear protein and proinflammatory cytokine.44–50 Homeostatic benefits of HMGB1 cytokine signaling range from dendritic cell (DC) maturation to wound healing. And several new studies link unintended HMGB1 signaling with pathogenesis in a number of diseases and conditions such as atherosclerosis, cancer, lupus, rheumatoid arthritis and sepsis.51–55 Consequently, HMGB1 has attracted considerable attention as a potential disease biomarker and therapeutic target. We hypothesize that a nucleic acid-based strategy could be used to elaborate inhibitors of undesired HMGB1 functionality. Recently, for example, Musumeci et al. synthesized a novel nucleic acid hybrid (a kinked DNA–PNA duplex) to reduce HMGB1 mediated cytokine signaling in mice.56 Our analysis aims to establish the potential of more structurally complex hybrids to serve as high affinity and selective HMGB1 ligands.
Results
Circular dichroism (CD) analysis of hybrid 4WJs
Each junction was scanned in the absence and presence of 1 mM MgCl2 in order to monitor the signature conformational shifts (i.e., open-x to stacked-x). The CD spectra of DNA 4WJs closely resemble that of B-form duplex DNA, with maxima/minima at ∼280 and 250 nm.57–61 As shown in Figure 3(A), the CD spectra for J1 display the expected bands in the absence (dashed line) and the presence of MgCl2 (solid line). The increase in amplitude of each peak in the presence of MgCl2 is the result of the screening effect by the metals ions that promotes the compaction of the phosphate backbones of each DNA. Compaction of the junction subsequently increased the overall level of base stacking within the junction lattice. Enhanced base stacking is reflected by an increase in the CD signals at 260 and 280 nm.61
Figure 3.
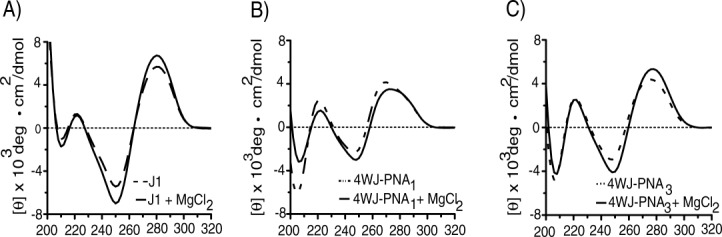
CD scans of 4WJs in the presence (solid line) and absence (dashed line) of 1 mM MgCl2. Panel (A) J1, (B) 4WJ-PNA1, and (C) 4WJ-PNA3.
The CD spectra of the hybrid 4WJs displayed interesting differences from those of J1. Changes in the CD spectrum of 4WJ-PNA1 for example oppose those in J1. As shown in Figure 3(B), the amplitude(s) of the PNA1 spectra decreased in the presence of MgCl2 (solid line). The signals for 4WJ-PNA1 were also blue-shifted relative to those in J1. 4WJ-PNA1 spectra possessed a minimum at 246 nm and a maximum at 270 (vs. 250 nm and 280 nm for J1). These data suggest that in the presence of MgCl2, the structure of 4WJ-PNA1 may convert between open and stacked conformers with the majority of the structures being in the open-x conformation. 4WJ-PNA3, on the other hand, possessed CD features that more closely resembled J1. The CD spectra of 4WJ-PNA3 display an increase in amplitude(s) in the presence of MgCl2 (solid line) indicating that the hybrid shifted readily from an open-x to stacked-x conformation [Fig. 3(C)]. The locations of the signal amplitudes of 4WJ-PNA3 were also closer to those for J1 as well (±5 nm). These data were based on at least three independent experiments.
CD analysis of 4WJs in the presence of DNA-binding proteins
CD analysis of each 4WJ was conducted in the presence of HMGB1b and H1 to monitor the structural changes upon protein recognition. Each assay was conducted in the presence of 1mM MgCl2 to more closely conform to physiological conditions. The binding trends for each 4WJ with both proteins were consistent, in that there was a reduction in signal at the CD maxima (θ 280 and 250 nm). The reduction in signal amplitude indicates that protein binding facilitates “unstacking” of the 4WJ. EMSA analyses and junction binding models suggest that unstacking of the stacked-x conformer (upon protein binding) facilitates a shift toward open conformers.62,63 Each panel in Figure 4 displays the spectra of a junction bound with HMGB1b and H1. Panel A) corresponds to J1, panel B) represents 4WJ-PNA1 and panel C) represents 4WJ-PNA3. HMGB1b reduced the CD signals at 280 nm for J1, 4WJ-PNA1 and 4WJ-PNA3 by 30, 17, and 28%, respectively. The corresponding 250 nm readings for HMGB1b and each junction were more similar. The CD signals for J1, 4WJ-PNA1 and 4WJ-PNA3 were 28, 25, and 27%, respectively. Histone H1 binding generated a relatively larger reduction in CD signal for each junction. The 280 nm readings for J1, 4WJ-PNA1 and 4WJ-PNA3 were reduced by 60, 25, and 31% in the presence of H1. The corresponding 250 nm readings for J1, 4WJ-PNA1 and 4WJ-PNA3 were 48, 25, and 31% in the presence of H1. Figure 5 displays a summary of the change(s) in ellipticity for each 4WJ in the presence of HMGB1b and H1. The ellipticity change at 280 nm is displayed in panel (A); the corresponding change at 250 nm is displayed in panel (B). HMGB1b signals are denoted with open icons; H1 signals are denoted with hatched icons. These data represent and average of at least three independent experiments. Scans were run of HMGB1b and H1 in the absence of 4WJs from 320 to 200 nm to ensure that the proteins did not influence ellipticity signals of each junction. The CD scans for both proteins are included in the Supporting Information.
Figure 4.
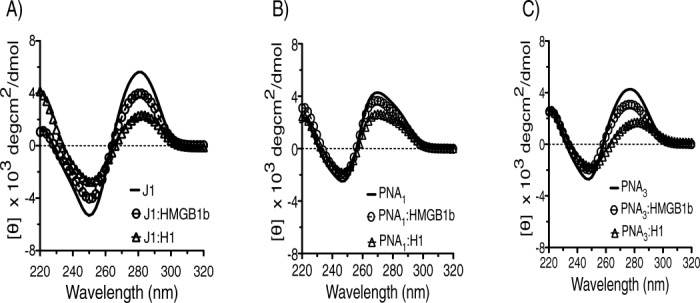
CD scans of 4WJS in the absence (solid line) and presence of DNA-binding proteins. HMGB1b:4WJ binding is represented by open circles. H1:4WJ binding represented by open triangles. Panel (A) J1, (B) 4WJ-PNA1, and (C) 4WJ-PNA3.
Figure 5.
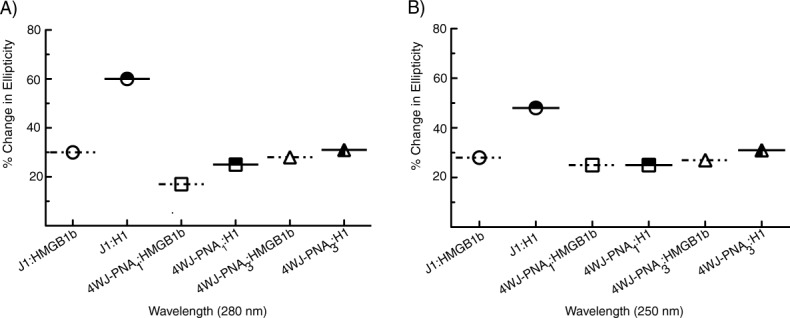
Maximum change in molar ellipticity of each 4WJ in the presence of HMGB1b (open icons) and histone H1 (hatched icons). Panel (A) 280 nm and (B) 250 nm. The data is based on the measurements displayed in Figure 4.
EMSA analysis of 4WJ:HMGB1b binding interactions
EMSAs were run to provide a more quantitative view of the binding affinity trends of HMGB1b toward each junction. The 4WJs were prepared using a fluorescein-labeled DNA strand mixed with excess DNA or PNA oligonucleotides as discussed in Materials and Methods. The fluorescent DNA strands are identified with green icons in Figure 1. EMSAs were run with each 4WJ and two proteins: HMGB1b and the HMGB1b alanine replacement mutant R26A (HMGB1b/R26A). Previous reports have shown that R26A has a significantly higher affinity for J1 than HMGB1b.34,64 Hence, we were interested in determining if the insertion of PNA strands alter/reduce the enhanced binding capacity R26A possesses toward immobilized 4WJs. R26A is also of interest because this mutant has been shown to bind duplex DNA under conditions where wild type HMGB1b does not bind double stranded DNA.34 Xin et al. used fluorescence binding and analytical ultracentrifugation analysis to show that HMGB1b binds J1 with a stoichiometry of four to one (4:1).35 The 4:1 complex has four protein monomers bound to each 4WJ, presumably in an open configuration. The complex typically migrates as a single band without intermediate binding species.
The EMSA data is displayed in Figure 6. Panels A, B and C represent J1, 4WJ-PNA1 and 4WJ-PNA3 analysis, respectively. In each gel, lane 1 contains the 4WJ (control without protein), lanes 2–11 represent the junction in the presence of increasing amounts of protein. The junction and protein are expressed in terms of molar ratio of protein to junction (P/J). As shown in Figure 6(A) both proteins bound J1 with high affinity. HMGB1b displayed a moderate affinity toward J1 at P/J molar ratios > 64:1 (lanes 8–9). Complete binding of HMGB1b to J1 was achieved upon increasing the amount of protein to great excess [P/J ratios of > 256:1, (lanes 10–11)]. Tight binding is indicated by the absence of unbound or “free” J1. As expected, R26A displayed a higher affinity toward J1 than HMGB1b. R26A formed initial complexes at lower protein ratios than HMGB1b; R26A formed complexes at molar ratios of 32:1 (lane 7) versus 64:1 (lanes 8) for HMGB1b. Complete binding of R26A to J1 was very similar to the wild type protein.
Figure 6.
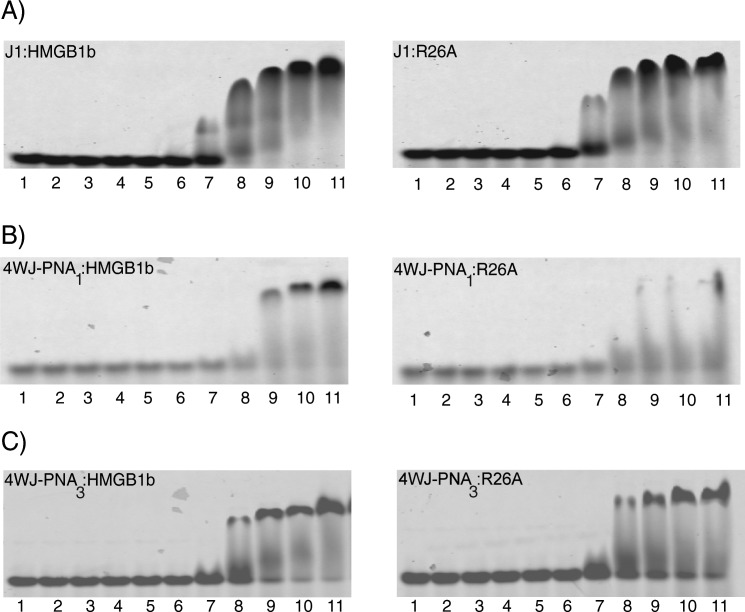
Electrophoretic mobility shift analysis of each 4WJ in the presence of HMGB1b and R26A. Panel (A) corresponds J1 analysis, (B) 4WJ-PNA1, and (C) 4WJ-PNA3 analysis. Lane 1, 4WJ (0.05 µM); lanes 2–11 represent each 4WJ incubated with each protein at protein/DNA ratios of 1:1, 2:1, 4:1, 8:1, 16:1, 32:1, 64:1, 128:1, 256:1, and 478:1.
HMGB1b and R26A bound each chimera with either significantly lower (4WJ-PNA1) or similar (4WJ-PNA3) affinity to J1. As shown in panel B, HMGB1b bound 4WJ-PNA1 to form an initial complex at a 128:1 ratio (lane 9). Complete binding of HMGB1b to 4WJ-PNA1 occurred at molar ratios of >256:1 (lanes 10–11). This trend confirms that HMGB1b possesses a lower affinity for 4WJ-PNA1 than for J1. Next, the binding characteristics of 4WJ-PNA1with R26A were investigated [panel B)]. Somewhat surprisingly, the binding profile for 4WJ-PNA1:R26A analysis could not be accurately characterized. R26A formed an initial complex with 4WJ-PNA1 at a protein molar ratio of 64:1 (lane 8). At higher protein ratios (lanes 9–11), there was apparent binding but no discrete complex could be detected. To address this issue, 4WJ-PNA1 was prepared using different protocols (i.e., changing the annealing temperature and stoichiometry of nucleic acids) but in each case R26A failed to form a stable 4:1 complex with this junction. The binding profile for each protein with 4WJ-PNA3 was very similar to that of J1. As shown in panel C), HMGB1b bound 4WJ-PNA3 to form strong but incomplete complexes at a P/J molar ratio of 64:1 (lane 8). Near complete binding of HMGB1b to 4WJ-PNA3 occurred at higher molar ratios (lanes 9–11). Although the bands for each complex were sharp, a slight amount of free 4WJ-PNA3 could be detected throughout the analyses. R26A displayed a similar binding profile toward 4WJ-PNA3. In this case, initial complexes formed at a molar ratio of 64:1 (lane 8); discrete complexes formed at higher molar ratios (lanes 9–11).
Summary of 4WJ:HMGB1b EMSAs
The EMSA data for HMGB1b and each junction were fit to a modified Langmuir binding isotherm to account for 4:1 binding stoichiometry. The formation of the complex is represented on the y-axis (fraction 4WJ bound) versus HMGB1b concentration (x axis), as shown in Figure 7. As expected, the apparent KD values of J1 and 4WJ-PNA3 were nearly identical (KD ∼ 6.0 µM). HMGB1b possessed the lowest relative affinity toward 4WJ-PNA1 (KD ∼ 16.0 µM). The 4WJ binding control protein, R26A, displayed the highest binding affinity toward J1 (KD ∼ 3.0 µM). Binding constants for R26A and the chimeric 4WJs could not be determined within reasonable error (R2 values < 0.900).
Figure 7.
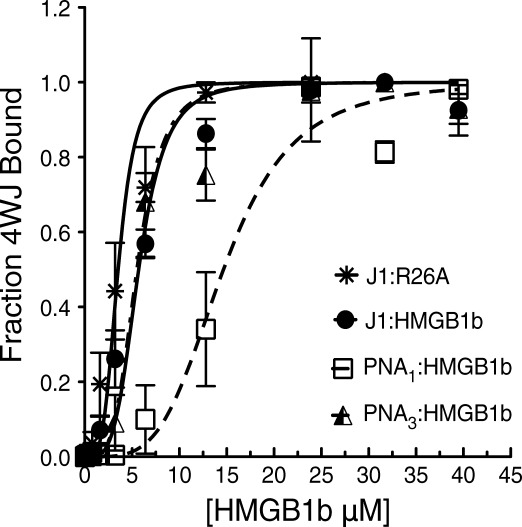
Nonlinear fitting of EMSAs. The binding profile of each 4WJ with HMGB1b is denoted with the icons: J1(closed circles), 4WJ-PNA1 (open squares) and 4WJ-PNA3 (hatched triangle). The binding profile of J1 with R26A is denoted with asterisks.
Discussion
The objective of this study was to evaluate the effects of inserting PNA oligonucleotide sequences into the protein-binding surface of an immobilized 4WJ. As expected, the CD amplitude for each hybrid 4WJ was diminished (and blue-shifted) versus J1 due to the presence of the achiral PNA strand. A similar CD profile is generated by chimeric PNA-DNA duplexes.56,65 With regard to specific spectroscopic features of each hybrid, 4WJ-PNA1 did not undergo a clear transition between an open-x and stacked-x conformation in the presence of magnesium ions. The divergent CD spectra for 4WJ-PNA1 may reflect the inability of this substrate to acquire a stacked conformation. A similar CD pattern was observed for mobile 4WJs in the presence of MgCl2.57
Current 4WJ and PNA-DNA structural data may shed more light on the structural differences between the hybrid 4WJs. To date, one 3D structure has been solved of a junction composed of four unique DNA sequences.23 The stacked-x conformer of this asymmetric junction possesses a rotation angle of 56.5° between each duplex. NMR analysis of a PNA-DNA duplex revealed that the hybrid duplex possessed a wider major groove and more narrow minor groove versus B-DNA duplexes.66 With regard to the chimeras, more specifically 4WJ-PNA1, the rotation angle may exceed 56.5°—an orientation that could facilitate unstacking and the elongation of the junction into more open structures as shown in Figure 8(A). Without high-resolution structural analysis, other scenarios are possible, including (i) PNA-DNA Hoogsteen-like contacts (between PNA and 104) and (ii) H-bonding between the amide backbone of PNA1 and a DNA backbone(s) within the junction lattice. On the other hand, 4WJ-PNA3 displayed a clear shift between conformers in the presence of magnesium, Figure 8(B). These results indicate that the PNA3 strand can be successfully incorporated into an immobilized junction without significantly perturbing the secondary structure of the junction.
Figure 8.
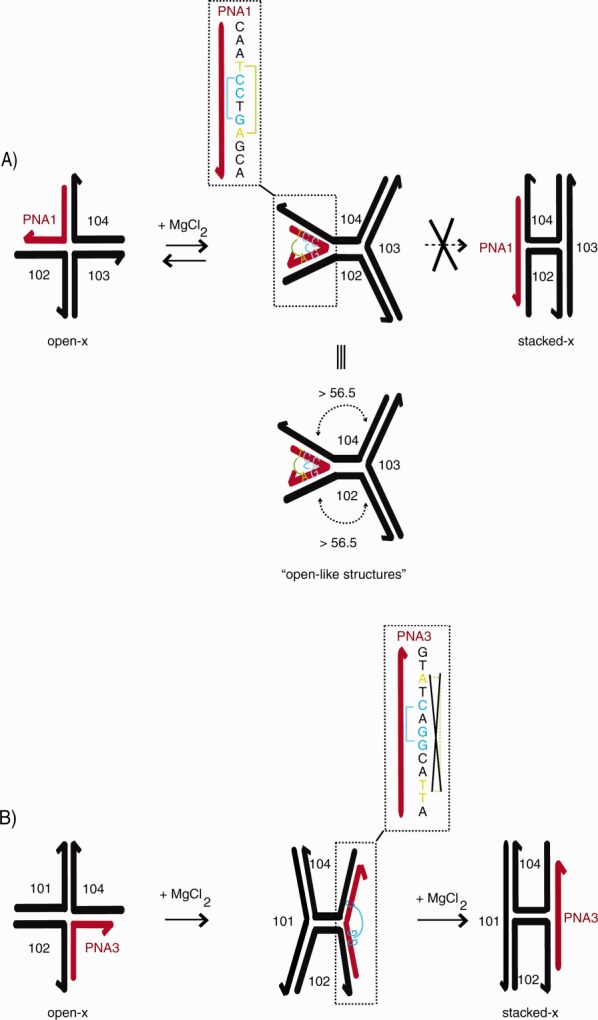
Schematic of proposed conformational changes of hybrid 4WJs. Panel (A) displays the formation of open and open-like structures of 4WJ-PNA1. Panel (B) displays the transition from open-x to stacked-x of 4WJ-PNA3.
CD analysis of each 4WJ in the presence of HMGB1b and H1 revealed that, despite its lack of structural transition, 4WJ-PNA1 is readily recognized. Both proteins bind and “unstack” 4WJ-PNA1 at nearly the same level as 4WJ-PNA3. This relationship is indicated by a change in ellipticity of ∼25% for both hybrids as shown in Figure 5. H1, however, displays a much stronger preference toward J1. H1 induces ellipticity reductions in J1 that range between 48 and 60%, as shown in Figure 5. The higher relative affinity of H1 for J1 is confirmed by EMSA data showing that H1 outcompetes HMGB1 for the native 4WJ, 4H.37 The difference in affinity may reflect increased electrostatic interactions between H1 and J1 (vs. the hybrid 4WJs). H1 possesses a positive charge ratio that is ∼5.5 times larger than the charge ratio of HMGB1b.64 Hence, H1 may be more strongly attracted to the additional phosphate bonds in J1 (64 phosphates vs. 48 in the hybrid 4WJs).
Finally, we used EMSA to determine if there were discernible binding affinity differences between hybrid 4WJs and HMGB1b. The resulting EMSA data clearly showed that HMGB1b binds J1 and 4WJ-PNA3 with nearly identical affinity (KD values of ∼6.0 µM). HMGB1b bound these substrates with nearly three-fold higher affinity than 4WJ-PNA1 (KD ∼ 16.0 µM). Moreover, the mutant protein HMGB1b/R26A was unable to form a stable (detectable) 4:1 complex with 4WJ-PNA1. In this case, it is clear that the presence of the PNA strand adversely effects protein binding. Thus insertion of a PNA strand may cause either significant (4WJ-PNA1) or minimal (4WJ-PNA3) effect(s) on the structure and protein recognition properties of an immobilized 4WJ. Recently, Musumeci et al. synthesized a novel nucleic acid chimera (a kinked DNA-PNA duplex) in order to functionally reduce HMGB1 mediated cytokine signaling in mice.56 This seminal report represents the first HMG-PNA binding analysis. However the protein-interacting sequence of their chimera consisted entirely of DNA with the PNA strands flanking. Thus the PNA strands did not influence HMGB1 binding-activity per se. A related study was reported by the Gombari laboratory—using PNA-DNA chimeras as “decoys” targeting transcriptions factors (i.e., NF-κB and Sp1).13,14,65 These studies aimed to use PNA-DNA decoys to attenuate gene expression. Their results indicate that the most effective decoys/chimeras contain PNAs in the flanking regions (ends) versus the protein-binding sequence of the duplex.13,14,65 The presence of the PNA within the duplex significantly alters the structure of DNA binding region and effectively eliminates protein recognition.44 In our case, the formation of a 4:1 complex (for both hybrids with HMGB1b) suggests that the PNA strand itself can be recognized by the protein-interacting shape sensitive surface. The affinity for HMG proteins for hybrid 4WJs is likely to reflect the fact that HMG box subunits bind the DNA minor groove with minimal sequence selectivity. Finally, our current studies clearly show that 4WJ hybrids such as 4WJ-PNA3 that closely resemble native junctions can be readily recognized. This investigation provides evidence that hybrid 4WJs have potential to serve as high affinity ligands against HMGB proteins.
Materials and Methods
Synthesis of PNA oligomers
Fmoc-protected PNA monomers A, C, G, and T were purchased from Panagene and used without further purification. Fmoc-Lys(Boc)-OH was obtained from Novabiochem and used without further purification. PNA oligomers were synthesized manually by standard Fmoc solid-phase peptide synthesis protocol using Rink amide resin (0.62 mmol g−1), HBTU as activating reagent and N,N-diisopropylethylamine as base. About 30 mg of resin was first swollen for 1 h with dichloromethane and downloaded overnight with Fmoc-Lys(Boc)-OH (5.7 mmol) to give a final loading of 0.19 mmol g−1. The unreacted amino sites were then blocked using Ac2O/DIEA/NMP (1:2:2). The Fmoc group of the Rink-amide resin and the growing peptide chain was removed by treatment with 20% of piperidine in DMF (2 × 8 min2). Upon completion of the last monomer coupling, PNA oligomers were cleaved from the resin using TFA/m-cresol (9:1) and precipitated with diethyl ether. The crude PNAs were purified by reversed phase HPLC with UV detection at 260 nm, using a C-18 column, and eluting with H2O + 0.1% TFA (eluent A) and CH3CN + 0.1% TFA (eluent B). Elution gradient: 0–100% CH3CN in 40 min and flow rate = 7 mL min−1. The resulting pure products were collected, lyophilized, and characterized by Maldi-TOF, which gave positive ions consistent with the final products.
PNA1: H-CAATCCTGAGCA-K-NH2 (MW = 3364.4); found m/z = 3387.21 (M+Na+)
PNA3: H-ATTCGGACTATG-K-NH2 (MW = 3410.4); found m/z = 3433.14 (M+Na+). MALDI-MS spectra and HPLC chromatographs that indicate high purity end PNA products are included in Supporting Information. PNA concentrations were determined spectrophotometrically using the following molar extinction coefficients at 260 nm: A = 13,700, C = 6600, G = 11,700, and T = 8600 M−1 cm−1.
Four-way junction oligonucleotides
The control 4WJ (J1) was composed of four DNA oligonucleotides purchased from Integrated DNA Technologies (IDT). The sequences of the four strands were: 101, 5′-CGCAATCCTGAGCACG-3′; 102, 5′-CGTGCTCACCGAATCGC-3′; 103, 5′-GCATTCGGACTATGGC-3′, and 104, 5′-GCCATAGTGGATTGCG. Fluorescein-labeled strands (101 and 103) were purified via HPLC (IDT). Nonlabeled strands were purified via denaturing polyacrylamide gels (IDT). All four-way junctions were formed by mixing a fluorescein-labeled strand (25 µM) with fivefold excess of the unlabeled strands (125 µM) in 50 mM Tris-HCl (pH 7.5) and 10 mM MgCl2. The oligonucleotides were incubated at 95°C for 2 min, followed by cooling to room temperature for 12–16 h. 4WJ-PNA1 was composed of PNA1: H-CAATCCTGAGCA-K-NH2 and oligonucleotides 102, 103, and 104. In this case, the labeled strand 103 was mixed with fivefold excess of unlabeled PNA1, 102, and 104. 4WJ-PNA3 was composed of PNA3: H-ATTCGGACTATG-K-NH2, 101, 102, and 104. In this case, the labeled strand 101 was mixed with 5-fold excess of unlabelled 102, PNA3, and 104.
To determine purity of each 4WJ, samples were loaded onto 15% Mini-PROTEAN TBE precast native polyacrylamide gels (BioRad) and run for 1–5 h (4°C). The gel running buffer was composed of 0.5 X TBE•MgCl2 buffer (45 mM Trisma, 45 mM boric acid, 1.0 mM EDTA, and 1 mM MgCl2), pH 8.0. The gels were subsequently scanned with a Typhoon 9400 Phosphorimager. Stable junctions were formed for J1 and 4WJ-PNA3; the major band (i.e., 4WJ band) migrated in the absence of significant upper and lower bands. The 4WJ-PNA1 displayed a detectable amount of a faster running species. However, the majority of the material (ca. <90%) were intact 4WJ (4WJ-PNA1). Images of junction control gels are provided in the Supporting Information.
Protein expression and purification
HMGB1b and HMGB1b/R26A from rat were expressed from pHB1-Escherichia coli Bl21(DE3)pLysS in accordance to the methods described by Chow et al.67 Each protein was purified via FPLC using an Econo-Pac CM cartridge (Bio-Rad). Crude proteins were loaded onto the CM cartridge in the presence of low salt buffer: A 50 mM Tris-HCl (pH 7.0), 50 mM NaCl and eluted with high salt buffer 50 mM Tris-HCl (pH 7.0), 500 mM NaCl using a linear gradient. Human histone H1 was purchased from New England Biolabs. The purity of each protein was monitored by resolution of each sample on 12% SDS-polyacrylamide (29:1 acrylamide:bisacrylamide) gels in Tris-Tricine buffer (150 V for 45 min) followed by staining for 12 h with Coomassie Brilliant Blue G-250.68 The concentration of purified proteins were determined by methods described previously by Pace et al.69 An image (SDS gel) displaying the purity of the proteins is included in the Supporting Information.
Circular dichroism analysis
Circular dichroism (CD) spectra were recorded using Jasco J-815 spectrometer. A 2.0 µM solution of each 4WJ was prepared in CD analysis buffer: 20 mM HEPES, 30 mM NH4Cl, 200 mM KCl, 2 mM DTT, and 10% glycerol. Magnesium chloride (1 mM) was added to the buffer in order to monitor the change in 4WJ conformation (i.e., shift from open-x to stacked-x). For 4WJ:protein binding analysis, 10 µM of each protein (HMGB1b and H1) was included along with 1 mM MgCl2. All spectra were measured in a 0.1 cm path-length quartz cuvette; spectra were recorded from 320 to 220 nm in 1.0 nm increments at 4°C. All CD data displayed in Figures 5 were based on at least three independent assays.
Electrophoretic mobility shift assays
To ensure maximum complex (4:1) formation, 0.05 µM of each 4WJ was incubated with HMGB1b and R26A at 4°C for 30 min in binding buffer. The binding buffer was composed of 20 mM Tris-HCl (pH 7.5), 10 mM NaCl, 1 mM MgCl and 10% (w/v) glycerol. The junction and protein are expressed in terms of molar ratio of protein to junction (P/J). P/J binding interactions were evaluated over a molar ratio range of 1:1 (0.05 µM:0.05 µM) to 480:1 (24.0 µM:0.05 µM). Lane 1 corresponds to 0.05 µM of 4WJ, lanes 2–11 represent each 4WJ incubated with each protein at protein/DNA ratios of 1:1, 2:1, 4:1, 8:1, 16:1, 32:1, 64:1, 128:1, 256:1, and 480:1. Each sample was loaded onto 15% Mini-PROTEAN TBE precast native polyacrylamide gels (BioRad). EMSAs were run using a buffer composed of 0.5 X TBE•MgCl2 buffer (45 mM Trisma, 45 mM boric acid, 1.0 mM EDTA, and 1 mM MgCl2), pH 8.0, at 4°C for 6–8 h. The gels were subsequently scanned with a Typhoon 9400 Phosphorimager.
Measurement of protein binding affinity
Fluorescently labeled 4WJs (0.05 µM) were incubated with a know range of protein concentrations (HMGB1b or R26A) in binding buffer. The binding data was analyzed by measuring the fraction of 4WJ bound (fb) (i.e., 4:1 complex formed) versus protein concentration and fitted by non-linear regression analysis to the equation:
where X is the individual protein concentration values that generate 4:1 complex formation (fb) and KA is the association constant. The equation represents a Langmuir binding model that has been modified to account for 4:1 binding stoichiometry (i.e., insertion of exponents). The dissociation constant (KD) is the reciprocal of KA. The KD values displayed in Figure 7 were based on at least three independent EMSAs (Fig. 6).
Supplementary material
Additional Supporting Information may be found in the online version of this article.
References
- 1.Egholm M, Buchardt O, Nielsen PE, Berg RH. Peptide nucleic acids (PNA). Oligonucleotide analogs with an achiral peptide backbone. J Am Chem Soc. 1992;114:1895–1897. [Google Scholar]
- 2.Weiler J, Gausepohl H, Hauser N, Jensen ON, Hoheisel JD. Hybridisation based DNA screening on peptide nucleic acid (PNA) oligomer arrays. Nucleic Acids Res. 1997;25:2792–2799. doi: 10.1093/nar/25.14.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egholm M, Buchardt O, Christensen L, Behrens C, Freier SM, Driver DA, Berg RH, Kim SK, Nordén B, Nielsen PE. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature. 1993;365:566–568. doi: 10.1038/365566a0. [DOI] [PubMed] [Google Scholar]
- 4.Demidov VV, Potaman VN, Frank-Kamenetskil MD, Egholm M, Buchard O, Sönnichsen SH, Nlelsen PE. Stability of peptide nucleic acids in human serum and cellular extracts. Biochem Pharmacol. 1994;48:1310–1313. doi: 10.1016/0006-2952(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 5.Lukeman PS, Mittal AC, Seeman NC. Two dimensional PNA/DNA arrays: estimating the helicity of unusual nucleic acid polymers. Chem Commun (Camb) 2004;7:1694–1695. doi: 10.1039/b401103a. [DOI] [PubMed] [Google Scholar]
- 6.Kleiner RE, Brudno Y, Birnbaum ME, Liu DR. DNA-templated polymerization of side-chain-functionalized peptide nucleic acid aldehydes. J Am Chem Soc. 2008;130:4646–4659. doi: 10.1021/ja0753997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitenstein M, Nielsen PE, Hölzel R, Bier FF. DNA-nanostructure-assembly by sequential spotting. J Nanobiotech. 2011;9:54. doi: 10.1186/1477-3155-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gasser G, Sosniak AM, Metzler-Nolte N. Metal-containing peptide nucleic acid conjugates. Dalton Trans. 2011;40:7061. doi: 10.1039/c0dt01706j. [DOI] [PubMed] [Google Scholar]
- 9.Hyrup B, Nielsen PE. Peptide nucleic acids (PNA): synthesis, properties and potential applications. Bioorg Med Chem. 1996;4:5–23. doi: 10.1016/0968-0896(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 10.Hanvey J, Peffer N, Bisi J, Thomson S, Cadilla R, Josey J, Ricca D, Hassman C, Bonham M, Au K. Antisense and antigene properties of peptide nucleic acids. Science. 1992;258:1481–1485. doi: 10.1126/science.1279811. [DOI] [PubMed] [Google Scholar]
- 11.Larsen HJ, Nielsen PE. Transcription-mediated binding of peptide nucleic acid (PNA) to double-stranded DNA: sequence-specific suicide transcription. Nucleic Acids Res. 1996;24:458–463. doi: 10.1093/nar/24.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen PE. Peptide nucleic acids (PNA) in chemical biology and drug discovery. Chem Biodivers. 2010;7:786–804. doi: 10.1002/cbdv.201000005. [DOI] [PubMed] [Google Scholar]
- 13.Mischiati C, Borgatti M, Bianchi N, Rutigliano C, Tomassetti M, Feriotto G, Gambari R. Interaction of the human NF-κB p52 transcription factor with DNA-PNA hybrids mimicking the NF-κB binding sites of the human immunodeficiency virus type 1 promoter. J Biol Chem. 1999;274:33114–33122. doi: 10.1074/jbc.274.46.33114. [DOI] [PubMed] [Google Scholar]
- 14.Borgatti M, Lampronti I, Romanelli A, Pedone C, Saviano M, Bianchi N, Mischiati C, Gambari R. Transcription factor decoy molecules based on a peptide nucleic acid (PNA)-DNA chimera mimicking Sp1 binding sites. J Biol Chem. 2003;278:7500–7509. doi: 10.1074/jbc.M206780200. [DOI] [PubMed] [Google Scholar]
- 15.Finotti A, Borgatti M, Bezzerri V, Nicolis E, Lampronti I, Dechecchi M, Mancini I, Cabrini G, Saviano M, Avitabile C, Romanelli A, Gambari R. Effects of decoy molecules targeting NF-kappaB transcription factors in Cystic fibrosis IB3-1 cells: recruitment of NF-kappaB to the IL-8 gene promoter and transcription of the IL-8 gene. Artif DNA PNA XNA. 2012;3:97–296. doi: 10.4161/adna.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwacha A, Kleckner N. Identification of double Holliday junctions as intermediates in meiotic recombination. Cell. 1995;83:783–791. doi: 10.1016/0092-8674(95)90191-4. [DOI] [PubMed] [Google Scholar]
- 17.Hoess R, Wierzbicki A, Abremski K. Isolation and characterization of intermediates in site-specific recombination. Proc Natl Acad Sci USA. 1987;84:6840–6844. doi: 10.1073/pnas.84.19.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitts PA, Nash HA. Homology-dependent interactions in phage λ site-specific recombination. Nature. 1987;329:346–348. doi: 10.1038/329346a0. [DOI] [PubMed] [Google Scholar]
- 19.Gopaul DN, Guo F, Van Duyne GD. Structure of the Holliday junction intermediate in Cre-loxP site-specific recombination. EMBO J. 1998;17:4175–4187. doi: 10.1093/emboj/17.14.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortiz-Lombardía M, González A, Eritja R, Aymamí J, Azorín F, Coll M. Crystal structure of a DNA Holliday junction. Nat Struct Biol. 1999;6:913–917. doi: 10.1038/13277. [DOI] [PubMed] [Google Scholar]
- 21.Ariyoshi M, Nishino T, Iwasaki H, Shinagawa H, Morikawa K. Crystal structure of the holliday junction DNA in complex with a single RuvA tetramer. Proc Natl Acad Sci USA. 2000;97:8257–8262. doi: 10.1073/pnas.140212997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hays FA, Watson J, Ho PS. Caution! DNA crossing: crystal structures of Holliday junctions. J Biol Chem. 2003;278:49663–49666. doi: 10.1074/jbc.R300033200. [DOI] [PubMed] [Google Scholar]
- 23.Khuu P, Ho PS. A rare nucleotide base tautomer in the structure of an asymmetric DNA junction. Biochemistry. 2009;48:7824–7832. doi: 10.1021/bi900829b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pikkemaat JA, van den Elst H, van Boom JH, Altona C. NMR studies and conformational analysis of a DNA four-way junction formed in a linear synthetic oligonucleotide. Biochemistry. 1994;33:14896–14907. doi: 10.1021/bi00253a029. [DOI] [PubMed] [Google Scholar]
- 25.Lilley DMJ. Structures of helical junctions in nucleic acids. Quart Rev Biophys. 2000;33:109–159. doi: 10.1017/s0033583500003590. [DOI] [PubMed] [Google Scholar]
- 26.Duckett DR, Murchie AI, Diekmann S, Kitzing von E, Kemper B, Lilley DM. The structure of the Holliday junction, and its resolution. Cell. 1988;55:79–89. doi: 10.1016/0092-8674(88)90011-6. [DOI] [PubMed] [Google Scholar]
- 27.Duckett DR, Murchie AI, Lilley DM. The role of metal ions in the conformation of the four-way DNA junction. EMBO J. 1990;9:583–590. doi: 10.1002/j.1460-2075.1990.tb08146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duckett DR, Murchie AI, Giraud-Panis MJ, Pöhler JR, Lilley DM. Structure of the four-way DNA junction and its interaction with proteins. Phil Trans R Soc Lond B. 1995;347:27–36. doi: 10.1098/rstb.1995.0005. [DOI] [PubMed] [Google Scholar]
- 29.Kallenbach NR, Ma RI, Wand AJ, Veeneman GH, van Boom JH, Seeman NC. Fourth rank immobile nucleic acid junctions. J Biomol Struct Dyn. 1983;1:159–168. doi: 10.1080/07391102.1983.10507432. [DOI] [PubMed] [Google Scholar]
- 30.Seeman NC, Kallenbach NR. Design of immobile nucleic acid junctions. Biophys J. 1983;44:201–209. doi: 10.1016/S0006-3495(83)84292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seeman NC, Maestre MF, Ma RI, Kallenbach NR. Physical characterization of a nucleic acid junction. Prog Clin Biol Res. 1985;172A:99–108. [PubMed] [Google Scholar]
- 32.Zhong M, Kallenbach NR. Conformation and thermodynamics of DNA “necks.” Models for three-arm branch formation in a duplex. J Mol Biol. 1993;230:766–778. doi: 10.1006/jmbi.1993.1199. [DOI] [PubMed] [Google Scholar]
- 33.Pohler JRG. HMG box proteins bind to four-way DNA junctions in their open conformation. EMBO J. 1998;17:817–826. doi: 10.1093/emboj/17.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taudte S, Xin H, Kallenbach NR. Alanine mutagenesis of high-mobility-group-protein-1 box B (HMG1-B) Biochem J. 2000;347:807–814. [PMC free article] [PubMed] [Google Scholar]
- 35.Xin H, Taudte S, Kallenbach NR, Limbach MP, Zitomer RS. DNA binding by single HMG box model proteins. Nucleic Acids Res. 2000;28:4044–4050. doi: 10.1093/nar/28.20.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varga-Weisz P, Zlatanova J, Leuba SH, Schroth GP, van Holde K. Binding of histones H1 and H5 and their globular domains to four-way junction DNA. Proc Natl Acad Sci USA. 1994;91:3525–3529. doi: 10.1073/pnas.91.9.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill DA, Reeves R. Competition between HMG-I (Y), HMG-1 and histone H1 on four-way junction DNA. Nucleic Acids Res. 1997;25:3523–3531. doi: 10.1093/nar/25.17.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill DA, Pedulla ML, Reeves R. Directional binding of HMG-I (Y) on four-way junction DNA and the molecular basis for competitive binding with HMG-1 and histone H1. Nucleic Acids Res. 1999;27:2135–2144. doi: 10.1093/nar/27.10.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Kokkola R, Tabibzadeh S, Yang R, Ochai M, Qiang X, Harris HE, Czura CJ, Wang H, Ulloa L, Wang H, Warren HS, Moldawer LL, Fink MP, Andersson U, Tracey KJ, Yang H. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med. 2003;9:37–45. [PMC free article] [PubMed] [Google Scholar]
- 40.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hreggvidsdottir HS, Ostberg T, Wähämaa H, Schierbeck H, Aveberger A-C, Klevenvall L, Palmblad K, Ottosson L, Andersson U, Harris HE. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86:655–662. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- 42.Weir HM, Kraulis PJ, Hill CS, Raine AR, Laue ED, Thomas JO. Structure of the HMG box motif in the B-domain of HMG1. EMBO J. 1993;12:1311–1319. doi: 10.1002/j.1460-2075.1993.tb05776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramakrishnan V, Finch JT, Graziano V, Lee PL, Sweet RM. Crystal structure of globular domain of histone H5 and its implications for nucleosome binding. Nature. 1993;362:219–223. doi: 10.1038/362219a0. [DOI] [PubMed] [Google Scholar]
- 44.Thomas J. HMG1 and 2: architectural DNA-binding proteins. Biochem Soc Trans. 2001;29:395. doi: 10.1042/bst0290395. [DOI] [PubMed] [Google Scholar]
- 45.Agresti A, Bianchi ME. HMGB proteins and gene expression. Curr Opin Genet Dev. 2003;13:170–178. doi: 10.1016/s0959-437x(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 46.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jianhua Li, Kokkola R, Tabibzadeh S, Yang R, Ochani M, Qiang X, Harris HE, Czura CJ, Wang H, Ulloa L, Wang H, Warree HS, Moldawer L, Fink MP, Andersson U, Tracey KJ, Yang H. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med. 2003;9:37. [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Wang H, Mason JM, Levine J, Yu M, Ulloa L, Czura CJ, Tracey KJ, Yang H. Recombinant HMGB1 with cytokine-stimulating activity. J Immun Meth. 2004;289:211–223. doi: 10.1016/j.jim.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 49.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 51.Porto A, Palumbo R, Pieroni M, Aprigliano G, Chiesa R, Sanvito F, Maseri A, Bianchi ME. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J. 2006;20:2565–2566. doi: 10.1096/fj.06-5867fje. [DOI] [PubMed] [Google Scholar]
- 52.Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, Lotze MT. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007;13:2836–2848. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 53.Andersson U, Rauvala H. Introduction: HMGB1 in inflammation and innate immunity. J Intern Med. 2011;270:296–300. doi: 10.1111/j.1365-2796.2011.02430.x. [DOI] [PubMed] [Google Scholar]
- 54.Andersson U, Erlandsson-Harris H. HMGB1 is a potent trigger of arthritis. J Intern Med. 2004;255:344–350. doi: 10.1111/j.1365-2796.2003.01303.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 56.Musumeci D, Bucci EM, Roviello GN, Sapio R, Valente M, Moccia M, Bianchi ME, Pedone C. DNA-based strategies for blocking HMGB1 cytokine activity: design, synthesis and preliminary in vitro/in vivo assays of DNA and DNA-like duplexes. Mol Biosyst. 2011;7:1742–1752. doi: 10.1039/c1mb05009e. [DOI] [PubMed] [Google Scholar]
- 57.Shida T, Iwasaki H, Shinagawa H, Kyogoku Y. Characterization and comparison of synthetic immobile and mobile Holliday junctions. J Biochem. 1996;119:653–658. doi: 10.1093/oxfordjournals.jbchem.a021292. [DOI] [PubMed] [Google Scholar]
- 58.Wu Q, Zhang W, Pwee K-H, Kumar PP. Rice HMGB1 protein recognizes DNA structures and bends DNA efficiently. Arch Biochem Biophys. 2003;411:105–111. doi: 10.1016/s0003-9861(02)00721-x. [DOI] [PubMed] [Google Scholar]
- 59.Zhang W, Wu Q, Pwee K-H, Jois SDS, Kini RM. Characterization of the interaction of wheat HMGa with linear and four-way junction DNAs. Biochemistry. 2003;42:6596–6607. doi: 10.1021/bi034280h. [DOI] [PubMed] [Google Scholar]
- 60.Kypr J, Kejnovská I, Renčiuk D, Vorlíčková M. Circular dichroism and conformational polymorphism of DNA. Nucleic Acids Res. 2009;37:1713–1725. doi: 10.1093/nar/gkp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Howell LA, Waller ZAE, Bowater R, O'Connell M, Searcey M. A small molecule that induces assembly of a four way DNA junction at low temperature. Chem Commun. 2011;47:8262–8264. doi: 10.1039/c1cc12922h. [DOI] [PubMed] [Google Scholar]
- 62.Fogg JM, Kvaratskhelia M, White MF, Lilley DMJ. Distortion of DNA junctions imposed by the binding of resolving enzymes: a fluorescence study. J Mol Biol. 2001;313:751–764. doi: 10.1006/jmbi.2001.5081. [DOI] [PubMed] [Google Scholar]
- 63.Khuu PA, Voth AR, Hays FA, Ho PS. The stacked-X DNA Holliday junction and protein recognition. J Mol Recognit. 2006;19:234–242. doi: 10.1002/jmr.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bell AJ, Chauhan S, Woodson SA, Kallenbach NR. Interactions of recombinant HMGB proteins with branched RNA substrates. Biochem Biophys Res Commun. 2008;377:262–267. doi: 10.1016/j.bbrc.2008.09.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romanelli A, Pedone C, Saviano M, Bianchi N, Borgatti M, Mischiati C, Gambari R. Molecular interactions with nuclear factor κB (NF-κB) transcription factors of a PNA–DNA chimera mimicking NF-κB binding sites. Eur J Biochem. 2001;268:6066–6075. doi: 10.1046/j.0014-2956.2001.02549.x. [DOI] [PubMed] [Google Scholar]
- 66.Eriksson M, Nielsen PE. Solution structure of a peptide nucleic acid-DNA duplex. Nat Struct Biol. 1996;3:410–413. doi: 10.1038/nsb0596-410. [DOI] [PubMed] [Google Scholar]
- 67.Chow CS, Barnes CM, Lippard SJ. A single HMG domain in high-mobility group 1 protein binds to DNAs as small as 20 base pairs containing the major cisplatin adduct. Biochemistry. 1995;34:2956–2964. doi: 10.1021/bi00009a027. [DOI] [PubMed] [Google Scholar]
- 68.Schägger H, Jagow von G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 69.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


