Abstract
Lactobacillus curvatus LTH 1174, a strain originating in fermented sausage, produces the antilisterial bacteriocin curvacin A. Its biokinetics of cell growth and bacteriocin production as a function of various concentrations of salt (sodium chloride) were investigated in vitro during laboratory fermentations using modified MRS medium. A model was set up to describe the effects of different NaCl concentrations on microbial behavior. Both cell growth and bacteriocin activity were affected by changes in the salt concentration. Sodium chloride clearly slowed down the growth of L. curvatus LTH 1174, but more importantly, it had a detrimental effect on specific curvacin A production (kB) and hence on overall bacteriocin activity. Even a low salt concentration (2%, wt/vol) decreased bacteriocin production, while growth was unaffected at this concentration. The inhibitory effect of NaCl was mainly due to its role as an aw-lowering agent. Further, it was clear that salt interfered with bacteriocin induction. Additionally, when 6% (wt/vol) sodium chloride was added, the minimum biomass concentration necessary to start the production of curvacin A (XB) was 0.90 g (cell dry mass) per liter. Addition of the cell-free culture supernatant or a protein solution as a source of induction factor resulted in a decrease in XB, an increase in kB, and hence an increase in the maximum attainable bacteriocin activity.
Fermentation of raw meat normally results in stable and safe end products with a long shelf life (40). This outcome is due mainly to the low end pH caused by the production of large amounts of lactic acid by the lactic acid bacteria (LAB) naturally present or intentionally added as starter culture. Other characteristics of raw fermented sausages also help prevent the outgrowth of spoilage and pathogenic bacteria; of these, the two most important are fast acidification during fermentation (usually achieved by addition of fermentable carbohydrates) and a steady decrease in water activity (aw) during fermentation and ripening of the sausage. For aw lowering, it is important that a sufficient amount of salt (2.4 to 3.0% [wt/wt] NaCl) be added to the meat batter (40, 45). Salt is used not only as a preventive measure against food spoilage and pathogenic bacteria but also as a flavor compound. The curing agent nitrite is added to the sausage batter to create the typical cured flavor, to produce color, to prevent lipid rancidity, and to inhibit the growth of salmonellae and clostridia (14, 40). These established methods, however, are not very selective and thus not only affect the undesirable microorganisms but also affect the functionality of the added meat starter cultures (42).
At present, consumers are requesting safe, natural food products as well as mild and light foods with a low acid, sugar, salt, or fat content (27). This trend has stimulated research on the additives used in meat processing, such as NaCl and curing agents. For instance, to decrease the high Na+ content of meat, attempts are being made to replace at least part of the NaCl with other salts such as potassium chloride or calcium chloride, without affecting the fermentation process or the quality of the end product (26). The request for more-natural products has also provided an incentive to search for safe, food-grade preservatives of biological origin (16, 60). Furthermore, some food-borne pathogenic bacteria, such as Listeria monocytogenes, can survive in fermented sausage despite the various hurdles they encounter, and they may pose a health risk to consumers (21, 35, 40). The spread of antibiotic resistance genes among listeriae and other pathogens is raising new concerns (61). Moreover, countries such as the United States have adopted a zero-tolerance policy for Listeria contamination of processed meat products (59). This policy has serious consequences, in particular for European exporting companies, since the European Union applies a tolerance of less than 100 cells of L. monocytogenes in fermented meat products. Hence, there is a need for novel and/or extra preventives in meat processing.
Bacteriocins produced by LAB are antibacterial peptides or proteins active against other gram-positive, mainly closely related bacterial species, including some undesirable spoilage bacteria and food-borne pathogens (17). The only legally approved bacteriocin in many countries is nisin, approved for use as a preservative in a limited number of food products (15). Unfortunately, this bacteriocin is not very efficient in a meat environment, because of its low solubility, uneven distribution, and lack of stability (60). An alternative and interesting approach for the use of bacteriocins in fermented sausages is the use of bacteriocinogenic LAB starter cultures adapted to the specific meat environment (43). Several bacteriocins produced by strains isolated from fermented sausages are highly active against listeriae (13, 29, 33, 48, 55, 66). Moreover, several investigators have already shown the possibility of using LAB strains that produce listericidal bacteriocins to reduce Listeria counts in fermented meat products (6, 7, 10, 11, 22, 33, 34, 38, 58, 66). This is one of the reasons why Listeria contamination of fermented sausage is very rare, although Listeria contamination of the raw meat materials does occur (56). Although the meat itself may be contaminated, it is also possible that the contamination occurs in the meat plant. Hence, one of the most important objectives remains the prevention of Listeria contamination through good manufacturing practice, thereby decreasing the hygiene risk. However, the presence of this organism in the food-processing environment seems inevitable. Although the risk of contamination can be reduced, it seems unlikely that it can be eliminated. In addition, in situ production of bacteriocins enhances the competitiveness of the starter organisms toward fortuitous flora and hence ensures a stable and safe end product (31, 66). Therefore, it is of the utmost importance to know how the growth and bacteriocin production of the functional starter cultures used are affected by various sausage ingredients such as salt.
Lactobacillus curvatus LTH 1174, a strain originating in fermented sausage, is the producer of the listericidal bacteriocin curvacin A and has been shown to be a successful starter strain for European sausage fermentations (66). Previously, it was also shown that this strain displays maximum bacteriocin activity levels under the temperature and pH conditions used for these fermentations (47). However, the effects of different sausage ingredients on the behavior of this strain have yet to be determined so as to estimate the bioavailability of bacteriocin in the meat. The present study was undertaken to examine and model the inhibitory effects of different NaCl concentrations in a modified de Man-Rogosa-Sharpe (mMRS) medium on the growth and bacteriocin production of the meat starter culture L. curvatus LTH 1174.
MATERIALS AND METHODS
Microorganisms and media.
L. curvatus LTH 1174 was used as the producer of the antilisterial bacteriocin curvacin A (63). A curvacin A-sensitive indicator organism, Listeria innocua LMG 13568, was used to determine bacteriocin activity levels (41). Strains were stored at −80°C in MRS medium and brain heart infusion medium (both from Oxoid, Basingstoke, United Kingdom), respectively, both containing 25% (vol/vol) glycerol as a cryoprotectant. To produce fresh cultures, the strains were propagated twice at 30°C for 12 h before experimental use. Solid medium for enumeration of cells was prepared by adding 1.5% (wt/vol) agar (Oxoid) to the broth. The overlays used for estimation of bacteriocin titers were prepared with 0.7% (wt/vol) agar.
mMRS was used as the fermentation medium for L. curvatus LTH 1174. The modification consisted in a doubling of the concentration of the complex nutrient source (bacteriological peptone [Oxoid], Lab Lemco [Oxoid], and yeast extract [VWR International, Darmstadt, Germany]). This was done to avoid a severe growth limitation of L. curvatus strain LTH 1174 due to nutrient depletion in standard MRS medium (44). In addition, calculations of the amino nitrogen content of this medium (9) indicated that this composition more closely simulates the actual sausage environment (14). Furthermore, maximum curvacin A activity was found when the complex nutrient source was doubled, while specific bacteriocin production and the specific apparent bacteriocin inactivation rate were comparable to those with standard MRS (J. Verluyten, W. Messens, F. Leroy, V. Schrijvers, and L. De Vuyst, unpublished data). All media and solutions were sterilized at 121°C for 20 min.
Fermentation experiments.
A series of in vitro fermentations were performed using mMRS supplemented with different concentrations of salt (0, 2, 4, and 6% [wt/vol] NaCl) to investigate their effects on both the growth and bacteriocin production of L. curvatus LTH 1174. The fermentations without added sodium chloride and those with 4% (wt/vol) NaCl added were performed in triplicate to show the reproducibility of the experiments. Standard deviations were calculated both on the experimental values and on the biokinetic parameters derived from the primary model. To determine whether the effect of the added NaCl was due solely to a reduction in aw, an additional fermentation was carried out in the presence of 9.9% (wt/vol) glycerol (sterilized separately) in the absence of added salt. Based on extrapolation of data from previous studies (12, 46), addition of 9.9% (wt/vol) glycerol to the basal growth medium should result in the same decrease in aw as addition of approximately 4% (wt/vol) NaCl (42).
Fermentations were carried out in a 15-liter laboratory fermentor (BiostatC; B. Braun Biotech International, Melsungen, Germany) with a working volume of 10 liters of mMRS as previously described (41). Briefly, temperature (25°C) and pH (held constant at 5.5) control was performed online (Micro-MFCS for Windows NT; B. Braun Biotech International). For preparation of the inoculum, 10 ml of MRS medium was inoculated with 0.5 ml of a freshly prepared L. curvatus LTH 1174 culture and then incubated at 30°C for 12 h. Five milliliters of this preculture was added to 100 ml of MRS medium. After 13 h of growth at 30°C, this culture was used to inoculate the fermentor.
To examine the effect of added induction factor (IF), two additional fermentations were performed using mMRS containing 6% (wt/vol) NaCl at a controlled temperature of 25°C and a constant pH of 5.5. To obtain a source of IF, a fermentation was performed in MRS at a controlled temperature of 22°C and a constant pH of 5.4, a set of conditions optimal for the production of curvacin A (47). After 24 h of growth, cells were removed by centrifugation (at 20,500 × g and 4°C for 30 min), and 50 ml of the cell-free culture supernatant (CFS; curvacin A activity, 1,600 arbitrary units [AU] ml−1) was used as a source of IF for the first fermentation containing 10 liters of mMRS. This fermentation using the CFS was also performed in triplicate, and standard deviations were calculated. As an alternative source of the IF, a precipitate of curvacin A and possibly its IF was prepared as follows. The pH of the CFS was adjusted to 6.5, 300 g of ammonium sulfate per liter (48% saturation) was added, and the suspension was stirred at 4°C for 12 h. After centrifugation (at 20,500 × g and 4°C for 30 min), the pellicle and pellet were harvested and dissolved in 50 mM sodium phosphate buffer at pH 6.5 (5 ml per liter of CFS). The second fermentation was carried out by adding 1.5 ml of this protein solution (ammonium sulfate precipitate; curvacin A activity, 153,600 AU ml−1) to the fermentor (10 liters of mMRS).
Assays.
At regular time intervals, samples were withdrawn aseptically from the fermentor to determine cell counts (CFU), biomass (cell dry mass [CDM]), levels of soluble bacteriocin activity in CFS, lactic acid concentrations, and residual glucose concentrations. Briefly, CDM was determined gravimetrically after membrane filtration (41); the amount of lactic acid produced and the residual glucose concentration were determined by high-performance liquid chromatography (18); and the level of bacteriocin activity was determined by a modified critical dilution method using L. innocua LMG 13568 as the indicator strain (18). The standard deviations for the CDM, glucose, and lactic acid measurements were 0.11, 0.04, and 0.02 g liter−1, respectively.
Primary and secondary modeling.
Primary modeling of cell growth, glucose consumption, lactic acid production, and bacteriocin production and inactivation was performed both to fit the data and to estimate the values of the biokinetic parameters representative of growth and curvacin A production. Abbreviations for biokinetic parameters are explained, and the equations used are given, in Table 1. These equations are the same as those reported by Messens et al. (47), except that bacteriocin production was made dependent on XB, defined as the minimum biomass concentration required for the onset of bacteriocin production due to induction (19, 44). For fermentations performed in triplicate, standard deviations were calculated on all biokinetic parameters estimated via primary modeling.
TABLE 1.
Equations used for primary model development
| Model | Equationa |
|---|---|
| Cell growth | dX/dt = {μmax [1 − (X/Xmax)]n − α}X when t > λ |
| Sugar consumption (glucose) | dS/dt = − [(1/YX/S)(dX/dt)] − mSX |
| Lactic acid production | dL/dt = − (YL/S)(dS/dt) |
| Bacteriocin production | dB/dt = [(kB)(dX/dt)] − [(kinact)(XB)] when X > XB |
Abbreviations: X, biomass concentration (in grams [CDM] per liter); t, time (in hours); λ, duration of the lag phase (in hours); μmax, maximum specific growth rate (in h−1); Xmax, maximum attainable biomass concentration (in grams [CDM] per liter); n, inhibition exponent; α, specific death rate (in h−1); S, residual glucose concentration (in grams of glucose per liter); YX/S, cell yield coefficient (in grams [CDM] per gram of glucose); ms, maintenance coefficient (in grams of glucose per gram of CDM per hour); L, lactic acid production (in grams of lactic acid per liter); YL/S, yield coefficient for the conversion of glucose into lactic acid (in grams of lactic acid per gram of glucose); B, bacteriocin activity in the CFS (in AU per liter); kB, specific bacteriocin production (in AU per gram of CDM); kinact, apparent rate of bacteriocin inactivation (in liters per gram of CDM per hour); XB, minimum biomass concentration for the onset of bacteriocin production (in grams [CDM] per liter).
The equations were integrated by the Euler integration technique in Microsoft Excel 97 (version 8.0a). All parameters needed for the modeling were estimated by manual adjustment until the best fit was obtained. This procedure has been followed previously and led to reliable results (41, 47). It has the advantage of avoiding the unrealistic fitting solutions that are sometimes generated by computational solving.
By use of secondary modeling, all biokinetic parameters (μmax, Xmax, n, YX/S, ms, YL/S, kB, and kinact) derived from the primary model were expressed as functions of the salt concentration. For this purpose, empirical models were used. Standard deviations were calculated where appropriate.
RESULTS
Fermentation profiles.
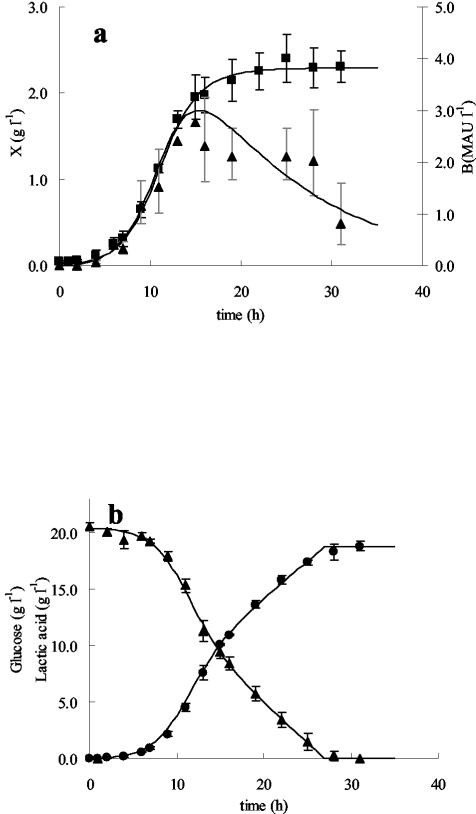
Figure 1 represents both the mean experimental and the mean modeled values of biomass concentrations and bacteriocin activities in the supernatant (Fig. 1a) and of residual glucose concentrations and lactic acid production levels (Fig. 1b) for a control fermentation carried out in mMRS at a controlled temperature of 25°C and a constant pH of 5.5 without added NaCl. The biokinetic parameters derived from the primary model are listed in Table 2. The profiles of the fermentations carried out in the presence of NaCl were similar. Empirical models were set up to show the relationship between the biokinetic parameters derived from the primary model and the salt concentration (Table 2 and Fig. 2). Details are described below.
FIG. 1.
Modeling of biokinetic parameters for L. curvatus LTH 1174 in mMRS at a controlled temperature of 25°C and a constant pH of 5.5. (a) Biomass (in grams [CDM] per liter) (▪) and bacteriocin production (in MAU per liter) (▴). (b) Residual glucose concentration (▴) and lactic acid formation (•) (both in grams per liter). Symbols represent experimental values; lines are drawn according to the model. Experimental values are averages from three fermentations. Error bars, standard deviations.
TABLE 2.
Values of biokinetic parameters and empirical-model equations showing the relationship between biokinetic parameters and sodium chloride concentrationsa
| Biokinetic parameters(primary model)b | Empirical-model equations (secondary model) |
|---|---|
| μmax = 0.52 ± 0.02 | μmax = 0.51 − 0.01(% NaCl)2 |
| Xmax = 2.30 ± 0.17 | Xmax = 2.39 ± 0.21 |
| n = 1.17 ± 0.06 | n = 1.20 − 0.06(% NaCl) |
| YX/S = 0.24 ± 0.01 | YX/S = 0.23 ± 0.01 |
| mS = 0.30 ± 0.03 | mS = 0.32 ± 0.03 |
| kB = 1.90 ± 0.10 | kB = 1.84 − 0.24(% NaCl) |
| kinact = 0.035 ± 0.005 | kinact = 0.041 ± 0.004 |
Calculated values that are negative are set to zero. Standard deviations are reported where appropriate.
Values for the fermentation carried out at a controlled temperature of 25°C and a constant pH of 5.5 without added sodium chloride. Abbreviations and dimensions are explained in the footnote to Table 1.
FIG. 2.
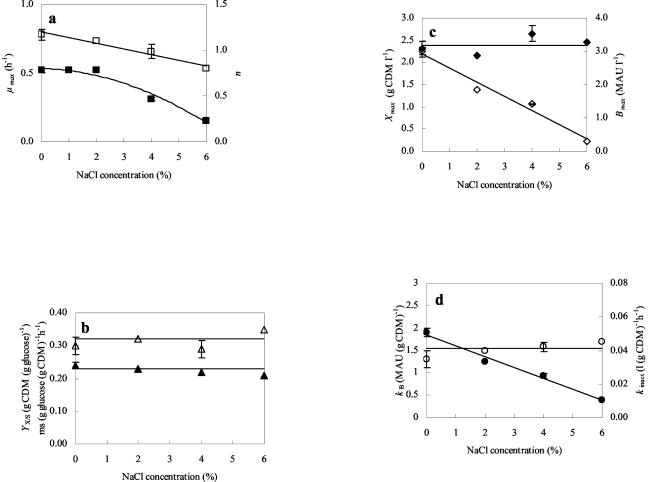
Secondary modeling of the biokinetic parameters derived from the primary model. Shown are the effects of different NaCl concentrations (expressed in percentages [wt/vol]) added to mMRS on the different parameters for L. curvatus LTH 1174 growing at a controlled temperature of 25°C and a constant pH of 5.5. (a) Bacterial growth (μmax) (▪) per hour and inhibition exponent (n) (□). (b) Sugar metabolism (YX/S) (▴), expressed in grams of CDM per gram of glucose, and mS (▵), expressed in grams of glucose per gram of CDM per hour. (c) Maximum biomass production (Xmax) (⧫), expressed in grams of CDM per liter, and maximum bacteriocin activity (Bmax) (◊), expressed in MAU per liter. (d) Bacteriocin production (kB) (•), expressed in MAU per gram of CDM, and apparent rate of bacteriocin inactivation (kinact) (○), expressed in liters per gram of CDM per hour. For 0 and 4% added NaCl, biokinetic parameters are means from three fermentations; error bars, standard deviations. Lines are drawn according to the model.
Effects of sodium chloride.
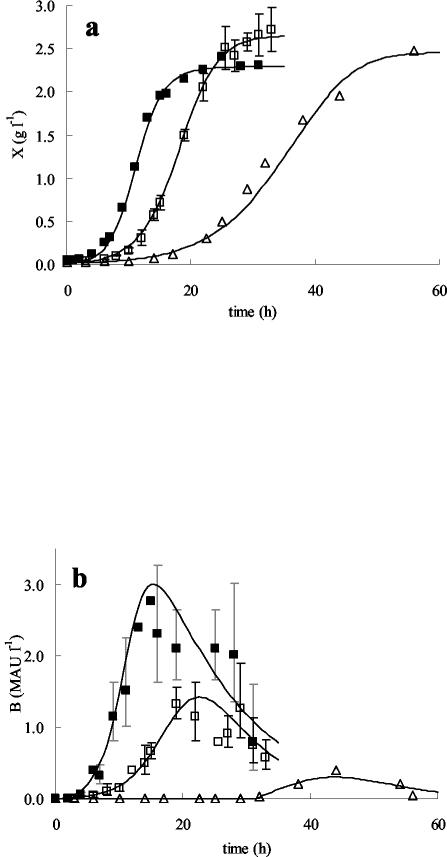
Addition of 2% (wt/vol) NaCl had no effect on μmax, while at higher salt concentrations, μmax decreased linearly with increasing salt concentrations (Fig. 2a). Addition of sodium chloride did not affect Xmax much. A slightly higher Xmax was found at high salt concentrations (4 and 6% [wt/vol] NaCl) (Fig. 3a), a finding that can be ascribed to the formation of cell aggregates as observed microscopically, probably caused by stress due to these high salt concentrations. Neither the cell yield coefficient (YX/S) nor the maintenance coefficient (ms) was changed by the addition of salt (Table 2 and Fig. 2b).
FIG. 3.
Effects of sodium chloride (concentrations expressed in percentages [wt/vol]) added to mMRS on the production of biomass (expressed in grams [CDM] per liter) (a) and bacteriocin production (expressed in MAU per liter) (b) as a function of time. Symbols: ▪, 0% NaCl; □, 4% NaCl; ▵, 6% NaCl. Symbols represent experimental values; lines are drawn according to the model. For 0 and 4% added NaCl, the experimental values and the model derived are means from three fermentations; error bars, standard deviations.
Modeling of the bacteriocin activity in the CFS for the various salt concentrations tested is represented in Fig. 3b. Note that for the fermentation without added sodium chloride, the error bars on the bacteriocin data are larger due to the increased uncertainty at high bacteriocin activities, as a result of the twofold dilution assay used. Bacteriocin activity was strongly affected by added salt. A severe drop in Bmax was observed even at a concentration of 2% (wt/vol) NaCl, while growth remained unaffected (Fig. 2c). At higher salt concentrations, a further decrease in bacteriocin activity was seen, and with 6% (wt/vol) added NaCl, Bmax reached only 12% of the activity observed without added salt (Fig. 2c and 3b). This was due to a linear decrease in kB with increasing NaCl concentrations (Fig. 2d). In addition, the minimum biomass concentration required for curvacin A production to start (XB) increased when 6% (wt/vol) NaCl was present in the medium. Up to an NaCl concentration of 4% (wt/vol), XB was nil, indicating that curvacin A production started from the beginning of fermentation. However, when 6% (wt/vol) NaCl was present, bacteriocin production started only at a biomass concentration of 0.90 g (CDM) liter−1 (Fig. 3). Apparently, a critical NaCl concentration exists above which NaCl clearly interferes with the induction of bacteriocin production. Probably the IF that was added to the fermentor with the inoculum was sufficient to initiate bacteriocin production from the beginning up to an NaCl concentration of 4% (wt/vol).
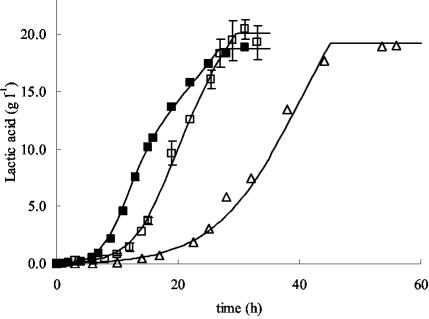
Glucose was consumed more slowly at higher concentrations of NaCl, resulting in retarded production of lactic acid (Fig. 4).
FIG. 4.
Effects of different concentrations of sodium chloride (expressed in percentages [wt/vol]) added to mMRS on lactic acid production. Symbols: ▪, 0% NaCl; □, 4% NaCl; ▵, 6% NaCl. Symbols represent experimental values; lines are drawn according to the model. For 0 and 4% added NaCl, the experimental values and the model derived are means from three fermentations; error bars (shown only for 4% added NaCl), standard deviations.
Effect of aw.
To determine whether a reduction in aw was solely responsible for the inhibitory effect of NaCl on bacterial growth and curvacin A production with L. curvatus LTH 1174 or if there were other (ionic) effects responsible, one additional fermentation was performed using glycerol as an aw-lowering agent.
When the different biokinetic parameters used for the primary model were compared, the values obtained for fermentation in the presence of glycerol (9.9%, wt/vol) were comparable to those obtained for fermentation with 4% (wt/vol) added NaCl. Only the value of Xmax (2.06 versus 2.65 ± 0.18 g [CDM] liter−1) was somewhat lower. The difference in Xmax may be ascribed to the formation of cell aggregates, resulting in a nonhomogenous fermentation liquor.
Effect of added IF.
To determine the effect of added IF on curvacin A production, fermentations in the presence of 6% (wt/vol) NaCl were performed by adding either CFS or protein solution (ASP) as a source of IF. These fermentations were compared with a fermentation in which no CFS or ASP was added. Bacteriocin activity was determined immediately after inoculation and addition of CFS or ASP. No inhibition of the indicator strain was observed, indicating that the amount of bacteriocin that was added in this way did not contribute to the observed inhibition during these experiments. Growth was comparable in the three cases, although less formation of aggregates was observed during the fermentations with added IF. This resulted in an Xmax of 2.02 ± 0.08 or 2.10 g (CDM) liter−1 with added CFS or ASP, respectively.
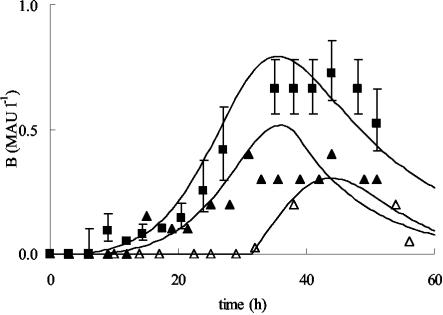
Modeling of bacteriocin activity for fermentations with and without the addition of IF (CFS or ASP) is represented in Fig. 5. Addition of IF resulted in an increase in Bmax from 0.30 mega arbitrary unit (MAU) liter−1 (no IF added) to 0.79 ± 0.10 or 0.52 MAU liter−1 when CFS or ASP was added, respectively. These bacteriocin activities were the result of specific bacteriocin production levels of 0.40 MAU (g of CDM)−1 (no IF added) and 0.62 ± 0.06 or 0.40 MAU (g of CDM)−1 when CFS or ASP was added, respectively, indicating that CFS was a better source of IF than ASP. Possibly the precipitation of the IF was not very successful, or the IF may form inactive complexes in a more isolated form. Another possibility is that other compounds present in CFS stimulate bacteriocin production. The values for kinact were comparable in the three cases. Addition of IF also affected the onset of curvacin A production, as reflected in the XB. When either CFS or ASP was added, bacteriocin production started from the beginning of the fermentation and XB was reduced to only 0.03 ± 0.03 g (CDM) liter−1, compared with 0.90 g (CDM) liter−1 when no IF was added. Finally, sugar metabolism was not affected by the addition of IF, resulting in comparable values for YX/S and mS.
FIG. 5.
Modeling of bacteriocin production (in MAU per liter) by L. curvatus LTH 1174 either with no IF added (▵) or with the addition of CFS (▪) or ASP (▴) in mMRS with 6% (wt/vol) added sodium chloride at a controlled temperature of 25°C and a constant pH of 5.5. Symbols represent experimental values; lines are drawn according to the model. For the fermentation with CFS added, the experimental values and the model derived are means from three fermentations; error bars, standard deviations.
DISCUSSION
In the past few years, suppression of food-borne pathogens such as L. monocytogenes in meat has become a major issue, and much work has been devoted to the selection of starter strains that are antagonistic to food pathogens (45). However, the production of a bacteriocin under laboratory conditions does not necessarily mean a sufficient and effective bacteriocin production and bioavailability in the food ecosystem. Severe limiting factors include, for instance, restricted nutrient availability for the bacteriocin-producing cells, adsorption of the bacteriocin onto meat particles, fats, and proteins, and/or the presence of endogenous proteases degrading the bacteriocin (60). In addition, meat processing technology may interfere with the bacteriocin production capacity of the starter cultures or cocultures used.
Salt affects the growth and bacteriocin production of LAB. In the case of L. curvatus LTH 1174, a low concentration of NaCl (2%, wt/vol) showed no effect on bacterial growth, while an inhibition that increased linearly was evident with higher salt concentrations. The strong negative effect of high salt concentrations on the growth of LAB has been reported previously (18, 54, 64). However, low concentrations of salt (1 to 2%, wt/vol) can sometimes enhance bacterial growth (23, 36, 64, 65).
Although a strong negative effect of salt was observed on the maximum specific growth rate (μmax) of L. curvatus LTH 1174, the maximum attainable biomass concentration (Xmax) did not decrease. This indicates that the aw constraints caused by NaCl addition may not impose large energetic burdens (37). In addition, replacing the sodium chloride with glycerol as an aw-lowering agent resulted in comparable inhibition; hence, the effect exerted by NaCl was probably due to its effect on aw. Although an ionic effect cannot be excluded, such an effect is not likely, because of the natural salt resistance of the LAB present in fermented meat and vegetable products (5, 36). However, Lactobacillus sakei CTC 494 shows a decreasing trend of Xmax with increasing NaCl concentrations (42). This may indicate that adding NaCl to the growth medium interferes with the efficiency of the substrate conversion into biomass for the latter strain. A linear decrease in the growth rate with aw values below the optimum is usually observed (46, 53).
Even at a concentration of 2% (wt/vol) NaCl, which did not affect the bacterial growth of L. curvatus LTH 1174, the maximum attainable bacteriocin activity (Bmax) decreased by almost 40% from that in the fermentation without added salt. This can be explained by the decrease in specific bacteriocin production (kB), since Xmax values were comparable. At higher NaCl concentrations, kB decreased further. In contrast, Uguen et al. (64) reported an increased lacticin 481 production when the osmolarity of the growth medium increased due to added NaCl. Also, for plantaricin S, the highest production is observed at a sodium chloride concentration of 2.5% (wt/vol) (39). On the other hand, the production of sakacin K by L. sakei CTC 494 is negatively affected by added NaCl (42), as is the case for the antilisterial carnobacteriocin B2 produced by Carnobacterium piscicola A9b (30). Although enterococci are more salt resistant, production of the enterocins A and B by Enterococcus faecium CTC 492 is also inhibited in the presence of NaCl (3). However, enterocin production can be increased in the meat matrix by the addition of a small amount of the bacteriocin, which acts as an inducer (4).
A possible explanation for the inhibition of bacteriocin production by NaCl is interference with the binding of the IF to its receptor (50). The IF is excreted by the producing strain, and when it reaches a critical concentration, the binding to its receptor initiates bacteriocin production. For C. piscicola A9b, the induction capacities of both acetate and carnobacteriocin B2 itself are negatively affected by the addition of NaCl (51). Moreover, for E. faecium CTC 492, addition of IF can overcome the inhibition of enterocin production (3, 50). Even at NaCl concentrations that do not affect growth, the induction of bacteriocin production decreases, indicating that higher concentrations of the inducer are necessary to sustain bacteriocin production (50). Likewise, curvacin A production by L. curvatus LTH 1174 is abolished at elevated temperatures (35°C) due to reduced synthesis of the cationic pheromone peptide Sap-Ph (19, 47). Possibly, addition of NaCl to the growth medium interferes with pheromone stability or binding to its receptor and/or reduces pheromone synthesis. Indeed, addition of CFS or ASP as a source of IF increased Bmax. In contrast, addition of IF could not reverse the negative effect of NaCl on sakacin P production (49), which was explained by the fact that sakacin P production is an “all or nothing process” (20).
Alternatively, in the actual sausage environment, the lower specific bacteriocin production may very well be counterbalanced by an increased relative bacteriocin activity, due to a higher susceptibility of pathogens in the presence of certain sausage ingredients such as salt. For instance, the addition of NaCl acts synergistically on the activity of curvacin A against Escherichia coli O157:H7 (24). Other strains of E. coli tested are also sensitive to nisin and curvacin A at a pH of <5.5 and in the presence of >3% (wt/vol) NaCl (25). For the enterotoxin-producing organism Bacillus cereus, a similar higher susceptibility to bacteriocin AS-48 is observed at an NaCl concentration of 5% (wt/vol) (1). For L. monocytogenes, a higher susceptibility to curvaticin 13 or nisin is also observed at higher NaCl concentrations (8, 62). Fewer bacteriocin molecules seem to be necessary to exert the same lethal effect. Moreover, during fermentation, a pH drop occurs, which will render certain species more susceptible to the bacteriocin that is produced in situ.
L. sakei and L. curvatus are the dominant LAB in European sausage starters (28). However, their adaptive responses to the meat environment differ in that L. curvatus is slightly more sensitive to salt than L. sakei (36), as is also shown in papers on L. curvatus LTH 1174 and L. sakei CTC 494 (42; this study). This difference may at least partially explain the dominance of L. sakei over L. curvatus in fermented sausages, as is often observed upon isolation of these strains from such products (2, 32, 52, 57). The use of bacteriocin-producing meat starter cultures, such as L. curvatus and L. sakei, for sausage fermentation enhances their competitiveness and contributes to a safer and more uniform end product. It is essential to understand the effects of different sausage-related environmental factors on the induction of curvacin A production in order to better understand the potential for application of this strain in the production of fermented sausages. The potential of this strain can be considerably broadened by the addition of CFS containing IF to achieve a higher level of bacteriocin production under unfavorable conditions. The addition of fermentation liquor or lyophilized whole-culture medium is an avenue to be investigated.
Acknowledgments
We acknowledge the financial support of the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT), in particular the STWW project “Functionality of Novel Starter Cultures in Traditional Fermentation Processes.” Also, the financial support of the Research Council of the Vrije Universiteit Brussel, the Fund for Scientific Research—Flanders, and various food companies is greatly appreciated.
Vincent Schrijvers and Tom De Winter are acknowledged for technical assistance. L. curvatus LTH 1174 was kindly provided by W. P. Hammes (Institut für Lebensmitteltechnologie, Universität Hohenheim, Stuttgart, Germany).
REFERENCES
- 1.Abriouel, H., M. Maqueda, A. Gálvez, M. Martinez-Bueno, and E. Valdivia. 2002. Inhibition of bacterial growth, enterotoxin production, and spore outgrowth in strains of Bacillus cereus by bacteriocin AS-48. Appl. Environ. Microbiol. 68:1473-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrighetto, C., L. Zampese, and A. Lombardi. 2001. RAPD-PCR characterization of lactobacilli isolated from artisanal meat plants and traditional fermented sausages of Veneto region (Italy). Lett. Appl. Microbiol. 33:26-30. [DOI] [PubMed] [Google Scholar]
- 3.Aymerich, M. T., M. G. Artigas, M. Garriga, J. M. Monfort, and M. Hugas. 2000. Effect of sausage ingredients and additives on the production of enterocins A and B by Enterococcus faecium CTC 492. Optimization of in vitro production and anti-listerial effect in dry fermented sausages. J. Appl. Microbiol. 88:686-694. [DOI] [PubMed] [Google Scholar]
- 4.Aymerich, M. T., M. Garriga, S. Costa, J. M. Monfort, and M. Hugas. 2002. Prevention of ropiness in cooked pork by bacteriocinogenic cultures. Int. Dairy J. 12:239-246. [Google Scholar]
- 5.Ballesteros, C., L. Palop, and I. Sánchez. 1999. Influence of sodium chloride concentration on the controlled lactic acid fermentation of “Almagro” eggplants. Int. J. Food Microbiol. 53:13-20. [DOI] [PubMed] [Google Scholar]
- 6.Benkerroum, N., A. Daoudi, and M. Kamal. 2003. Behaviour of Listeria monocytogenes in raw sausages (Merguez) in presence of a bacteriocin-producing lactococcal strain as a protective culture. Meat Sci. 63:479-484. [DOI] [PubMed] [Google Scholar]
- 7.Berry, E. D., M. B. Liewen, R. W. Mandigo, and R. W. Hutkins. 1990. Inhibition of Listeria monocytogenes by bacteriocin-producing Pediococcus during the manufacture of fermented semidry sausage. J. Food Prot. 53:194-197. [DOI] [PubMed] [Google Scholar]
- 8.Bouttefroy, A., M. Linder, and J. B. Millière. 2000. Predictive models of the combined effects of curvaticin 13, NaCl and pH on the behaviour of Listeria monocytogenes ATCC 15313 in broth. J. Appl. Microbiol. 88:919-929. [DOI] [PubMed] [Google Scholar]
- 9.Bridson, E. Y. 1998. The Oxoid manual, 8th ed. Oxoid, Basingstoke, United Kingdom.
- 10.Callewaert, R., M. Hugas, and L. De Vuyst. 2000. Competitiveness and bacteriocin production of enterococci in the production of Spanish-style dry fermented sausages. Int. J. Food Microbiol. 57:33-42. [Google Scholar]
- 11.Campanini, M., I. Pedrazzoni, S. Barbuti, and P. Baldini. 1993. Behaviour of Listeria monocytogenes during the maturation of naturally and artificially contaminated salami: effect of lactic acid bacteria starter cultures. Int. J. Food Microbiol. 20:169-175. [DOI] [PubMed] [Google Scholar]
- 12.Chandler, R. E., and T. A. McMeekin. 1989. Modelling growth response of Staphylococcus xylosus to changes in temperature and glycerol concentration/water activity. J. Appl. Bacteriol. 66:543-548. [DOI] [PubMed] [Google Scholar]
- 13.Cintas, L. M., P. Casaus, L. S. Håvarstein, P. E. Hernández, and I. F. Nes. 1997. Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl. Environ. Microbiol. 63:4321-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dainty, R., and H. Blom. 1995. Flavour chemistry of fermented sausages, p. 176-193. In G. Campbell-Platt and P. E. Cook (ed.), Fermented meats. Blackie Academic & Professional, London, United Kingdom.
- 15.Delves-Broughton, J., P. Blackburn, R. J. Evans, and J. Hugenholtz. 1996. Applications of the bacteriocin, nisin. Antonie Leeuwenhoek 69:193-202. [DOI] [PubMed] [Google Scholar]
- 16.De Vuyst, L. 2000. Technology aspects related to the application of functional starter cultures. Food Technol. Biotechnol. 38:105-112. [Google Scholar]
- 17.De Vuyst, L., and E. J. Vandamme. 1994. Antimicrobial potential of lactic acid bacteria, p. 91-142. In L. De Vuyst and E. J. Vandamme (ed.), Bacteriocins of lactic acid bacteria: microbiology, genetics and applications. Blackie Academic & Professional, London, United Kingdom.
- 18.De Vuyst, L., R. Callewaert, and K. Crabbé. 1996. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology 142:817-827. [DOI] [PubMed] [Google Scholar]
- 19.Diep, D. B., L. Axelsson, C. Grefsli, and I. F. Nes. 2000. The synthesis of the bacteriocin sakacin A is a temperature-sensitive process regulated by a pheromone peptide through a three-component regulatory system. Microbiology 146:2155-2160. [DOI] [PubMed] [Google Scholar]
- 20.Eijsink, V. G. H., M. B. Brurberg, P. H. Middelhoven, and I. F. Nes. 1996. Induction of bacteriocin production in Lactobacillus sake by a secreted peptide. J. Bacteriol. 178:2232-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Encinas, J.-P., J.-J. Sanz, M.-L. Garcia-Lopez, and A. Otero. 1999. Behaviour of Listeria spp. in naturally contaminated chorizo (Spanish fermented sausage). Int. J. Food Microbiol. 46:167-171. [DOI] [PubMed] [Google Scholar]
- 22.Foegeding, P. M., A. B. Thomas, D. H. Pilkington, and T. R. Klaenhammer. 1992. Enhanced control of Listeria monocytogenes by in situ-produced pediocin during dry fermented sausage production. Appl. Environ. Microbiol. 58:884-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gänzle, M. G., M. Ehmann, and W. P. Hammes. 1998. Modeling of growth of Lactobacillus sanfranciscensis and Candida milleri in response to process parameters of sourdough fermentation. Appl. Environ. Microbiol. 64:2616-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gänzle, M. G., S. Weber, and W. P. Hammes. 1999. Effect of ecological factors on the inhibitory spectrum and activity of bacteriocins. Int. J. Food Microbiol. 46:207-217. [DOI] [PubMed] [Google Scholar]
- 25.Gänzle, M. G., C. Hertel, and W. P. Hammes. 1999. Resistance of Escherichia coli and Salmonella against nisin and curvacin A. Int. J. Food Microbiol. 48:37-50. [DOI] [PubMed] [Google Scholar]
- 26.Gimeno, O., I. Astiasarán, and J. Bello. 2001. Influence of partial replacement of NaCl with KCl and CaCl2 on microbiological evolution of dry fermented sausages. Food Microbiol. 18:329-334. [DOI] [PubMed] [Google Scholar]
- 27.Gould, G. W. 1996. Industry perspectives on the use of natural antimicrobials and inhibitors for food applications. J. Food Prot. 1996(Suppl.):S82-S86. [DOI] [PubMed]
- 28.Hammes, W. P., and C. Hertel. 1996. Selection and improvement of lactic acid bacteria used in meat and sausage fermentation. Lait 76:159-168. [Google Scholar]
- 29.Herranz, C., P. Casaus, S. Mukhopadhyay, J. M. Martinez, J. M. Rodriguez, I. F. Nes, P. E. Hernandez, and L. M. Cintas. 2001. Enterococcus faecium P21: a strain occurring naturally in dry-fermented sausages producing the class II bacteriocins enterocin A and enterocin B. Food Microbiol. 18:115-131. [Google Scholar]
- 30.Himelbloom, B., L. Nilsson, and L. Gram. 2001. Factors affecting production of an antilisterial bacteriocin by Carnobacterium piscicola strain A9b in laboratory media and model fish systems. J. Appl. Microbiol. 91:506-513. [DOI] [PubMed] [Google Scholar]
- 31.Hugas, M. 1998. Bacteriocinogenic lactic acid bacteria for the preservation of meat and meat products. Meat Sci. 49(Suppl. 1):S139-S150. [PubMed] [Google Scholar]
- 32.Hugas, M., M. Garriga, M. T. Aymerich, and J. M. Monfort. 1993. Biochemical characterization of lactobacilli from dry fermented sausages. Int. J. Food. Microbiol. 18:107-113. [DOI] [PubMed] [Google Scholar]
- 33.Hugas, M., M. Garriga, M. T. Aymerich, and J. M. Monfort. 1995. Inhibition of Listeria in dry fermented sausages by the bacteriocinogenic Lactobacillus sake CTC 494. J. Appl. Bacteriol. 79:322-330. [Google Scholar]
- 34.Hugas, M., B. Neumeyer, F. Pagés, M. Garriga, and W. P. Hammes. 1996. Comparison of the antilisterial potential of bacteriocin producing lactobacilli in fermenting sausages. Fleischwirtschaft 76:649-652. [Google Scholar]
- 35.Johnson, J. L., M. P. Doyle, and R. G. Cassens. 1990. Listeria monocytogenes and other Listeria spp. in meat and meat products: a review. J. Food Prot. 53:81-91. [DOI] [PubMed] [Google Scholar]
- 36.Korkeala, H., T. Alanko, and T. Tiusanen. 1992. Effect of sodium nitrite and sodium chloride on growth of lactic acid bacteria. Acta Vet. Scand. 33:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krist, K. A., T. Ross, and T. A. McMeekin. 1998. Final optical density and growth rate; effects of temperature and NaCl differ from acidity. Int. J. Food Microbiol. 43:195-203. [DOI] [PubMed] [Google Scholar]
- 38.Lahti, E., T. Johansson, T. Honkanen-Buzalski, P. Hill, and E. Nurmi. 2001. Survival and detection of Escherichia coli O157:H7 and Listeria monocytogenes during the manufacture of dry sausage using two different starter cultures. Food Microbiol. 18:75-85. [Google Scholar]
- 39.Leal-Sánchez, M. V., R. Jiménez-Diaz, A. Maldonado-Barragán, A. Garrido-Fernández, and J. L. Ruiz-Barba. 2002. Optimization of bacteriocin production by batch fermentation of Lactobacillus plantarum LPCO10. Appl. Environ. Microbiol. 68:4465-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leistner, L. 1995. Stable and safe fermented sausages world-wide, p. 160-175. In G. Campbell-Platt and P. E. Cook (ed.), Fermented meats. Blackie Academic & Professional, London, United Kingdom.
- 41.Leroy, F., and L. De Vuyst. 1999. Temperature and pH conditions that prevail during fermentation of sausages are optimal for production of the antilisterial bacteriocin sakacin K. Appl. Environ. Microbiol. 65:974-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leroy, F., and L. De Vuyst. 1999. The presence of salt and curing agent reduces bacteriocin production by Lactobacillus sakei CTC 494, a potential starter culture for sausage fermentation. Appl. Environ. Microbiol. 65:5350-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leroy, F., and L. De Vuyst. 2000. Sakacins, p. 589-610. In A. S. Naidu (ed.), Natural antimicrobial systems. CRC Press LLC, Boca Raton, Fla.
- 44.Leroy, F., and L. De Vuyst. 2001. Growth of the bacteriocin-producing Lactobacillus sakei CTC 494 in MRS broth is strongly reduced due to nutrient exhaustion: a nutrient depletion model for the growth of lactic acid bacteria. Appl. Environ. Microbiol. 67:4407-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lücke, F.-K. 1998. Fermented sausages, p. 441-483. In B. J. B. Wood, (ed.), Microbiology of fermented foods. Blackie Academic & Professional, London, United Kingdom.
- 46.McMeekin, T. A., R. E. Chandler, P. E. Doe, C. D. Garland, J. Olley, S. Putros, and D. A. Ratkowsky. 1987. Model for combined effect of temperature and salt concentration/water activity on the growth rate of Staphylococcus xylosus. J. Appl. Bacteriol. 62:543-550. [DOI] [PubMed] [Google Scholar]
- 47.Messens, W., J. Verluyten, F. Leroy, and L. De Vuyst. 2002. Modelling growth and bacteriocin production by Lactobacillus curvatus LTH 1174 in response to temperature and pH values used for European sausage fermentation processes. Int. J. Food Microbiol. 81:41-52. [DOI] [PubMed] [Google Scholar]
- 48.Messi, P., M. Bondi, C. Sabia, R. Battini, and G. Manicardi. 2001. Detection and preliminary characterization of a bacteriocin (plantaricin 35d) produced by a Lactobacillus plantarum strain. Int. J. Food Microbiol. 64:193-198. [DOI] [PubMed] [Google Scholar]
- 49.Møretrø, T., I. M. Aasen, I. Storrø, and L. Axelsson. 2000. Production of sakacin P by Lactobacillus sakei in a completely defined medium. J. Appl. Microbiol. 88:536-545. [DOI] [PubMed] [Google Scholar]
- 50.Nilsen, T., I. F. Nes, and H. Holo. 1998. An exported inducer peptide regulates bacteriocin production in Enterococcus faecium CTC 492. J. Bacteriol. 180:1848-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nilsson, L., M. K. Nielsen, Y. Ng, and L. Gram. 2002. Role of acetate in production of an autoinducible class IIa bacteriocin in Carnobacterium piscicola A9b. Appl. Environ. Microbiol. 68:2251-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parente, E., S. Grieco, and M. A. Crudele. 2001. Phenotypic diversity of lactic acid bacteria isolated from fermented sausages produced in Basilicata (Southern Italy). J. Appl. Bacteriol. 90:943-952. [DOI] [PubMed] [Google Scholar]
- 53.Passos, F. V., H. P. Fleming, D. F. Ollis, H. M. Hassa, and R. M. Felder. 1993. Modeling the specific growth rate of Lactobacillus plantarum in cucumber extract. Appl. Microbiol. Biotechnol. 40:143-150. [Google Scholar]
- 54.Rozés, N., and C. Peres. 1996. Effect of oleuropein and sodium chloride on viability and metabolism of Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 45:839-843. [Google Scholar]
- 55.Sabia, C., G. Manicardi, P. Messi, S. de Niederhäusern, and M. Bondi. 2002. Enterocin 416K1, an antilisterial bacteriocin produced by Enterococcus casseliflavus IM 416K1 isolated from Italian sausages. Int. J. Food Microbiol. 75:163-170. [DOI] [PubMed] [Google Scholar]
- 56.Samelis, J., and J. Metaxopoulos. 1999. Incidence and principal sources of Listeria spp. and Listeria monocytogenes contamination in processed meats and a meat processing plant. Food Microbiol. 16:465-477. [Google Scholar]
- 57.Samelis, J., J. Metaxopoulos, M. Vlassi, and A. Pappa. 1998. Stability and safety of traditional Greek salami: a microbiological ecology study. Int. J. Food Microbiol. 44:69-82. [DOI] [PubMed] [Google Scholar]
- 58.Schillinger, U., M. Kaya, and F. K. Lücke. 1991. Behaviour of Listeria monocytogenes in meat and its control by a bacteriocin-producing strain of Lactobacillus sake. J. Appl. Bacteriol. 70:473-478. [DOI] [PubMed] [Google Scholar]
- 59.Shank, F. R., E. L. Elliot, I. K. Wachsmuth, and M. E. Losikoff. 1996. The US position on Listeria monocytogenes in foods. Food Control 7:229-234. [Google Scholar]
- 60.Stiles, M. E. 1996. Biopreservation by lactic acid bacteria. Antonie Leeuwenhoek 70:331-345. [DOI] [PubMed] [Google Scholar]
- 61.Teuber, M. 1999. Spread of antibiotic resistance with food-borne pathogens. Cell. Mol. Life Sci. 56:755-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas, L. V., and J. W. T. Wimpenny. 1996. Investigation of the effect of combined variations in temperature, pH, and NaCl concentrations on nisin inhibition of Listeria monocytogenes and Staphylococcus aureus. Appl. Environ. Microbiol. 62:2006-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tichaczek, P. S., J. Nissen-Meyer, I. F. Nes, R. F. Vogel, and W. P. Hammes. 1992. Characterization of the bacteriocins curvacin A from Lactobacillus curvatus LTH 1174 and sakacin P from L. sake LTH 673. Syst. Appl. Microbiol. 15:460-468. [Google Scholar]
- 64.Uguen, P., J. Hamelin, J. P. Le Pennec, and C. Blanco. 1999. Influence of osmolarity and the presence of an osmoprotectant on Lactococcus lactis growth and bacteriocin production. Appl. Environ. Microbiol. 65:291-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vignolo, G. M., M. N. de Kairuz, A. A. P. de Ruiz Holgado, and G. Oliver. 1995. Influence of growth conditions on the production of lactocin 705, a bacteriocin produced by Lactobacillus casei CRL 705. J. Appl. Bacteriol. 78:5-10. [Google Scholar]
- 66.Vogel, R. F., B. S. Pohle, P. S. Tichaczek, and W. P. Hammes. 1993. The competitive advantage of Lactobacillus curvatus LTH 1174 in sausage fermentations is caused by formation of curvacin A. Syst. Appl. Microbiol. 16:457-462. [Google Scholar]