Abstract
Etodolac, a nonsteroidal antiinflammatory drug, widely used in arthritis is associated with gastric ulceration and irritation due to presence of free carboxylic group. The current investigation reports synthesis of mutual amide prodrug of etodolac by masking free carboxylic group with glucosamine, a nutritional supplement for treatment of arthritis. Confirmation and characterization of the structure of the synthesized prodrug done by elemental and spectroscopy analysis, melting point, determination of migration parameters (Rf, RM, and Rt) by using thin layer chromatography and high performance liquid chromatography, respectively. Partition coefficient and solubility study confirms its lipophilic character so can be suitable candidate for controlled release delivery. In vitro hydrolytic studies of prodrug confirms good rate of hydrolysis in blood plasma, fecal matter, and simulated intestinal fluid while stable in gastric simulated fluid. In vivo pharmacological screening performed on animals. Prodrug with respect to etodolac shows good analgesic, antiinflammatory, and antiarthritic activity. The prodrug was assessed for their probable damaging effects by ulcerogeniticity and histopathological analysis. The histopathological studies showed less ulceration in the gastric region when treated with prodrug, thereby proving the prodrug to be better in action as compared to etodolac and are advantageous in having less gastrointestinal side effects.
Keywords: Mutual amide prodrug, etodolac, glucosamine, chemical and pharmacological characterization
Nonsteroidal antiinflammatory drugs (NSAIDs) are widely employed for the treatment of chronic inflammatory diseases, such as arthritis. Long term clinical use of most of the available acidic NSAIDs is strongly limited by their side effects notably gastric irritation, ulceration, bleeding, perforation, and in some cases may develop from relatively mild to more serious and potentially life threatening states[1,2]. These gastroenteropathic side effects are generally believed to result from the direct contact effect, which can be attributed to the potentiation of local irritation produced by a free carboxylic group in the molecular structure by local blockage of prostaglandin biosynthesis in the gastrointestinal tract[3]. NSAIDs could not be used as up to its potential, because of its adverse reactions offered due to presence of free carboxylic acid group[4,5]. Gastric mucosal injury problems have been overcome by derivatization of carboxylic functional group of NSAIDs into ester and amide prodrug[4,5,6,7,8,9,10,11].
Etodolac (EC), (RS)-2-(1,8-Diethyl-4,9-dihydro-3H-pyrano[3,4-b] indol-1-yl) acetic acid is a racemic acetic acid derivative and nonselective cyclooxygenase (COX) inhibitor with potential analgesic and antiinflammatory activities. It is effective in the treatment of osteoarthritis, gout, rheumatoid arthritis and traumatic injury[12]. EC like other NSAIDs produces gastrointestinal (GI) side effects[13]. So it is desired to develop a mutual prodrug of EC of the need to overcome related GI side effects.
Glucosamine (GLU) is an amino sugar which is body's basic starting material for production of natural joint component like critical joint lubricant and shock absorber. It has been widely touted as being an effective arthritis treatment and wound healing property[14,15].
Numerous reports of development of mutual amide prodrug using GLU with NSAIDs other than EC was reported[16,17,18]. Yet no attempts were made to develop mutual amide prodrug using GLU with EC for possible use in management of arthritis with less or no gastric side effects. Thus, present work aims to synthesize mutual amide prodrug of EC by temporarily masking of free carboxylic group of EC with GLU to stabilize prodrug (EC-GLU) in gastric region and further aimed to characterize on chemical and pharmacological basis. Various proteolytic enzymes especially amidase will help in release of EC and GLU by hydrolysis of peptide linkage of EC-GLU in basic pH of intestinal specifically colonic region. So as to produce minimized GIT disturbances while producing synergistic antiinflammatory, analgesic, and antiarthritic activities.
MATERIALS AND METHODS
EC and GLU were gift samples from Windlas Biotech Limited, Dehradun India. Dicyclohexylcarbodiimide (DCC) was purchased from Hi-media Laboratories Pvt. Ltd., Mumbai, India. Triethyl amine, benzene, methanol, dimethyl sulfoxide (DMSO), sodium bicarbonate were purchased from S. D. Fine-Chem Ltd., Mumbai, India. Diethylether, HPLC-grade methanol, acetonitrile and water were procured from Merck, Mumbai, India. Distilled water was used throughout the study.
Synthesis of mutual prodrug:
The synthesis of mutual prodrug (EC-GLU) was performed in three steps (fig. 1) First step deals with activation of carboxylic acid functional group of EC (10 mol/ml) by using DCC (10 mol/ml) in ethanol at 0-5° for 20 min, resulting a reactive intermediate etodolac-o-acylisourea. Glucosamine solution (10 mol/ml) was prepared with DMSO as cosolvent (4 ml), 2 ml of triethyl amine was added dropwise at 0° and volume was made upto 20 ml with methanol. In third step, equimolar amount of both prepared solution of GLU and etodolac-o-acylisourea were mixed with proper stirring at 0° for 24 h. The white precipitate formed as side product in reaction mixture was filtered off and remaining washed with 10% NaHCO3 and distilled water to remove other impurities. Further extraction with ether was done and crude product was obtained after vacuum evaporation of ether layer. The product was recrystallized with methanol and dried under vacuum.
Fig. 1.
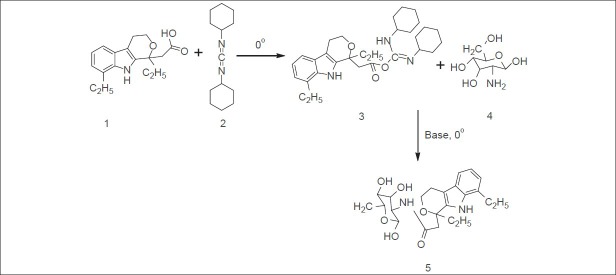
Scheme of synthesis of mutual prodrug from etodolac and glucosamine.
1=Etodolac; 2=dicyclohexylcarbodiimide; 3=etodolac o-acylisourea; 4=glucosamine and 5=ETO-GLU complex.
Characterization and preformulation studies of the synthesized prodrug:
Physical properties of synthesized prodrug, viz., yields, color, odor, aqueous solubility, Rf, Rm and melting point were observed. Solubility of 10 mg of prodrug was checked in demineralized water, methanol, phosphate buffer saline (PBS, pH 6.8) and methanol:PBS at pH 6.8 (80:20) at 37±10° in glass test tubes. Test tube is gently shaken and solubility was observed. In case of any observed insoluble fraction, the known amount of solvent was further added to ascertain the solubility of the compound. The synthesized prodrug was subjected to thin layer chromatography in order to check confirmation of product and their purity. The prepared plates of silica gel G adsorbents were dried and activated. The solvent system methanol:dichloromethane:benzene (4:1.5:0.5), visualizing agent ninhydrin was used. A dark pink spot on heating at 121° for 2-3 min in day light observed for Rf and Rm value calculation. Further confirmation and purity of compound checked on Qualisil Gold C18 column injecting on HPLC (LC 2010HT liquid chromatograph, Shimadzu, Japan). The melting point of the drug and synthesized prodrug was determined by capillary fusion method, by using calibrated thermometer and melting point apparatus (S. M. Scientific Instruments Pvt. Ltd., New Delhi). The λmax was determined at double beam UV/Vis spectrophotometer (UV-1700 Pharmspec, Shimadzu, Japan) by using software UV probe ver 2.33. Partition coefficient was determined in n-octanol/phosphate buffer of pH 7.4. The elemental analysis of EC-GLU was performed in Central Drug Research Institute, Lucknow, India, using Carlo-Erba Model 1108 Analyzer. It was carried out to find the percentage of C, H, and N in the prodrug. The IR spectra of the compounds were obtained on Spectrum two (FTIR spectormeter, Perkin Elmer, USA) by using software spectrum ver 10.3.02. The 1H NMR and mass analysis of the EC and EC-GLU were done on NMR spectrophotometer (Jeol) at 300 MHz using CDCl3 as solvent and on FAB mass spectrometer (Jeol S×102/DA-6000 mass spectrometer at IIT, Kanpur, India).
Protein binding study:
Protein binding study of EC-GLU is performed by using cellophane membrane. Prodrug solution (100 μg/ml) was made in phosphate buffer (PBS) at pH 7.4. After washing cellophane membrane with PBS, it was tied at the opening end of dialysis tube containing (6%) egg albumin. Then the whole assembly was dipped into the drug solution and placed on a magnetic stirrer with 100 rpm, at 37°. Sampling of drug solution was done at definite intervals. Withdrawn sample was diluted further with appropriate amount of phosphate buffer and monitored on UV spectrscopy at 280 nm.
In vitro hydrolysis study:
Hydrolysis of prodrug was studied in simulated gastric fluid (SGF, pH 1.2), simulated intestinal fluid (SIF, pH 7.4), PBS (pH 6.8) containing fresh rat fecal content (20% w/v, to provide amidase) and 80% human plasma (pH 7.4). Prodrug solution (100 μg/ml) was prepared in SGF at 37°. Five milliliter solution was withdrawn from the prodrug solution at 15 min time interval (0-90 min) followed by addition of methanol to make up the volume. Upon hydrolysis sample was shaken for 5 min with equal amount of methanol to extract free EC. After centrifugation at 2500 rpm for 5 min all clear supernatant obtained for different time intervals were injected into the column of HPLC. Mobile phase methanol:water (80:20) was used with flow rate of 0.8 ml/min, UV detector (D2 lamp) for the quantification of free etodolac at 280 nm. The same procedure was fallowed for SIF and 80% human plasma for hydrolysis of EC-GLU. For reversion study in rat feces, sample was processed by protein precipitation technique using appropriate amount of acetonitrile.
Pharmacological screening:
For all in vivo screening Sprague Dawley rats (100-200 g) and Swiss albino mice (20-25 g) were randomly divided into six groups each of 6, three males and three females, including a control and a two standard group for EC and EC-GLU. The selected animals were housed in acrylic cages at standard environmental conditions at 25±2°, relative humidity of 45-55%, in a well ventilated room, fed with standard rodent diet and water ad libitum. All the animals were acclimatized for a week before experiment. All the experiments were carried out under the guidelines and approval of Institutional Animal Ethics Committee (IAEC/PSIT/1273/ac/09), Pranveer Singh Institute of Technology, Kanpur, India.
Antiinflammatory activity:
In vivo antiinflammatory action of prodrug was determined by hind paw edema method using carrageenan (0.1 ml, 1% w/v) as phlogistic agent[19]. The initial volume of right hind paw of rat was measured by plethysmometer without administration of drug. A 1% sodium carboxymethyl cellulose (CMC) suspension containing EC and EC-GLU (equivalent to 20 mg/kg body weight) was administered orally to the standard groups. Control group administers only 1% CMC suspension. After 30 min of administration of the drug and prodrug, carrageenan solution in normal saline was injected into the planter surface of right hind paw of each animal. The volume of swelling of right hind paw of each rat was measured after 0.5, 1, 2, 4, and 6 h. The mean increase in the volume of the right hind paw of rat was compared with control and standard. The percent inhibition of paw edema was calculated as, percent inhibition = (1-Vt/Vc) ×100, where, Vt is mean relative change in paw edema volume in test group, Vc is mean relative change in paw edema volume in control group.
Analgesic activity:
The analgesic activity of the EC and EC-GLU was determined by acetic acid induced writhing method in mice[20]. Swiss albino mice were divided into three groups (n=6 in each group). Dose equivalent to 20 mg/kg body weight was administered to control and standard groups. Three hours after treatment, 0.6% (v/v) acetic acid solution (10 ml/kg) was injected to mice intraperitoneally. Total number of writhes, which was a parameter of chemically induced pain (i.e., constriction of abdomen, turning of trunk, and extension of hind limbs), was counted for 20 min. The analgesic effect was expressed as percent reduction of writhes in comparison with the control.
Antiarthritic activity:
Arthritis was induced in rats by the intraplantar injection of 0.1 ml of Complete Freund's Adjuvant (CFA) in the left hind paw[21]. The adjuvant contained heat-killed Mycobacterium tuberculosis (H37Rv strain, Tuberculosis Research Centre, ICMR, Chennai) in sterile paraffin oil (10 mg/ml). The paw volume of all the animal groups was measured by plethysmograph at 0, 7, 14, 21 days after the injection of Freund's complete adjuvant.
Ulcerogenic activity:
Gastrointestinal toxicity of the EC-GLU was observed and compared with the parent drug by measuring mean ulcer index[22]. The rats were divided into six groups, each comprising six animals, including a control and standard group. The control group was administered orally by 1% CMC. Standard EC and EC-GLU were administered orally (at 10 times higher dose) as a suspension with 1% CMC daily for 5 days. The rats were fasted after the administration of last dose, thereafter they were sacrificed by decapitation and the stomachs were removed, opened and washed with distilled water. The lesions on the gastric mucosa were counted by visual examination using a binocular magnifier. Ulcers greater than 0.5 mm were recorded. The size of ulcers was measured by Microimage process software (DA1-180M v 2.01; Sunny International United Co., Ltd, Zhejiang, China), using an Olympus SP 350 camera (Olympus, Tokyo, Japan). Ulcers were scored as: 0 for normal colored stomach; 0.5 for red coloration; 1 for spot ulcers; 1.5 for hemorrhagic streaks; 2 for ulcers of 3 mm up to 5 mm; and 3 for ulcers of 5 mm and greater[22] and mean ulcer index calculated as, UI=[1×(number of lesions of grade 1)+2×(number of lesions of grade 2)+3×(number of lesions of grade 3)]/10.
Histopathological study:
The histopathological studies of stomach of rats were carried out using hemotoxylin and eosin stain. The stomach tissues of rats were removed from the rats and fixed in 10% normal saline for at least 48 h. Further processed routinely and the tissues were embedded in paraffin wax. Histological sections were cut at 5-6 μm and stained with routine hematoxylin and eosin, observed for necrosis and ulcer.
RESULTS AND DISCUSSION
Coupling reagent based amide synthesis is used for synthesis because of easy step synthesis with direct activation of carboxylic group of EC to generate its active form which can react with amine group of GLU directly in presence of a base as catalyst. No heating was required in this procedure and yield was found more than 77%. Initial characterization of EC and EC-GLU were performed by TLC and HPLC. Rf for EC, EC-GLU were found 0.80±0.08 and 0.85±0.06, respectively. The synthesized compound was of white color with melting point of 120-122°. The practical values for C, H and N (61.97, 7.38, and 6.38%) in elemental analysis of EC-GLU were found near to theoretical values i.e., 61.58, 7.20, and 6.25%. By spectral analysis the structure of prodrug was confirmed. UV: λmax280 nm, FTIR (cm−1): 3460 (OH str.), 3315 (NH str. Amide), 3057 (aromatic CH str.), 2930 and 2911 (CH str. for CH3 and CH2), 1707 (C = O), 1649 (amide I), 1546 (amide II), 1292 (OH bending, sec. alcohol). 1H-NMR (δ) (CDCl3): 8.21 (t, CONH), 7.82 (s, NH), 7.28 (s, ArH), 2.02 (s, OH), 1.48 (q, CH2), 1.55 (s, CH2), 1.01 (t, CH3). By mass spectroscopy (MS-FAB) the M + value 448 confirms molecular formula and molecular weight of prodrug, is C23H32N2O7 and 448.22.
The synthesized prodrug was subjected to solubility, partition coefficient and hydrolytic studies. EC-GLU showed moderate solubility in boiling water, methanol, better solubility in PBS (pH 6.8) and good solubility in methanol: PBS at pH 6.8 (80:20). The enhancement in solubility of prodrug in comparison to standard drug is might be due to presence of amide linkage. Due to this transcellular absorption by lipid membrane permeation might be limited in gastric region. This would facilitate delivery of intact prodrug to lower intestinal region. Here C18 column is used as the stationary phase had more affinity for nonpolar drugs. Rt value for EC and EC-GLU was found 5.5 and 12.8 min, respectively. Increment in log P value of EC-GLU (1.35) observed in comparison to EC (1.02). Higher log P and Rt value for EC-GLU with respects to parent drug supports its more lipophilic nature.
Protein binding for EC-GLU was 41% less than EC indicates more availability of the EC-GLU for hydrolysis in plasma and the required dose will be less so as to minimize dose related effect on prostaglandin formation.
Hydrolytic studies of the prodrug in SGF, SIF, PBS (pH 6.8) containing fresh rat fecal content and 80% human plasma were performed. The free form of EC was observed 8, 62, 68, and 79%, respectively after hydrolysis. Fig. 2 illustrates comparative hydrolytic behavior of EC-GLU in different media. In SGF negligible amount of prodrug hyrolysis observed, indicating valid stability of prodrug in gastric pH. The t1/2 value of EC-GLU in SIF at pH 7.4 is 142 min. Moreover hydrolysis of prodrug occurs at basic pH-releasing active EC and GLU for therapeutic action. Prodrug in PBS at colonic pH with rat's fresh fecal content indicates its better hydrolysis due to presence of enzyme amidase. In 80% human plasma t1/2 value for prodrug was found to be 71 min. It is due to presence of different active proteolytic enzyme to break amide linkage.
Fig. 2.
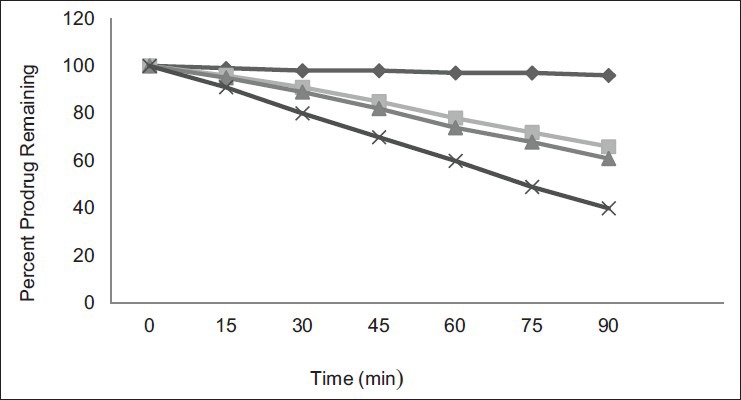
Hydrolytic pattern of prodrug.
In vitro hydrolytic pattern of mutual amide prodrug in SGF, SIF, PBS with rat feces (pH 6.8) and 80% human plasma. SGF (-♦-); SIF(-■-); pH 6.8 PBS (-▲-); human plasma (-×-)
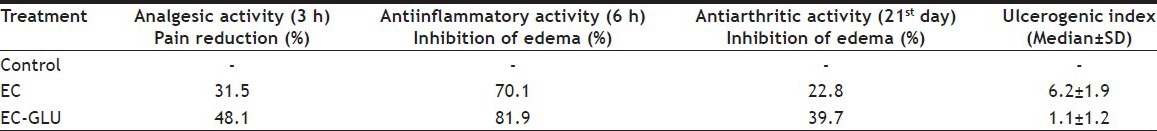
The synthesized prodrug along with EC was evaluated for analgesic, antiinflammatory, ulcerogenic, antiarthritic activity, and histopathology study. Table 1 reports result of pharmacological screenings. In analgesic study, significant reduction in number of writhes were observed after treatment with EC and EC-GLU. The result indicates initially (after 2 h of administration) advantage of EC over EC-GLU but after 3 h equimolar dose of EC-GLU to EC has better analgesic activity. It may be due to stability of prodrug in gastric pH. The antiinflammatory activities after 6 h oral dosing of EC-GLU and EC shows inhibition of edema 70.1 and 81.9%. GLU has antiinflammatory property apart from antiarthritic activity[23], responsible for synergistic effect on percentage inhibition of edema. EC-GLU shows better antiarthritic activity with respect to EC on 21st day. Value for ulcerogenic index shows notable difference between EC-GLU and EC. The minimized ulcerogenic index of prodrug might be due to inhibition of direct contact of carboxyl group of the drug to the gastric mucosa, which is mainly responsible for the damage. It is also due to negligible hydrolysis in stomach (pH 1.2) region as well.
TABLE 1.
RESULTS OF IN VIVO STUDIES
The stomach walls of rat in ulcerogenic study are presented in fig. 3. The animals treated with only EC have hemorrhagic spots, deep ulceration, and necrotic cells while animals treated with EC-GLU has small red spots but no necrosis. On comparing histopathology of the stomachs of control rats (fig. 4a) and those treated with drug (fig. 4b), and prodrug (fig. 4c), more severe hemorrhage, ulcers, and necrosis were evident in the drug group than the prodrug group.
Fig. 3.
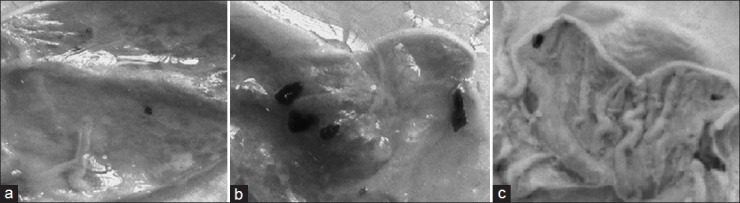
Photographs of rat stomachs in ulcerogenic activity.
Evaluation of ulcerogenic activity in the stomachs of rats treated with (a) drug vehicle 1% w/v CMC, (b) EC (200 mg kg−1 body weight), (c) mutual prodrug EC-GLU (equivalent to 200 mg kg−1 body weight.
Fig. 4.
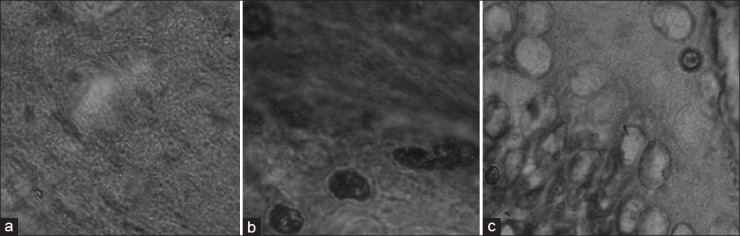
Histopathology of rat stomach.
Histopathology of Sprague Dawley rat stomach after 14 days treated with (a) 1% w/v CMC (b) EC (200 mg kg−1 body weight) (c) mutual prodrug EC-GLU (equivalent to 200 mg kg−1 body weight).
In conclusion, the synthesized prodrug had increased solubility, synergistic antiinflammatory and antiarthritic activity with lower toxicity and less ulcerogenic activity than the parent drug EC. Thus, this prodrug approach solves not only the formulation problem of EC (lower aqueous solubility, BCS class II drug) but also reduces gastric adverse effects.
Footnotes
Pandey, et al.: Mutual Prodrug Synthesis of Etodolac and Glucosamine
REFERENCES
- 1.Champion GD, Feng PH, Azuma T, Caughey DE, Chan KH, Kashiwazaki S, et al. NSAID induced gastrointestinal damage. Epidemiology risk and prevention, with an evaluation of the role of Misoprostol: An Asia-Pacific perspective and consensus. Drugs. 1997;53:61–9. doi: 10.2165/00003495-199753010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Schoen RT, Vender RJ. Mechanisms of nonsteroidal antiinflammatory drug-induced gastric damage. Am J Med. 1989;86:449–58. doi: 10.1016/0002-9343(89)90344-6. [DOI] [PubMed] [Google Scholar]
- 3.Cioli V, Putzolu S, Rossi V, Carrandino C. The role of direct tissue contact in the production of gastrointestinal ulcers by antiinflammatory drugs in rats. Toxicol Appl Pharmacol. 1979;50:283–9. doi: 10.1016/0041-008x(79)90153-4. [DOI] [PubMed] [Google Scholar]
- 4.Mishra A, Veerasamy R, Jain PK, Dixit VK, Agrawal RK. Synthesis, characterization and pharmacological evaluation of amide prodrugs of ketorolac. Eur J Med Chem. 2008;43:2464–72. doi: 10.1016/j.ejmech.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Azeem AZ, Abdel-Hafez AA, El-Karamany GS, Hassan HF. Chlorzoxazone esters of some nonsteroidal antiinflammatory (NSAI) carboxylic acids as mutual prodrug: Design, synthesis, pharmacological investigations and docking studies. Biol Med Chem. 2009;17:3665–70. doi: 10.1016/j.bmc.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 6.Bonina F, Puglia C, Santagati NA, Saija A, Tomaino A, Tita B. Oligoethylene ester derivatives of ketoprofen, naproxen and diclofenac as oral prodrug: A pharmacological evaluation. Pharmazie. 2002;57:552–5. [PubMed] [Google Scholar]
- 7.Halen PK, Chagti KK, Giridhar R, Yadav MR. Substituted aminoalcohol ester analogs of indomethacin with reduced toxic effects. Med Chem Res. 2007;16:101–1. [Google Scholar]
- 8.Mecit OU, Burcu C, Alis KE, Duygu AA, Nil UE, Guler YO, et al. Stable ester and amide conjugates of some NSAIDs as analgesic and antiinflammatory compounds with improved biological activity. Turk J Chem. 2011;35:427–9. [Google Scholar]
- 9.Shanbhag VR, Crider AM, Gokhale R, Harpani A, Dick RM. Ester and amide prodrug of ibuprofen and naproxen: Synthesis, antiinflammatory activity, and gastrointestinal toxicity. J Pharm Sci. 1992;81:149–4. doi: 10.1002/jps.2600810210. [DOI] [PubMed] [Google Scholar]
- 10.Ravichandran V, Mishra A, Agrawal RK, Dixit VK. Synthesis, characterization and pharmacological evaluation of amide prodrug of flubiprofen. J Braz Chem Soc. 2008;19:89–100. [Google Scholar]
- 11.Lohade AA, Jain P, Iyer KR. Parallel combinatorial synthesis and in vitro evaluation of ester and amide prodrug of flurbiprofen, ibuprofen and ketoprofen. Indian J Pharm Educ Res. 2009;43:140–9. [Google Scholar]
- 12.Savita V, Trivedi P, Chaturvedi SC. Dextran-etodolac conjugates: synthesis, in vitro and in vivo evaluation. Acta Pol Pharm Drug Res. 2009;66:201–6. [PubMed] [Google Scholar]
- 13.Liang TH, Hsu PN. Double-blind, randomised, comparative trial of etodolac SR versus diclofenac in the treatment of osteoarthritis of the knee. Curr Med Res Opin. 2003;19:336–41. doi: 10.1185/030079903125001866. [DOI] [PubMed] [Google Scholar]
- 14.Rubin BR, Talent JM, Kongtawelert P, Pertusi RM, Forman MD, Gracy RW. Oral polymeric N-acetyl-D-glucosamine and osteoarthritis. J Am Osteopath Assoc. 2001;101:339–44. [PubMed] [Google Scholar]
- 15.Dhaneshwar SS, Ghodeswar BC, Bhojani MR. Synthesis and biological evaluation of glucosamine conjugate prodrug of flurbiprofen. Indian Drugs. 2003;40:156–9. [Google Scholar]
- 16.Ghodeswar BC, Pophalikar RN, Bhojani MR, Nagpal D, Dhaneshwar SS. Synthesis and pharmacological evaluation of mutual prodrug of some nonsteroidal antiinflammatory drugs with glucosamine. Indian J Pharm Sci. 2004;66:773–7. [Google Scholar]
- 17.Gairola N, Nagpal D, Dhaneshwar SS, Dhaneshwar SR, Chaturvedi SC. Synthesis, hydrolysis, kinetics and pharmacodynamic profiles of novel prodrug of flubiprofen. Indian J Pharm Sci. 2005;67:369–73. [Google Scholar]
- 18.Patil SJ, Shirote PJ. Synthesis and evaluation of carrier linked Prodrug of Ketoprofen with Glucosamine. J Pharm Res. 2012;5:954–7. [Google Scholar]
- 19.Brodie DA, Cook PG, Bauer BJ, Dagle GE. Indomethacin- induced intestinal lesions in the rat. Toxicol Appl Pharmacol. 1970;17:615–24. doi: 10.1016/0041-008x(70)90036-0. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh MN. 2nd ed. Calcutta: Scientific Book Agency; 1984. Fundamentals of Experimental Pharmacology. [Google Scholar]
- 21.Vogel GH, Vogel WH. 2nd ed. Berlin: Springer-Verlag; 1997. Drug Discovery evaluation pharmacological assays. [Google Scholar]
- 22.Rainsford KD. Comparative studies of gastric ulcerogenesis by nonsteroid antiinflammatory drugs. Proc R Soc Med. 1977;70:4–10. [PMC free article] [PubMed] [Google Scholar]
- 23.Yomogida S, Hua J, Sakamoto K, Nagaoka I. Glucosamine suppresses interleukin-8 production and ICAM-1 expression by TNF-alpha-stimulated human colonic epithelial HT-29 cells. Int J Mol Med. 2008;22:205–1. [PubMed] [Google Scholar]


