Abstract
For the first time, a soil bacterium, designated Pseudomonas aeruginosa, was isolated based on its ability to grow on tyrosol as a sole source of carbon and energy. During growth on tyrosol, this strain was capable of promoting the formation of a significant amount of hydroxytyrosol and trace quantities of parahydroxyphenyl acetic acid and 3,4-dihydroxyphenyl acetic acid. The products were confirmed by high-performance liquid chromatography and gas chromatography-mass spectrometry analyses. Using an optimized tyrosol concentration of 2 g liter−1, the maximal hydroxytyrosol yield (80%) was achieved after a 7-h reaction in a growth experiment. To enhance the formation of hydroxytyrosol and prevent its degradation, a resting-cell method using P. aeruginosa was performed. The growth state of the culture utilized for biomass production, the carbon source on which the biomass was grown, the concentration of the biomass, and the amount of tyrosol that was treated were optimized. The optimal yield of hydroxytyrosol (96%) was obtained after a 7-h reaction using 4 g of tyrosol liter−1 and 5 g of cells liter−1 pregrown on tyrosol and harvested at the end of the exponential phase. This proposed procedure is an alternative approach to obtain hydroxytyrosol in an environmentally friendly way. In addition, the reaction is easy to perform and can be adapted to a bioreactor for industrial purposes.
Many companies in the pharmaceutical, chemical, and food sectors are interested in the development of biotransformations utilizing enzymes as biocatalysts, either free, immobilized, or in whole cells (20). The products of such bioconversions are considered natural, since the European Community legislation includes products that are produced by living cells or enzymes using starting materials from a natural source under the term “natural products” (17).
Among useful biotransformations, hydroxylation of aromatic compounds stands out as a fundamental reaction due to its many uses in the manufacture of high-added-value compounds, namely, orthodiphenol compounds (28). Hydroxytyrosol (3,4-dihydroxyphenylethanol) is present in virgin olive oil (2), on which it confers chemical stability (21). It is also the most abundant orthodiphenol compound occurring in olive mill wastewaters (30). These wastewaters are the most problematic effluents on the southern shore of the Mediterranean Sea (29). Hydroxytyrosol has powerful antioxidant properties and presents several interesting aspects for human health (32). For example, results in vitro demonstrated that hydroxytyrosol inhibits human low-density lipoprotein oxidation (3), scavenges free radicals (31), inhibits platelet aggregation (26) and leucotriene production for human neutrophils (13), and confers cell protection (21). It has also been demonstrated that hydroxytyrosol acts in vitro as an antibacterial agent against both gram-positive and gram-negative bacteria (5). Recently, hydroxytyrosol has been reported to have good bioavailability, which encourages its addition to the diet (22).
Hydroxytyrosol is not commercially available. Several methods have been developed to produce it by means of chemical synthesis (10) through conversion of oleuropein (6), by enzymatic synthesis using tyrosinase as a biocatalyst (16), and by bench scale purification from olive mill wastewater (9). Recently, Bolanos et al. (7) reported the production of hydroxytyrosol from the liquid-solid waste of two-phase olive processing, “alperujo,” by hydrothermal treatment. Other developed protocols, as well as industrial applications of hydroxytyrosol, are patented and therefore inaccessible (11, 12, 14).
In the present investigation, we have developed the first whole-cell catalysts for synthesizing relatively large quantities of hydroxytyrosol. The present approach has several advantages over the enzymatic synthesis of hydroxytyrosol using mushroom tyrosinase (16). The enzymatic procedure has disadvantages, namely, the high cost of the enzyme; the pronounced instability of the enzyme, especially in the presence of oxygen; and the need to supply a reductant to prevent rapid quinone formation. In addition, pure hydroxytyrosol is obtained only after further preparative chromatography to eliminate ascorbic acid and other intermediates (16). The novel biosynthesis method developed in the present study could prove useful for laboratory applications, as well as for possible industrial exploitation.
MATERIALS AND METHODS
Chemicals.
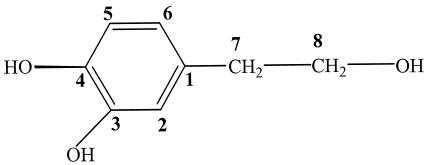
Tyrosol was purchased from Fluka. Hydroxytyrosol, used as a standard, was purified from olive mill wastewater in our laboratory as follows. One volume of olive mill wastewater was extracted twice with 2 volumes of ethyl acetate. After evaporation of the solvent, 1 g of the obtained extract was filtered on a C18 silica gel (liChroprep RP-18; 25- to 40-μm) column (2.5 by 70 mm) under medium pressure. Elution of phenolic compounds was carried out with the same gradient solvent used in high-performance liquid chromatography (HPLC) (see below). The flow rate was adjusted to 0.3 ml min−1, and 4.5-ml fractions were collected. These fractions were measured by optical density at 280 nm (OD280), and the chromatogram (OD versus fraction number) was represented (data not shown). The first separated peak corresponds to pure hydroxytyrosol. Its structure was established by means of spectroscopic techniques (mass spectrometry [MS] and 1H nuclear magnetic resonance [NMR]), which gave the following data. EI-MS m/z (relative intensity): 154 (M+, 61), 153 (20), 136 (48), 123 (100), 105 (63), 87 (64), and 77 (66). 1H NMR (270 MHz, CD3OD) δ: 2.66 (2H, t, J7-8 = 7.3 Hz, H7), 3.66 (2H, t, J8-7 = 7.3 Hz, H8), 6.52 (1H, dd, J6-5 = 8, J6-2 = 2.1 Hz, H6), 6.64 (1H, d, J2-6 = 2.1 Hz, H2), 6.67 (1H, d, J5-6 = 8 Hz, H5). The MS and NMR analyses were performed using a spectrograph, Kratos apparatus type MS 25 magnetic sector, and a Geol apparatus, 270 MHz, respectively. These spectroscopic data agreed with those described by Capasso et al. (9, 10). The chemical structure of hydroxytyrosol is shown in Fig. 1.
FIG. 1.
Chemical structure of hydroxytyrosol.
Bacterial-strain isolation.
For bacterial isolation, a soil enrichment technique was used. Soil (1 g), regularly irrigated for 5 years with olive mill wastewater (100 m3 ha−1), was mixed with 100 ml of minimal medium containing 0.1 g of tyrosol liter−1 and 1 g of glucose liter−1 as the carbon sources. The culture obtained was incubated overnight in an orbital shaker at 180 rpm and 30°C. Each day, 5 ml from the inoculated flask was used to inoculate fresh medium (100 ml) in which the concentration of tyrosol was increased (0.1 g liter−1 was added) with decreasing glucose concentration. This procedure was repeated daily until the tyrosol concentration reached 1 g liter−1 while the glucose was completely removed from the medium composition. Fresh medium containing 1 g of tyrosol liter−1 was inoculated several times until the substrate was completely metabolized. The disappearance of tyrosol was confirmed by thin-layer chromatography (TLC) analysis. Aliquots (0.1 ml) of 10−2 to 10−8 dilutions were plated onto tyrosol (1 g liter−1) minimal agar medium and incubated overnight at 30°C. Single colonies were picked and used for screening.
Culture conditions.
Bacteria were grown in a minimal medium containing (in grams liter−1) Na2HPO4, 2.44; KH2PO4, 1.52; (NH4)2SO4, 1.5; MgSO4 · 0.7H2O, 0.2; CaCl2 · H2O, 0.05; and 10 ml of trace element solution, which contained (wt/vol) EDTA, 5%; ZnSO4 · 0.7H2O, 2.2%; CaCl2, 0.55%; MnCl2 · 0.5H2O, 0.5%; (NH4)6Mo7O24 · 0.4H2O, 0.11%; CuSO4 · 0.5H2O, 0.16%; CoCl2 · 0.6H2O, 0.16%. The pH of the medium was adjusted to 7.2. The carbon substrates were filter sterilized, and solid media were prepared by the addition of 1.5% agar. Cultures (25 ml) were inoculated with 107 cells pregrown in Luria-Bertani broth and incubated in an orbital shaker at 180 rpm and 30°C. The growth rate was followed by measuring the changes in turbidity at 600 nm using a Shimadzu UV-visible-light spectrophotometer (UV-160A).
DNA extraction and PCR amplification.
Total DNA was isolated from the isolated strain by using the alkaline-lysis method with certain modifications. The universal primers Fd1 and Rd1 (34) were used to obtain a PCR product of ∼1.5 kb corresponding to base positions 8 to 1542 based on Escherichia coli numbering of the 16S DNA (33). A 50-μl reaction mixture contained 50 mg of genomic DNA, 1 μl of each primer, 5 μl of 10× buffer, 200 μl of deoxynucleoside triphosphate, 3.5 mM MgCl2 and 2.5 U of Taq polymerase (Promega). PCR was carried out by an initial denaturation at 94°C for 1 min, followed by cycles of annealing at 55°C for 30 s, extension at 72°C for 45 s (depending on the lengths of the amplified fragments), and denaturation at 94°C for 30 s, followed by cycles of annealing at 55°C for 30 s, extension at 72°C for 45 s, and finally an extension cycle of 72°C for 10 min.
Quantitative determination of hydroxytyrosol formation by resting cells.
The biomass obtained after centrifugation (7,000 × g at 4°C for 20 min) was washed twice in saline phosphate buffer (4.2 mM Na2HPO4, 2.2 mM KH2PO4, 0.9 mM NaCl, 1.9 mM NH4Cl) before being resuspended in the same buffer. The bioconversion reaction was monitored in a 150-ml conical flask containing 25 ml of buffer supplemented with a sterile solution of tyrosol and incubated on a rotary shaker at 30°C. Samples (1 ml) were withdrawn periodically and centrifuged at 7,000 × g (10 min). The supernatant was analyzed directly by HPLC for substrate and intermediary-metabolite determination. The yield of hydroxytyrosol produced was calculated as follows: yield of hydroxytyrosol = (hydroxytyrosol concentration produced [grams liter−1]/initial tyrosol concentration [grams liter−1]) × 100.
HPLC and TLC analyses.
HPLC was performed on a Shimadzu C-R6A liquid chromatograph. The separation was carried out in a C18 column (length, 250 mm; internal diameter, 4.6 mm; Waters Chromatography). Compounds were eluted with a gradient, acetonitrile (70%)- H3PO4 (0.1%), in which the concentration of acetonitrile varied as follows: 0 min, 10%; 0 to 20 min, increase to 50%; 20 to 25 min, 50%; 25 to 30 min, decrease to 10%. The column temperature was maintained at 40°C, and the flow rate was 0.5 ml min−1. Sample detection was achieved at 280 nm with a Shimadzu SPD-6AUV detector connected to a Shimadzu C-R6A integrator. The injection volume was 20 μl. Compounds were identified and quantified by comparison of retention times and peak areas with those of authentic samples, i.e., tyrosol was purchased from Fluka and hydroxytyrosol was purified in our laboratory from olive mill wastewater. TLC was monitored using silica gel sheets (Kieselgel 60 F254; 0.2-mm layers; Merck) eluted with toluene-ethyl acetate-acetic acid (7:2:1 [vol/vol/vol]). The constituents were visualized by exposure to UV light and revealed by resublimed iodine fumes.
GC-MS analysis.
GC-MS was performed with a Hewlett-Packard model 5872A chromatograph apparatus equipped with a capillary HP5MS column (length, 30 m; internal diameter, 0.32 mm; film thickness, 0.32 μm). The carrier gas was He, used at a 1.7-ml min−1 flow rate. The oven temperature program was as follows: 1 min at 100°C, from 100 to 260°C at 4°C min−1, and 10 min at 260°C. A sample from the culture medium (1 ml) was extracted with ethyl acetate, and 100 μl of bis-(trimethylsilyl)-acetamide was added to 100 μl of the organic extract. The solution obtained was incubated 20 min at 80°C. The ethyl acetate and bis-(trimethylsilyl)-acetamide were evaporated under an N2 current, and the residue was redissolved in ethyl acetate (1 ml) and analyzed by GC-MS.
RESULTS
Isolation of hydroxytyrosol-producing strains.
To isolate different tyrosol-tolerant microorganisms, an enrichment culture method was used according to the protocol described in Materials and Methods. This enrichment culture was designed to select strains able to grow on tyrosol as a sole carbon and energy source. The substrate transformation was followed by TLC and HPLC analyses. Based on their morphologies, six different tyrosol-tolerant strains were isolated. These bacteria were then screened for the ability to produce hydroxytyrosol in the presence of tyrosol. In order to select the most efficient bacterium, the concentration of hydroxytyrosol produced by each strain was determined by the HPLC technique (data not shown), and one strain appeared to produce the greatest quantity of hydroxytyrosol. This selected strain was identified by 16S ribosomal DNA sequence analysis as Pseudomonas aeruginosa.
Biotransformation of tyrosol to hydroxytyrosol by P. aeruginosa.
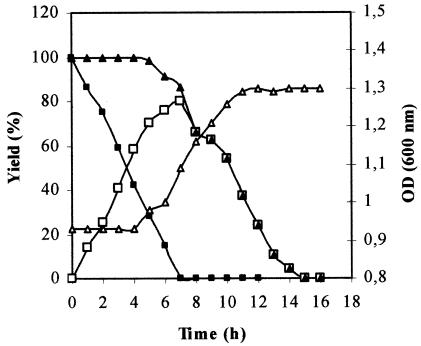
The formation of hydroxytyrosol by P. aeruginosa was assessed by the addition of tyrosol to a culture previously grown to stationary phase on tyrosol. Tyrosol (2 g liter−1) was added to the culture, and the conversion was monitored by HPLC analysis of samples withdrawn at different times. Figure 2 shows the time course of tyrosol depletion and transient accumulation of hydroxytyrosol in the medium during the growth of P. aeruginosa. During the first 4 h of incubation, the OD600 remained constant. This adaptation phase, which varied from 4 to >15 h depending on the initial concentration of tyrosol (data not shown), could be explained by the toxicity of tyrosol to the isolated strain. During this period, P. aeruginosa transformed tyrosol to hydroxytyrosol without the consumption of aromatic compounds. After this lag phase, the hydroxytyrosol concentration in the medium increased, but the degradation of aromatic compounds started, and consequently, an increase in the OD600 was observed. After 7 h, hydroxytyrosol was degraded and trace amounts of 3,4-dihydroxyphenyl acetic acid and parahydroxyphenyl acetic acid appeared in the medium as intermediates. The identities of these compounds were confirmed by GC-MS analysis. Hydroxytyrosol was completely removed from the culture after 15 h. The maximal hydroxytyrosol concentration (1.6 g liter−1) was achieved at 7 h, resulting in a yield of 80%. At that moment, the culture medium contained only the hydroxytyrosol produced. It should be stressed that this productivity was accomplished with an optimized tyrosol concentration of 2 g liter−1.
FIG. 2.
Time course of the growth of P. aeruginosa strain in minimal medium (see Materials and Methods). ▵, OD600; ▪, tyrosol; □, hydroxytyrosol; ▴, aromatic compounds.
Optimization of conditions for hydroxytyrosol production by cell suspensions.
To improve the yield of hydroxytyrosol and to prevent its subsequent degradation by the isolated strain, further bioconversion studies were performed using a resting-cell procedure. Cells produced in minimal-medium culture were harvested by centrifugation, washed, suspended in saline phosphate buffer, and then incubated aerobically at 30°C in the presence of tyrosol (as described in Materials and Methods). Under these conditions, biomass duplication was prevented. The relationships between the production of hydroxytyrosol and (i) the growth state of the culture utilized for biomass production, (ii) the carbon source on which the biomass was grown, (iii) the concentration of the biomass, and (iv) the amount of tyrosol that was treated were examined.
Cells from tyrosol-grown cultures were harvested for hydroxytyrosol production experiments in the middle of the exponential phase (OD600 = 0.5), at the end of the exponential phase (OD600 = 1), and in the stationary phase (OD600 = 1.1) of growth; washed; and resuspended at a concentration of 2 g liter−1 (wet weight) in saline phosphate buffer. The different types of cells were incubated aerobically in an orbital shaker at 180 rpm and 30°C in the presence of 2 g of tyrosol liter−1. The decrease in the substrate concentration and the increase of the hydroxytyrosol metabolite in each medium were monitored by frequent sample analysis by HPLC. The results obtained showed that the maximum level of hydroxytyrosol (1.4 g liter−1) was accumulated in the medium using cells harvested at the end of the exponential phase of growth. This corresponded to a bioconversion yield of 70%. However, for cells harvested in the middle of the exponential phase and in the stationary phase of growth, yields of 21 and 42%, respectively, were obtained.
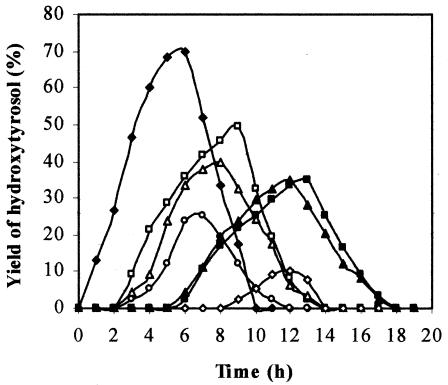
The effect of the carbon source on which the biomass was grown on the bioconversion rates was then examined. Cells (2 g liter−1 [wet weight]) harvested at the end of the exponential phase of cultures grown on glucose, tyrosol, catechol, parahydroxy benzoic acid, parahydroxyphenyl acetic acid, paracoumaric acid, and 3,4-dihydroxyphenyl acetic acid were incubated in the presence of 2 g of tyrosol liter−1. Figure 3 shows that the maximum level of hydroxytyrosol production (70%) was achieved with a shorter incubation time in experiments carried out with tyrosol-grown cells. As an example, 8% of hydroxytyrosol was obtained with cells produced on glucose as a carbon source.
FIG. 3.
Time courses of hydroxytyrosol formation with cells of P. aeruginosa strain pregrown on tyrosol (♦), 4-hydroxyphenyl acetic acid (□), 3,4-dihydroxyphenyl acetic acid (▵), catechol (○), paracoumaric acid (▴), parahydroxy benzoic acid (▪), and glucose (⋄).
In order to examine the effect of the tyrosol concentration on the formation of hydroxytyrosol, cells were resuspended at 2 g liter−1 (wet weight) in saline-phosphate buffer with different concentrations of tyrosol (1, 2, 3, 4, 5, and 6 g liter−1). The optimal tyrosol concentration should be considered to be that which allows higher yields of hydroxytyrosol to be obtained, and afterwards, a delayed decrease in the metabolite. Figure 4 shows the variation in the level of hydroxytyrosol produced with the treated tyrosol concentration. The maximum yield of hydroxytyrosol increased with the amount of tyrosol used for concentrations up to 4 g liter−1. An excess of tyrosol concentration (>4 g liter−1) was detrimental to the increase in the quantity of hydroxytyrosol produced. This fact can probably be explained by the toxicity of the tyrosol and/or hydroxytyrosol produced. Similar results were reported by Narbad and Gasson (25), who studied the metabolism of ferulic acid via vanillin. These researchers reported that the bioconversion product (vanillin) was found to be toxic at certain concentrations.
FIG. 4.
Time courses of hydroxytyrosol formation by resting cells of P. aeruginosa strain incubated with increasing concentrations of tyrosol. ♦, 1 g/liter; □, 2 g/liter; ▴, 3 g/liter; ▵, 4 g/liter; ▪, 5 g/liter; ○, 6 g/liter.
Thus, the optimal tyrosol concentration is 4 g liter−1, which resulted in a bioconversion yield of 82%. Figure 4 shows that for tyrosol concentrations higher than 3 g liter−1, the bioconversion reaction is stopped after reaching its maximal product level. Consumption of the hydroxytyrosol produced had not begun after >24 h.
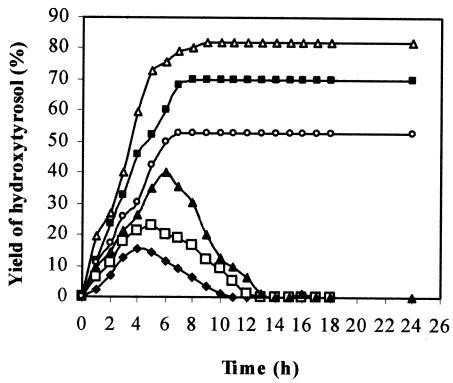
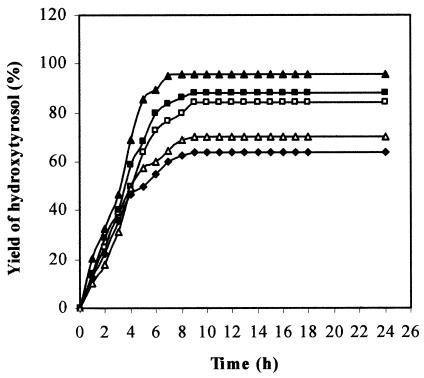
Using 4 g of tyrosol liter−1 and cells pregrown on tyrosol and harvested at the end of the exponential phase of growth, the effect of the biomass concentration on the bioconversion yield was examined next. In Fig. 5, the time courses of hydroxytyrosol production at different biomass concentrations are shown. An excellent result (96% hydroxytyrosol produced) is obtained with 5 g of cells liter−1 (wet weight) after an incubation time of 7 h. At that time, the culture medium contained only hydroxytyrosol, which was confirmed by HPLC analysis. For hydroxytyrosol isolation, the culture medium was acidified at pH 2 and extracted twice with ethyl acetate. Pure hydroxytyrosol was obtained after solvent evaporation under reduced pressure. The chemical structure of the hydroxytyrosol produced was confirmed by GC-MS and 1H NMR analyses as described in Materials and Methods.
FIG. 5.
Time courses of hydroxytyrosol formation using 4 g of tyrosol/liter and different concentrations of resting cells of P. aeruginosa strain: ♦, 3 g/liter; □, 4 g/liter; ▴, 5 g/liter; ▪, 6 g/liter; ▵, 7 g/liter.
DISCUSSION
Because of increasing demand for natural food additives, there is considerable interest in utilizing microbial transformation for producing natural antioxidants, especially orthodiphenolic compounds (15). It has been proved that hydroxytyrosol, an orthodiphenol that occurs in olives and its derivatives, presents several interesting aspects for human health that would allow its potential application in food, as well as in the cosmetic and pharmaceutical industries (27, 22). This polyphenolic compound is not commercially available (10). We investigated its production by microbial transformation using synthetic tyrosol as a precursor. Tyrosol, occurring naturally in olive mill wastewater (24), would be an excellent starting material for microbial conversion to hydroxytyrosol. Tyrosol can also be obtained from olive cake (18) and olive stones (19), in both cases by means of steam treatment.
Tyrosol and hydroxytyrosol account for >80% of the olive mill wastewater phenolic extract. Bioconversion of tyrosol into hydroxytyrosol in the extract by P. aeruginosa would increase its antioxidant effect.
During the screening of microorganisms able to utilize tyrosol as a sole carbon and energy source, a strain, further characterized as P. aeruginosa, was isolated. This strain was found to be capable of transiently accumulating a significant amount of hydroxytyrosol, which resulted from orthohydroxylation of tyrosol. Thus, Burkhead et al. (8) reported that Pseudomonas species are among the best-known microorganisms that can carry out specific hydroxylation of phenolic compounds. Mason (23) has noted that monooxygenases are involved in the aromatic-ring hydroxylation of a wide range of aromatic compounds and that the most frequently encountered aromatic monooxygenases are of the p-hydroxybenzoate hydroxylase type. These enzymes, which can be present in Pseudomonas strains, add a single hydroxyl group to an already hydroxylated substrate to generate a product with vicinal hydroxyl groups. In a previous work (34), a gene encoding phenol hydroxylase, which catalyzes the hydroxylation of phenol and other aromatic compounds, was detected and sequenced in Pseudomonas pseudoalcaligenes strain MH1.
The maximal yield of hydroxytyrosol obtained with a P. aeruginosa strain in batch growth experiments was high. However, this product is subject to fast degradation by growing P. aeruginosa cells. Therefore, in order to improve the bioconversion yield and prevent its further degradation, we investigated the inhibition of cell growth by using suspensions of resting cells at high density. This method was used by Barghini et al. (4) for vanillin production. Our study showed interesting features, and a significant improvement in the hydroxytyrosol yield was achieved. Under optimal conditions, 3.84 g of hydroxytyrosol was obtained after 7 h of incubation of P. aeruginosa cells in 1 liter of phosphate buffer medium in the presence of 4 g of tyrosol. This corresponds to a hydroxytyrosol formation rate of 4.49 mmol min−1 mg of protein−1. Furthermore, this process can be extrapolated for large-scale production. A simple ethyl acetate extraction produced pure hydroxytyrosol from the reaction medium.
Several chromatographic purifications of hydroxytyrosol from olive mill wastewater have been reported. The method developed by Capasso et al. (10) consisted of silica gel chromatography, C8 reverse-phase chromatography, preparative TLC chromatography, and a crystallization technique. In that study, the researchers produced only 91 mg of pure hydroxytyrosol per liter of olive mill wastewater. Chemical synthesis of hydroxytyrosol was performed by the reduction of 3,4-dihydroxyphenyl acetic acid, followed by a chromatographic purification. The precursor is very expensive, and the method uses toxic reagents which must be removed (10). Hydroxytyrosol was produced from olive oil extraction by-products: (i) the liquid-solid waste of two-phase olive processing, alperujo, by hydrothermal treatment using acid or alkaline catalysts (7) and (ii) the liquid effluent generated from the three-phase olive oil extraction process by solvent extraction (9). These processes presented some disadvantages, such as the complexity of the two substrates (high-strength black wastes containing oils and polyphenols of high molecular mass) and the need for hydrothermal treatment, a large amount of solvent, and a further step of chromatographic purification of the hydroxytyrosol. Furthermore, the concentration of hydroxytyrosol in these by-products depends upon several factors, such as the olive maturation and conservation methods (1).
The bacterial synthesis of hydroxytyrosol using P. aeruginosa that we performed is environmentally friendly and can be adapted to a bioreactor for industrial applications. In comparison with the other methods reported in the literature, our procedure appears to be more convenient and is interesting for several reasons. It consists of one cheap step, easy to perform and not time-consuming; it produces a high yield (96%) of hydroxytyrosol without using toxic reagents; and it does not require additives or a cofactor-regenerating system. These results suggest that our production method for hydroxytyrosol could be used as an alternative in the search for a replacement for synthetic antioxidant food additives.
Acknowledgments
This research was supported by “Contrats Programmes Secrétariat d'Etat à la Recherche Scientifique et à la Technologie,” Tunisia.
We thank Hèdi Aouissaoui and Mohamed Hammami for their help in HPLC and GC-MS analyses.
REFERENCES
- 1.Amiot, M. J., A. Fleuriet, and J. J. Macheix. 1986. Importance and evolution of phenolic compounds in olive during growth and maturation. J. Agric. Food Chem. 34:823-830. [Google Scholar]
- 2.Angerosa, F., N. d'Alessandro, P. Kostantinou, and I. Giacinto. 1995. GC-MS evaluation of phenolic compounds in virgin olive oil. J. Agric. Food Chem. 43:1802-1807. [Google Scholar]
- 3.Aruoma, O. I., M. Deiana, A. Jenner, B. Halliwell, H. Kaur, S. Banni, F. P. Corongiu, M. A. Dessi, and R. Aeschbach. 1998. Effects of hydroxytyrosol found in extra virgin olive oil on oxidative DNA damage and low-density lipoprotein oxidation. J. Agric. Food Chem. 46:5181-5187.
- 4.Barghini, P., F. Montebove, M. Ruzzi, and A. Schiesser. 1998. Optimal conditions for bioconversion of ferulic acid into vanillic acid by Pseudomonas fluorescens BF13 cells. Appl. Microbiol. Biotechnol. 49:309-314. [Google Scholar]
- 5.Bisignano, A., A. Tomaino, R. Lo Cascio, G. Crisafi, N. Uccella, and A. Saija. 1999. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 51:971-974. [DOI] [PubMed] [Google Scholar]
- 6.Briante, R., F. La Cara, M. P. Tonziello, F. Febraio, and R. Nucci. 2001. Antioxidant activity of the main bioactive derivatives from oleuropein hydrolysis by hyperthermophilic β-glycosidase. J. Agric. Food Chem. 49:3198-3203. [DOI] [PubMed] [Google Scholar]
- 7.Bolanos, F. J., G. Rodriguez, R. Rodriguez, A. Heredia, R. Guillen, and A. Jimenez. 2002. Production in large quantities of highly purified hydroxytyrosol from liquid-solid waste of two-phase olive oil processing or “Alperujo.” J. Agric. Food Chem. 50:6804-6811. [DOI] [PubMed] [Google Scholar]
- 8.Burkhead, K. D., S. W. Peterson, and P. L. Bolen. 1994. Microbial hydroxylation. I. Hydroxylation of aniline by Aspergillus alliaceus, A. albertensis and A. terreus. J. Ind. Microbiol. 13:233-237. [Google Scholar]
- 9.Capasso, R., A. Evidente, and C. Visca. 1994. Production of hydroxytyrosol from olive oil vegetation waters. Agrochimica 38:166-171. [Google Scholar]
- 10.Capasso, R., A. Evidente, S. Avolio, and F. Solla. 1999. A highly convenient synthesis of hydroxytyrosol and its recovery from agricultural waste waters. J. Agric. Food Chem. 47:1745-1748. [DOI] [PubMed] [Google Scholar]
- 11.Crea, R. March 2002. Method of obtaining a hydroxytyrosol-rich composition from vegetation water. U.S. patent WO0218310.
- 12.Crea, R. June 2003. Hydroxytyrosol-rich composition from vegetation water and method of use thereof. U.S. patent US2003108651.
- 13.De la Puerta, R., V. Ruiz-Gutiérrez, and J. R. Hoult. 1999. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 57:445-449. [DOI] [PubMed] [Google Scholar]
- 14.De Tomas, B. F. A., D. A. F. Ferreres, D. G. J. C. Espin, V. M. Garcia, H. J. Wichers, and R. C. Soler. May 2003. Enzymatic synthesis of antioxidant hydroxytyrosol. U.S. patent EP1310562.
- 15.Edlin, D. A. N., A. Narbad, J. R. Dickinson, and D. Lloyd. 1995. The biotransformation of simple phenolic compounds by Brettanomyces anomalus. FEMS Microbiol. Lett. 125:311-316. [Google Scholar]
- 16.Espin, J. C., C. Soler-Rivas, E. Cantos, F. A. Tomas-Barberan, and H. J. Wichers. 2001. Synthesis of the antioxidant hydroxytyrosol using tyrosinase as biocatalyst. J. Agric. Food Chem. 49:1187-1193. [DOI] [PubMed] [Google Scholar]
- 17.Eyal, S., R. Uzi, and S. Yuval. 2000. Isolation of a Bacillus sp. capable of transforming isoeugenol to vanillin. J. Biotechnol. 78:1-9. [DOI] [PubMed] [Google Scholar]
- 18.Felizon, B., J. Fernandez-Bolanos, A. Heredia, and A. Guillen. 2000. Steam-explosion pretreatment of olive cake. J. Am. Oil Chem. Soc. 77:15-22. [Google Scholar]
- 19.Fernandez-Bolanos, J., B. Felizon, M. Brenes, A. Guillen, and A. Heredia. 1998. Hydroxytyrosol and tyrosol as the main compounds found in the phenolic fraction of steam-exploded olive stones. J. Am. Oil Chem. Soc. 75:1-7. [Google Scholar]
- 20.Lilly, M. D., and J. M. Woodley. 1996. A structured approach to design and operation of biotransformation processes. J. Ind. Microbiol. 17:24-29. [Google Scholar]
- 21.Manna, C., P. Galletti, V. Cucciolla, O. Moltedo, A. Leone, and V. Zappia. 1997. The protective effect of olive oil polyphenols (3,4-dihydroxyphenyl)-ethanol counteracts reactive oxygen metabolite-induced cytotoxicity in Caco-cells. J. Nutr. 127:286-292. [DOI] [PubMed] [Google Scholar]
- 22.Manna, C., P. Galletti, G. Maisto, V. Cucciolla, S. D'Angelo, and V. Zappia. 2000. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2-cells. FEBS Lett. 470:341-344. [DOI] [PubMed] [Google Scholar]
- 23.Mason, J. R. 1998. Oxygenase catalyzed hydroxylation of aromatic compounds: simple chemistry by complex enzymes. Int. Ind. Biotechnol. 8:19-24. [Google Scholar]
- 24.Mulinacci, N., A. Romani, C. Galardi, P. Pinell, C. Giaccherini, and F. F. Vincieri. 2001. Polyphenolic content in olive oil waste waters and related olive samples. J. Agric. Food Chem. 49:3509-3514. [DOI] [PubMed] [Google Scholar]
- 25.Narbad, A., and M. J. Gasson. 1998. Metabolism of ferulic acid via vanillin using a novel CoA-dependent pathway in a newly-isolated strain of Pseudomonas fluorescens. Microbiology 144:1397-1405. [DOI] [PubMed] [Google Scholar]
- 26.Petroni, A., M. Blasevich, M. Salami, N. Papini, G. F. Montedoro, and C. Galli. 1995. Inhibition of platelet aggregation and eicosanoid production by phenolic component of olive oil. Thromb. Res. 78:151-160. [DOI] [PubMed] [Google Scholar]
- 27.Salami, M., C. Galli, L. De Angelis, and F. Visioli. 1995. Formation of F2-isoprostanes in oxidized low density lipoprotein: inhibitory effect of hydroxytyrosol. Pharmacol. Res. 31:275-279. [DOI] [PubMed] [Google Scholar]
- 28.Salvo, J. J., D. P. Mobley, D. W. Brown, L. A. Caruso, A. P. Yake, J. L. Spivack, and D. K. Dietrich. 1990. Biosynthesis of p-hydroxylated aromatics. Biotechnol. Prog. 6:193-197. [Google Scholar]
- 29.Sayadi, S., and R. Ellouz. 1995. Roles of lignin peroxidase and manganese peroxidase from Phanerochaete chrysosporium in the decolorization of olive mill wastewaters. Appl. Environ. Microbiol. 61:1098-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sayadi, S., N. Allouche, M. Jaoua, and F. Aloui. 2000. Detrimental effects of high molecular-mass polyphenols on olive mill waste water biotreatment. Proc. Biochem. 35:725-735. [Google Scholar]
- 31.Visioli, F., G. Bellomo, and C. Galli. 1998. Free radical scavenging properties of olive oil polyphenols. Biochem. Biophys. Res. Commun. 247:60-64. [DOI] [PubMed] [Google Scholar]
- 32.Visioli, F., A. Poli, and C. Galli. 2002. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 22:65-75. [DOI] [PubMed] [Google Scholar]
- 33.Winker, S., and C. R. Woese. 1991. Definition of the domains Archaea, Bacteria and Eucarya in terms of small subunit ribosomal RNA characteristics. Syst. Appl. Microbiol. 13:161-165. [DOI] [PubMed] [Google Scholar]
- 34.Zouari, H., S. Moukha, M. Labat, and S. Sayadi. 2002. Cloning and sequencing of a phenol hydroxylase gene of Pseudomonas pseudoalcaligenes strain MH1: a bacterium able to mineralize various aromatic compounds. Appl. Biochem. Biotechnol. 102-103:261-276. [DOI] [PubMed] [Google Scholar]