Abstract
Background:
Nanotechnology plays a remarkable role in the field of the treatment of Lymphomas associated with tumor.
Objective:
The purpose of this study is to determine and to compare the tumor uptake, biodistribution and pharmacokinetics of radiolabeled etoposide and etoposide loaded nanoparticles in Dalton's Lymphoma tumor bearing mice and healthy mice.
Materials and Methods:
Etoposide loaded nanoparticles were prepared by nanoprecipitation technique using the poly (lactic-co-glycolic) acid (PLGA) in the presence of Pluronic F 68 (F 68) as a stabilizer and characterized by particle size analyzer, zeta potential and transmission electron microscope. Etoposide and etoposide loaded nanoparticles were labeled with Technetium-99m (Tc-99m) by the direct method and various quality control tests were carried out. The labeling parameters like labeling efficiency, stability, etc., were optimized to get high labeling efficiency as well as stability of the labeled formulations. Tc-99m labeled formulations were administered intravenously in Balb C mice and their biodistribution and pharmacokinetics were determined.
Results:
Mean size of the etoposide loaded PLGA nanoparticles was found to be 105.1 nm. The concentration of both free etoposide and nanoparticles increased with time and showed higher tumor concentrations of both free etoposide and nanoparticles increased with time and showed higher retention, indicating their applicability in effective and prolonged tumor therapy. Nuclear scintigraphic images confirm the presence of labeled complexes at the site of tumor for 24 h at higher concentration than in the normal muscles.
Conclusion:
This study indicated higher tumor affinity and targeting properties of etoposide loaded nanoparticles than free etoposide.
KEY WORDS: Biodistribution, etoposide, pharmacokinetics, polymeric nanoparticles, tumor uptake
One of the most promising applications of nanoparticles is their use as carriers for anticancer drugs.[1] The association of a drug to polymeric nanoparticles modifies the drug pharmacokinetic parameters specifically the distribution profile of the drug-carrier itself.[2] Nanoparticles have easy accessibility in the body and can be transported to different body sites through the systemic circulation of blood. Targeting the drug through selective polymeric nanoparticles as carrier can enhance therapeutic efficacy of drugs. Once in the bloodstream, these nanoparticles are rapidly opsonized and massively cleared by the fixed macrophages of the mononuclear phagocyte system (MPS) organs such as Kuppfer cells of liver and macrophages of spleen.[3,4] When associated with nanoparticles, drugs concentrate mainly in the liver and spleen and are precluded to exert their acute toxicity in other organs. This was demonstrated earlier in mice treated with doxorubicin incorporated into poly (isohexylcyanoacrylate) nanospheres.[5] Such affinity of MPS macrophages for endocytosis/phagocytosis provides an opportunity to efficiently deliver therapeutic agents to these cells, using the polymeric conventional nanoparticles. This biodistribution study of polymeric nanoparticles can benefit for the chemotherapeutic treatment of MPS localized tumors such as hepatocarcinoma or hepatic metastasis arising from the digestive tract or gynecological cancers, bronchopulmonary tumors, myeloma and leukemia.[6]
One of the most suitable methods of studying the biodistribution and pharmacokinetics is to label these nanoparticles with radioisotopes like Technetium-99m (Tc-99m) and measure the biodistribution of radioactivity in various tissues and to perform gamma imaging of the whole body at a predetermined time periods.[7,8,9] Biodistribution, tumor uptake and anti-tumor efficacy for doxorubicin incorporated into poly (butylcynoacrylate) (PBCA) nanocapsules and poly (ethylene glycol) (PEG)/poly (lactide) (PLA) nanoparticles showed the highest accumulation of nanoparticles preferentially in the tumor cells.[10,11,12] Authors have previously demonstrated that etoposide loaded polymeric nanoparticles have better distribution and long residence time as compared with free etoposide in healthy mice after Tc-99m radiolabeling of nanoparticles and etoposide.[13] The objective of the present study is to prepare and characterize the etoposide loaded polymeric nanoparticle, radiolabel the same with Tc-99m and to study its biodistribution, pharmacokinetic and affinity for the target tissue in Dalton's lymphoma tumor bearing mice.
Materials and Methods
Materials
Poly (lactide-co-glycolide), PLGA (Purasorb® 85/15, Mol.wt. 10000) was procured from Purac chemicals, The Netherlands. Pluronic F 68 was purchased from Sigma-Aldrich Chemicals, USA. Etoposide was taken from Dabur Research Foundation, Sahibabad, U.P, India. Tc-99m was obtained from Regional Center for Radiopharmaceuticals, Board of Radiation and Isotope Technology, Institute of Nuclear Medicine and Allied Sciences (INMAS), Brig. S. K. Mazumdar Marg, Delhi, India, which was eluted from Molybdenum. Stannous chloride dihydrate was purchased from Sigma Chemicals. Triple distilled water was used in the preparation of nanoparticles. Instant thin layer chromatography (ITLC) plates were purchased from Gelman Science Inc., Ann Arbor, MI. All other materials such as acetone, acetonitrile, sodium hydrogen phosphate, acetic acid, pyridine and sodium chloride were procured from Spectrochem, Mumbai, India.
Preparation of nanoparticles
Etoposide loaded nanoparticles with PLGA 85/15 were prepared by nanoprecipitation method[14] using acetone as solvent. Pluronic F 68 was used as a stabilizing agent. Polymer PLGA (50 mg) and etoposide (5 mg) were dissolved in organic solvent and added slowly under stirring to aqueous phase containing F 68 (1.0% w/v) as stabilizer. After the formation of milky dispersion, organic solvent was evaporated under reduced pressure at 35°C for approximately 1 h (Rotavapor, Buchi, Switzerland). The entire dispersion was centrifuged at 14000 rpm at 25°C for 10 min (Cooling Compufuge, Remi, Mumbai) in three cycles. Supernatant liquid was analyzed for free drug content and the sediment constituting nanoparticles was dried by freezing.
Characterization of nanoparticles
Particle size and zeta potential measurement
Average particle diameter, polydispersity index and zeta potential for each batch of prepared nanoparticles was determined by using Zetasizer 3000HS (Malvern Instruments, UK). For zeta potential measurement nanoparticles formulations were diluted with water. Values reported are the mean and standard deviation of three batches of nanoparticles. Morphological examination was performed using Transmission electron microscopy (Morgagni, Philips, Netherlands) following negative staining with phosphotungstic acid (0.5%). Samples were also examined using Atomic Force Microscopy (Nanoscope II, USA) using contact mode.
Drug content and encapsulation efficiency
Freeze dried powder of nanoparticles was weighed and dissolved in acetonitrile. Etoposide was estimated by ultraviolet-visible spectrophotometer at 240 nm using a combination of acetonitrile (ACN):triple distilled water (TDW) (1:1) as medium. Drug content was calculated by the following equation:[15]
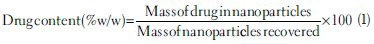
For entrapment efficiency, 1 ml of nanodispersion was taken and centrifuged in 3 cycles at 14,000 rpm for 10 min/cycle. Free drug present in the dispersion will remain in the supernatant and nanoparticles settle to the bottom. Supernatant was collected and analyzed for etoposide using above mentioned analytical method.[15]

Radiolabeling of nanoparticles with Tc-99m
Radiolabeling of etoposide and etoposide loaded formulation prepared with PLGA 85/15 was done by direct method using the stannous chloride as a reducing agent.[7] 1 ml of etoposide solution and nanoparticle dispersions was mixed individually with 50 μl of stannous chloride (2 mg/ml). To adjust the pH of this mixture to 7.0, 10 μl of sodium hydrogen carbonate (0.5 M) solution was added. Then, 0.1 ml of Tc-99m-pertechnetate solution (2 mCi) was added to each preparation, mixed well and incubated for 15 min at room temperature. Final radioactivity present in the preparation was checked using the dose calibrator. Effect of the amount of stannous chloride, final pH of the preparation and incubation time on labeling efficiency were optimized by changing one parameter at a time and performing quality control tests for the labeled complex as described earlier.[13,16] For optimizing the amount of stannous chloride required for high labeling efficiency and low radio colloids, a range of 25-400 μg of stannous chloride was used. Similarly, pH of the reaction mixture was varied between 4 and 8.
Determination of labeling efficiency
Quality control tests were performed as per the reported methods.[7] The labeling efficiency of etoposide and formulation was determined by ascending thin layer chromatography (TLC) using ITLC strips coated with silica gel (Gelman Science Inc., Ann Arbor, MI, USA). The ITLC strips were used to determine free technetium and percentage of radio colloids in the preparation. Based on these two parameters labeling efficiency of the preparation were calculated.[17,18]
ITLC strips were spotted with 1-2 μl of labeled complex at 1 cm above the bottom. These strips were eluted using acetone as a solvent system to determine free Tc-99m-pertechnetate and reduced/hydrolyzed (R/H) Tc-99m. The solvent front was allowed to reach up to a height of approximately 6-8 cm from the origin and the strip was cut horizontally into two halves. Radioactivity in each half was determined by well type gamma ray spectrometer (Gamma ray spectrophotometer, Type GRS23C, Electronics Corporation of India Ltd., Mumbai). The free pertechnetate present in the preparation migrates to the top portion (Rf value = 0.9-1.0) of the ITLC strip, leaving the radio colloids (R/H technetium) along with the labeled complex at the point of application. The presence of radio colloids was determined by developing ITLC strip using pyridine: acetic acid: water in the ratio of 3:5:1.5. R/H Tc-99m present in the preparation remained at the point of application while both the free Tc-99m-pertechnetate as well as labeled complex migrated up with the solvent front.
Stability study of labeled complexes
Stability of the Tc-99m labeled etoposide and formulation was determined in vitro in rabbit serum, normal saline by ascending TLC technique. The labeled complex (0.1 ml) was incubated with freshly collected rabbit serum (0.9 ml) at room temperature. The samples were taken from this at regular intervals up to 24 h and ITLC was carried out using above mentioned solvent systems. These strips were counted for radioactivity in gamma ray spectrometer and percentage labeling efficiency was calculated for etoposide and formulations.[13]
Biodistribution, pharmacokinetics and tumor uptake study
Studies were carried out after the prior approval and in accordance with the rules and regulations of the Animal Ethics Committee of INMAS, New Delhi. Male strain mice (25-30 g) were used for biodistribution and pharmacokinetic studies. The Dalton's lymphoma solid (DLS) tumor cells were maintained in the peritoneum of Balb C mice. Animals were kept in cages at a constant temperature and humidity. Water and feed were given ad libitum.
The DLS tumor cells were maintained in the peritoneum of Balb C mice in the ascites form by serial weekly passages. Exponentially growing cells were harvested and tumor cells of 5 × 106 per mouse were injected subcutaneously in the thigh of right hind leg of the Strain A mice. After 8-10 days, a palpable tumor in the volume range of 1.0 ± 0.1 cm3 was observed and used for further studies.
Tc-99m labeled etoposide and etoposide loaded PLGA nanoparticles (100 μl) containing around 200 μCi of radioactivity were injected into the tail vein of healthy and tumor bearing mice. For each injected preparation, three mice were used per time point. The mice were sacrificed 1, 4 and 24 h post-injection. Before sacrificing those mice, at specified time points, mice were anesthetized with excess amount chloroform and blood samples were obtained by cardiac puncture and placed in pre-weighed plastic tubes. The heart, liver, lungs, muscle, bone (femur), kidneys, spleen and brain were isolated.
Along with these organs, tumor was excised from the right hind leg of the tumor induced mice. As a control; muscle from the right hind leg of a healthy animal, which was administered with the same preparation, was used. All the organs/tissues collected were thoroughly rinsed with saline, placed in pre-weighed plastic tubes and weighed. The radioactivity was determined as mentioned above.
Gamma scintigraphy
Gamma imaging was performed for mice after administering intravenously (i.v.) 200 μl of Tc-99m labeled etoposide and PLGA formulation containing 200 μCi of radioactivity. The animals were anesthetized by chloroform prior to imaging. Mice were fixed on a wooden board and imaging was performed on a single photon emission computerized tomography (SPECT, LC 75-005, Diacam, Siemens, USA) after 4 and 24 h of administration.
Statistical analysis
The results of the in vivo biodistribution studies were evaluated by t-test with P ≤ 0.05 as the minimal level of significance. Pharmacokinetic parameters were assessed using a non-compartmental technique with the software program WinNonlin (version 2.1) Pharsight® St. Louis, Missouri, USA. Data was fitted to the model i.v. bolus for i.v. administration. Pharmacokinetic parameters like area under the curve (AUC), mean residence time (MRT) and t1/2 were calculated using this software.
Results and Discussion
Preparation and characterization of nanoparticles
Nanoprecipitation method produced fine dispersion containing nanoparticles without any agglomeration and the procedure was optimized at lab scale for different parameters in the current study.[15] The technique of nanoprecipitation followed by freeze drying yielded good powder particles with excellent redispersibility in aqueous media. Effect of formulation variables such as stabilizer concentration, amount of polymer and the drug was studied. These parameters were found to affect particle size, zeta potential, drug content and entrapment efficiency of nanoparticles. The data from these studies is published previously and optimized formulations was chosen from these trials for further studies.[15] Formulations (prepared with PLGA 85/15 in the presence of Pluronic F 68 as stabilizer) were characterized for different physico-chemical parameters and are given in Table 1. Mean size and polydispersity index values indicate narrow size and homogenous distribution of the particles. Transmission electron microscopy photograph of nanoparticles confirms the round shape of nanoparticles, which are dispersed without any agglomeration in the aqueous media [Figure 1]. Percent recovery of the formulations prepared with these polymers was high and ranged from 85.99 to 96.71. Entrapment efficiency for the formulations prepared with PLGA was about 79% and Zeta potential values indicate the stability of the prepared nanodispersions.
Table 1.
Formulation characters for the optimized PLGA formulation
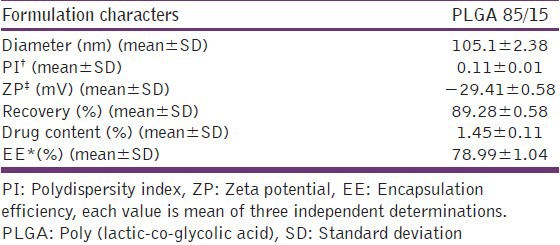
Figure 1.
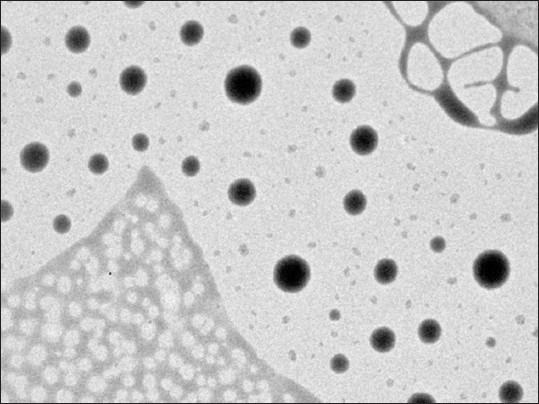
Transmission electron microscopy image of etoposide formulation prepared with poly (lactic-co-glycolic acid) 85/15 (×32,000)
Radiolabeling and labeling efficiency
In the current study, etoposide and PLGA formulation were labeled with Tc-99m with a high labeling efficiency. During this process of radiolabeling, the pertechnetate existing in its heptavalent oxidation state was reduced to a lower valence state by stannous chloride and the pH was adjusted to 6.5 before addition of etoposide or nanoparticles dispersion.[13] This is an established process for radiolabeling of drugs as well as formulations presented in many studies.[10,11] Different variables such as the amount of stannous chloride, pH for radiolabeling, incubation time, stability of radiolabeled complexes in different media were studied extensively for various drug and formulations and were optimized to suit current study for etoposide and nanoparticles. The results of these studies were published previously.[13]
After conducting preliminary trials, the pH to be adjusted for the reduced Tc-99m to obtain high labeling efficiency was optimized to 6.5. The amount of stannous chloride used for reducing the pertechnetate plays a significant role in the labeling process as higher amount results in formation of radio colloids, which are undesirable and lower amounts of stannous chloride results in poor labeling efficiency. In the present study, the optimum amount of stannous chloride required for high labeling efficiency, with a low amount of free and R/H Tc-99m was found to be 100 μg for all preparations. Incubation time in which maximum percentage of labeling occurs was also optimized as 15 min after the addition of Tc-99m-pertechnetate to the preparation. For optimizing above parameters at each time point, quality control tests were carried out by TLC using ITLC strips.[10,17,18,19]
As a part of quality control, radiolabeled preparations were checked for their in vitro stability in the presence of rabbit serum and normal saline. These conditions were selected for stability study to mimic in vivo environment such as serum proteins and physiological pH. All preparations are stable in rabbit serum and normal saline for 24 h. In these media, at all-time points Tc-99m labeled preparations has shown more than 96% radiolabeling the stability of the labeled complexes in different conditions indicates the usefulness of the label as a marker for the biodistribution studies. The materials and the methods used were already established for various drugs to maintain good stability of the labeled complexes.[17,18,19]
Biodistribution, pharmacokinetics and tumor uptake study
Biodistribution pattern of free etoposide and etoposide loaded PLGA nanoparticles in the body of the tumor bearing mice is similar to that of the healthy mice with few exceptions. Radioactivity of nano formulation measured from blood was more in tumor bearing mice (4.10% A/g) than healthy mice (2.49% A/g) at 1 h post-injection. Furthermore, residence time of nanoparticles in blood is more compared with free etoposide. This might be due the faster clearance of free etoposide from the body [Figure 2] compared to formulation. After 24 h of study, PLGA formulation is still present in blood circulation of mice and the radioactivity shown by formulation was 32 times more than that of free etoposide at the same time. Similar observation was presented in a study done for doxorubicin and doxorubicin loaded nanoparticles. The concentration of doxorubicin delivered through nanoparticles after i.v. injection was significantly higher than doxorubicin solution after 2 h post-injection.[10,11]
Figure 2.
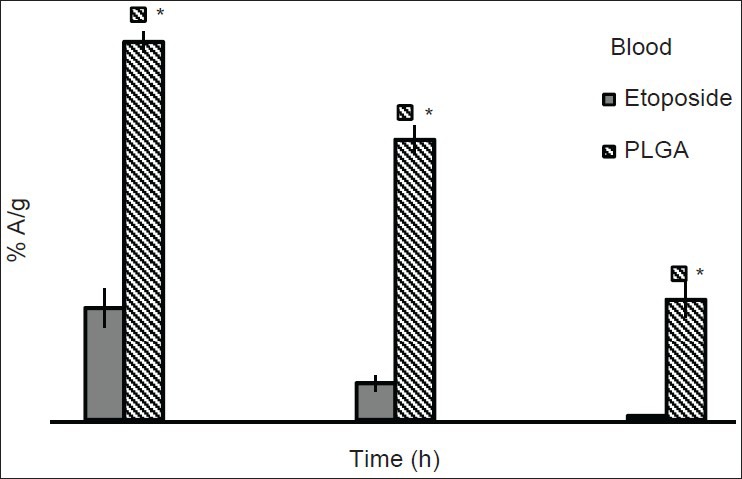
Comparative biodistribution profiles of drug and formulation in blood of Dalton's lymphoma solid tumor induced mice (n = 3). * P ≤ 0.01
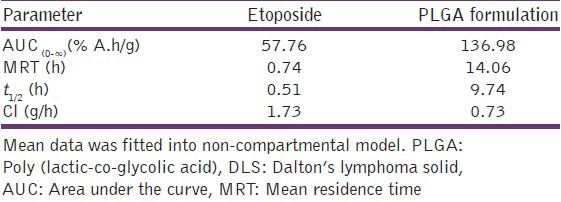
The pharmacokinetic parameters after non-compartmental modeling of Tc-99m labeled etoposide and nanoparticles are given in Table 2. There is a significant difference in all pharmacokinetic parameters between free etoposide and PLGA formulation, (etoposide: t1/2-0.51 h, MRT-0.74 h, AUC0-∞-57.76% A.h/g, Cl-1.73 g/h), PLGA formulation: (t1/2-9.74 h, MRT-14.06 h, AUC0-∞-136.97% A.h/g, Cl-0.73 g/h). Significant increase in t1/2 of PLGA formulation confirms that the nanoparticles significantly enhanced the circulation half-life of etoposide in blood. The same observation was reported for doxorubicin loaded nanoparticles.[10] MRT for nanoparticles is higher than free etoposide, demonstrating their long circulating characteristics. PLGA nanoparticles were prepared by using Pluronic F 68 as stabilizer. Pluronic F 68 has significantly reduced the zeta potential of nanoparticles formed by masking the surface charge of nanoparticles and creating hydrophilic surface on the particle, which inturn enhanced the residence time of nanoparticles. Other pharmacokinetic parameter MRT, AUC0-∞ were also higher for PLGA formulation, whereas clearance was significantly lower.
Table 2.
Pharmacokinetic parameters for etoposide and nanoparticle formulations in DLS tumor induced mice after intravenous administration
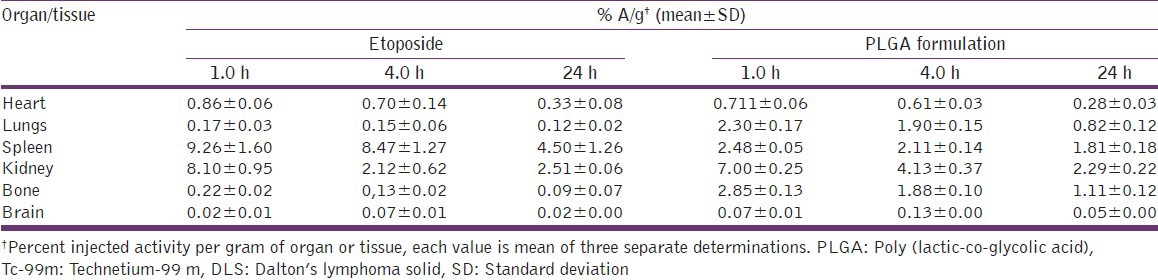
After 1 h of injection, greater concentrations of free etoposide were found in heart, kidney, liver and spleen than formulation. In the heart of tumor mice, uptake of the formulation was less when compared with free etoposide at all the time points of a study indicating the potential to reduce cardiotoxicity associated with etoposide therapy. Lungs have shown significant uptake of nanoparticles formulations than etoposide, but the uptake is less when compared with the normal mice. After administration of nanoparticles and free etoposide through i.v. injection, there is rapid distribution to different organs of the body of mice especially to liver, kidney, spleen, etc., This is observed for healthy as well as tumor bearing mice, but the concentrations in each organ were different for etoposide and formulation.
The highest radioactivity was found in liver after 1 h of administration of free etoposide and PLGA formulation [Figure 3] in tumor bearing mice. After 4 h, free etoposide concentrations started decreasing indicating its elimination from the body, but nanoparticles were present in liver even after 24 h also as indicated by the presence of radioactivity. Spleen uptake of etoposide was higher initially and decreased with time. Spleen capture of the formulation was less when compared to free etoposide. For formulation, around 2.47% A/g was found in spleen at 1 h post-injection where as for etoposide % A/g found was around 9.20. In kidney also, free etoposide got eliminated from the body earlier than the formulation. Presence of higher amount of PLGA nanoparticles in liver, spleen and kidney might be due to the phagocytic uptake by macrophages. This could be efficient in passive targeting of drugs through nanoparticles to various organs/tissues of the body.[20,21] Brain uptake was comparatively more for formulation than free etoposide. However radioactivity measured in the brain was very less. Biodistribution of radiolabeled PLGA nanoparticles in healthy and tumor induced mice in various organs is shown in Tables 3 and 4. Distribution of radiolabeled etoposide in tumor mice is also shown in Table 4. The body distribution and pharmacokinetic data of etoposide and PLGA nanoparticles formulation clearly signifies the advantageous role of nanoparticles in enhancing the circulation time in blood. The slow and prolonged clearance of nanoparticles from different organs can be useful in local chemotherapy of tumors, wherein the tumor cells are exposed to the nanoparticles containing etoposide for a longer time and are provide greater anti-tumor activity compared with i.v. administration of etoposide.[10,11,19] The similar results were observed across the studies in which different types of nanoparticles like PEG-modified gelating nanoparticles, bovine serum albumin nanospheres were used.[20,21]
Figure 3.
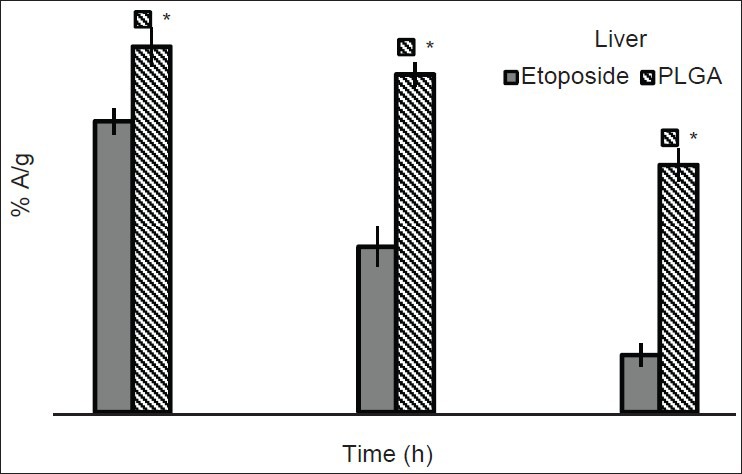
Comparative biodistribution profiles of drug and formulation in the liver of Dalton's lymphoma solid tumor induced mice after intravenous administration (n = 3). * P ≤ 0.01
Table 3.
Biodistribution of Tc-99m labeled PLGA nanoparticles in healthy mice
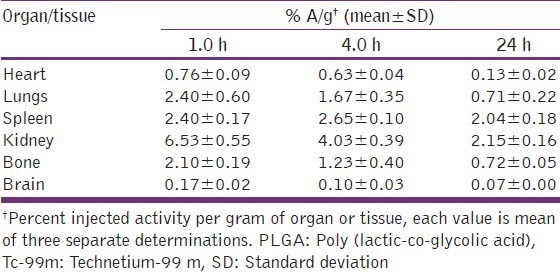
Table 4.
Biodistribution of Tc-99m labeled etoposide and PLGA nanoparticles in DLS tumor induced mice
Tumor uptake of etoposide and nanoparticles
The tumor uptake of etoposide and nanoparticles was studied in DLS tumor induced mice at 1, 4 and 24 h post-injection of the preparations. The uptake of free etoposide and nanoparticles increased with time up to 4 h and then decreased by 24 h. PLGA nanoparticles showed significantly high tumor uptake compared with free etoposide at all-time points studied. The tumor concentration of PLGA nanoparticles was 4.07 folds high at 1 h post-injection, 7.11 fold high at 4 h post-injection and 13.4 fold high at 24 h post-injection [Figure 4]. Radioactivity for free etoposide and formulation in tumor muscle was higher than normal muscle, which was used as control at all-time points [Figure 5]. This observation supports the hypothesis that nanoparticles can accumulate in the tumor tissue by enhanced permeability and retention (EPR) effect.[27] The EPR effect can be attributed to two factors with regard to the long-circulating Nanoparticulate drugs, which (a) escape the vasculature through abnormally leaky tumor blood vessels (b) are subsequently retained in the tumor tissue due to lack of effective tumor lymphatic drainage.[10,22,23,24]
Figure 4.
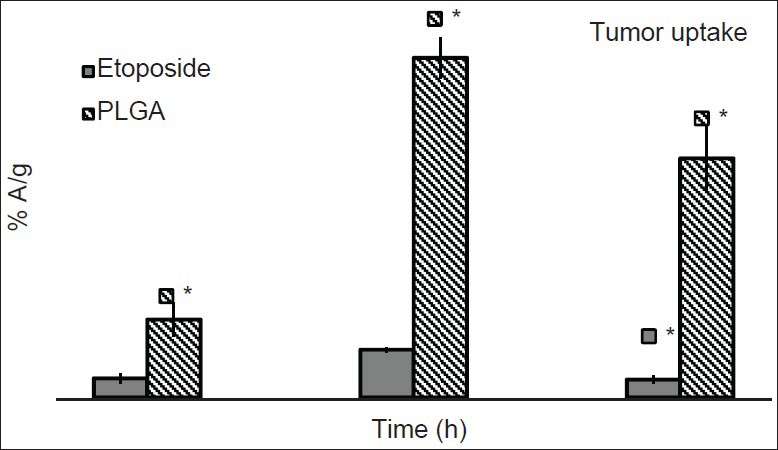
Comparative profile of drug and formulation in tumor muscle of Dalton's lymphoma solid tumor bearing mice (n = 3). * P ≤ 0.01
Figure 5.
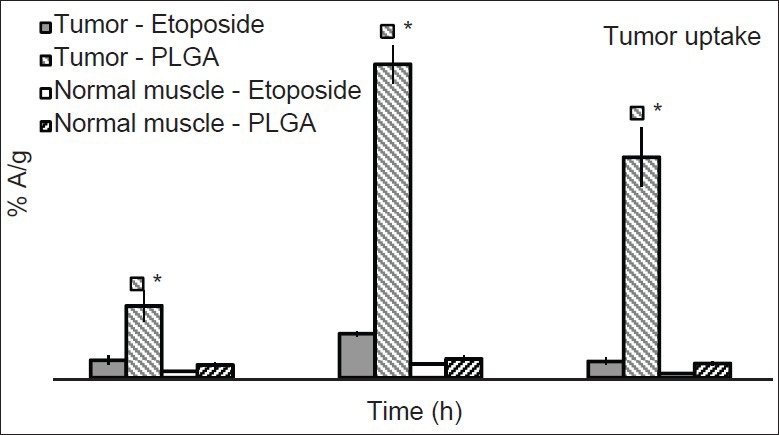
Comparative profiles of drug and formulations in tumor muscle and normal muscle (n = 3). * P ≤ 0.01
Etoposide amount was high in tumor muscle at all-time points when compared with the same muscle in the healthy mice. As the tumor muscles are highly perfused and the vasculature is highly permeable than normal muscles, therefore free etoposide amount is high in the tumor. Free etoposide levels decreased in tumor after 24 h. Whereas, PLGA formulation has shown radioactivity in the tumor after 24 h confirming the presence of formulation in tumor. This is the advantage with nanoparticle formulation as they are available at the tumor for more time to release drug there by showing anti-tumor activity for a longer time, which can reduce the frequency of dosing and also providing targeted drug delivery.[25,26]
Gamma scintigraphy
Gamma scintigraphic images were taken after i.v. administration of etoposide and formulation to DLS tumor induced mice. Whole body of the mice was viewed under gamma camera. As a control, normal mice administered with free etoposide and PLGA formulations were used and radioactivity was measured under gamma camera. Figure 6 shows image taken after 4 h of administration of preparations, which clearly shows more radioactivity than 24 h image [Figure 7]. Circled portions indicate radioactivity present in tumor or normal tissue/muscle. It is evident from the figure that the maximum radioactivity of PLGA formulation is in tumor bearing mice than in healthy mice. In all the cases studied, maximum radioactivity was present in liver. All the above presented results on the biodistribution, pharmacokinetics and tumor uptake of etoposide and PLGA nanoparticles were confirmed with scintigraphy images showing that the radioactivity shown by nanoparticles in DLS tumor mice was more when compared with radioactivity showed by free etoposide. Gamma scintigraphy and imaging found to be an important technique for observing radioactivity in the body of animals and to understand the distribution of various compounds, formulations like Tc-99m-EC-Guanine biodistribution in animals.[27]
Figure 6.
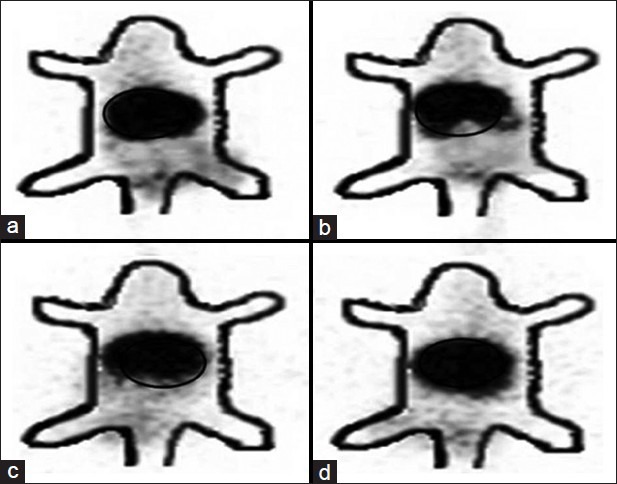
Gamma Scintigraphic images taken 4 h after intravenous administration of Technetium-99 m labeled complexes. (a) Etoposide loaded poly (lactic-co-glycolic acid) formulation in Dalton's lymphoma solid tumor induced mice. (b) Free etoposide in DLS tumor induced mice. (c) Etoposide loaded PLGA formulation in healthy mice. (d) Free etoposide in healthy mice. Circled portions indicate radioactivity present in tumor and normal muscle/tissue. Darker the color more the radioactivity present at that part
Figure 7.
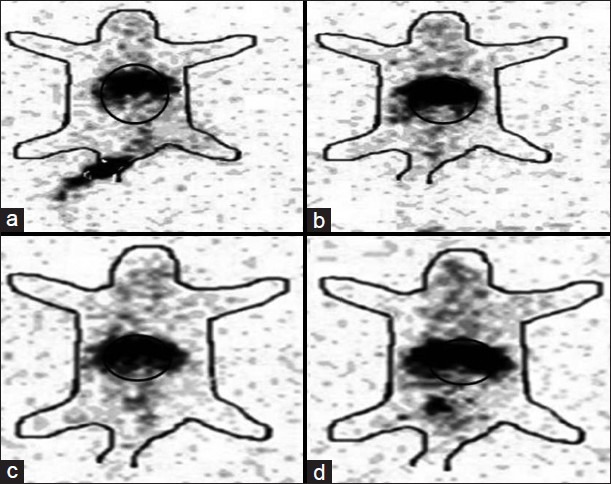
Gamma scintigraphic image of Dalton's lymphoma solid (DLS) tumor induced and normal mice 24 h after intravenous administration. (a)Etoposide loaded poly (lactic-co-glycolic acid) formulation in DLS tumor induced mice. (b) Free etoposide in DLS tumor induced mice. (c) Etoposide loaded PLGA formulation in healthy mice. (d) Free etoposide in healthy mice. Circled portions indicate radioactivity present in tumor and normal muscle/tissue. Darker the color more the radioactivity present at that part
Conclusions
Studies presented in the current article indicate that the administration of PLGA nanoparticles, leads to prolonged retention of PLGA nanoparticles in plasma with increased distribution to tumor tissues when compared to etoposide alone. Outcome of the study revealed a good agreement between the responses and set objective. The slow and prolonged clearances of Etoposide loaded PLGA nanoparticles from different organs can be utilized for local chemotherapy of tumors with further surface modifications of nanoparticles. This study signifies that etoposide loaded PLGA nanoparticles is a better delivery system to deliver etoposide to DLS tumor in high concentrations for a prolonged period of time and is expected to provide greater anti-tumor effect and tumor regression.
Acknowledgments
We are grateful to Dr. R.P. Tripathi, Director, Institute of Nuclear Medicine and Allied Sciences (INMAS), Delhi for providing necessary facilities for radiolabeling experiments, tumor development and maintenance and biodistribution studies. Scholarship provided by the Council of Scientific and Industrial Research (CSIR, New Delhi) is sincerely acknowledged. Authors are grateful to Indian Institute of Chemical Technology (IICT, Hyderabad) and All India Institute of Medical Sciences (AIIMS, New Delhi) for extending their help in analyzing samples by Zeta Sizer, transmission electron microscopy respectively.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hyung Park J, Kwon S, Lee M, Chung H, Kim JH, Kim YS, et al. Self-assembled nanoparticles based on glycol chitosan bearing hydrophobic moieties as carriers for doxorubicin: In vivo biodistribution and anti-tumor activity. Biomaterials. 2006;27:119–26. doi: 10.1016/j.biomaterials.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 2.Leroux JC, Doelker E, Gurny R. New York: Marcel Dekker; 1996. Microencapsulation Methods and Industrial Applications. [Google Scholar]
- 3.Grislain L, Couvreur P, Lenaerts V, Roland M, Deprez-Decampeneere D, Speiser P. Pharmacokinetics and distribution of a biodegradable drug-carrier. Int J Pharm. 1983;15:335–45. [Google Scholar]
- 4.Moghimi SM, Hunter AC. Capture of stealth nanoparticles by the body's defences. Crit Rev Ther Drug Carrier Syst. 2001;18:527–50. [PubMed] [Google Scholar]
- 5.Verdun C, Brasseur F, Vranckx H, Couvreur P, Roland M. Tissue distribution of doxorubicin associated with polyisohexylcyanoacrylate nanoparticles. Cancer Chemother Pharmacol. 1990;26:13–8. doi: 10.1007/BF02940287. [DOI] [PubMed] [Google Scholar]
- 6.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631–51. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 7.Babbar A, Kashyap R, Chauhan UP. A convenient method for the preparation of 99mTc-labelled pentavalent DMSA and its evaluation as a tumour imaging agent. J Nucl Biol Med. 1991;35:100–4. [PubMed] [Google Scholar]
- 8.Bhatnagar A, Singh AK, Babbar A, Soni NL, Singh T. Renal imaging with 99Tc (m)-dextran. Nucl Med Commun. 1997;18:562–6. doi: 10.1097/00006231-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Arulsudar N, Subramanian N, Mishra P, Sharma RK, Murthy RS. Preparation, characterisation and biodistribution of 99mTc-labeled liposome encapsulated cyclosporine. J Drug Target. 2003;11:187–96. doi: 10.1080/10611860310001615415. [DOI] [PubMed] [Google Scholar]
- 10.Reddy LH, Murthy RS. Pharmacokinetics and biodistribution studies of doxorubicin loaded poly (butyl cyanoacrylate) nanoparticles synthesized by two different techniques. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148:161–6. doi: 10.5507/bp.2004.029. [DOI] [PubMed] [Google Scholar]
- 11.Reddy LH, Sharma RK, Murthy RS. Enhanced tumour uptake of doxorubicin loaded poly (butyl cyanoacrylate) nanoparticles in mice bearing Dalton's lymphoma tumour. J Drug Target. 2004;12:443–51. doi: 10.1080/10611860400011406. [DOI] [PubMed] [Google Scholar]
- 12.Yu JJ, Lee HA, Kim JH, Kong WH, Kim Y, Cui ZY, et al. Bio-distribution and anti-tumor efficacy of PEG/PLA nano particles loaded doxorubicin. J Drug Target. 2007;15:279–84. doi: 10.1080/10611860701357235. [DOI] [PubMed] [Google Scholar]
- 13.Snehalatha M, Venugopal K, Saha RN, Babbar AK, Sharma RK. Etoposide loaded PLGA and PCL nanoparticles II: Biodistribution and pharmacokinetics after radiolabeling with Tc-99m. Drug Deliv. 2008;15:277–87. doi: 10.1080/10717540802006500. [DOI] [PubMed] [Google Scholar]
- 14.Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55:R1–4. [Google Scholar]
- 15.Snehalatha M, Venugopal K, Saha RN. Ethoposide loaded PLGA and PCL nanoparticles I: Preparation and effect of formulation variable. Drug Deliv. 2008;15:267–75. doi: 10.1080/10717540802006500. [DOI] [PubMed] [Google Scholar]
- 16.Theobald AE. New York: Gordon and Breach Science; 1990. Textbook of Radiopharmacy: Theory and Practice. [Google Scholar]
- 17.Mishra P, Babbar A, Chauhan UP. A rapid instant thin layer chromatographic procedure for determining radiochemical purity of 99Tcm-IDA agents. Nucl Med Commun. 1991;12:467–9. [PubMed] [Google Scholar]
- 18.Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G, et al. Development and characterization of 99mTc-timolol maleate for evaluating efficacy of in situ ocular drug delivery system. AAPS PharmSciTech. 2009;10:540–6. doi: 10.1208/s12249-009-9238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy LH, Sharma RK, Chuttani K, Mishra AK, Murthy RR. Etoposide-incorporated tripalmitin nanoparticles with different surface charge: Formulation, characterization, radiolabeling, and biodistribution studies. AAPS J. 2004;6:e23. doi: 10.1208/aapsj060323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santhi K, Dhanaraj SA, Koshy M, Ponnusankar S, Suresh B. Study of biodistribution of methotrexate-loaded bovine serum albumin nanospheres in mice. Drug Dev Ind Pharm. 2000;26:1293–6. doi: 10.1081/ddc-100102311. [DOI] [PubMed] [Google Scholar]
- 21.Kaul G, Amiji M. Biodistribution and targeting potential of poly (ethylene glycol)-modified gelatin nanoparticles in subcutaneous murine tumor model. J Drug Target. 2004;12:585–91. doi: 10.1080/10611860400013451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuitmaker JJ, Feitsma RI, Journée-De Korver JG, Dubbelman TM, Pauwels EK. Tissue distribution of bacteriochlorin a labelled with 99mTc-pertechnetate in hamster Greene melanoma. Int J Radiat Biol. 1993;64:451–8. doi: 10.1080/09553009314551641. [DOI] [PubMed] [Google Scholar]
- 23.Molina-Trinidad EM, de Murphy CA, Ferro-Flores G, Murphy-Stack E, Jung-Cook H. Radiopharmacokinetic and dosimetric parameters of 188Re-lanreotide in athymic mice with induced human cancer tumors. Int J Pharm. 2006;310:125–30. doi: 10.1016/j.ijpharm.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 24.Schluep T, Cheng J, Khin KT, Davis ME. Pharmacokinetics and biodistribution of the camptothecin-polymer conjugate IT-101 in rats and tumor-bearing mice. Cancer Chemother Pharmacol. 2006;57:654–62. doi: 10.1007/s00280-005-0091-7. [DOI] [PubMed] [Google Scholar]
- 25.Müller RH, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47:3–19. doi: 10.1016/s0169-409x(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 26.Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res. 1999;16:1564–9. doi: 10.1023/a:1018983904537. [DOI] [PubMed] [Google Scholar]
- 27.Yang DJ, Ozaki K, Oh CS, Azhdarinia A, Yang T, Ito M, et al. (99m) Tc-EC-guanine: Synthesis, biodistribution, and tumor imaging in animals. Pharm Res. 2005;22:1471–9. doi: 10.1007/s11095-005-6157-8. [DOI] [PubMed] [Google Scholar]


