Abstract
Purpose
An isolated perfused pig heart model has recently been proposed for the development of novel methods in standard clinical magnetic resonance (MR) scanners. The original set-up required the electrical system to be within the safe part of the MR-room, which introduced significant background noise. The purpose of the current work was to refine the system to overcome this limitation so that all electrical parts are completely outside the scanner room.
Methods
Four pig hearts were explanted under terminal anaesthesia from large white cross landrace pigs. All hearts underwent cardiovascular magnetic resonance (CMR) scanning in the MR part of a novel combined 3T MR and x-ray fluoroscopy (XMR) suite. CMR scanning included real-time k-t SENSE functional imaging, k-t SENSE accelerated perfusion imaging and late gadolinium enhancement imaging. Interference with image quality was assessed by spurious echo imaging and compared to noise levels acquired while operating the electrical parts within the scanner room.
Results
Imaging was performed successfully in all hearts. The system proved suitable for isolated heart perfusion in a novel 3T XMR suite. No significant additional noise was introduced into the scanner room by our set-up.
Conclusions
We have substantially improved a previous version of an isolated perfused pig heart model and made it applicable for MR imaging in a state of the art clinical 3T XMR imaging suite. The use of this system should aid novel CMR sequence development and translation into clinical practice.
Keywords: cardiovascular magnetic resonance imaging, coronary artery disease, isolated heart perfusion, Langendorff, pig, translational research
Introduction
Cardiovascular magnetic resonance (CMR) is being used increasingly for the diagnosis and assessment of coronary artery disease (CAD) as it offers assessment of myocardial function, viability, and perfusion in a single examination [1–3]. It also compares favourably with other non-invasive methods [4–8]. New sequences, advances in hardware, and post-processing are constantly being developed and require substantial preclinical and translational research before making their way into clinical practice [9]. We have recently introduced an isolated blood perfused heart model to facilitate this process [10]. This model allows control of regional blood flow, oxygenation and workload in order to validate novel MR derived parameters against gold standards, which can expedite translation of new sequences into clinical practice. This system has already been exploited for validation of quantitative MR perfusion imaging [11] using a dual bolus injection scheme for myocardial blood flow (MBF) quantification [12]. However, the original set-up required the electrical system to be within the MR room, which introduced significant background noise [10]. The purpose of the current work was to refine the system to overcome this limitation so that all electrical parts are completely outside the scanner room.
Methods
Redevelopment of the perfusion system
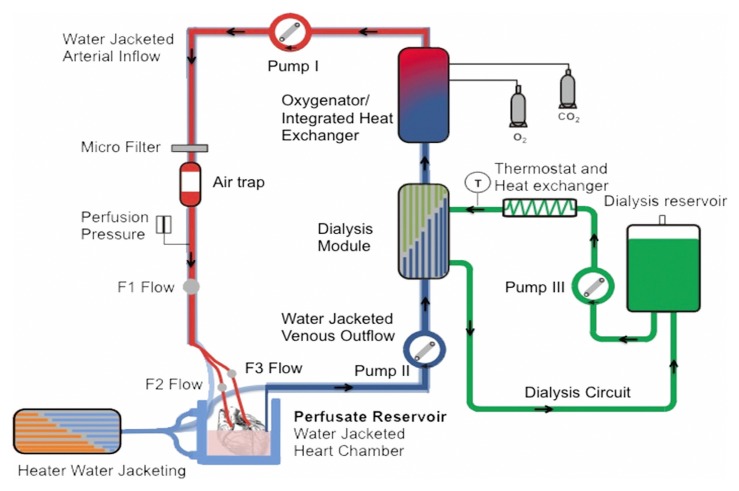
The perfusion circuit of the system remained the same as previously described (Fig. 1) [10]. In brief, it consists of a main circuit to supply the heart with haemoperfusate, a dialysis module to clear out contrast agent and an oxygenator with integrated heat exchanger (Dideco D 100, Mirandola, Modena, Italy) to warm and reoxygenate the haemoperfusate.
Fig. 1.
Schematic view of the perfusion system reproduced from Ref. [10]: roller pump 2 transports the deoxygenated blood through a dialysis module (exchange of metabolites and contrast agent clearance) and then through a blood oxygenator with integrated heat exchanger. The oxygenated blood is then pumped into the heart via roller pump 1
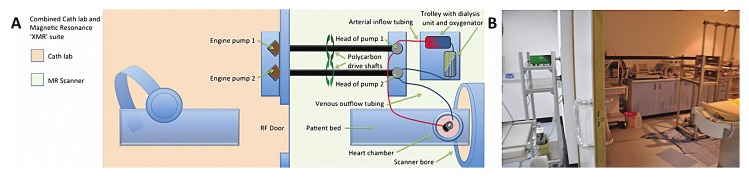
The perfusate consisted of autologous whole blood (1.5 ± 0.2 L), diluted with Krebs–Henseleit solution (1 L, 9.6 g dry mass, Sigma, St. Louis, MO, USA, Krebs–Henseleit buffer K-3753). All equipment used at the scanner was made of MR-compatible materials [13], which did not influence the homogeneity of the main magnetic field. In contrast to the previous set-up where all electrical and magnetic parts (control-unit, power-supply, pump engines, pacer etc.) were kept approximately 6 metres away from the magnet outside the 5 Gauss line but within the scanner room. The new set-up allowed us to operate the system through wave-guides from a neighbouring room (in our case, the X-ray room of a combined 3T XMR suite) (Fig. 2). All electrical and magnetic parts and thus potentially interfering equipment remained in the X-ray room [10]. The pump engines in the X-ray room were connected to the custom-made MR compatible pump heads in the scanner room using polycarbon drive shafts and custom made connectors that were fitted in the wave-guides (Fig. 2).
Fig. 2.
The new set-up allows the operation of the system through wave guides from the X-ray room (Cath lab) of the XMR suite. All electrical and magnetic parts remain in the X-ray room. The pump engines are connected to the custom-made MR compatible pump heads using polycarbon drive shafts through custom made connectors that are fitted in the wave guides
Animal experiments
Hearts were supplied by Harlan Laboratories, UK. All experiments were performed according to protocols approved by the U.K. Home Office in accordance with the U.K. Animals (Scientific Procedures) Act of 1986 and in compliance with the World Medical Association Declaration of Helsinki regarding ethical conduct of research involving animals. Hearts were harvested from Large White Cross Landrace pigs weighing between 40 and 55 kg (average weight of 47 ± 7 kg) as previously described [10]. In brief sedation was performed with ketamine (10 mg/kg i.m.) and xylazine (0.3 mg/kg i.m.). Alphaxolone was used for general intravenous anaesthesia (1.5 mg/kg i.v.). After being heparinised (5000 IU), exsanguination was commenced through the superior vena cava and the heart was removed after transection of the great heart vessels. It was immediately immersed in iced 0.9% saline solution, and intra-coronary infusion of cold (4 °C) cardioplegia infusion (Martindale Pharmaceuticals, Romford, Essex, UK) was performed. Back in the MR-suite, catheters were inserted into the left main and right coronary artery for reperfusion. To simulate cardiac workload, a pressure balloon was inserted through the aortic valve into the left ventricle. After the hearts were cannulated, pressure controlled perfusion of the coronary arteries was started around 50 mmHg. Over approximately 5 min, pressure was slowly increased to accommodate perfusion values around 0.8 mL/min/g. In the event of ventricular fibrillation, electrical defibrillation was performed. In one heart, the imaging was performed while the right coronary artery was occluded.
CMR imaging
Cardiovascular magnetic resonance: MR imaging was performed in a state of the art hybrid clinical 3 Tesla XMR suite (Achieva, Philips, Best, The Netherlands). For signal reception, a large flex interventional coil array was positioned around the heart chamber, which was then placed in the magnet.
MR data were acquired in short axis and long axes (2-chamber, 3-chamber, and 4-chamber view) of the LV. We used a k-t real time fast field echo (FFE) sequence with a repetition time of 3.12 ms, echo time of 1.87 ms, flip angle 15°, spatial resolution at 2.5×2.5×10 mm3 for CINE imaging. For perfusion MR imaging, we used a saturation recovery gradient echo pulse sequence accelerated with k-t BLAST with a repetition time of 2.7 ms, echo time of 0.9 ms, flip angle 20°, spatial resolution at 1.3×1.3×8 mm3. CLEAR was used for homogeneity correction. Perfusion-CMR was performed using a dual bolus scheme (5 mL of neat (0.07 mmol/mL) and 5 mL of dilute (0.007 mmol/mL) gadobutrolum (Gadovist, Schering, Berlin, Germany) [12]. Late gadolinium-enhanced cardiovascular magnetic resonance (LGE-CMR) was performed in identical slice orientations using conventional methods with a repetition time of 4.9 ms, echo time of 2.4 ms, flip angle of 15°, and spatial resolution at 1.7×1.7×8 mm3. Post-processing of perfusion images was performed with dedicated software (Circle Cardiovascular Imaging Suite 12, Release 3.4.0 (84) Calgary, Alberta, Canada) and a dedicated feature tracking prototype software (Diogenes MRI, Tomtec, Germany) [14].
Interference with image quality
All equipment within and close to the MR-scanner was made of MR-compatible materials to avoid adverse effects on the function of the MR-scanner (e.g., significant image artefacts or noise). The main source of potential image degradation would be radiofrequency (rf)-interference with the MR-scanner from the remaining equipment (e.g., control-unit, power-supply, pumps, pacer etc.). In order to test the potential rf-interference, MR-images without rf-excitation were acquired: the resulting noise images allowed the detection of any spurious rf-signal within the bandwidth used by the MR image acquisition. For this, noise images with a bandwidth of 190 kHz were acquired at four different centre frequencies ensuring an rf sweep across a 760-kHz range around the MR resonance frequency. This large frequency range covers all frequencies that can be acquired in different MR experiments. Such noise scans were obtained with the novel set-up running and compared to the background noise with the pump engine running inside the Faraday cage (but outside the 5 Gauss line). As an additional reference, we performed one experiment without any equipment as reference.
Signal to noise ratio: Signal-to-noise ratios (SNRs) for each sequence and different cardiac segments were calculated by dividing each mean signal-intensity (SI) by the standard deviation (SD) of the background noise (measured in the air around the heart).
Results
Intra-coronary haemoperfusion was performed in 4 hearts. Hearts were defibrillated 2–6 times with 30 Joule to reach stable electrical activity with synchronous ventricular contraction.
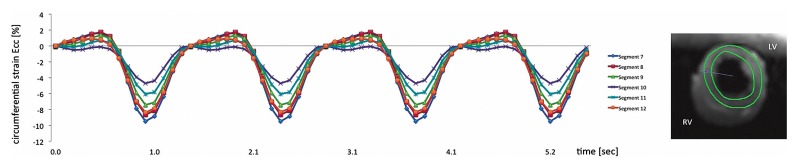
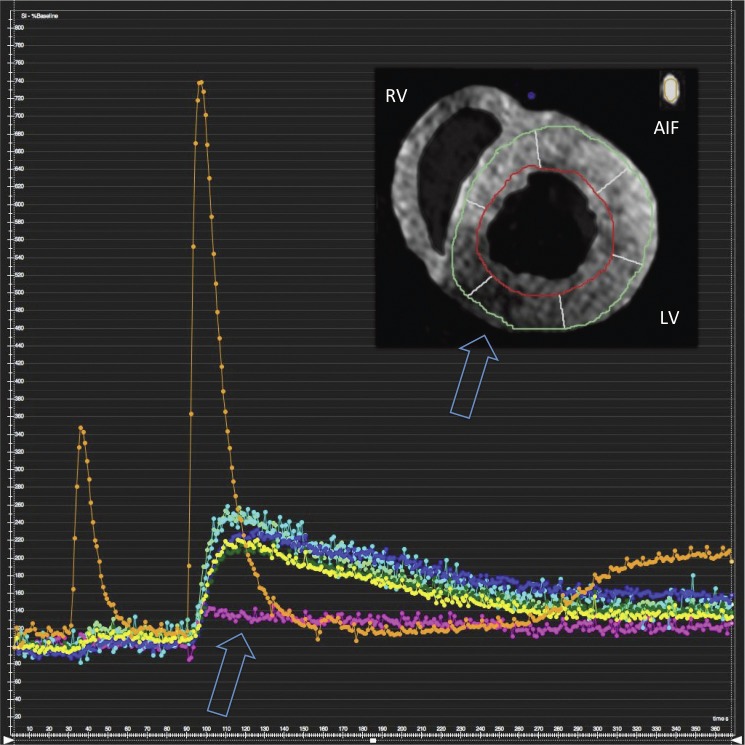
Cine imaging, first pass perfusion imaging, and LGE imaging were successfully performed in all hearts with our newly developed set-up in a clinical 3T XMR suite. Fig. 3 and 4 show an example of myocardial contraction and perfusion of one heart after the RCA was deliberately occluded. Circumferential strain values (Ecc) as obtained with myocardial feature tracking during intra ventricular balloon inflation at 50 mmHg were significantly lower in the inferior wall (Fig. 3). Signal intensity curve analysis during first pass perfusion imaging revealed a perfusion defect in the inferior wall corresponding to the reduced strain values (Fig. 4).
Fig. 3.
Cardiovascular magnetic resonance feature tracking circumferential strain values on a segmental basis using real-time radial k-t SENSE functional imaging over 4 heart beats. There was reduced strain in the inferior wall corresponding to an occluded RCA as compared to remote segments. LV, left ventricle; RV, right ventricle
Fig. 4.
Myocardial first pass dual bolus [12] perfusion imaging showing a perfusion defect in the area of an occluded RCA. Images have been segmented for better visibility. LV, left ventricle; RV, right ventricle; AIF, arterial input function (taken from the inflow tubing)
Interference with image quality
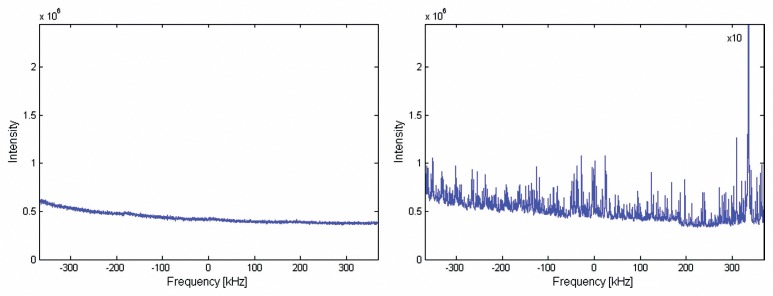
The acquired noise images showed no rf-interference from the equipment outside the Faraday cage. In particular no increase of noise levels was found with the novel set-up running as compared to background noise levels without any additional equipment. With the unshielded pump engine running in the room, overall noise levels increased significantly by a factor of 8. In addition, a high rf-signal was observed at 330 kHz above the MR-resonance frequency that would potentially result in strong image artefacts (Fig. 5). The noise level and the additional spurious rf-signal can be reduced to original values (reference measurement without any instruments) when the pump motor is installed outside the Faraday cage driving the mechanical component of the pump at the scanner through wave guides.
Fig. 5.
The figure shows a comparison between spurious echo noise scans with A: operating our newly developed set-up within the MR scanner room and B: operating the electrical equipment outside the 5 Gauss line within the scanner room. Background noise levels were 8 times higher running the pump within the scanner room and additionally there was high intensity at 330 kHz that would potentially result in image artefacts. There was no difference between running the novel set-up and background noise levels in the scanner room without any equipment (data not shown)
Signal to noise ratio. The SNR values for the different sequences used were as follows: k-t real time FFE CINE 23 ± 10, k-t BLAST perfusion 26 ± 13, and LGE imaging 21 ± 7.
Discussion
The current work demonstrates substantial improvement of a previous version of an isolated perfused pig heart model [10].
First, we were able to reduce noise levels associated with the heart model to a level that is comparable to background noise. A previous preliminary version of our model required the operation of electrical parts of the model (control-unit, power-supply, pump-engines, pacer etc.) within the MR scanner room (outside the 5 Gauss line) that led to an increase of noise levels [10]. For such a set-up with additional electronic equipment inside the Faraday cage, the increase of noise level depends on the experimental set-up, i.e. the position of the additional electronic equipment and the pacing leads with respect to the MR-receive coils determining the rf-coupling and thus the level of spurious rf-signal in the acquired MR-images. Elimination of this excess noise will potentially deliver more reliable and reproducible results.
Second, the novel set-up enabled us to keep the perfusion circuit closely to the scanner, which has previously been validated and shown to be within a near normal physiological range [10]. In particular, there was no need to increase the dead space of the perfusion system by lengthening the tubing. This is important in order to keep the performance as close as possible to normal physiology when testing novel imaging or interventions. The visible heart project used such an ex vivo set-up for endoscopic visualisation of trans-catheter pulmonary valve implantation using high resolution cameras in a human donor heart that was not suitable for transplantation [15]. This endoscopic imaging technique provides a true gold-standard and training platform for new device development and implantation. A similar project, called the Physio Heart, was used for trans-catheter aortic valve implantation in slaughterhouse pig hearts [16]. This full-working heart set-up was described as very close to human physiology with excellent performance for up to 4 h [17]. The current set-up now enables us to perform state-of-the art CMR imaging in such a model within an XMR environment. This infrastructure gives us the flexibility to test new device implantations within the isolated hearts and image their effect on haemodynamic parameters and tissue perfusion and viability with CMR. Within the X-ray part of the XMR suite, direct imaging with endoscopic cameras or ultra-sound imaging is also feasible. Whilst several XMR applications seem interesting for future projects, it is important to note that the system we describe in this paper can be operated in any MR scanner to validate novel MR methodology, independently of an XMR suite.
Currently, our model is designed to operate isolated pig hearts. However, adapting it to use different types of animals in order to address specific scientific questions, for example, the model of extensive collateralisation evident in certain breeds of dogs, seems feasible. Even the study of human donor hearts that are not suitable for transplantation has been described by Eggen and colleagues [18]. They studied pacing induced electrical ventricular asynchrony using CMR in an isolated perfused human heart. Another application is the validation of quantitative myocardial perfusion [11]. The heart model described could close the gap between basic phantom experiments [19] used for very controlled identification and development of suitable sequences and quantification algorithms [20], small animal models for perfusion quantification [21], and the final translational step into patients.
Limitations
The current study investigated the feasibility of a novel perfusion system to be used in a clinical hybrid X-ray fluoroscopy and 3T CMR “XMR” unit and did not test a specific scientific hypothesis. Future studies need to be designed to test novel imaging developments or interventions in this multi-modality imaging set-up. Even though the current model has certain aspects that are superior to living animal set-ups, it was not designed to replace living animal preparations. Initial validation of novel techniques may potentially benefit from a very controlled environment before translating the results into a more realistic scenario such as living animals and patients.
Conclusions
We have substantially improved and tested our animal platform for validation of novel imaging techniques. The infrastructure we have available and the design of our set-up bring the promise of testing, validating, and comparing numerous imaging techniques and novel interventional devices in a very controlled and reproducible fashion.
This model should facilitate rapid translation of novel developments from bench to bedside and into clinical practice.
Acknowledgments
Andreas Schuster is a British Heart Foundation (BHF) Clinical Research Fellow (FS/10/029/28253) and received grant support from the BHF (RE/08/003) and the Biomedical Research Centre (BRC-CTF 196). Amedeo Chiribiri was supported by the Centre of Excellence in Medical Engineering, funded by the Wellcome Trust and Engineering and Physical Sciences Research Council (EPSRC) (grant number WT 088641/Z/09/Z). The authors thank Michael Kelly and Philip Halsted for technical support and David Burt and Michael Walker for procedural support.
Footnotes
Conflict of interest. Tobias Schaeffter served as a Consultant to Philips Healthcare. Eike Nagel received minor consultancy fees from Philips Healthcare and major grant support from Philips Healthcare and Bayer Schering Pharma. The other authors declare that they have no competing interests’.
Contributor Information
Andreas Schuster, 1Division of Imaging Sciences and Biomedical Engineering, King’s College London British Heart Foundation (BHF) Centre of Excellence, National Institute of Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust, Wellcome Trust and Engineering and Physical Sciences Research Council (EPSRC) Medical Engineering Centre, The Rayne Institute, St. Thomas’ Hospital, London, UK; 2Department of Cardiology and Pulmonology and Heart Research Center, Georg-August-University, Göttingen, Germany.
Amedeo Chiribiri, 1Division of Imaging Sciences and Biomedical Engineering, King’s College London British Heart Foundation (BHF) Centre of Excellence, National Institute of Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust, Wellcome Trust and Engineering and Physical Sciences Research Council (EPSRC) Medical Engineering Centre, The Rayne Institute, St. Thomas’ Hospital, London, UK.
Masaki Ishida, 1Division of Imaging Sciences and Biomedical Engineering, King’s College London British Heart Foundation (BHF) Centre of Excellence, National Institute of Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust, Wellcome Trust and Engineering and Physical Sciences Research Council (EPSRC) Medical Engineering Centre, The Rayne Institute, St. Thomas’ Hospital, London, UK.
Geraint Morton, 1Division of Imaging Sciences and Biomedical Engineering, King’s College London British Heart Foundation (BHF) Centre of Excellence, National Institute of Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust, Wellcome Trust and Engineering and Physical Sciences Research Council (EPSRC) Medical Engineering Centre, The Rayne Institute, St. Thomas’ Hospital, London, UK.
Matthias Paul, 1Division of Imaging Sciences and Biomedical Engineering, King’s College London British Heart Foundation (BHF) Centre of Excellence, National Institute of Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust, Wellcome Trust and Engineering and Physical Sciences Research Council (EPSRC) Medical Engineering Centre, The Rayne Institute, St. Thomas’ Hospital, London, UK.
Shazia T. Hussain, 1Division of Imaging Sciences and Biomedical Engineering, King’s College London British Heart Foundation (BHF) Centre of Excellence, National Institute of Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust, Wellcome Trust and Engineering and Physical Sciences Research Council (EPSRC) Medical Engineering Centre, The Rayne Institute, St. Thomas’ Hospital, London, UK.
Boris Bigalke, 1Division of Imaging Sciences and Biomedical Engineering, King’s College London British Heart Foundation (BHF) Centre of Excellence, National Institute of Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust, Wellcome Trust and Engineering and Physical Sciences Research Council (EPSRC) Medical Engineering Centre, The Rayne Institute, St. Thomas’ Hospital, London, UK; 3Department of Cardiology, Eberhard-Karls-University, Tübingen, Germany.
Divaka Perera, 4King’s College London BHF Centre of Excellence, NIHR Biomedical Research Centre and Department of Cardiology, Guy’s and St. Thomas’ NHS Foundation Trust, London, UK.
Tobias Schaeffter, 1Division of Imaging Sciences and Biomedical Engineering, King’s College London British Heart Foundation (BHF) Centre of Excellence, National Institute of Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust, Wellcome Trust and Engineering and Physical Sciences Research Council (EPSRC) Medical Engineering Centre, The Rayne Institute, St. Thomas’ Hospital, London, UK.
Eike Nagel, 1Division of Imaging Sciences and Biomedical Engineering, King’s College London British Heart Foundation (BHF) Centre of Excellence, National Institute of Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust, Wellcome Trust and Engineering and Physical Sciences Research Council (EPSRC) Medical Engineering Centre, The Rayne Institute, St. Thomas’ Hospital, London, UK.
References
- 1.Bettencourt N, Chiribiri A, Schuster A, Nagel E. Assessment of myocardial ischemia and viability using cardiac magnetic resonance. Curr Heart Fail Rep. 2009 Sep;6(3):142–153. doi: 10.1007/s11897-009-0021-9. [DOI] [PubMed] [Google Scholar]
- 2.Morton G, Schuster A, Perera D, Nagel E. Cardiac magnetic resonance imaging to guide complex revascularization in stable coronary artery disease. Eur Heart J. 2010 Sep;31(18):2209–2215. doi: 10.1093/eurheartj/ehq256. [DOI] [PubMed] [Google Scholar]
- 3.Schuster A, Morton G, Chiribiri A, Perera D, Vanoverschelde JL, Nagel E. Imaging in the management of ischemic cardiomyopathy: special focus on magnetic resonance. J Am Coll Cardiol. 2012 Jan 24;59(4):359–370. doi: 10.1016/j.jacc.2011.08.076. [DOI] [PubMed] [Google Scholar]
- 4.Nagel E, Lehmkuhl HB, Bocksch W, Klein C, Vogel U, Frantz E, Ellmer A, Dreysse S, Fleck E. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999 Feb 16;99(6):763–770. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 5.Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–489. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 6.Bandettini WP, Arai AE. Advances in clinical applications of cardiovascular magnetic resonance imaging. Heart. 2008 Nov;94(11):1485–1495. doi: 10.1136/hrt.2007.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DC, Simonetti OP, Harris KR, Holly TA, Judd RM, Wu E, Klocke FJ. Magnetic resonance versus radionuclide pharmacological stress perfusion imaging for flow-limiting stenoses of varying severity. Circulation. 2004 Jul 6;110(1):58–65. doi: 10.1161/01.CIR.0000133389.48487.B6. [DOI] [PubMed] [Google Scholar]
- 8.Schwitter J, Nanz D, Kneifel S, Bertschinger K, Büchi M, Knüsel PR, Marincek B, Lüscher TF, von Schulthess GK. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation. 2001 May 8;103(18):2230–2235. doi: 10.1161/01.cir.103.18.2230. [DOI] [PubMed] [Google Scholar]
- 9.Attili AK, Schuster A, Nagel E, Reiber JH, van der Geest RJ. Quantification in cardiac MRI: advances in image acquisition and processing. Int J Cardiovasc Imaging. 2010 Feb;26(Suppl 1):27–40. doi: 10.1007/s10554-009-9571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster A, Grünwald I, Chiribiri A, Southworth R, Ishida M, Hay G, Neumann N, Morton G, Perera D, Schaeffter T, Nagel E. An isolated perfused pig heart model for the development, validation and translation of novel cardiovascular magnetic resonance techniques. J Cardiovasc Magn Reson. 2010 Sep 17;12:53. doi: 10.1186/1532-429X-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuster A, Chiribiri A, Ishida M, Paul M, Hussain S, Nooralipour NZ, et al. Quantitative assessment of myocardial perfusion by magnetic resonance imaging in the isolated porcine heart. J Cardiovasc Magn Reson. 2011;13:P55. doi: 10.1186/1532-429X-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishida M, Schuster A, Morton G, Chiribiri A, Hussain S, Paul M, Merkle N, Steen H, Lossnitzer D, Schnackenburg B, Alfakih K, Plein S, Nagel E. Development of a universal dual-bolus injection scheme for the quantitative assessment of myocardial perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011 May 24;13:28. doi: 10.1186/1532-429X-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shellock FG, Crues JV 3rd. MR Safety and the American College of Radiology White Paper. AJR Am J Roentgenol. 2002 Jun;178(6):1349–1352. doi: 10.2214/ajr.178.6.1781349. [DOI] [PubMed] [Google Scholar]
- 14.Schuster A, Kutty S, Padiyath A, Parish V, Gribben P, Danford DA, Makowski MR, Bigalke B, Beerbaum P, Nagel E. Cardiovascular magnetic resonance myocardial feature tracking detects quantitative wall motion during dobutamine stress. J Cardiovasc Magn Reson. 2011 Oct 12;13:58. doi: 10.1186/1532-429X-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quill JL, Laske TG, Hill AJ, Bonhoeffer P, Iaizzo PA. Images in cardiovascular medicine. Direct visualization of a transcatheter pulmonary valve implantation within the visible heart: a glimpse into the future. Circulation. 2007 Nov 27;116(22):e548. doi: 10.1161/CIRCULATIONAHA.107.728667. [DOI] [PubMed] [Google Scholar]
- 16.de Weger A, van Tuijl S, Stijnen M, Steendijk P, de Hart J. Images in cardiovascular medicine. Direct endoscopic visual assessment of a transcatheter aortic valve implantation and performance in the Physioheart, an isolated working heart platform. Circulation. 2010 Apr 6;121(13):e261–e262. doi: 10.1161/CIR.0b013e3181d9b879. [DOI] [PubMed] [Google Scholar]
- 17.de Hart J, de Weger A, van Tuijl S, Stijnen JM, van den Broek CN, Rutten MC, de Mol BA. An ex vivo platform to simulate cardiac physiology: a new dimension for therapy development and assessment. Int J Artif Organs. 2011 Jun;34(6):495–505. doi: 10.5301/IJAO.2011.8456. [DOI] [PubMed] [Google Scholar]
- 18.Eggen MD, Bateman MG, Rolfes CD, Howard SA, Swingen CM, Iaizzo PA. MRI assessment of pacing induced ventricular dyssynchrony in an isolated human heart. J Magn Reson Imaging. 2010 Feb;31(2):466–469. doi: 10.1002/jmri.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiribiri A, Schuster A, Ishida M, Hautvast G, Zarinabad N, Morton G, Otton J, Plein S, Breeuwer M, Batchelor P, Schaeffter T, Nagel E. Perfusion phantom: An efficient and reproducible method to simulate myocardial first-pass perfusion measurements with cardiovascular magnetic resonance. Magn Reson Med. 2013 Mar 1;69(3):698–707. doi: 10.1002/mrm.24299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida M, Morton G, Schuster A, Nagel E, Chiribiri A. Quantitative assessment of myocardial perfusion MRI. Curr Cardiovasc Imag Rep. 2010;3:65–73. [Google Scholar]
- 21.Makowski M, Jansen C, Webb I, Chiribiri A, Nagel E, Botnar R, Kozerke S, Plein S. First-pass contrast-enhanced myocardial perfusion MRI in mice on a 3-T clinical MR scanner. Magn Reson Med. 2010 Dec;64(6):1592–8. doi: 10.1002/mrm.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]