Abstract
Objective
To assess the efficacy and safety of laser-assisted percutaneous coronary interventions (PCI) in an unselected population.
Methods
One hundred consecutive patients, who underwent a laser assisted PCI between January 2008 and March 2012, were included in the present study. Fifty-one patients underwent laser ablation for thrombus vaporization (Group 1), 36 patients for neointima/plaque debulking (Group 2) and 13 patients for lesion compliance modification in calcified lesions (Group 3).
Results
The rate of in-hospital serious events was 2%. The cumulative laser success was 82%, and it was significantly higher for Group 1 and Group 2 in comparison with Group 3 (p = 0.001). Furthermore, the need for repeat revascularization was significantly higher in the Group 3 compared with the others two groups (46% vs. 8% for Group 1 and 11% for Group 2, p = 0.03). The MACE rate was 14%. There was a trend toward a higher MACE rate in the Group 3 compared with others two groups (p = 0.05).
Conclusions
Laser ablation is an effective and safe tool for complex PCI. Patients underwent laser for thrombus vaporization or for neointima/plaque debulking had better immediate success and better outcome at follow-up than patients underwent laser for lesion compliance modification.
Keywords: excimer laser, percutaneous transluminal coronary angioplasty, stents
Introduction
Nowdays, cardiac interventionalists perform more and more complex percutaneous coronary intervention (PCI), but technical challenges remain despite advances in equipment and technique. Since the first intravascular laser intervention, the technique has been significantly improved by the use of optimized wavelength, the development of flexible multifiber catheters and an additional saline “flush and bathe” technique [1, 2]. Several coronary indications for laser use are currently accepted. Treatment of acute myocardial infarction (AMI) and thrombus-laden coronary lesion are one of the most relevant because 308-nm pulsed-wave ultraviolet excimer laser light can vaporize thrombus, suppress platelet aggregation, and, unlike other thrombectomy devices, ablates the underlying plaque [3–8]. The efficacy of laser for in-stent restenosis has been suggested in several studies [9–12]. There are some evidences about the usefulness of laser in the treatment of degenerated saphenous vein grafts [4, 13]. Other applications of the device are total occlusions, as long as crossable by a guidewire [14], moderately calcified and balloon refractory lesions [14, 15].
The aim of this study was to assess the efficacy and safety of laser-assisted PCI in an unselected population. Furthermore, we analyzed major cardiovascular adverse event rate and target vessel revascularization at midterm follow-up.
Methods
Patient selection
Out of 102, we selected 100 patients who underwent a laser assisted PCI in the cath lab of Potenza San Carlo Hospital, from January 2008 to March 2012 for the following indications: 51 patients for thrombus vaporization [16, 17] (Group 1), 36 patients for neointima/plaque debulking for in-stent restenosis or saphenous vein graft lesions (Group 2), and 13 patients for lesion compliance modification in moderate-highly calcified lesions (Group 3). All patients provided informed consent for the procedure and subsequent data collection and analysis for research purposes. Procedural anticoagulation and antiplatelet therapy followed standard protocols. Aspirin was continued indefinitely, and thienopyridine was prescribed for at least 1 month after bare metal stent implantation and at least 12 months after drug eluting stent implantation.
Coronary intervention
The laser ablation was carried out with the excimer laser system (CVX-300®, Spectranetics, Colorado Springs, CO) using a pulsed xenon-chlorine mid-ultraviolet wave length (wave length: 308 nm, pulse duration 135 ns and output of 165 mJ/pulse). The laser catheters with concentric tips and a size of 0.9 mm, 1.4 mm, 1.7 mm, and 2.0 mm were used, depending on the vessel size. For safety reason, catheter laser diameter was never >50% of the reference vessel diameter. Lasering was started with a delivery rate of 25 Hz and an energy density of 45 mJ/mm2, and was increased if necessary as described by Dörr et al. [7]. During the lasering, the saline flush-bathe technique was applied to facilitate laser transmitted pressure wave. The laser catheter was moved forward at speed of 0.2 to 0.5 mm. The safe pulse-and-retreat technique [7] was routinely applied. In case of incomplete ablation, additional passes were carried-out also with a bigger catheter. Coronary interventions were finalized by stenting the target lesion using the standard technique. Procedural costs were also computed.
Data collection, end points, and study definitions
Clinical follow-up was performed by telephone contact or office visit at 1, 6, and 12 months after the index procedure. Angiographic follow-up was clinically driven or scheduled at the operator discretion. Angiographic success was defined as a final residual stenosis less than 20% with TIMI flow grade 3 [18]. Laser success was defined as complete crossing of target lesion by laser catheter, a decrease in the diameter stenosis >20%, and a final TIMI 3 flow without any major coronary complication (distal embolization, major dissection, vessel perforation) [18]. A successful PCI was defined when an angiographic success was achieved without major clinical complications (e.g., death, AMI, emergency coronary artery bypass surgery) during hospitalization [19]. In patients undergone laser for thrombus vaporization, we evaluated the thrombus score [20] and myocardial blush grade [21], at baseline, after laser ablation and at the end of procedure. The clinical end points analyzed were periprocedural AMI, death, after-discharge AMI, target vessel revascularization, target lesion revascularization, and major adverse cardiac events (MACE). MACE were defined as a composite of death, AMI, and target vessel revascularization during the follow-up period and were evaluated on a per-patient basis. All deaths were considered cardiac, unless otherwise documented. We defined post-procedural AMI as elevation of biomarker values >5×99th percentile upper reference limit [22]. Nonprocedural or after-discharge AMI was defined as an elevation of troponin above the upper range limit in combination with at least 1 of the following: symptoms of ischemia, electrocardiographic changes indicative of new ischemia, or the development of pathological Q waves on electrocardiogram. Angiographic stent restenosis was consider a diameter stenosis of ≥50% within the 5-mm borders proximal or distal to the stent edge. We defined target lesion revascularization as repeat revascularization within the stent or within the 5-mm borders proximal or distal to the stent edge at the follow-up angiogram. Target lesion revascularization was considered ischemic-driven if associated with a positive functional study result and/or ischemic symptoms and a target lesion diameter stenosis of ≥50% by visual estimation, or a target lesion diameter stenosis of ≥70% with or without documented ischemia. Quantitative coronary angiographic analysis was performed based upon visual assessment.
Statistical analysis
Categorical variables are presented as percentages and were compared with chi-squared or Fisher exact test, as appropriate. Continuous variables are reported as mean±standard deviation or median and were compared with ANOVA. Pre- and post-procedure TIMI flow, blush grade, and thrombus score were compared using a Wilcoxon matched-pairs signed-ranks test. A pvalue of <0.05 was considered to be statistically significant, and all reported p-values are 2-sided. Statistical analysis was performed using SPSS version 18 (SPSS, Chicago, IL, USA).
Results
Table I summarizes the principal clinical and angiographic features of the predefined three groups. In the thrombus vaporization group, 23 patients received a laser for stent thrombosis and 28 patients received laser for coronary thrombus resistant to manual catheter-aspiration during AMI (Fig. 1). In the neointima/plaque debulking group, 15 patients underwent laser for a non-focal stent restenosis (Fig. 2), of which 8 had drug eluting stent restenosis, and 21 patients for a saphenous vein graft disease, in which the filter could not be positioned due to ostial or distal localization of the stenosis (n = 8), diffuse disease (n = 9), or completely occluded graft (n = 4). Thirteen patients underwent laser-assisted PCI for hard lesion compliance modification: 6 had uncrossable lesion with balloon, 5 had an undilatable balloon lesion, one had a drug-eluting stent restenosis unable to be expanded with appropriate noncompliant-balloon, and the last one received a laser for unexpanded stent.
Table I.
Baseline clinical characteristics of 100 patients underwent laser-assisted percutaneous coronary intervention
| Thrombus vaporization (N = 51) | Plaque debulking (N = 36) | Compliance modification (N = 13) | P-value | |
|---|---|---|---|---|
|
| ||||
| Age (years) | 59±12 | 64±8 | 67±10 | 0.12 |
| Male gender | 86 | 75 | 71 | 0.51 |
| Previous MI | 32 | 37 | 43 | 0.85 |
| Previous CABG | 11 | 75 | 29 | 0.001 |
| Previous PCI | 29 | 55 | 43 | 0.14 |
| DM | 7 | 30 | 57 | 0.004 |
| Hypertension | 54 | 70 | 86 | 0.17 |
| Dyslipidemia | 36 | 60 | 57 | 0.18 |
| Family history | 29 | 50 | 14 | 0.14 |
| Smoking | 64 | 70 | 43 | 0.44 |
| Angina | 15 | 65 | 57 | 0.007 |
| ACS-NSTEMI | 30 | 35 | 43 | 0.849 |
| STEMI | 55 | 0 | 0 | 0.019 |
| Ejection fraction | 48±8 | 48±9 | 54±10 | 0.94 |
| Multivessel disease | 46 | 90 | 86 | 0.02 |
| Lesion location | ||||
| LAD | 46 | 16 | 57 | 0.001 |
| LCX | 4 | 16 | 0 | |
| RCA | 43 | 10 | 43 | |
| SVG | 7 | 58 | 0 | |
| TIMI-flow 3 | 25 | 35 | 100 | 0.01 |
|
| ||||
| Data are presented as percentages or means±standard deviation, unless otherwise specified. MI = myocardial infarction; CABG = coronary artery bypass graft; PCI = percutaneous coronary intervention; DM = diabetes mellitus; ACS-NSTEMI = acute coronary syndrome-non ST-elevation myocardial infarction; LAD = left anterior descending; LCX = left circumflex; RCA = right coronary artery; SVG = saphenous vein graft; TIMI=thrombolysis in myocardial infarction | ||||
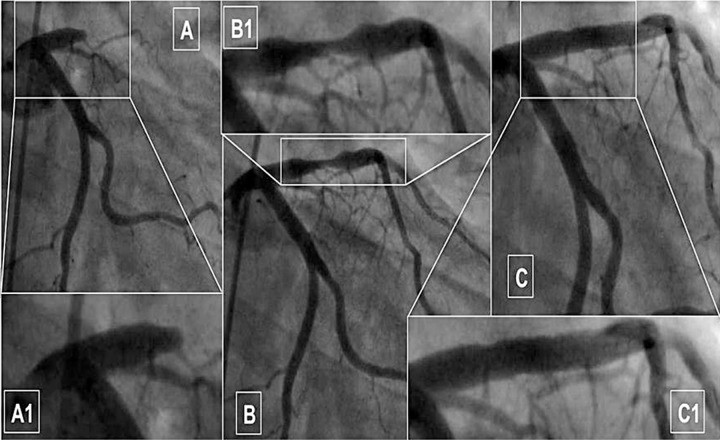
Fig. 1.
Case of dethrombosis after failure of manual thromectomy, during anterior ST elevation myocardial infarction: thrombotic occlusion at the proximal segment of left anterior descending artery (A, magnified in A1); angiographic result after excimer laser angioplasty using 1.7 mm catheter (fluence 25, rate 45 Hz) (B, magnified in B1); good final result after bare metal stent (C)
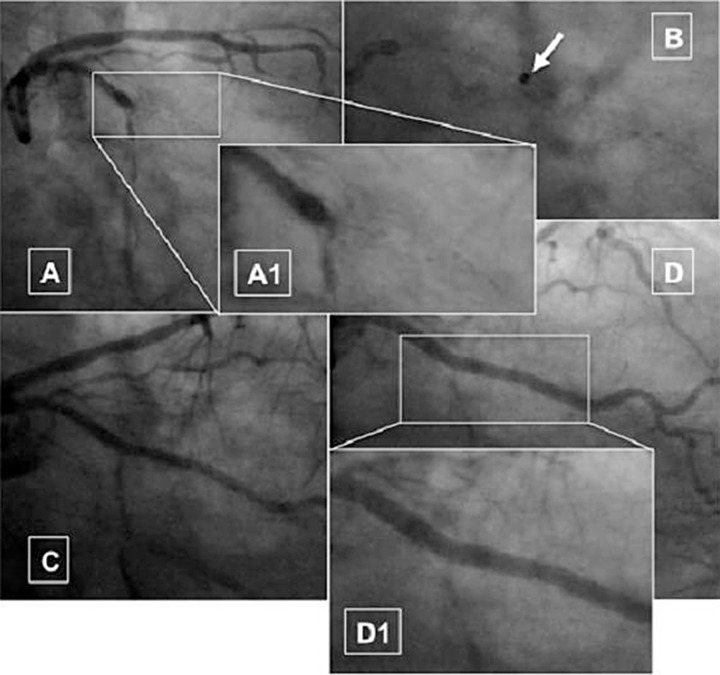
Fig. 2.
Case of plaque debulking: occlusive in stent restenosis of bare metal stent in the proximal segment of obtuse marginal branch (A, magnified in A1); neo-intima debulking using 1.4 and 1.7 mm laser catheters (fluence 25, rate 45 Hz) (arrow in B); immediate angiographic result after lasering (C); final result after two drug eluting stents in partial overlapping (D, magnified in D1)
Quantitative coronary analysis, procedural findings, and in-hospital events
Table II shows procedural findings and quantitative coronary analysis. In Group 1, TIMI flow increased from 1.14±1.25 to 2.46±0.89 (p < 0.001) and blush grade from 1.02±1.29 to 2.41±0.97 (p < 0.001) after laser use. Conversely, thrombus score decreased from 4.20±1.40 to 1.36±1.51 (p < 0.001). In Group 2, the minimal lumen diameter increased from 0.25±0.35 to 2.83±0.76 (p = 0.001) after laser; accordingly, diameter stenosis decreased from 92±10 to 14±21 (p = 0.001).
Table II.
Angiographic procedural characteristics and follow-up data of 100 patients underwent laser-assisted percutaneous coronary intervention
| Thrombus vaporization (N = 51) | Plaque debulking (N = 36) | Compliance modification (N = 13) | P-value | |
|---|---|---|---|---|
|
| ||||
| QCA pre | ||||
| Lesion length | 25±15 | 27±16 | 15±4 | 0.13 |
| % Stenosis | 95±8 | 92±10 | 89±10 | 0.31 |
| MLD | 0.19±0.28 | 0.25±0.35 | 0.31±0.30 | 0.54 |
| RVD | 3.11±0.40 | 3.08±0.59 | 2.79±0.24 | 0.25 |
| QCA post | ||||
| % Stenosis | 11±18 | 14±21 | 30±44 | 0.15 |
| MLD | 2.91±0.66 | 2.83±0.76 | 2.14±1.38 | 0.06 |
| RVD | 3.29±0.35 | 3.25±0.54 | 2.90±0.32 | 0.10 |
| Procedural characteristics | ||||
| Stent implantation | 93 | 85 | 72 | 0.27 |
| Rotablator | 0 | 0 | 23 | 0.001 |
| Laser catheter size | 0.45 | |||
| 0.9 mm | 18 | 10 | 29 | |
| 1.4 mm | 43 | 50 | 29 | |
| 1.7 mm | 39 | 30 | 42 | |
| 2.0 mm | 0 | 10 | 0 | |
| Laser Hz | 25.5±8.3 | 25.2±1.1 | 54.3±27.6 | 0.001 |
| Laser mJ/mm2 | 44.1±9.1 | 45.2±1.1 | 54.3±25.1 | 0.08 |
| Laser success | 86 | 89 | 43 | 0.001 |
| Procedural success | 96 | 95 | 71 | 0.05 |
| Complication | 14 | 5 | 14 | 0.56 |
|
| ||||
| Data are presented as percentages or means±standard deviation, unless otherwise specified. MLD = minimal lumen diameter; RVD = reference vessel diameter | ||||
There was a trend toward a smaller post-procedure minimal lumen diameter and higher residual stenosis in the Group 3 compared with the others two group. Out of 87 patients receiving a stent, 52% received a drug eluting stent. Eighteen patients had a laser failure: unable to achieve the target lesions in 5 patients for excess vessel tortuosity, ineffective ablation in 11 patients, and 2 effective ablations but complicate with a major dissection. Laser success was significant higher for the Group 1 and Group 2 in comparison with Group 3 (p = 0.001). There was a strong trend toward higher procedural success rate for the Group 1 (96%) and Group 2 (95%) in comparison with Group 3 (71%; p = 0.05). Laser success and procedural success in the 21 patients with saphenous vein graft disease were, respectively, 86% and 91%. Procedural complications were recorded in 11 patients, of which only two had a clinical consequence. Four patients had a distal embolization, two during a PCI, without a distal protection, on friable, plaque-burden lesions of saphenous vein graft and the other two, during a primary PCI. Two patients had minor coronary perforation and two a small coronary dissection; both complications sealed with stents. These patients were asymptomatic at follow-up. Two patients had spiral dissection, during PCI for diffusely calcified lesions on right coronary artery, that evolved in Q-wave MI due to vessel occlusion. The last dissection occurred to a 45-year-old female, during a primary PCI on ostial left anterior descending, after laser with a 1.7-mm catheter for a manual catheter resistant thrombus. The dissection associated with a minor coronary perforation has been treated with conventional bare-metal stent. This patient after 3 months underwent angiography that showed a severe stenosis associated with a coronary pseudo-aneurysm on the ostial left anterior descending. The patient underwent a successful coronary artery bypass with a left internal mammary for the left anterior descending, and a computer tomography showed the complete closure of the pseudo-aneurysm [23].
The rate of in-hospital serious events was 2%: two Q-wave AMI, in patients with spiral dissection. Nine patients had an asymptomatic but significant post-PCI AMI. The median cost of a procedure was 9.150±2.680 Euros.
Follow-up analysis
The median follow-up was 526±263 days. The MACE rate was 14%. Only two patients died at follow-up (one of sudden death and another of an end-stage heart failure). No patient had an AMI at follow-up. Twelve patients had a target lesion revascularization, of which three underwent coronary by-pass and nine a second PCI. There was a trend toward a higher MACE rate in the Group 3 compared with others two groups (p = 0.05). The need for repeat revascularization was significantly higher in Group 3 compared with the other two groups (46% vs. 8% for Group 1 and 11% for Group 2, p = 0.03). Of note, MACE rate in the patients with saphenous vein graft disease was 5%.
The higher MACE rate was found in women and in patients with a less complex lesion, but this could be a bias related to the low number (n = 5) of B1 type lesion. Conversely, patients underwent laser for AMI had significant lower MACE. We also found a positive trend between target vessel revascularization and a laser catheter diameter used in the patients whose procedure was successful (n = 82); this could be related to the higher risk of vessel injury with bigger catheter diameter.
Discussion
In the present study, the low rate of in-hospital serious event demonstrated that laser was a safe tool for complex PCI. In our series, the “flush-and-bathe” laser technique resulted in 82% of laser success, significantly lower in patients with solid lesions (43% for lesion compliance modification group) than patients with softer lesions (86% for thrombus-laden lesion vaporization group and 89% for neointima/plaque debulking). Patients in the lesion compliance modification group also had a lower procedural success rate (71% versus 96% and 95%) and a higher rate of target vessel revascularization (46% versus 8% and 11%). The higher target vessel revascularization rate in the compliance modification group may be related to the higher diabetes mellitus rate and to a smaller final minimal lumen diameter.
The literature on this topic is not unique. One study made a clear distinction between calcified and noncalcified lesions, with respective procedural success rate of 79% and 96% (p < 0.05) [24]. A large study conducted on this topic showed that, in calcified lesions, laser success rate improved using X80 catheter at high energy (up to 80 mJ/mm2 and 80 Hz) if compared with standard technique (respectively 92% and 69%; p ≤ 0.001), without increase in complications [15]. A recent published paper, including 60 all comer patients, showed, if compared with our study, a similar procedural success for overall population (93%), lesion vaporization group (91%), and for neointima/plaque debulking group (100%) but a higher success (89%) in lesion compliance modification group [25]. However, the same authors reported lower MACE rate at 6 months follow-up in the first two groups compared with the lesion compliance modification group (respectively, 17%, 18.7%, and 33.3%). The highest energies used to modify calcified lesion compliance may induce vessel injury, leading to a higher target vessel revascularization in the follow-up.
In present study, the patients underwent laser for thrombus vaporization had excellent results: 96% of procedural success, 10% MACE, and 8% target vessel revascularization rate. The CARMEL trial carried out in patients underwent laser for AMI showed an excellent immediate results: procedural success 91% with a 5% of complication rate [3]. Our data, on this subset of patients, are similar to that of Ambrosini et al., reporting the largest single experience, with a 99% of procedural success and 95% event free survival after 6 months of follow-up [8].
In our study, the laser and procedural success in patients with saphenous vein graft disease were, respectively, 86% and 91%, with a 5% MACE rate. A large “pre-stent era” study, conducted in 496 patients underwent laser and balloon angioplasty for saphenous vein graft disease showed a good immediate result (92% clinical success and 6.1% acute complication rates) but a high restenosis rate (55%) at 6 months follow-up [26]. A second study, conducted in the “stent era”, on 31 patients with AMI underwent laser assisted PCI on saphenous vein graft, reported laser and procedure success, respectively, of 87% and 84%, with a 3% of laser related complications and 13% of in-hospital MACE [13].
In our study, fifteen patients underwent laser for in stent restenosis with a 100% laser success without procedural complication, but with a 27% of follow-up target vessel revascularization rate. The LARS trial, including 400 patients with bare metal stent restenosis, reported a procedural success rate of 92% with 4.9% laser-related coronary dissection [11]. Mehran et al. [12], comparing clinical results at a six-month follow-up of laser plus angioplasty vs. angioplasty alone in patients with restenosed stent, showed a trend for lower target vessel revascularization rate in the first group (21% vs. 38%, p = 0.08). Currently, no large study evaluated the effect of laser for drug eluting stent restenosis.
Although Spectranetics laser received the approval for treatment of many complex lesions, it still does not have a widespread application in PCI. The first reason could be the absence of multicenter randomized studies comparing laser ablation safety and long-term efficacy with standard procedure. A main limitation of the present study is the low number of patients which do not allow drawing any definitive conclusion on long-term outcome. Second, there are some disparity in the technical utilization of laser between center and single operators; third, laser assisted PCI is an expensive procedure (the median cost of a laser-assisted PCI in our hospital was >9.000 Euros).
In conclusion, the present study showed that laser is a powerful device for thrombus vaporization, especially in thrombus resistant to manual aspiration catheters, and for neointima/plaque debulking in patients with in-stent restenosis or saphenous vein graft disease. More studies are needed to demonstrate the safety and efficacy of the “flush and bathe” laser technique for lesion compliance modification in moderate-highly calcified lesions. The new laser “explosion” technique may be applied in this subset of patients to improve procedural success [27].
Contributor Information
Giandomenico Tarsia, 1Heart and Vessels Department, San Carlo Hospital, Potenza, Italy.
Mario De Michele, 2Division of Cardiology, Moscati Hospital, Aversa, Italy.
Nicola Viceconte, 1Heart and Vessels Department, San Carlo Hospital, Potenza, Italy.
Kensuke Takagi, 3Interventional Cardiology Unit, San Raffaele Hospital-Emo GVM Centro Cuore Columbus, Milan, Italy; 4New Tokyo Hospital, Tokyo, Japan.
Carmine Biscione, 5Division of Cardiology, Department of Internal Medicine, University of Rome ‘Tor Vergata’, Rome, Italy.
Giuseppe Del Prete, 1Heart and Vessels Department, San Carlo Hospital, Potenza, Italy.
Domenico Polosa, 1Heart and Vessels Department, San Carlo Hospital, Potenza, Italy.
Roccoaldo Osanna, 1Heart and Vessels Department, San Carlo Hospital, Potenza, Italy.
Pasquale Lisanti, 1Heart and Vessels Department, San Carlo Hospital, Potenza, Italy.
References
- 1.Deckelbaum LI, Natarajan MK, Bittl JA, Rohlfs K, Scott J, Chisholm R, Bowman KA, Strauss BH. Effect of intracoronary saline infusion on dissection during excimer laser coronary angioplasty: a randomized trial. The Percutaneous Excimer Laser Coronary Angioplasty (PELCA) Investigators. Journal of the American College of Cardiology. 1995 Nov 1;26(5) doi: 10.1016/0735-1097(95)00330-4. [DOI] [PubMed] [Google Scholar]
- 2.Tcheng JE, Wells LD, Phillips HR, Deckelbaum LI, Golobic RA. Development of a new technique for reducing pressure pulse generation during 308-nm excimer laser coronary angioplasty. Catheterization and cardiovascular diagnosis. 1995 Jan 1;34(1) doi: 10.1002/ccd.1810340306. [DOI] [PubMed] [Google Scholar]
- 3.Dahm JB, Ebersole D, Das T, Madyhoon H, Vora K, Baker J, Hilton D, Topaz O. Prevention of distal embolization and no-reflow in patients with acute myocardial infarction and total occlusion in the infarct-related vessel: a subgroup analysis of the cohort of acute revascularization in myocardial infarction with excimer laser-CARMEL multicenter study. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2005 Jan 1;64(1) doi: 10.1002/ccd.20239. [DOI] [PubMed] [Google Scholar]
- 4.Ebersole D, Dahm JB, Das T, Madyoon H, Vora K, Baker J, Hilton D, Alderman E, Topaz O. Excimer laser revascularization of saphenous vein grafts in acute myocardial infarction. The Journal of invasive cardiology. 2004 Apr 1;16(4) [PubMed] [Google Scholar]
- 5.Dahm JB, Topaz O, Woenckhaus C, Staudt A, Möx B, Hummel A, Felix SB. Laser-facilitated thrombectomy: a new therapeutic option for treatment of thrombus-laden coronary lesions. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2002 Jul 1;56(3) doi: 10.1002/ccd.10200. [DOI] [PubMed] [Google Scholar]
- 6.Ilkay E, Karaca I, Yavuzkir M, Akbulut M, Pekdemir M. The effect of interventional treatment in acute myocardial infarction on ST resolution: a comparison of coronary angioplasty with excimer laser angioplasty. Angiology. 2005 Jul-Aug;56(4) doi: 10.1177/000331970505600403. [DOI] [PubMed] [Google Scholar]
- 7.Dörr M, Vogelgesang D, Hummel A, Staudt A, Robinson DM, Felix SB, Dahm JB. Excimer laser thrombus elimination for prevention of distal embolization and no-reflow in patients with acute ST elevation myocardial infarction: results from the randomized LaserAMI study. International journal of cardiology. 2007 Mar 2;116(1) doi: 10.1016/j.ijcard.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Ambrosini V, Cioppa A, Salemme L, Tesorio T, Sorropago G, Popusoi G, Stabile E, Medolla A, Cangella F, Agrusta M, Picano E, Rubino P. Excimer laser in acute myocardial infarction: single centre experience on 66 patients. International journal of cardiology. 2008 Jun 23;127(1) doi: 10.1016/j.ijcard.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 9.Batyraliev TA, Pershukov IV, Niyazova-Karben ZA, Karaus A, Calenici O, Guler N, Eryonucu B, Temamogullari A, Ozgul S, Akgul F, Sengul H, Dogru O, Demirbas O, Timoshin IS, Gaigukov AV, Petrakova LN, Peresypko MK, Sidorenko BA International Invasive Cardiology Research Group. Current role of laser angioplasty of restenotic coronary stents. Angiology. 2006 Jan-Feb;57(1) doi: 10.1177/000331970605700104. [DOI] [PubMed] [Google Scholar]
- 10.Noble S, Bilodeau L. High energy excimer laser to treat coronary in-stent restenosis in an underexpanded stent. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2008 May 1;71(6) doi: 10.1002/ccd.21490. [DOI] [PubMed] [Google Scholar]
- 11.Köster R, Hamm CW, Seabra-Gomes R, Herrmann G, Sievert H, Macaya C, Fleck E, Fischer K, Bonnier JJ, Fajadet J, Waigand J, Kuck KH, Henry M, Morice MC, Pizzulli L, Webb-Peploe MM, Buchwald AB, Ekström L, Grube E, Al Kasab S, Colombo A, Sanati A, Ernst SM, Haude M, Serruys PW, et al. Laser angioplasty of restenosed coronary stents: results of a multicenter surveillance trial. The Laser Angioplasty of Restenosed Stents (LARS) Investigators. Journal of the American College of Cardiology. 1999 Jul 1;34(1) doi: 10.1016/s0735-1097(99)00167-9. [DOI] [PubMed] [Google Scholar]
- 12.Mehran R, Dangas G, Mintz GS, Waksman R, Abizaid A, Satler LF, Pichard AD, Kent KM, Lansky AJ, Stone GW, Leon MB. Treatment of in-stent restenosis with excimer laser coronary angioplasty versus rotational atherectomy: comparative mechanisms and results. Circulation. 2000 May 30;101(21) doi: 10.1161/01.cir.101.21.2484. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Karim AR, Banerjee S, Brilakis ES. Percutaneous intervention of acutely occluded saphenous vein grafts: contemporary techniques and outcomes. The Journal of invasive cardiology. 2010 Jun 1;22(6) [PubMed] [Google Scholar]
- 14.Taylor K, Harlan K, Branan N. Small 0.7 mm diameter laser catheter for chronic total occlusions, small vessels, tortuous anatomy, and balloon-resistant lesions - development and initial experience. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2006 Aug 1;2(2) [PubMed] [Google Scholar]
- 15.Bilodeau L, Fretz EB, Taeymans Y, Koolen J, Taylor K, Hilton DJ. Novel use of a high-energy excimer laser catheter for calcified and complex coronary artery lesions. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2004 Jun 1;62(2) doi: 10.1002/ccd.20053. [DOI] [PubMed] [Google Scholar]
- 16.Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F. Thrombus aspiration during primary percutaneous coronary intervention. The New England journal of medicine. 2008 Feb 7;358(6) doi: 10.1056/NEJMoa0706416. [DOI] [PubMed] [Google Scholar]
- 17.Tarsia G, De Michele M, Polosa D, Biondi-Zoccai G, Costantino F, Del Prete G, Osanna RA, Innelli P, Sisto F, Sheiban I, Lisanti P. Manual versus nonmanual thrombectomy in primary and rescue percutaneous coronary angioplasty. Heart and vessels. 2010 Jul 1;25(4) doi: 10.1007/s00380-009-1198-2. [DOI] [PubMed] [Google Scholar]
- 18.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, Jacobs AK, Kern MJ, King SB, 3rd, Morrison DA, O'Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B American College of Cardiology/American Heart Association Task Force on Practice Guidelines; ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). Journal of the American College of Cardiology. 2006 Jan 3;47(1) doi: 10.1016/j.jacc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Topaz O. Laser. In: Topol EJ, editor. Textbook of Interventional Cardiology. Philadelphia: WB Saunders Company; 2003. pp. 665–700. [Google Scholar]
- 20.Gibson CM, de Lemos JA, Murphy SA, Marble SJ, McCabe CH, Cannon CP, Antman EM, Braunwald E TIMI Study Group. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 substudy. Circulation. 2001 May 29;103(21) doi: 10.1161/01.cir.103.21.2550. [DOI] [PubMed] [Google Scholar]
- 21.van 't Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998 Jun 16;97(23) doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 22.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasch P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012 Oct 16;126(16) doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 23.Biscione C, Mariano E, Sergi D, Tarsia G, Viceconte N, Bernardo V, Mango R, Del Prete G, Romeo F. Large coronary aneurysm following laser angioplasty of the left anterior descending coronary artery. Journal of cardiovascular medicine (Hagerstown, Md.) 2012 May 1;13(5) doi: 10.2459/JCM.0b013e328352909f. [DOI] [PubMed] [Google Scholar]
- 24.Vandormael M, Reifart N, Preusler W, Schwarz F, Storger H, Hofman M, et al. Six month follow-up following excimer laser coronary angioplasty, rotational atherectomy and balloon angioplasty for complex lesions: ERBAC study. Circulation. 1994;90:213A. doi: 10.1161/01.cir.96.1.91. [DOI] [PubMed] [Google Scholar]
- 25.Niccoli G, Giubilato S, Conte M, Belloni F, Cosentino N, Marino M, Mongiardo R, Crea F. Laser for complex coronary lesions: impact of excimer lasers and technical advancements. International journal of cardiology. 2011 Jan 21;146(2) doi: 10.1016/j.ijcard.2010.10.092. [DOI] [PubMed] [Google Scholar]
- 26.Bittl JA, Sanborn TA, Yardley DE, Tcheng JE, Isner JM, Chokshi SK, Strauss BH, Abela GS, Walter PD, Schmidhofer M, et al. Predictors of outcome of percutaneous excimer laser coronary angioplasty of saphenous vein bypass graft lesions. The Percutaneous Excimer Laser Coronary Angioplasty Registry. The American journal of cardiology. 1994 Jul 15;74(2) doi: 10.1016/0002-9149(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 27.Viceconte N, Biscione C, Tarsia G, Osanna R, Polosa D, Del Prete A, Lisanti P, Gaudio C. Laser "explosion" technique for treatment of unexpanded coronary stent. International journal of cardiology. 2011 Jun 16;149(3) doi: 10.1016/j.ijcard.2011.03.021. [DOI] [PubMed] [Google Scholar]