Abstract
The development of atherosclerosis is a multifactorial process. The purpose of the study was to examine three genetic polymorphisms playing a role in the metabolic processes underlying the disease. We compared the data of 348 atherosclerotic non-diabetic patients with 260 atherosclerotic diabetic patients and 384 healthy controls. We analyzed the prevalence of myocardial infarction and stroke in three different groups of patients carrying different polymorphisms. It was proved that if the mutant TT eNOS Glu298ASP variant is present, a significantly higher number of myocardial infarctions can be observed than in patients carrying heterozygote GT or normal GG genotype. We proved that in the case of MTHFR 677CT heterozygote variants, the occurrence of myocardial infarction is significantly higher and the difference is also significant in case of the 677TT homozygote variant. It was verified that among patients with the mutant TNF-α AA genotype the occurrence of cardiovascular events was significantly higher. Screening the genetically high risk groups on the long run should be considered as an early detection opportunity that may give better chances for prevention and treatment. Understanding the inflammatory mechanisms of the atherosclerosis may give new therapeutical targets to pharmacologists.
Keywords: eNOS G298T, MTHFR C677T, TNF-α G308A, polymorphism, atherosclerosis
Introduction
The development of atherosclerosis is a multifactorial process, where the clinical pattern is determined by environmental and genetic factors. Except for the classical risk factors of the atherosclerosis (hypertension [1], lipid-metabolic disorders [2], diabetes [3–5] and smoking [6]) the clinical signs can be influenced by the genetic variants (polymorphisms) [7] of the enzymes responsible for the endothelial cell function [8] and for the thrombotic factors. Inflammatory reaction [9–12] follows the primary endothelial injury [13–15] than a generalized process starts resulting in atherosclerotic plaque [16] development in the small and large arteries of the organ, leading to the occlusion of the artery. Diverse clinical presentations include myocardial infarction [17], peripheral arterial occlusion, dysbasia and gangrene. Besides the treatment of symptomes [18–20], it is important to explore the metabolic, inflammatory and genetic causes of the disease.
The purpose of the study was to examine three genetic polymorphisms of functional genes coding enzymes playing a role in the metabolic processes and to clarify their role in the vascular pathologys.
The nitric oxide (NO) produced by the endothelial nitric oxide synthase (eNOS) has an atheroprotective effect [21]. Previous studies have shown that the eNOS 298 Glu/Asp polymorphism causes low NO production, which results in hypertension, thrombus development and changes in the vascular tone due to the endothelial dysfunction, finally leading to atherosclerosis [22–25]. The aim of our investigation was to define eNOS Glu298Asp (GT) polymorphism in healthy, atherosclerotic non-diabetic and diabetic patients. We searched for a connection between acute myocardial infarction, stroke and the polymorphisms of the eNOS 298 Asp/Asp and eNOS 298 Glu/Asp.
It has been known for a long time that high homocystein level of the plasma can destroy the endothel cells of the small arteries, which can often be the first step towards atherosclerosis, [26–28]. The methylene-tetrahydrofolate reductase (MTHFR) C677T enzyme plays an important role in the high plasma homocystein levels and in the folate metabolism. The TT genotype is usually associated with higher homocystein levels, so it is supposed to increase the risk of atherosclerosis. In our examination, we looked for a link between stroke, myocardial infarction and the occurrence of TT/MTHRF polymorphism.
The TNF-α plays an important role in the pathophysiology of vascular diseases [29] by affecting inflammatory cascade [30, 31], lipid metabolism, obesity and insulin resistance. As the degree of the TNF-α expression is highly influenced by genetic factors, it is important to know how likely atherosclerosis develops in different diseases. The promoter region of this gene contains several polymorphisms, which influence the TNF-α level. In our examination, we have set the aim on the TNF-α 308GA polymorphisms in atherosclerotic, diabetic and healthy patients and we have tried to identify their association with myocardial infarction and stroke.
Materials and Methods
In this examination, we analyzed 992 patients’ data, out of which 608 had been treated at the Cardiovascular Department of the Semmelweis University from November 2003 until June of 2005. All these patients underwent vascular surgical reconstruction because of their atherosclerotic disease – this was the only inclusion criteria of the examination. They had carotid-, thoracic-, abdominal-, or lower limb-revascularization or aneurysm repair. The common cause was atherosclerosis. We compared the data of 348 atherosclerotic non-diabetic patients and 260 diabetic patients with the 384 healthy control samples. We analyzed the frequency of myocardial infarction and stroke in the case of different polymorphisms. All the 608 patients were divided into four groups: (1) atherosclerotic diabetic patients with myocardial infarction (n = 101), (2) atherosclerotic diabetic patients with stroke (n = 108), (3) atherosclerotic non-diabetic patients with myocardial infarction (n = "118) and (4) atherosclerotic non-diabetic patients witch stroke (n = 106). The occurrence of all the hetero- and homozygote variants of the analyzed genes were tested in every group and the results were compared to the healthy control samples.
Isolation of DNA samples
DNA was extracted from the buffy coat fraction of centrifuged blood using the QIAmp Blood Kit (Qiagen).
SNP analysis of eNOS G298T and MTHFR C677T
The following primers and probes were designed by LightCycler probe design software 2.0:
eNOS: sense primer: 5´-CAGCTCTGCATTCAGCAC-3´; antisense primer: 5´-CATCCCAC-CCAGTCAATC-3´ sensor: 5´-LCRed640-GTTCTGGGGGCTCATCTGGG-PH-3´ (designed for the wild type allele); anchor: 5´-CAGCTCGGGGGGCAGAAGGAA-FL-3´;MTHFR: sense primer: 5´-AGGCCAGCCTCTCCTGACTG-3´; antisense primer: 5´-AGGACGGTGCGGTGAGAGTG- 3´; sensor: 5´-CGGGAGCCGATTTCATCA-FL-3´; anchor: 5´-LCRed640-CGCAGCTTTTCTTTGAGGCTGACA-PH.
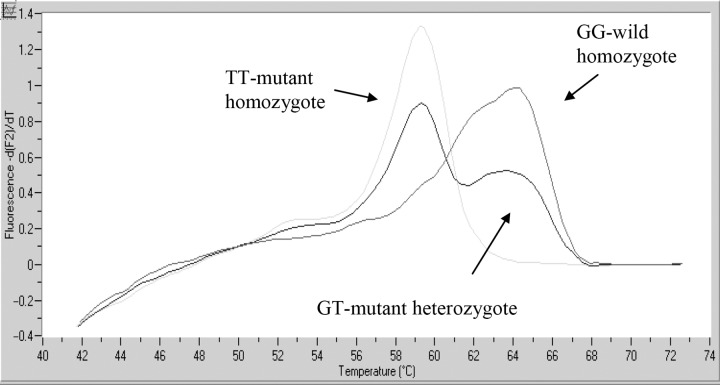
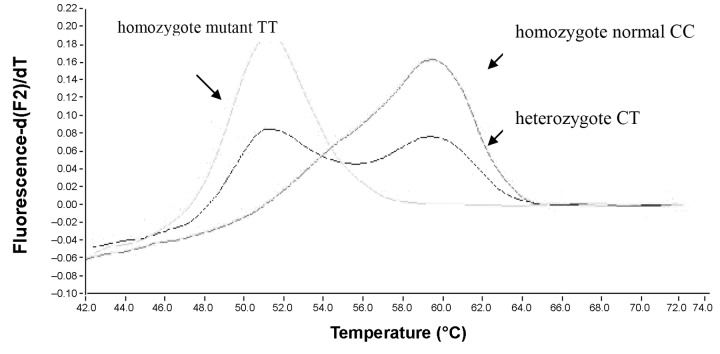
Amplification of 50-ng DNA template with primers (0.5 μM each) and fluorescent probes (0.2 μM each) was performed in LightCycler instrument (Roche Diagnostics GmbH) using LightCycler FastStart DNA Master HybProbe mix (Roche Diagnostics GmbH). The Mg-Cl2 concentration was 2 mM. The cycling conditions of eNOS fragment were 95 °C and 10 min followed by 45 cycles of 95 °C, 10 s; 65 °C; 5 s; and 72 °C, 5 s. The thermal profile of the MTHFR PCR was 95 °C and 10 s followed by 45 cycles of 95 °C, 3 s; 52 °C, 5 s; and 72 °C, 13 s. For the analysis of the melting curves at the end of the PCR,temperature was raised to 95 °C, lowered to 45 °C and then slowly raised to 85 °C whilst monitoring the fluorescence intensity. The negative first derivative of the melting curves created by the software (LC vs.3.5) made possible the distinction of the genotypes (Figs 1 and 2).
Fig. 1.
eNOS G298T DNA melting curve analysis, eNOS G298T polymorphism determination
Fig. 2.
MTHFR C677T polymorphism melting curve analysis
Sequencing
Sequencing analyses were performed with the PCR products using the primers described in the SNP analysis. The sequencing reactions were carried out by Big-Dye terminator cycle sequencing kit v.3.1. (Applied Biosystems, Foster City, CA, USA) and the reaction products run in ABI-PRISM 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
TNF-a genotype analysis/SNP analysis
For TNF-α 308GA polymorphisms analysis, the next PCR primer was used: 5´-AGGCAATAGGTTGAGGGCCAT-3´ and 5´-TCCTCCCTGCTCCGATTCCG-3´.
The 308 GA polymorphism analysis was performed by Fernandez-Real et al. [32]. We analysed the data statistically; we used two sides Student t-probe, odds’ ratio, chi-square probe, average and scattering (SD) and graphical presentation. Those differences were significant, where the statistical deviance was p < 0.05.
The author of this article has certified that he complies with the principles of ethical publishing [33].
Results
There was no significant difference: between the age of the patients in the compared groups. The mean age was 70 years in the diabetic group and 68 years in the atherosclerotic group. The youngest patient was 40 and the oldest patient was 93 years old. Half of the diabetic patients were treated by oral antidiabetics,while third of them were on insulin and 20% was only kept on diet. All patients had significantly higher cholesterol (5.2–5.5 mmol/L), LDL (3.6–3.6) and triglicerid (2.2–1.9 mmol/L) levels, in comparison with the the control group (3, 2.5 and 1 mmol/L). There was no difference in the occurrence of prior myocardial infarction between the diabetic and atherosclerotic non-diabetic groups (39.0% vs. 38.9%), but the stroke prevalence was significantly higher among the diabetic patients (20.0% vs. 38.0%, p < 0.05).
eNOS analysis
We specified the eNOS polymorphisms by DNA melting curve analysis at 384 healthy controls, 348 atherosclerotic non-diabetic and 260 diabetic patients. A representative DNA melting curve is shown in Fig. 1, where the different genotypes can be identified. The wild Glu/ Glu is GG genotype and the mutant Asp/Asp is TT genotype amino acid sequence.
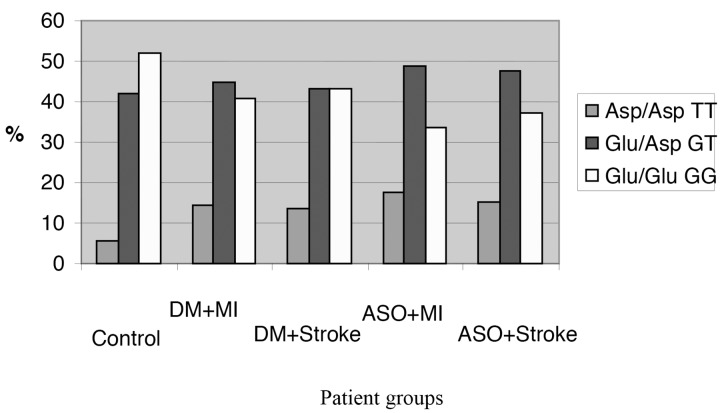
We examined the occurrence of the wild (GG), the mutant (TT) and the heterozygote (GT) allele in different illness-combinations. We verified that in the Asp 298 allele homozygote TT variants, NO production is decreased and endothelial dysfunction can occur more often, resulting in higher risk for atherosclerosis. We have confirmed that in the case of eNOS 298 mutant TT variants, the prevalence of the myocardial infarction (MI) is significantly higher compared to the patients carrying the homozygote GG normal or heterozygote GT variants. Our tests show that the frequency of the eNOS 298 TT mutation in the control group is 5.7%. Among the diabetic patients with prior MI the frequency is 14.8% (p < 0.05); while in the diabetic stroke patients it is 12.9% (p < 0.05). In the atherosclerotic non-diabetic MI patients, the allele occurrence is 16.9% (p < 0.05); while in the non-diabetic stroke group, we found it in 15.09%-of the patient (p < 0.05), we have also compared the homozygote TT to the homozygote GG frequency (Fig. 3). The case–control analysis (odds ratio) showed increased relative risk. The homozygote TT mutant allele carrying patients in the diabetic group have the risk of 3.34 (1.61–6.92) for MI and a risk of 2.72 (1.31–5.66) for stroke. In the atherosclerotic non-diabetic group, the MI risk is higher, 4.56 (2.29–9.08), but the stroke-risks are elevated, as well OR 3.74 (1.82–7.71). Thus, high risk patients, who were Asp 298 homozygotes (TT), showed four times higher risk for MI than patients with normal GG allele. One of the important results of our study is that we proved a close connection between Glu298Asp polymorphisms, MI and stroke risk. We could verify a new gene polymorphism on the NOS3 gene and the Glu298Asp mutation proved to be a major risk factor for MI. This finding seems to be important but further confirmation is necessary in other patient groups [34].
Fig. 3.
eNOS polymorphisms in the five groups. In the atherosclerotic non-diabetic patient group, the rate of the mutant TT homozygote genotype is significantly higher to the control group (Abbreviations: DM = diabetes, MI = myocardial infarction, ASO = atherosclerosis)
MTHFR analysis
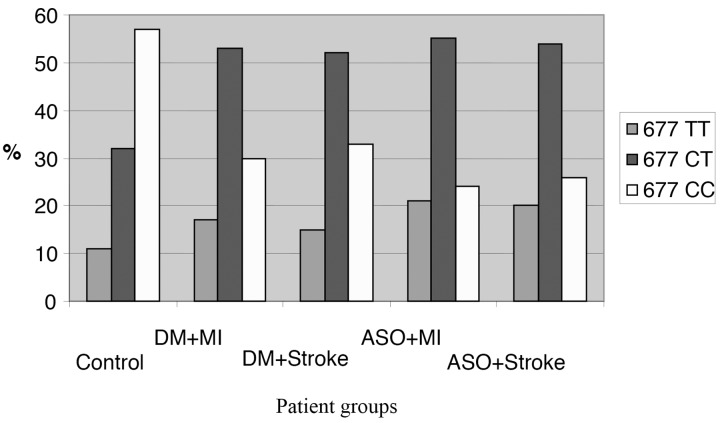
The MTHFR gene polymorphism were analyzed in 384 controls, 260 diabetic and 348 atherosclerotic non-diabetic patients was analyzed by melting curve analysis with DNA LightCycler. We concluded that in the case of mutant MTHFR C677T variants the priorMI occured significantly more often. In our samples, the MTHFR 677 CT mutations occurred in 32% in the control group; while among the diabetic patients with prior MI the frequency was 53.5% (p < 0.05); and in the diabetic stroke group it was 51.8% (p < 0.05). Among the nondiabetic atherosclerotic patients with prior MI, the CT occurence was 55.1% (p < 0.05) while among the stroke patients 53.7% carried the mutation, which is a significant difference (p < 0.05) compared to our controls.
The homozygote TT variant is less frequent in the the diabetic group but the difference is still significant, the frequency was 16.8% in the prior MI group (p < 0.05) and 14.8% (p < 0.05) in the prior stroke group. At the atherosclerotic non-diabetic patients group, the occurrence is highly significant again, in the prior MI group it is 21.2% (p < 0.05) and in the stroke patients,19.8% (p < 0.05) (Fig. 4).
Fig. 4.
MTHFR polymorphisms in the five groups. In the atherosclerotic non-diabetic patient group, the rate of the mutant 677TT homozygote genotype and 677 CT heterozygote genotype is significantly higher to the wild type (Abbreviations: DM = diabetes, MI = myocardial infarction, ASO = atherosclerosis)
When we examine all the mutant alleles (TT+CT) in every four patients’ groups in the relation to the normal CC variant, at every count, we found significant difference (p < 0.05). In our study, we have established that the MTHFR C677T allele is a risk factor of stroke. Also, a relevant difference could be proved for the risk of MI. The sample number of this study was not enough to verify the hypothesis that MTHFR genotypes influence the development of the coronary sclerosis; more and larger studies are necessary to examine the connection between the the genetic and environmental factors. In this study, we can only establish that the MTHFR C677T polymorphism is associated with a significantly higher prevalence of MI and stroke. The case–control analysis of the diabetic, homozygote TT mutant allele carrying patients showed that the risk of MI was 2.95 (1.51–5.79) and the risk of stroke was 2.31 (1.18–4.52). In the atherosclerotic nondiabetic group, the MI risk was higher, 4.65 (2.48–8.72) and the stroke-risk was also elevated OR 3.91 (2.04–7.49) compared to the healthy controls.
TNF-α analysis
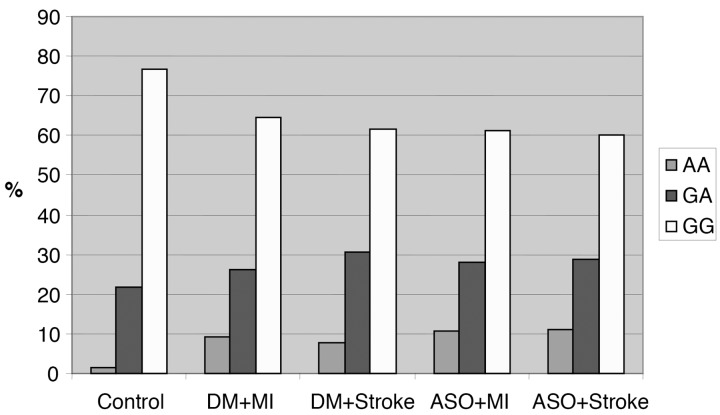
In our study, we have established that the mutant TNF-α AA allele carrying patients have a significant higher risk for cardiovascular events than the control group. We could verify that in the healthy control samples the wild TNF-α 308 GG genotype occurrence was 76.7%, the heterozygote (308 GA) was 21.7%, while the mutant homozygote (308 AA) genotype accounted for only in 1.6%. Our data show that in diabetic patients MI group (examined group 2) the mutant 308 AA frequency was elevated to 9.2% (p < 0.05). In the examined group 3, the TNF-α genotype distribution was the following: the AA homozygote high-risk mutant in 7.7% (p < 0.05), while the GA homozygote in 30.7%, against in the control groups, 61.6%. In all patients, carrying the A allele, the TNF-α level can be higher, causing an elevated risk for cardiovascular events. In the examined groups 4 and 5 there were atherosclerotic non-diabetic patients, MI and stroke patients. We could establish that the AA genotype occurrence in this group was higher (10.7%) (p < 0.05) than in the control groups (1.6%) (Fig. 5).
Fig. 5.
TNF-α polymorphisms in the five groups. In the atherosclerotic non-diabetic patient group, the rate of the mutant 308AA homozygote genotype is significantly higher to the control group (Abbreviations: DM = diabetes, MI = myocardial infarction, ASO = atherosclerosis)
The heterozygote GA allele occurrence was elevated at atherosclerotic patients (28%, 28.9%), in contrast to the 21.7% in the control group, At the odds ratio examination, we found an extreme high risk for MI and stroke in TT mutation carrying patients. Among the atherosclerotic non-diabetic patients, the MI risk was OR 6.714 (1.725–25.553) and the OR of stroke was 6.714 (1.725–25.553). Among the atherosclerotic patients, the MI risk was high, OR 8.173 (2.242–29.559), while the stroke prevalence was the highest, OR 8.703 (2.152–34.988) [35].
Discussion
The study resulted in the following new assumptions:
1. In patients carrying the eNOS Glu298Asp mutant homozygote TT variant, the MI occurrence was significantly higherthan in the heterozygote GT and normal GG variant (TT allele: control group 5.7%, MI group 16.9%, p < 0.05, OR: 4.56).
2. In the case of MTHFR 677 CT heterozygote variant, the MI prevalence was much higher (CT genotype: control group, 32%; MI group, 55.1%; p < 0.05; OR: 4.13), while in case of homozygote TT genotype the difference was still significant (TT allele: control group, 10.9%; MI group, 21.2%; p < 0.05; OR: 4.65).
3. Among the TNF-α AA allele carrying patients, the risk for cardiovascular events was significantly elevated (AA allele: control group, 1.6%; MI group, 10.7%; p < 0.05, OR: 8.17).
Screening the endangered or genetically high risk groups should be considered on the long run – an early detection of a susceptibility of the disease gives better chances for prevention and treatment. Understanding the inflammatory mechanisms of the atherosclerosis may give new therapeutic targets to pharmacologists. Therapeutic possibility of the statins today is available; they have anti-inflammatory, antithrombotic and plack-stabilisatory effects. The anti-thrombotic medications like acetylsalicylic acid and clopidogrel are part of the anti-inflammatory treatment as well. In certain cases, the high level of plasma homocystein can be reduced with giving acidum folicum. Further clinical and pharmacological investigations are needed to identify the key-molecules in different pathomechanisms (tumor, inflammation) in order to find validated therapeutic targets.
Acknowledgements
The author wishes to thank Gyorgy Acsady and Gyorgy Keri for the help in formulating the study design, preparing and evaluating; the National Oncological Institute Pathogenetic Department for the Laboratory measurements; and Peter Vargha for statistical analysis.
Footnotes
Place and date of defence: Budapest, June 15, 2011
PhD School: Semmelweis University School of PhD Studies, Budapest, Hungary
Tutor: György Acsády, MD, PhD, DSc
References
- 1.L Szollár. Az atherosclerosis. In: Szollár L, editor. Kórélettan. Budapest: Semmelweis Kiadó; 2005. pp. 304–328. [Google Scholar]
- 2.Meskó É, Szollár L. Az arteriosclerosis kórélettana. In: Meskó É, Farsang Cs, Pécsvárady Zs, editors. Belgyógyászati angiologia. Budapest: Medintel Könyvkiadó; 1999. pp. 55–67. [Google Scholar]
- 3.Hosszúfalusi N. A hyperglycaemia szerepe a diabetes mellitus késôi szövôdményeinek kialakulásában. LAM . 2000;10(1):40–48. [Google Scholar]
- 4.Gy Jermendi. Diabetes mellitus és az artérias érbetegségek. Hippocrates. 2001;3(5):319–314. [Google Scholar]
- 5.Nieszner É, Bárdos P, Baranyi É, Préda I. A diabetes mellitus cardiovascularis szövôdményei és diagnózisuk. LAM . 2005;15(10):722–729. [Google Scholar]
- 6.Lusis AJ. Atherosclerosis. Nature. 2000 Sep 14;407(6801) doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szalai Cs. Atherosclerosis. Genetikai alapok. 2009. pp. 1–18 . [Google Scholar]
- 8.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004 Jun 1;109(21 Suppl 1) doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 9.Blake GJ, Ridker PM. Inflammatory mechanisms in atherosclerosis: from laboratory evidence to clinical application. Italian heart journal : official journal of the Italian Federation of Cardiology. 2001 Nov 1;2(11) [PubMed] [Google Scholar]
- 10.Corti R, Hutter R, Badimon JJ, Fuster V. Evolving concepts in the triad of atherosclerosis, inflammation and thrombosis. Journal of thrombosis and thrombolysis. 2004 Feb 1;17(1) doi: 10.1023/B:THRO.0000036027.39353.70. [DOI] [PubMed] [Google Scholar]
- 11.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002 Mar 5;105(9) doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 12.Ross R. Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999 Jan 14;340(2) doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 13.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004 Jun 15;109(23 Suppl 1) doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 14.DeGraba TJ. Immunogenetic susceptibility of atherosclerotic stroke: implications on current and future treatment of vascular inflammation. Stroke; a journal of cerebral circulation. 2004 Nov 1;35(11 Suppl 1) doi: 10.1161/01.STR.0000143788.87054.85. [DOI] [PubMed] [Google Scholar]
- 15.Giese NA, Marijianowski MM, McCook O, Hancock A, Ramakrishnan V, Fretto LJ, Chen C, Kelly AB, Koziol JA, Wilcox JN, Hanson SR. The role of alpha and beta platelet-derived growth factor receptor in the vascular response to injury in nonhuman primates. Arteriosclerosis, thrombosis, and vascular biology. 1999 Apr 1;19(4) doi: 10.1161/01.atv.19.4.900. [DOI] [PubMed] [Google Scholar]
- 16.Jaeger BR, Labarrere CA. [Fibrinogen and atherothrombosis: vulnerable plaque or vulnerable patient?]. Herz. 2003 Sep 1;28(6) doi: 10.1007/s00059-003-2497-5. Article in German. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000 May 9;101(18) doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 18.Gardner M, Palmer J, Manrique C, Lastra G, Gardner DW, Sowers JR. Utility of aspirin therapy in patients with the cardiometabolic syndrome and diabetes. Journal of the cardiometabolic syndrome. 2009 Spring;4(2) doi: 10.1111/j.1559-4572.2008.00037.x. [DOI] [PubMed] [Google Scholar]
- 19.Geisler T, Bhatt DL. The role of inflammation in atherothrombosis: current and future strategies of medical treatment. Medical science monitor : international medical journal of experimental and clinical research. 2004 Dec 1;10(12) [PubMed] [Google Scholar]
- 20.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, Thomas D MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004 May 8;363(9420) doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 21.Colombo MG, Paradossi U, Andreassi MG, Botto N, Manfredi S, Masetti S, Biagini A, Clerico A. Endothelial nitric oxide synthase gene polymorphisms and risk of coronary artery disease. Clinical chemistry. 2003 Mar 1;49(3) doi: 10.1373/49.3.389. [DOI] [PubMed] [Google Scholar]
- 22.Hingorani AD, Liang CF, Fatibene J, Lyon A, Monteith S, Parsons A, Haydock S, Hopper RV, Stephens NG, O'Shaughnessy KM, Brown MJ. A common variant of the endothelial nitric oxide synthase (Glu298-->Asp) is a major risk factor for coronary artery disease in the UK. Circulation. 1999 Oct 5;100(14) doi: 10.1161/01.cir.100.14.1515. [DOI] [PubMed] [Google Scholar]
- 23.Rossi GP, Cesari M, Zanchetta M, Colonna S, Maiolino G, Pedon L, Cavallin M, Maiolino P, Pessina AC. The T-786C endothelial nitric oxide synthase genotype is a novel risk factor for coronary artery disease in Caucasian patients of the GENICA study. Journal of the American College of Cardiology. 2003 Mar 19;41(6) doi: 10.1016/s0735-1097(02)03012-7. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clinical medicine & research. 2006 Mar 1;4(1) doi: 10.3121/cmr.4.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marroni AS, Metzger IF, Souza-Costa DC, Nagassaki S, Sandrim VC, Correa RX, Rios-Santos F, Tanus-Santos JE. Consistent interethnic differences in the distribution of clinically relevant endothelial nitric oxide synthase genetic polymorphisms. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2005 May 1;12(3) doi: 10.1016/j.niox.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Selhub J, Jacques PF, Bostom AG, D'Agostino RB, Wilson PW, Belanger AJ, O'Leary DH, Wolf PA, Schaefer EJ, Rosenberg IH. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. The New England journal of medicine. 1995 Feb 2;332(5) doi: 10.1056/NEJM199502023320502. [DOI] [PubMed] [Google Scholar]
- 27.Bova I, Chapman J, Sylantiev C, Korczyn AD, Bornstein NM. The A677V methylenetetrahydrofolate reductase gene polymorphism and carotid atherosclerosis. Stroke; a journal of cerebral circulation. 1999 Oct 1;30(10) doi: 10.1161/01.str.30.10.2180. [DOI] [PubMed] [Google Scholar]
- 28.Mazza A, Giugliano D, Motti C, Cortese C, Andreotti F, Marra G, Nulli A. Glycemia, MTHFR genotype and low homocysteine in uncomplicated type 2 diabetic patients. Atherosclerosis. 2000 Mar 1;149(1) doi: 10.1016/s0021-9150(99)00421-9. [DOI] [PubMed] [Google Scholar]
- 29.Elkind MS, Cheng J, Boden-Albala B, Rundek T, Thomas J, Chen H, Rabbani LE, Sacco RL. Tumor necrosis factor receptor levels are associated with carotid atherosclerosis. Stroke; a journal of cerebral circulation. 2002 Jan 1;33(1) doi: 10.1161/hs0102.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodwin BL, Pendleton LC, Levy MM, Solomonson LP, Eichler DC. Tumor necrosis factor-alpha reduces argininosuccinate synthase expression and nitric oxide production in aortic endothelial cells. American journal of physiology. Heart and circulatory physiology. 2007 Aug 1;293(2) doi: 10.1152/ajpheart.01100.2006. [DOI] [PubMed] [Google Scholar]
- 31.Monraats PS, Pires NM, Schepers A, Agema WR, Boesten LS, de Vries MR, Zwinderman AH, de Maat MP, Doevendans PA, de Winter RJ, Tio RA, Waltenberger J, 't Hart LM, Frants RR, Quax PH, van Vlijmen BJ, Havekes LM, van der Laarse A, van der Wall EE, Jukema JW. Tumor necrosis factor-alpha plays an important role in restenosis development. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005 Dec 1;19(14) doi: 10.1096/fj.05-4634com. [DOI] [PubMed] [Google Scholar]
- 32.Fernández-Real JM, Gutierrez C, Ricart W, Casamitjana R, Fernández-Castañer M, Vendrell J, Richart C, Soler J. The TNF-alpha gene Nco I polymorphism influences the relationship among insulin resistance, percent body fat, and increased serum leptin levels. Diabetes. 1997 Sep 1;46(9) doi: 10.2337/diab.46.9.1468. [DOI] [PubMed] [Google Scholar]
- 33.Szél Á, Merkely B, Hüttl K, Gál J, Nemes B, Komócsi A. Statement of ethical publishing and scientific authorship. Int Med Appl Sci . 2010;2:101–102. [Google Scholar]
- 34.Szabó GV, Kunstár A, Acsády Gy. Methylentetrahydrofolate reductase and nitric oxide synthase polymorphism in patients with atherosclerosis and diabetes. Pathology oncology research : POR. 2009 Dec 1;15(4) doi: 10.1007/s12253-009-9163-z. [DOI] [PubMed] [Google Scholar]
- 35.Szabó GV, Acsády Gy. Tumornecrosis-factor-α 308 GA Polymorphism in atherosclerotic patients. Pathol Oncol Res. 2011;17:853–857. doi: 10.1007/s12253-011-9393-8. [DOI] [PubMed] [Google Scholar]