Abstract
Treatment of rheumatoid arthritis by intraarticular administration of anti-inflammatory drugs encapsulated in drug delivery systems, such as liposomes/niosomes and lipogelosomes/niogelosomes, prolongs the residence time of the drugs in the joint. It was therefore anticipated that liposome/niosome entrapment would enhance the efficacy of drugs in the inflammatory sides. Liposomes are good candidates for the local delivery of therapeutic agents, such as diclofenac sodium (DFNa), for intraarticular delivery. Drugs for parenteral delivery must be sterile, and radiation sterilization is a method recognized by pharmacopoeias to achieve sterility of drugs. However, irradiation might also affect the performance of drug delivery systems. One of the most critical points is irradiation dose, because certain undesirable chemical and physical changes may accompany with the treatment, especially with the traditionally applied dose of 25 kGy. The present study aims to determine the effects of gamma irradiation on DFNa-loaded liposomes/niosomes and lipogelosomes/niogelosomes for the treatment of rheumatoid arthritis.
Keywords: diclofenac sodium, gamma irradiation, lipogelosomes, liposomes, niogelosomes, niosomes, rheumatoid arthritis, sterilization
Introduction
Liposomes are phospholipid bilayer membrane-bound vesicles that can encapsulate a wide variety of substances either within their lipid membranes or their central aqueous cores [1]. Gamma irradiation is an approved sterilization technique for some pharmaceuticals [2–10] and has turned out to be an interesting and promising technique also for the sterilization of liposomes. Zuidam et al. [11] have published a review of the different sterilization techniques for liposomes, and they concluded that gamma irradiation can be accepted as a safe and convenient sterilization technique for liposomes, and more studies are necessary. The use of gamma rays for the sterilization of pharmaceutical raw materials and dosage forms is an alternative method for sterilization [12]. Currently, gamma irradiation includes high penetrating power, low chemical reactivity, low measurable residues, and small tempereature rise. A minimum absorbed dose of 25 kGy is regarded as adequate for the purpose of sterilizing pharmaceutical products without providing any biological validation [13]. However, chemical degradation of the phospholipids may take place during gamma irradiation, and production of new radiolytic products during the irradiation process is one of the major problem of the radiosterilization. Therefore, the principal problem in radiosterilization is to determine and to characterize these physical and chemical changes originating from high-energy radiation [12]. Stark [14] and Albertini and Rustichelli [15] presented reviews summarizing the effects of gamma irradiation on lipids and liposomal structure. In some studies, peroxidation of unsaturated phospholipids and formation of lysophospholipids, free fatty acids, phosphatidic acid, and different hydrocarbon compounds have been observed [16]. Efforts have been done to reduce the degradation by addition of radical scavengers or by freezing and lyophilization [17, 18]. The physical properties of gamma-irradiated liposomes (size, bilayer rigidity, and permeability) are less sensitive to changes than the chemical structure [17, 19]. Furthermore, there has been a reported increased physical stability due to a resulting increased electrostatic repulsion between the liposomes preventing especially the neutral liposomes from aggregation and fusion [17, 20]. In this study, the effects of gamma irradiation liposome/niosome and lipogelosome/niogelosome formulations prepared for the purpose of RA treatment are investigated in more detail. Physical/chemical analyses (organoleptic controls, pH, particle size, viscosity, in vitro release, and electron spin rezonance [ESR] analysis) of the formulations are followed by microbiological characterization (sterility, apyrogenicity, and sterility assurance level [SAL] determination) of the subsequent produced formulations. Furthermore, stability tests were carried out under normal (60% relative humidity and 25 °C temperature) and accelerated (75% relative humidity and 40 °C temperature) test conditions for 3 months.
This work complies information about the studies developed in order to find out if gamma radiation could be applied as a sterilization method of liposome/niosome and lipogelosome/niogelosome formulations containing diclofenac sodium (DFNa).
Materials and Methods
Dimyristoyl phosphatidilcholine (DMPC), diclofenac sodium (DFNa), and SUR I (hegza decyl 3-polyglycerole) (L’Oreal) were kindly provided by Nattermann Phospholipids (Köln, Gemany), Deva (İstanbul, Turkey), L’oreal (France), respectively. Dicethyl phosphate (DCP) and cholesterol (CHOL) were obtained from Sigma Chemical Co. (St. Louis, USA). Chloroform was purchased from Merck (Germany). All other chemicals were of analytical grade.
All investigations including (organoleptic properties, TLC, pH, viscosity, in vitro release, ESR studies, and microbiological tests) were performed on formulations irradiated at four different dose levels (5, 10, 25, and 50 kGy). Unirradiated samples were used as controls to detect physicochemical, and antimicrobial activity changes resulting from the action of ionizing radiation on studied samples.
Irradiation procedure
All irradiations were performed under normal conditions (25 °C, 60% relative humidity) in dark using a 60Co gamma cell (4523 Ci, Hungary) supplying a dose rate of 1.28 kGy·h−1 as an ionizing radiation source at the Sarayköy Gamma Irradiation Facility of Turkish Atomic Energy Agency in Ankara.
Preparation of liposomes/niosomes
Liposome and niosome dispersions were prepared by film technique [21]. Briefly, liposomes and niosomes were prepared by dissolving 40 µmol·mL−1 of phospholipids and/or the surfactant in 30-mL chloroform in a round-bottom flask. The chloroform was removed using a rotary evaporator (Buchi 461, Switzerland) under reduced pressure to form a thin film (7:1:2 molar ratio) over the wall of the flask. The dried film was then hydrated over a water bath with 10 mM HEPES (pH 7.4) containing 10 mg·mL−1 DFNa [8].
Preparation of lipogelosomes/niogelosomes
Lipogelosome and niogelosome formulations were prepared by incorpoation of liposomes and niosomes in structured vehicles. Carbopol 940 (C 940) at the concentration of 1% (w/v) in distilled water [22] and CMC-Na (carboxymethylcellulose sodium) at the concentration of 2.5% (w/v) [23] were used as gel-forming agents because of their good bioadhesive properties. For the preparation of lipogelosomes/niogelosomes, gel formulations of C 940 and CMC-Na and liposomal/niosomal DFNa were mixed in 1:1 ratio on weight basis (Table I) [8].
Table I.
Summary of the liposome/niosome and lipogelosome/niogelosome formulations
| Formulation | Composition | Ratio | Code |
|---|---|---|---|
| Liposome | (DMPC–CHOL–DCP) | (7:1:2) | L |
| Niosome | (SUR I–CHOL–DCP) | (7:1:2) | N |
| Lipogelosome | (DMPC–CHOL–DCP) + C-940 | (7:1:2) + (1:1) (w/w) | LJ |
| Niogelosome | (SUR I–CHOL–DCP)+ C-940 | (7:1:2) + (1:1) (w/w) | NJ |
The summary of DFNa-loaded lipogelosome and niogelosome formulations are given in Table I.
Organoleptic properties
Organoleptic properties (odor, apperance, clearity, and color) of all formulations were performed before and after gamma irradiation.
TLC studies
Samples of 20 µg·mL−1 liposome, niosome, lipogelosome, or niogelosome dispersions were dropped to the GF 254 silikagel plates which were activated at 110 °C for 60 min in an oven. The plates were dried and developed in chloroform–methanol–water (65:25:4) solvent system. Formulations were made visible by immersing the plates into lodine tank, the spots were determined under UV lamp at 254 nm before and after gamma irradiation.
pH measurements
pH measurements of the control (unirradiated) and irradiated formulations were performed using pH-meter (Inolab, Germany) before and after irradiation.
Particle size measurement
Mean particle size and size distributions of the liposome and niosome dispersions were measured by dynamic light scattering method (Malvern Mastersizer 2000, UK) before and after irradiation.
Determination of encapsulation efficiency
The preperation of liposome/niosome formulations containing DFNa was described in Section 2.2. After the hydration of dried film with 10-mM HEPES (pH 7.4) containing 10 mg·mL−1 DFNa, free DFNa was removed by centrifugation three times at 17,500 rpm for 45 min in each. The pellets that were obtained after the centrifugations were treated with detergent (Triton X-100 in 10 mM HEPES [pH 7.4] buffer), and then final clear solutions were analyzed for drug content spectrophotometrically at λ = 303 nm. Encapsulation efficiency was calculated as a fraction of drug in the liposome/niosome pellets expressed as the percentage of the total drug content. The in vitro characterization and in vitro release studies are given in our previous studies [8].
Determination of liposomal phospholipid
Determination of lipid content gives an idea about the efficiency of the method of preparation of liposomes. Liposomal phospholipid content was determined by the colorimetric method of Rouser et al. [24].
In vitro release of DFNa from liposome and niosome formulations
In vitro release of DFNa from liposome and niosome formulations was measured spectrophotometrically (Schimadzu UV 160A, Japan) at λ = 275 nm by incubation of 0.1 mL of liposome and niosome formulations in 10 mL 10 mM HEPES (pH 7.4) buffer at 37 °C in mild shaking water bath, before and after irradiation. Samples were withdrawn at fixed time intervals, and no interference of the empty formulations was detected at this wavelength.
In vitro release of DFNa from lipogelosome and niogelosome formulations
In vitro release properties of liposome and niosome dispersions were evaluated by using Franz-type diffusion cell before and after irradiation. About 0.5 g of sample was introduced into a donor compartment separated by a cellophane membrane (Thomas Sci. Comp., USA) from the receptor compartment, 20 mL of HEPES buffer (pH 7.4). The whole assembly was placed in a water bath, maintained at 37 °C and continuously well stirred. Care was paid to remove any air bubble from the under side of the membrane and the receiving solution. One-milliliter samples were removed from the receiver compartment at specified time intervals, i.e., partial sampling and refilled with an equal volume fresh buffer. All samples were analyzed for DFNa content spectrophotometrically at the λ = 275 nm.
Viscosity measurements
Viscosity measurements of lipogelosomes and niogelosomes were performed by using rheometer (Brookfield, USA) at 25 °C and 37 °C. The shear rate increased from 0 to 100 rpm. The viscosity was determined from the flow curve obtained at different values of shear rate.
ESR measurements
ESR measurements of DFNa-loaded formulations were carried out using Bruker EMX 113 spectrometer operating at 9.5 Ghz. The spectrophotometer operating conditions adapted during the experiments are given in Table II.
Table II.
ESR spectrometer operating conditions adopted throughout the experiments
| Central field | 350.0 mT |
| Sweep width | 20 mT |
| Microwave frequency | 9.85 GHz |
| Microwave power | 1 mW |
| Modulation frequency | 100 kHz |
| Modulation amplitude | 0.1 mT |
| Receiver gain | 6.3×103 |
| Sweep time | 83.89 s |
| Time constant | 327.68 s |
| Conversion time | 81.92 s |
| Temperature | RT (room temperature) |
Sterility test
For the sterility test, two media were used [fluid thioglycolate medium (FTM) and soybean-casein digest medium (SCDM)]. One hundred microliters of the formulations was inoculated to FTM and SCDM medium. They were incubated for 14 days at 35 °C, respectively. After 14 days, the tubes that are turbite were considered as non-sterile, and the tubes that are clear were considered as sterile.
Pyrogen (LAL) test
The gel-clot method for assay of bacterial endotoxins (the most common pyrogens) was examined for the above-mentioned formulations.
SAL determinations
The formulations were infected with Bacillus pumilus spore suspension [6 × 106 colony-forming unit (cfu·mL−1)] and irradiated with various radiation dose levels (1, 5, 10, 25, and 50 kGy) and incubated in TSB (tryptic soy broth) plates at 35–37 °C for 2 weeks. About 1 kGy irradiation dose was added to SAL determination experiments for obtaining the microorganism death graphics. Bacillus pumilus colonies were enumerated, and cfu in 1 mL were calculated. SAL 10−6 dose was calculated from the logaritmic microorganism death graphics.
Stability tests
For stability testing, irradiatiated samples (5, 10, 25, and 50 kGy) were stored at accelerated (60% relative humidity, 25 °C temperature) and normal (4–8 °C) conditions protected from light and in well-sealed containers for 3 months. Particle size, encapsulation efficiency, and phospholipid content were monitored under stability test conditions.
Results and Discussion
Liposomes are currently under investigation in various fields of science and technology, being used as a model system of membrane structure and function and in numerous applications in agriculture, food industry, drug delivery systems, and DNA transfection [19]. The DFNa-loaded formulations coaded L, N, LJ, and NJ are formulated for the purpose of intraarticular delivery, which requires sterile and apyrogenic injection. Irradiation is an established method of sterilization for pharmaceutical products. Gamma radiation, characterized by deep penetration and low dose rates, effectively kills microorganisms throughout the pharmaceutical formulations, products, and their packaging. However, sterilization of these systems has been problematic, with degradation of the liposomes being reported after sterilization using the various techniques available [25, 26]. This study describes the investigation of the effects of ionizing radiation to liposome/niosome and lipogelosome/niogelosome formulations containing DFNa following gamma irradiation.
Color change in the irradiated substances is the simplest and most helpful observation to get information about possible radiolytic intermediates produced in these substances upon irradiation. As no color change was observed in the irradiated formulations in the applied dose region of 5–50 kGy, it can be concluded that either radiolytic intermediates were not produced by irradiation in studied samples or formed intermediates do not exhibit any absorption in the visible region. The negative result in color change of the present work was consistent with the results reported in the literature for similar compounds [12]. With regard to the physical characteristics, there were no significant changes in the organoleptic properties of the formulations before or after irradiation. These results are in agreement with the previous studies [25].
RF values determined by TLC method of control and irradiated samples were found to be significantly different (p < 0.05) and are given in Table III. RF values of formulation changed with the increasing radiation dose levels, which might be due to the presence of degradation products originating from phospholipids and other components in the formulations. The degradation products cannot be identified with TLC analysis, further analytical methods, such as HPLC, might be studied to identify the degradation products.
Table III.
TLC results of formulations before and after gamma irradation
| Formulation |
RF |
||||
|---|---|---|---|---|---|
| Dose rate (kGy) |
|||||
| 0 | 5 | 10 | 25 | 50 | |
| L | 0.327 ± 0.045 | 0.333 ± 0.051 | 0.334 ± 0.048 | 0.340 ± 0.053 | 0.343 ± 0.045 |
| N | 0.412 ± 0.036 | 0.424 ± 0.055 | 0.427 ± 0.043 | 0.433 ± 0.035 | 0.437 ± 0.039 |
| LJ | 0.311 ± 0.019 | 0.323 ± 0.021 | 0.329 ± 0.023 | 0.331 ± 0.031 | 0.335 ± 0.041 |
| NJ | 0.407 ± 0.032 | 0.411 ± 0.019 | 0.417 ± 0.013 | 0.421 ± 0.023 | 0.427 ± 0.024 |
Experimental results showed that pH of all the formulations decreased siginificantly (p < 0.05) (Table IV). The lower pH measured after irradiation of the formulations might be caused by the degradation of the phospholipids and the formation of acidic degradation products, such as distearoylphosphatidic acid (DSPA) and fatty acids [17].
Table IV.
Measured pH values for control and irradiated formulations (n: 6)
| Formulation | pH |
||||
|---|---|---|---|---|---|
| Dose rate (kGy) |
|||||
| 0 | 5 | 10 | 25 | 50 | |
| L | 7.45 ± 0.01 | 7.40 ± 0.02 | 7.38 ± 0.02 | 7.24 ± 0.02 | 7.22 ± 0.01 |
| N | 7.40 ± 0.03 | 7.40 ± 0.03 | 7.39 ± 0.01 | 7.23 ± 0.01 | 7.22 ± 0.02 |
| LJ | 7.42 ± 0.01 | 7.40 ± 0.02 | 7.40 ± 0.03 | 7.30 ± 0.03 | 7.23 ± 0.02 |
| NJ | 7.39 ± 0.04 | 7.41 ± 0.02 | 7.37 ± 0.02 | 7.31 ± 0.02 | 7.29 ± 0.01 |
The size of the liposome and niosome formulations was not affected by gamma irradiation as determined by dynamic light scattering. The particle size of irradiated and non-irradiated formulations was essentially the same (Table V).
Table V.
Particle size and distribution results for control and irradiated formulations (n: 6)
| Formulation | Particle size (nm)a |
||||
|---|---|---|---|---|---|
| Dose rate (kGy) |
|||||
| 0 | 5 | 10 | 25 | 50 | |
| L | 239 ± 0.011 | 235 ± 0.010 | 237 ± 0.014 | 241 ± 0.017 | 240 ± 0.015 |
| N | 279 ± 0.012 | 276 ± 0.012 | 281 ± 0.010 | 273 ± 0.009 | 277 ± 0.008 |
|
aL and N formulations were dispersed in gel
formulations that is why the LJ and NJ results were the same
with the L and N formulations | |||||
The encapsulation efficiency of L and N formulations did not change as shown in Table VI; however, phospholipid content of L formulation was decreased with gamma irradiation (Table VII). That change in phospholipid content might be a sign of the onion peeling effect. All results were in agreement with each other.
Table VI.
Encapsulation efficiency results for control and irradiated formulations (n: 6)
| Formulation | Encapsulation efficiency (%)a |
||||
|---|---|---|---|---|---|
| Dose rate (kGy) |
|||||
| 0 | 5 | 10 | 25 | 50 | |
| L | 10.8 ± 0.1 | 10.9 ± 0.1 | 10.5 ± 0.5 | 10.9 ± 0.4 | 10.8 ± 0.2 |
| N | 9.6 ± 0.1 | 9.7 ± 0.2 | 9.6 ± 0.1 | 9.9 ± 0.2 | 9.3 ± 0.1 |
|
aL and N formulations were dispersed in gel formulations that is why the LJ and NJ results were the same with the L and N formulations | |||||
Table VII.
Phospholipid contents of control and irradiated formulations (n: 6)
| Formulation | Phospholipid content (%)a |
||||
|---|---|---|---|---|---|
| Dose rate (kGy) |
|||||
| 0 | 5 | 10 | 25 | 50 | |
| L | 97.3 ± 2.2 | 95.3 ± 3.1 | 94.5 ± 0.5 | 92.9 ± 2.4 | 88.8 ± 1.3 |
| aL formulation was dispersed in gel that is why the LJ results were the same with the L formulations | |||||
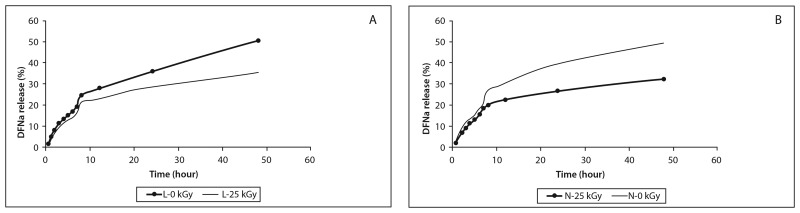
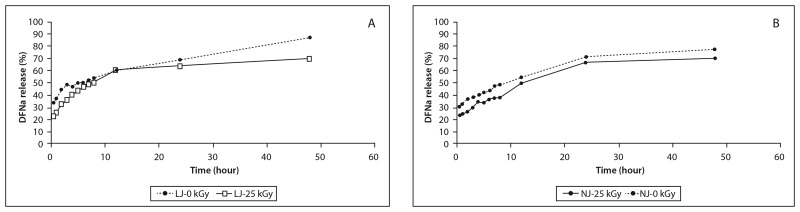
The drug release profiles of DFNa from the formulations were examined in 10 mM HEPES (pH = 7.4) buffer. The total amount of DFNa was released in 48 h from the formulations. As is expected, liposome and niosome formulations showed higher release of DFNa than lipogelosome and niogelosome formulations. The lipogelosome and niogelosome formulations significantly (p < 0.05) prolonged the DFNa release compared with the unirradiated and irradiated formulations (Figs 1 and 2). The release of DFNa from the lipogelosome and niogelosome formulations is a combination of the release of free, surface-bound, and encapsulated drug through the micellar network channel structures of the gel. Also, the release rate of a drug dissolved in the liquid phase of lipogelosome and niogelosome formulations may be affected by the type and concentration of the gelling agent and by the processing conditions [27]. Release profiles of the L, N, LJ, and NJ formulations were altered after irradiation. The release rates were significantly increased with the irradiation process. These results might imply radiation-induced decrease in bilayer fluidity. The changes in permeability and fluidity in the bilayer were found to be dependent on the radiation dose in a biphasic fashion in previous studies, and our results are in agreement with the literature [25].
Fig. 1.
In vitro release of DFNa from L (A) and N (B) formulations
Fig. 2.
In vitro release of DFNa from LJ (A) and NJ (B) formulations
Lipogelosome and niogelosome formulations showed a higher dynamic viscosity than liposome and niosome formulations due to the presence of the miceller network structure of gel in lipogelosomes and niogelosomes. As the shear rate increased, the viscosity of the lipogelosome and niogelosome formulations decreased. All the formulations displayed a non-Newtonian behavior at both 25 °C and 37 °C [8]. The difference between the viscosities of lipogelosome and niogelosome formulations was found to be statistically significant (p < 0.05). The dynamic viscosity of the lipogelosome and niogelosome formulations was significantly lowered upon irradiation process (Figs 3 and 4). These changes might be responsible for the shortening of polymer chains and structural degradion, depending upon the irradiation dose levels.
Fig. 3.
Rhelogical behavior (viscosity) of DFNa-loaded LJ formulation before and after gamma irradiation at (A) 25 °C and (B) 37 °C
Fig. 4.
Rhelogical behavior (viscosity) of DFNa-loaded NJ formulation before and after gamma irradiation at (A) 25 °C and (B) 37 °C
As is expected, unirradiated and irradiated formulations (liposome, niosome, lipogelosome, and niogelosome) were observed to be not exhibiting ESR signals. It is likely due to the unstable natures of the radical species produced upon gamma irradiation in solutions where radical–radical recombination is very fast.
According to the sterility test results, the negative control showed no growth, denoting sterility of the culture medium. The positive control showed bacterial growth signifying suitability of the medium for growth of aerobic and anaerobic bacteria. According to the sterilitiy test results, there was no microbial growth observed within 2 weeks with both of the media (Table VIII).
Table VIII.
Results of sterility test of liposomes/niosomes and lipogelosomes/niogelosomes
| Formulation | Microbiological growth |
|
|---|---|---|
| FTM (37 °C) | SCDM (25 °C) | |
| L | (–) | (–) |
| N | (–) | (–) |
| LJ | (–) | (–) |
| NJ | (–) | (–) |
| (−): no growth | ||
Pyrogen test results which indicate that the bacterial endotoxins (most common pyrogens) showed there was no clothing for all raw materials.
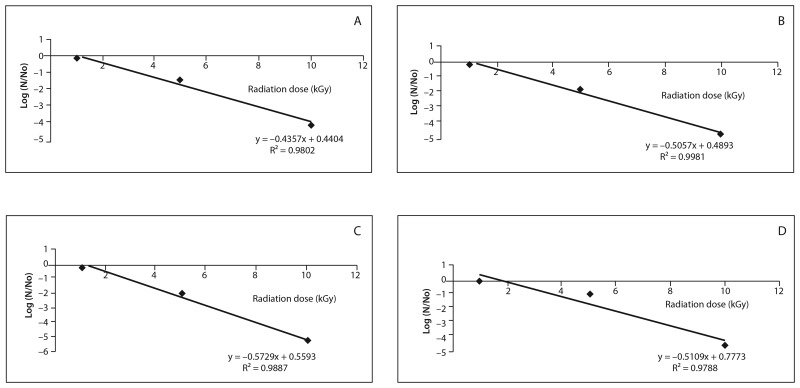
SAL of L, N, LJ, and NJ were found as 13.8, 15.2, 14.3, and 14.9 kGy, respectively (Fig. 5).
Fig. 5.
Microorganism death graphics (SAL). (A) L, (B) N, (C) LJ, (D) NJ
Those results showed that the coded formulations might be sterilized with the lower radiation doses after the validation of gamma irradiation, in comparison with the recommended radiation dose of 25 kGy in pharmacopeias.
According to the stability studies performed at room (25 °C) and refrigerator (4 °C) temperatures, the particle size distribution of L and N formulations did not change with gamma irradiation and stability test conditions (Table IX). Encapsulation of unirradited L formulations did not change as the irradiated ones in both of the stability conditions up to 1 month. However, the encapsulation efficiency of L formulation significantly decreased at room temperature in comparison with the formulations which were kept at 4 °C (Table X). The reason for those results might be the effect of gamma irradiation which might be more intensive at higher temperatures. According to the encapsulation efficiency results of N formulation, gamma irradiation effect on N formulation was higher than L formulation, the encapsulation efficiency results were significantly lower. The phospholipid content of the unirradiated formulations did not change at 4 °C; however, the phospholipid content of the irradiated formulations decreased (p < 0.05) over 3 months (Table XI). The stability test might be useful for the determination of optimum sterilization conditions with gamma irradiation for DFNa-loaded formulations.
Table IX.
Stability test results for particle size distribution of unirradiated and irradiated formulations
| Time (month) | Particle size (nm)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Refrigerator (4 °C) |
Room temperature (25 °C) |
|||||||||
| Formulation |
Formulation |
|||||||||
| L |
L (5 kGy) |
L (10 kGy) |
L (25 kGy) |
L (50 kGy) |
L |
L (5 kGy) |
L (10 kGy) |
L (25 kGy) |
L (50 kGy) |
|
| 1 | 235 ± 0.013 | 233 ± 0.015 | 235 ± 0.011 | 231 ± 0.011 | 230 ± 0.014 | 238 ± 0.013 | 230 ± 0.011 | 229 ± 0.010 | 234 ± 0.013 | 235± 0.016 |
| 2 | 234 ± 0.010 | 228 ± 0.010 | 231 ± 0.010 | 227 ± 0.010 | 232 ± 0.016 | 233 ± 0.010 | 234 ± 0.012 | 233 ± 0.014 | 237 ± 0.012 | 234 ± 0.014 |
| 3 | 230 ± 0.011 | 232 ± 0.014 | 231 ± 0.010 | 229 ± 0.012 | 235 ± 0.010 | 235 ± 0.014 | 233 ± 0.011 | 237 ± 0.015 | 230 ± 0.011 | 235 ± 0.012 |
| N |
N (5 kGy) |
N (10 kGy) |
N (25 kGy) |
N (50 kGy) |
N |
N (5 kGy) |
N (10 kGy) |
N (25 kGy) |
N (50 kGy) |
|
| 1 | 272 ± 0.015 | 271 ± 0.011 | 270 ± 0.010 | 271 ± 0.013 | 266 ± 0.010 | 275 ± 0.010 | 276 ± 0.011 | 271 ± 0.010 | 275 ± 0.010 | 277± 0.011 |
| 2 | 275 ± 0.012 | 270 ± 0.013 | 275 ± 0.011 | 273 ± 0.012 | 273 ± 0.015 | 269 ± 0.012 | 271 ± 0.012 | 269 ± 0.012 | 274 ± 0.013 | 272 ± 0.017 |
| 3 | 271 ± 0.014 | 275 ± 0.014 | 277 ± 0.015 | 279 ± 0.010 | 279 ± 0.012 | 271 ± 0.018 | 273 ± 0.015 | 272 ± 0.016 | 271 ± 0.010 | 275 ± 0.011 |
| aL and N formulations were dispersed in gel formulations that is why the LJ and NJ results were the same with the L and N formulations | ||||||||||
Table X.
Stability test results for encapsulation efficiency of unirradiated and irradiated formulations
| Time (month) | Encapsulation efficiency (%)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Refrigerator (4 °C) |
Room temperature (25 °C) |
|||||||||
| Formulation |
Formulation |
|||||||||
| L |
L (5 kGy) |
L (10 kGy) |
L (25 kGy) |
L (50 kGy) |
L |
L (5 kGy) |
L (10 kGy) |
L (25 kGy) |
L (50 kGy) |
|
| 1 | 10.8 ± 0.3 | 10.7 ± 0.1 | 10.7 ± 0.5 | 10.5 ± 0.4 | 10.6 ± 0.2 | 10.6 ± 0.1 | 10.5 ± 0.3 | 10.5 ± 0.1 | 10.4 ± 0.4 | 10.6 ± 0.3 |
| 2 | 10.7 ± 0.5 | 10.0 ± 0.8 | 10.5 ± 0.3 | 10.1 ± 0.6 | 10.2 ± 0.1 | 10.3 ± 0.2 | 8.9 ± 0.2 | 10.1 ± 0.3 | 9.9 ± 0.1 | 10.2 ± 0.1 |
| 3 | 10.7 ± 0.4 | 9.3 ± 0.4 | 10.0 ± 0.5 | 9.9 ± 0.2 | 9.8 ± 0.1 | 10.4 ± 0.2 | 8.1 ± 0.1 | 9.5 ± 0.5 | 8.6 ± 0.2 | 8.8 ± 0.4 |
| N |
N (5 kGy) |
N (10 kGy) |
N (25 kGy) |
N (50 kGy) |
N |
N (5 kGy) |
N (10 kGy) |
N (25 kGy) |
N (50 kGy) |
|
| 1 | 9.6 ± 0.1 | 9.5 ± 0.1 | 9.6 ± 0.1 | 9.7 ± 0.2 | 9.4 ± 0.1 | 9.6 ± 0.2 | 9.7 ± 0.2 | 9.5 ± 0.3 | 9.3 ± 0.4 | 9.1± 0.1 |
| 2 | 9.5 ± 0.5 | 9.3 ± 0.1 | 9.4 ± 0.6 | 9.2 ± 0.4 | 9.1 ± 0.5 | 9.6 ± 0.2 | 9.3 ± 0.3 | 9.1 ± 01 | 9.0 ± 0.2 | 9.0 ± 0.1 |
| 3 | 9.5 ± 0.3 | 9.1 ± 0.3 | 9.0 ± 0.1 | 9.0 ± 0.4 | 9.0 ± 0.2 | 9.0 ± 0.2 | 8.7 ± 0.2 | 8.7 ± 0.1 | 8.6 ± 0.2 | 8.7 ± 0.4 |
| aL and N formulations were dispersed in gel formulations that is why the LJ and NJ results were the same with the L and N formulations | ||||||||||
Table XI.
Stability test results for phospholipid content of unirradiated and irradiated formulations
| Time (month) | Phospholipid content (%)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Refrigerator (4 °C) |
Room temperature (25 °C) |
|||||||||
| Formulation |
Formulation |
|||||||||
| L |
L (5 kGy) |
L (10 kGy) |
L (25 kGy) |
L (50 kGy) |
L |
L (5 kGy) |
L (10 kGy) |
L (25 kGy) |
L (50 kGy) |
|
| 1 | 98.47 ± 1.26 | 95.47 ± 1.53 | 93.47 ± 1.22 | 91.47 ± 1.81 | 90.63 ± 1.14 | 88.92 ± 1.88 | 87.15 ± 1.19 | 85.11 ± 1.52 | 83.27 ± 1.59 | 82.33 ± 1.87 |
| 2 | 95.28 ± 1.17 | 94.13 ± 1.08 | 90.01 ±1.12 | 91.22 ± 1.19 | 86.14 ± 1.22 | 86.92 ± 1.61 | 84.90 ± 1.43 | 83.75 ± 1.38 | 80.62 ± 1.90 | 77.26 ± 1.29 |
| 3 | 94.45 ± 1.42 | 93.27 ± 1.55 | 91.45 ± 1.71 | 90.45 ± 1.38 | 83.11 ± 1.29 | 77.17 ± 1.34 | 75.28 ± 1.41 | 74.43 ± 1.55 | 71.26 ± 1.82 | 68.46 ± 1.15 |
| aL formulation was dispersed in gel that is why the LJ results were the same with the L formulation | ||||||||||
Conclusions
In this study, the effectiveness of gamma irradiation on DFNa-loaded formulations, L, N, LJ, and NJ, was investigated. Our results indicated that organoleptic properties did not change after gamma irradiation. TLC analysis indicated that the RF values of the DFNa-loaded formulations were significantly changed upon irradiation, which might indicate the degradation products. Those products might be analyzed with more sophisticated analytical methods, such as HPLC. pH of all formulations were significantly lowered upon irradiation process. Particle size distribution and encapsulation efficiency of L and N formulations were not affected by gamma irradiation. However, the phospholipid content of L formulations decreased which might be an indication for the damage of lipid bilayers. DFNa is entrapped in the liposomal core, that is why encapsulation efficiency was not affected while phospholipid content decreased. Release profiles of the L, N, LJ, and NJ formulations were altered, and the release rates were significantly increased with the irradiation process. The damage in lipid bilayers might facilitate the release of DFNa which is entrapped in the aqueous core in liposome/niosome and dispersed in lipogelosome/niogelosome formulations. Dynamic viscosity of the lipogelosome and niogelesome formulations were significantly lowered upon irradiation process. According to the sterility and pyrogen test results, there was no bacterial growth observed for all of the formulations. SAL of L, N, LJ, and NJ were found as 13.8, 15.2, 14.3, and 14.9 kGy, respectively, and all the SAL results were under the pharmacopeial obligatory dose rate which is 25 kGy. Stability test results indicated that the average particle size of the formulations did not change during the stability test conditions. Encapsulation efficiency of control and irradiated formulations were decreased both at room temperature and refrigerator temperature after 1 month. Gamma irradiation affects some of the physicochemical properties of DFNa-loaded formulations especially over 25 kGy. However, formulations can be sterilized safely by gamma irradiation method at predetermined SAL doses with minimum physical and chemical changes. The most important issue in radiation sterilization is to provide the optimum sterilization conditions. Further studies are needed to investigate the stability of the formulation upon gamma irradiation, like HPLC determinations, and improvement studies, like cryopreservation with different types of cryoprotectants.
Acknowledgements
We gratefully acknowledge the support of the Scientific and Technological Research Council of Turkey (TÜBİTAK) for their support (project no: SBAG 107 S 140). We also would like to thank Deva, L’oreal, and Phospholipid GmbH for supplying DFNa, SUR I, and phospholipids, respectively.
Contributor Information
Selcan Turker, 1Department of Radiopharmacy, Faculty of Pharmacy, Hacettepe University, Ankara, Turkey.
A. Yekta Özer, 1Department of Radiopharmacy, Faculty of Pharmacy, Hacettepe University, Ankara, Turkey.
Ekrem Kiliç, 2Department of Pharmaceutical Microbiology, Faculty of Pharmacy, Hacettepe University, Ankara, Turkey.
Meral Özalp, 2Department of Pharmaceutical Microbiology, Faculty of Pharmacy, Hacettepe University, Ankara, Turkey.
Seyda Çolak, 3Department of Physics Engineering, Faculty of Engineering, Hacettepe University, Ankara, Turkey.
Mustafa Korkmaz, 3Department of Physics Engineering, Faculty of Engineering, Hacettepe University, Ankara, Turkey.
References
- 1.Harrington KJ, Rowlinson-Busza G, Uster PS, Vile RG, Peters AM, Stewart JS. Single-fraction irradiation has no effect on uptake of radiolabeled pegylated liposomes in a tumor xenograft model. Int J Radiat Oncol Biol Phys. 2001 Mar 15;49(4):1141–1148. doi: 10.1016/s0360-3016(00)01444-9. [DOI] [PubMed] [Google Scholar]
- 2.Erdoğan S, Özer AY, Ekizoğlu M, Özalp M, Çolak Ş, Korkmaz M. Gamma irradiation of liposomal phospholipids. FABAD J Pharm Sci. 2006;31:182–190. [Google Scholar]
- 3.Olguner G. Radiation sterilization of sulfonamides and comparison with the other sterilization techniques. MSci Thesis. Turkey: Hacettepe University, Institute of Health Sciences; 2000. [Google Scholar]
- 4.Berk F, Özer AY. Radiation sterilization of medical devices. FABAD J Pharm Sci. 1999;24:223–231. [Google Scholar]
- 5.Berk F. Tek Kullanımlık Tıbbi Malzemelerin Gama Radyasyonu ile Sterilizasyonu ve Diğer Yöntemlerle Karşılaştırılması, MSci Thesis. Turkey: Hacettepe University Institute of Health Sciences; 2002. [Google Scholar]
- 6.Olguner Mercanoğlu G, Özer AY, Çolak Ş, Korkmaz M, Özalp M, Ekizoğlu M, Barbarin N, Tilquin B. Radiosterilization of sulfonamides: I: Determination of the effects of gamma irradiation on solid sulfonamids. Radiat Phys Chem. 2004;69:511–520. [Google Scholar]
- 7.Olguner Mercanoğlu G, Özer AY, Özalp M, Ekizoğlu M. Radiosterilization of sulfonamides II: Determination of the effects of gamma irradiation on commercial sulfanomide preparations. Turkish J Pharm Sci. 2007;4:159–170. [Google Scholar]
- 8.Türker S, Erdoğan S, Ozer AY, Ergün EL, Tuncel M, Bilgili H, Deveci S. Scintigraphic imaging of radiolabelled drug delivery systems in rabbits with arthritis. Int J Pharm. 2005 May 30;296(1-2):34–43. doi: 10.1016/j.ijpharm.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Liman V, Özer AY, Çolak S, Korkmaz M, Kılıç E, Özalp M. The effects of gamma radiation sterilization on cefazolin sodium. FABAD J Pharm Sci. 2005;30:124–132. [Google Scholar]
- 10.Naki N. Kozmetik ve Kozmetik Hammaddelerinin Gama Radyasyonla Sterilizasyon/Dekontaminasyonu Üzerinde Çalışmalar, MSci Thesis. Turkey: Hacettepe University, Institute of Health Sciences; 2003. [Google Scholar]
- 11.Zuidam NJ, Versluis C, Vernooy EA, Crommelin DJ. Gamma-irradiation of liposomes composed of saturated phospholipids: effect of bilayer composition, size, concentration and absorbed dose on chemical degradation and physical destabilization of liposomes. Biochim Biophys Acta. 1996 Apr 3;1280(1):135–148. doi: 10.1016/0005-2736(95)00275-8. [DOI] [PubMed] [Google Scholar]
- 12.Phillips GO, Power DM, Sewart M. Effects of gamma irradiation on sodium sulphacetamide. Radiat Res. 1971 May;46(2):236–250. [PubMed] [Google Scholar]
- 13.Fernández-Carballido A, Puebla P, Herrero-Vanrell R, Pastoriza P. Radiosterilisation of indomethacin PLGA/PEG-derivative microspheres: protective effects of low temperature during gamma-irradiation. Int J Pharm. 2006 Apr 26;313(1-2):129–35. doi: 10.1016/j.ijpharm.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 14.Stark AA. Oxidative metabolism of glutathione by gamma-glutamyl transpeptidase and peroxisome proliferation: the relevance to hepatocarcinogenesis. A hypothesis. Mutagenesis. 1991 Jul;6(4):241–245. doi: 10.1093/mutage/6.4.241. [DOI] [PubMed] [Google Scholar]
- 15.Albertini G, Rustichelli F. Effects of gamma irradiation on the liposomal structure. In: Gregoriadis G, editor. Liposome Technology; Liposomes Preparation and Related Techniques. Boca Raton: CRC Press; 1993. pp. 399–428. [Google Scholar]
- 16.Benson RS. Use of radiation in biomaterials science. Nucl Instrum Methods Phys Res Sect B. 2002;191:752–757. [Google Scholar]
- 17.Zuidam NJ, Gouw HK, Barenholz Y, Crommelin DJ. Physical (in) stability of liposomes upon chemical hydrolysis: the role of lysophospholipids and fatty acids. Biochim Biophys Acta. 1995 Nov 22;1240(1):101–110. doi: 10.1016/0005-2736(95)00180-5. [DOI] [PubMed] [Google Scholar]
- 18.Bushell JA, Claybourn M, Williams HE, Murphy DM. An EPR and ENDOR study of gamma- and beta-radiation sterilization in poly (lactide-co-glycolide) polymers and microspheres. J Control Release. 2005 Dec 10;110(1):49–57. doi: 10.1016/j.jconrel.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Samuni AM, Barenholz Y, Crommelin DJ, Zuidam NJ. Gamma-irradiation damage to liposomes differing in composition and their protection by nitroxides. Free Radic Biol Med. 1997;23(7):972–979. doi: 10.1016/s0891-5849(97)00123-8. [DOI] [PubMed] [Google Scholar]
- 20.Dam AM, Gazso LG, Kaewpila S, Maschek I. Radiation treatment of pharmaceuticals. Radiat Phys Chem. 1996;47:515–517. [Google Scholar]
- 21.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965 Aug;13(1):238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 22.Farshi F, Özer AY, Tavassoli S, Sungur A, Hıncal AA. A clinical trial: In vivo studies on dexamethasone sodium phosphate liposomes in the treatment of human aphthous stomatitis. J Liposome Res. 1996;6:699–712. [Google Scholar]
- 23.Paavola A, Kilpeläinen I, Yliruusi J, Rosenberg P. Controlled release injectable liposomal gel of ibuprofen for epidural analgesia. Int J Pharm. 2000 Apr 10;199(1):85–93. doi: 10.1016/s0378-5173(00)00376-8. [DOI] [PubMed] [Google Scholar]
- 24.Rouser G, Fkeischer S, Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 25.Mohammed AR, Bramwell VW, Coombes AG, Perrie Y. Lyophilisation and sterilisation of liposomal vaccines to produce stable and sterile products. Methods. 2006 Sep;40(1):30–38. doi: 10.1016/j.ymeth.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 26.El-Ridy MS, Mostafa DM, Shehab A, Nasr EA, Abd El-Alim S. Biological evaluation of pyrazinamide liposomes for treatment of Mycobacterium tuberculosis. Int J Pharm. 2007 Feb 7;330(1-2):82–88. doi: 10.1016/j.ijpharm.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Realdon N, Zotto MD, Ragazzi E, Fini GD. Drug release from lipogels according to gelling conditions and mechanical treatment. Drug Dev Ind Pharm. 1996;22:125–134. [Google Scholar]