Abstract
Acute radiation sickness (ARS) is expected to occur in astronauts during large solar particle events (SPEs). One parameter associated with ARS is the hematopoietic syndrome, which can result from decreased numbers of circulating blood cells in those exposed to radiation. The peripheral blood cells are critical for an adequate immune response, and low blood cell counts can result in an increased susceptibility to infection. In this study, Yucatan minipigs were exposed to proton radiation within a range of skin dose levels expected for an SPE (estimated from previous SPEs). The proton-radiation exposure resulted in significant decreases in total white blood cell count (WBC) within 1 day of exposure, 60% below baseline control value or preirradiation values. At the lowest level of the blood cell counts, lymphocytes, neutrophils, monocytes and eosinophils were decreased up to 89.5%, 60.4%, 73.2% and 75.5%, respectively, from the preirradiation values. Monocytes and lymphocytes were decreased by an average of 70% (compared to preirradiation values) as early as 4 h after radiation exposure. Skin doses greater than 5 Gy resulted in decreased blood cell counts up to 90 days after exposure. The results reported here are similar to studies of ARS using the nonhuman primate model, supporting the use of the Yucatan minipig as an alternative. In addition, the high prevalence of hematologic abnormalities resulting from exposure to acute, whole-body SPE-like proton radiation warrants the development of appropriate countermeasures to prevent or treat ARS occurring in astronauts during space travel.
Introduction
Exposure to space radiation in a major solar particle event (SPE) may place astronauts at significant risk for acute radiation sickness (ARS), skin injury and/or a compromised immune defense. Unpredictable, highly energetic SPEs originate from magnetically disturbed regions of the sun, which sporadically emit bursts of energetic charged particles (1, 2). Normally, large SPEs occur only once or twice in a solar cycle; however, during the 22nd solar cycle, four comparable extremely large SPEs occurred in a 4 month period. The types of radiation emitted during SPEs are predominately protons (3).
The high skin doses that would occur during exposure to SPE radiation result from the relatively low energy of most of the SPE protons, which have limited penetrating ability in tissue. Thus, exposure to SPE radiation results in an inhomogeneous total-body dose distribution, with considerably higher doses delivered to the skin and subcutaneous tissues than those delivered to internal organs, as described by Cengel et al. (4). The doses that could have been received by astronauts during large SPEs have been estimated by modeling 3 large historic SPEs (5). During extravehicular activity (EVA), skin doses are estimated to be as high as 32 Gy and in the blood-forming organs (BFO), 1.4 Gy. Inside the spacecraft, the doses are estimated to be ∼3 Gy to the skin and 0.5 Gy to the BFO (5). From the modeling of past SPEs, it has been concluded that the probable radiation environment of astronauts will lead to the following doses during EVA: >5 Gy to the skin and the maximum deep dose is estimated at 2 Gy (F. Cucinotta, NASA-Space Radiation Program Element, http://www.nasa.gov/exploration/humanresearch/elements/research_info_element-srpe.html). The National Aeronautics and Space Administration (NASA) has determined that the likelihood of acute risks during internal vehicle activity (IVA) is very small; however, there are scenarios during lunar, trans-lunar or Mars EVAs in which ARS may occur.
ARS represents a constellation of clinical toxicities that varies both qualitatively and quantitatively with radiation dose, dose rate, quality and individual radiation sensitivity (3). The stages of the ARS include the prodromal stage (nausea, vomiting and diarrhea), the latent stage, the manifest illness stage and the recovery or death stage. The prodromal phase occurs within a few hours post-exposure, with a latency time inversely correlated with dose. There are three classic ARS syndromes that can lead to death; these are the hematopoietic syndrome, the gastrointestinal syndrome and the cerebrovascular syndrome. Symptoms of the cerebrovascular syndrome include severe nausea and emesis, diarrhea, loss of consciousness, convulsive seizures, and coma. The mechanisms involved in the cerebrovascular syndrome are unclear. Both the hematopoietic and gastrointestinal syndromes are thought to result from the cell killing effects of radiation in the stem cells of critical self-renewing tissues. The destruction of hematopoietic progenitor cells ultimately results in reduced numbers of mature, circulating blood cells. Lymphopenia has been used in the past as an indicator of the systemic effects of radiation exposure, as lymphocytes are known to be the most sensitive blood cell type to radiation. Lymphocytes mediate the adaptive immune response, and they have a life span of weeks to years. Neutrophil granulocytes are the most abundant phagocyte (other circulating phagocytes include eosinophils and monocytes), and they are the first line of defense in the body. Encompassing 50–60% of the circulating white blood cells (WBCs) in humans, these cells have a short life span and are constantly being renewed. Neutropenia commonly occurs in patients receiving chemotherapy or radiation therapy and may result in infections, fever and impairment of immune system mechanisms. While wound healing is suppressed in the space environment (6), platelet count depression contributes to bleeding caused by hemorrhage. For the gastrointestinal syndrome, the destruction of stem cells in the gut leads to a depopulation of the epithelial lining of the gastrointestinal tract. The symptoms resulting from the loss of the epithelial lining include diarrhea, emaciation and dehydration. For astronauts, it is not expected that the doses received will be sufficiently high to result in death from the gastrointestinal or cerebrovascular syndrome, but the doses received may be sufficient to cause hematopoietic cell loss, immune system dysfunction and symptoms of the ARS such as vomiting. If such ARS symptoms do occur in astronauts, it is expected that they would be accompanied by skin damage, due to high skin doses from SPEs, which can lead to burns.
A combined U.S. Air Force (USAF)/NASA project was conducted from 1963–1969, to measure the biological effects of space radiation (with varying energies of proton radiation) and calculate the relative biological effectiveness (RBE) values using approximately 2,000 primates (7). Late radiation effects included leukemia (8) and endometriosis (9). The authors suggest that the occurrence of radiation induced endometriosis is a systemic rather than local disease, with deficiencies in the immune system as likely contributors; however, immune parameters were not reported. The acute effects after whole-body proton, γ or X irradiation at energies and doses found in the space environment, were measured. During these studies, ARS symptoms reported include malaise with depression (10), loss of appetite and diarrhea (10, 11). Blood samples were taken from Rhesus monkeys within days post-irradiation to evaluate the hematopoietic tissue injury. A dose-dependent reduction in circulating WBC counts after proton or γ irradiation, with a greater depression in the proton-irradiated animals, was reported (11). A dose-rate effect on proton-radiation induced acute mortality was also reported (10).
As the studies reported here were designed to mimic EVA conditions for astronauts during a large SPE, it was decided that the skin doses to be used would range from 5–10 Gy. The SPE exposures delivered at the James M. Slater, M.D. Proton Treatment and Research Center were created to match the electron irradiations (used as the control radiation modality) described by Cengel et al. (4).
In the current study, we investigated SPE-like proton radiation on peripheral blood cell counts as early as 4 h postirradiation to 90 days postirradiation in a porcine model utilizing the Yucatan minipig. The Yucatan minipig was used in our previous study investigating electron radiation induced skin toxicity (12). In the current study, Yucatan minipigs were exposed to proton radiation to characterize the time and dose response of radiation induced changes in the hematopoietic system. The data presented here are similar to the data from the large-scale primate study, suggesting that the Yucatan minipig may be an alternative to the nonhuman primate in radiobiology research and a suitable large animal model to study the physiological symptoms associated with ARS.
Materials and methods
Animals
Yucatan minipigs aged 8–10 weeks were purchased from Sinclair BioResources (Auxvasse, MO). Animals were acclimated for 7 days in the Loma Linda University Medical Center (LLUMC) animal facility, and were housed individually, given water ad libitum and fed standard mini-piglet chow twice daily. The animal care and treatment procedures were approved by the Institutional Animal Care and Use Committee of the LLUMC and the University of Pennsylvania.
Upon acclimation, the animals were randomly assigned to 4 groups with 3 animals per group and exposed to proton radiation at doses of 0 (sham-irradiation control), 5.0, 7.7 and 10.0 Gy. Animals were evaluated once daily for at least 90 days after irradiation.
Irradiation
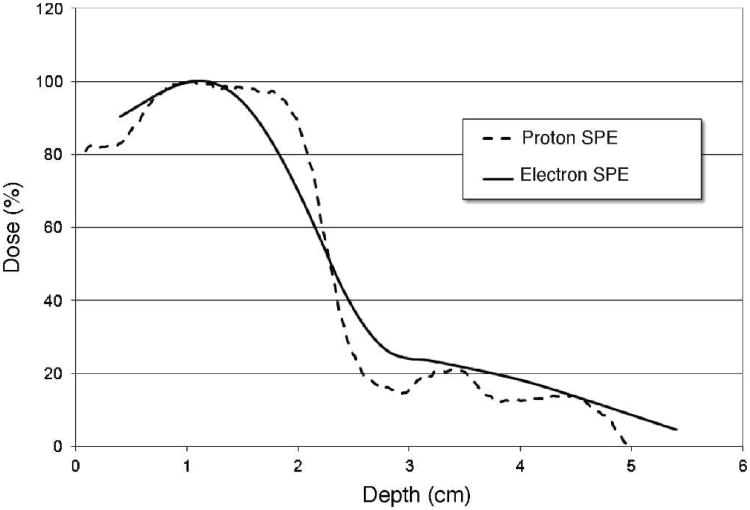
For the proton radiation exposures (referred to as proton SPE), a custom designed double scattering system was developed and built to allow for the delivery of 50-cm diameter radiation field with a radiation flatness of 3.5% or better. This system was installed on one of the research beam-lines available at LLUMC and was tuned to deliver approximately 5 Gy/h. A clinical modulator wheel was used to create a fully modulated 155 MeV/n proton beam, while radiation dose was prescribed at a depth of 1.1 cm in water along the central beam axis. A 2 stage bolus at the level of the animal chamber and beam weighting allowed for the creation of a custom depth dose profile to match the combined 6 + 12 MeV electron beam (referred to electron SPE) described by Cengel et al. (4), which itself was developed to mimic the dose profile of an SPE. This setup delivered a maximum proton range of 5.0 cm (approximately 80 MeV) at the entrance of the animal cage and a distribution of proton energies below 80 MeV (due to the beam modulation), which is reflective of the space environment where the maximum proton energy is accompanied by a significant number of lower energy protons. Characterization of the proton beam was completed using radiographic film, radiochromic film and ionization chambers. Figure 1 shows the dose distribution profile of the proton SPE beam developed at LLUMC (dashed line), measured using Gafchromic film (EDR-2) verified with a plane parallel ionization chamber, and the electron SPE beam profile (solid line), measured with a plane parallel ionization chamber (PPC40, IBA-Dosimetry) in a plastic water phantom. Field flatness was established using radiochromic film at both the external and internal surface of the minipig cage. The dose at the prescription point was calibrated using an Exradin T1 ionization chamber. Animals were irradiated to 5.0, 7.7 and 10.0 Gy per side with the animal cage rotated at 50% dose delivery per side. Dose delivery was verified with TLD dosimeters that were attached to the animal.
FIG. 1.
Dose distribution profiles. The percentage of the maximum dose is illustrated for the proton SPE beam (dashed line), developed at LLUMC to match the combined 6 + 12 MeV electron SPE beam [described by Cengel et al. (4), solid line].
A Geant4 based Monte Carlo dose modeling software tool was utilized to estimate dose to specific organs and a completed description will be published separately. Estimates of the median dose, mean dose and percentage of organ volume receiving 2 Gy for an animal with a nominal 5 Gy skin dose are shown in Table 1. We have included organs of skin, body (representing integral body dose), total lymphovascular volume (TLV) and bone marrow (marrow cavities of skull, spine, ribs, pelvis and proximal femur/humerus). TLV is essentially the entirety of the body that receives blood or lymphatic flow and is defined as “from the dermis inward”, i.e., almost the whole body, but without the part of the skin that has no vessels.
TABLE 1. Monte Carlo Derived Estimated Doses to the Integral Body, Skin, Total Lymphovascular Volume (TLV), and Bone Marrow (BM) in an Animal Receiving a 5 Gy Skin Dose.
| Median dose(Gy)/5 Gy skin | Mean dose(Gy)/5 Gy skin | Organ volume (%)2 Gy/5 Gy skin | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Proton SPE | Electron SPE | Proton SPE | Electron SPE | Proton SPE | Electron SPE | |
| Body | 1.01 | 0.99 | 1.95 | 1.67 | 37.3 | 33.5 |
| Skin | 4.81 | 4.41 | 4.74 | 4.32 | 95.4 | 96.9 |
| TLV | 0.85 | 0.85 | 1.60 | 1.41 | 30.1 | 27.2 |
| BM | 0.42 | 0.52 | 0.80 | 0.82 | 11.8 | 10.2 |
Note. The percentage of the organ volume receiving 2 Gy is shown in the far right columns.
Blood Cell Count Analyses
At 4 h and 1, 4, 14, 30 and 90 days after radiation exposure, whole-blood samples were collected from each anesthetized animal (cranial vena cava) and placed into a lavender top collection tube containing EDTA and kept at ambient temperature during preparation for transport. Samples were then transported at 4°C to Antech Diagnostics (Irvine, CA) and analyzed using a Bayer Advia 120 Hematology Analyzer (using the porcine specific profile) within 24 h of the blood sample collection. It has been previously determined that the blood cell counts are stable and reliable when comparing blood cell counts analyzed immediately after collection or within/up to 24 h after collection (13).
Data and Statistical Analyses
The mean counts of WBCs, lymphocytes, neutrophils, monocytes, eosinophils, red blood cells (RBCs) and platelets of all animals before irradiation were calculated and used as the baseline control values for the respective blood cell types (n = 12). For each animal at each time point after irradiation, the count of each blood cell type was divided by the respective baseline control value and the result was expressed as fractions of control for all further analyses. The blood cell count at each time point for each dose group consists of 3 data points (n = 3), except for the baseline control value (obtained prior to radiation exposure), or 0 Gy dose group, which consists of 12 data points (n = 12). The blood cell count data for animals irradiated with 0 (sham radiation control), 5.0, 7.7 and 10.0 Gy of proton radiation were compared by one-way ANOVA followed by Tukey's test using SigmaPlot software (Version 12) to evaluate the blood cell count changes at different time points after irradiation.
The relationship between radiation dose and counts of each blood cell type was determined for each time point after irradiation by fitting the data to a linear-quadratic model (y = e–aD–bD2) using SigmaPlot graphics software (SPSS Inc., Chicago, IL), in which D is the radiation dose and y is the blood cell count expressed as a fraction of control.
Results
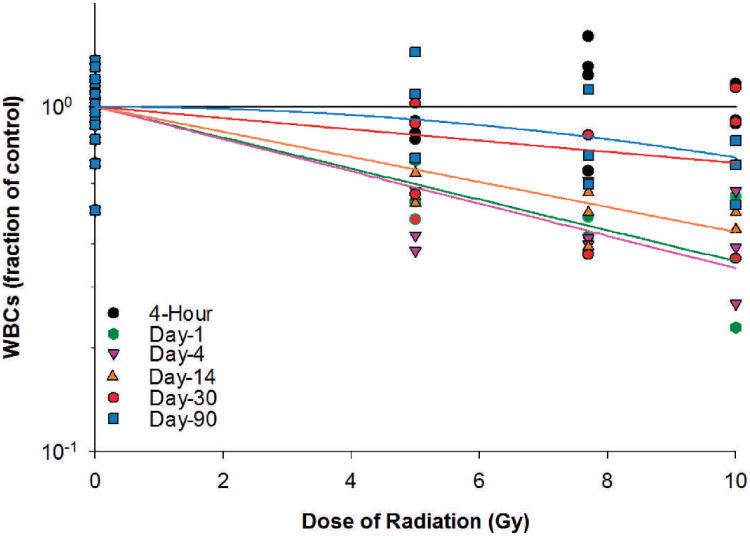
The present study was undertaken to determine the effect of proton SPE radiation on peripheral blood cells using Yucatan minipigs as an experimental model system. The WBC, lymphocyte, neutrophil, monocyte, eosinophil, RBC and platelet count data (expressed as fraction of control) at different time points after irradiation were compared to the respective baseline levels to evaluate the blood cell count changes after irradiation. The blood cell count data were also fitted using a linear-quadratic model to analyze the dose-response relationship for the WBCs, lymphocytes, neutrophils, monocytes, eosinophils, RBCs and platelets. In the irradiated Yucatan minipigs, the WBC count decreased within a day after irradiation by 43.4, 57.4 and 59.3%, respectively, for the 5, 7.7 and 10 Gy radiation dose groups (Table 2). Between day 1 and day 4 after irradiation, the WBC count reached the lowest level, which was 54.2, 57.4 and 59.3% below the baseline level for the 5, 7.7 and 10 Gy radiation dose groups. The WBC count recovered slowly thereafter. By day 30, the WBC count in the irradiated animals was only 17.7%, 38.3% and 20.0% below the baseline level for the 5, 7.7 and 10 Gy radiation dose groups. By day 90, the WBC count recovered fully for the 5 Gy radiation dose group while remaining 18.7 and 33.5% below the baseline level for the 7.7 and 10 Gy radiation dose groups. As for the dose-response relationships at different time points after the radiation exposure, significant radiation dose responses for WBCs were observed in irradiated animals on days 1, 4, 14, 30 and 90 after irradiation (Fig. 2). The linear component of the dose-response curve slope was 0.103, 0.108, 0.084, 0.038 and 0.003, respectively, for days 1, 4, 14, 30 and 90 after irradiation. The steepest slope was observed on day 4 after irradiation. The quadratic component of the dose-response curve slope was negligible (≤7.647 ×10−15) for all time points investigated.
TABLE 2. WBC Counts in Animals Irradiated with Protons.
| WBC counts (fraction of control) in pigs irradiated at dose shown below | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 5 Gy | 7.7 Gy | 10 Gy | ||||
|
|
|
|
||||
| Time after irradiation | Mean ± SE | Change | Mean ± SE | Change | Mean ± SE | Change |
| Preirradiation | 1.00 ± 0.08 | N/A | 1.00 ± 0.08 | N/A | 1.00 ± 0.08 | N/A |
| 4h | 0.85 ± 0.03 | −15.0% | 1.38 ± 0.11 | 38.1% | 0.99 ± 0.09 | −0.8% |
| Day 1 | 0.57 ± 0.07 | −43.4%# | 0.43 ± 0.03 | −57.4%** | 0.41 ± 0.09 | –59.3%* |
| Day 4 | 0.46 ± 0.06 | −54.2%* | 0.47 ± 0.07 | −52.6%* | 0.41 ± 0.09 | −59.1%* |
| Day 14 | 0.57 ± 0.04 | −42.8%# | 0.48 ± 0.05 | −51.7%* | 0.54 ± 0.07 | −46.2%# |
| Day 30 | 0.82 ± 0.14 | −17.5% | 0.62 ± 0.13 | −38.3% | 0.80 ± 0.23 | −20.0% |
| Day 90 | 1.08 ± 0.21 | 7.9% | 0.81 ± 0.16 | −18.7% | 0.67 ± 0.08 | −33.5% |
Note. Statistical significance for comparison with the preirradiation value by the Tukey test is indicated by symbols of
(P < 0.10),
(P < 0.05) and
(P < 0.01), respectively.
FIG. 2.
WBCs in Yucatan minipigs after proton irradiation. The dose-response curve for each time point after irradiation is fitted using a linear-quadratic model.
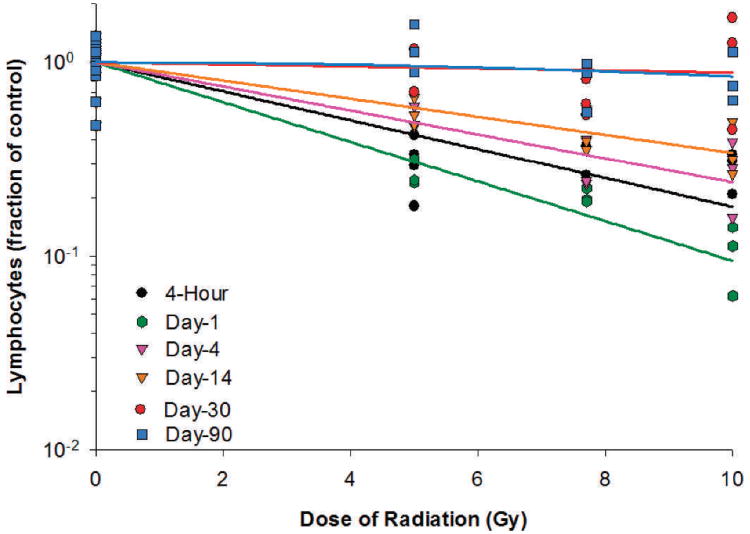
Lymphocyte counts decreased significantly as early as 4 h after irradiation for the 5, 7.7 and 10 Gy radiation dose groups (Table 3). On day 1 after irradiation, the lymphocyte count reached the lowest level, which was 73.0, 79.7 and 89.5% below the baseline level for the 5, 7.7 and 10 Gy radiation dose groups, respectively. Lymphocyte counts recovered slowly thereafter. By day 30, the lymphocyte count in the irradiated animals was not more than 34.5% below the baseline level for the three radiation dose groups. By day 90, the lymphocyte count recovered fully for the 5 Gy radiation dose group and was not more than 19.5% below the baseline level for the two higher radiation dose groups. For the dose-response relationships at different time points after radiation exposure, significant radiation dose responses for lymphocytes were observed in irradiated animals at 4 h and on days 1, 4 and 14 postirradiation (Fig. 3). The dose responses on days 30 and 90 were not statistically significant. The linear component of the linear quadratic dose-response curve slope was 0.172, 0.236, 0.143, 0.108, respectively, for 4 h and days 1, 4 and 14 after irradiation. The steepest slope was observed on day 1 after irradiation. The quadratic component of the dose-response curve slope was 0.002 for day 90 and negligible (≤ 1.030 ×10−18) for all other time points investigated.
TABLE 3. Lymphocyte Counts in Animals Irradiated with Protons.
| Lymphocyte counts (fraction of control) in pigs irradiated at dose shown below | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 5 Gy | 7.7 Gy | 10 Gy | |||||
|
|
|
|
|||||
| Time after irradiation | Mean ± SE | Change | Mean ± SE | Change | Mean ± SE | Change | |
| Preirradiation | 1.00 ± 0.07 | N/A | 1.00 ± 0.07 | N/A | 1.00 ± 0.07 | N/A | |
| 4h | 0.27 ± 0.05 | −73.0%*** | 0.33 ± 0.04 | −66.6%*** | 0.28 ± 0.04 | −71.6%** | |
| Day 1 | 0.27 ± 0.02 | −73.2%*** | 0.20 ± 0.01 | −79.7%*** | 0.11 ± 0.02 | −89.5%*** | |
| Day 4 | 0.50 ± 0.05 | −50.5%* | 0.29 ± 0.05 | −70.6%*** | 0.28 ± 0.07 | −72.2%** | |
| Day 14 | 0.56 ± 0.05 | −44.1%# | 0.44 ± 0.07 | −55.9%** | 0.36 ± 0.07 | −64.1%* | |
| Day 30 | 0.86 ± 0.15 | −14.2% | 0.66 ± 0.08 | −34.5% | 1.13 ± 0.36 | 13.4% | |
| Day 90 | 1.20 ± 0.20 | 19.5% | 0.81 ± 0.13 | −19.5% | 0.84 ± 0.15 | −15.8% | |
Note. Statistical significance for comparison with the preirradiation value by the Tukey test is indicated by symbols of
(P < 0.10)
(P <0.05)
(P < 0.01) and
(P < 0.01), respectively.
FIG. 3.
Lymphocytes in Yucatan minipigs after proton irradiation. The dose-response curve for each time point after irradiation is fitted using a linear-quadratic model.
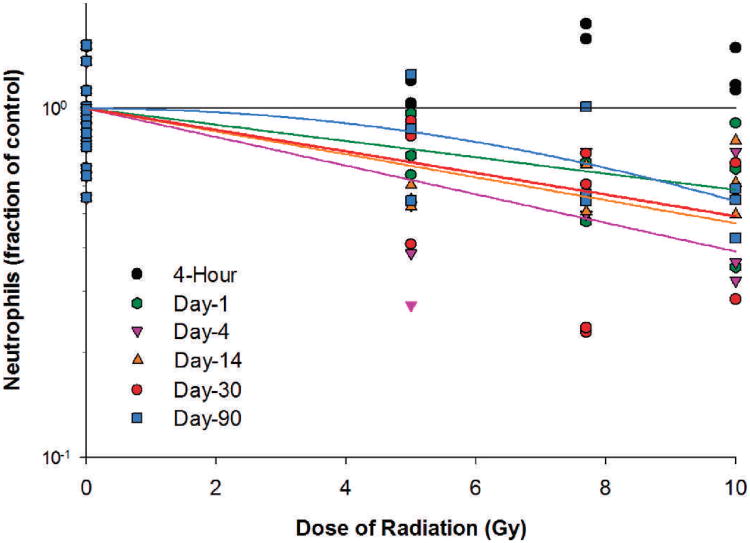
Neutrophil counts increased by 8.7%, 79.8% and 26.3% for the 5, 7.7 and 10 Gy radiation dose groups, respectively, at 4 h after irradiation (Table 4). Following the initial increase, the neutrophil count decreased to 21.7–42.1% below baseline for the three radiation dose groups on day 1 after irradiation. Between days 4 and 14 postirradiation, the neutrophil counts were at their lowest levels of 60.4%, 52.5% and 52.1% below baseline, respectively, for the 5, 7.7 and 10 Gy dose groups. For the dose-response relationships at different time points after irradiation, significant radiation dose responses for neutrophils were observed in irradiated animals on days 1, 4, 14, 30 and 90 after irradiation (Fig. 4). The linear component of the linear quadratic dose-response curve slope was 0.054, 0.094, 0.076, 0.071 and 5.946 ×10−19, respectively, for days 1, 4, 14, 30 and 90 after irradiation. The steepest slope was observed on day 4 after irradiation. The quadratic component of the dose-response curve slope was 0.006 for day 90 and negligible (≤ 1.005 ×10−18) for all other time points investigated.
TABLE 4. Neutrophil Counts in Animals Irradiated with Protons.
| Neutrophil counts (fraction of control) in pigs irradiated at dose shown below | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 5 Gy | 7.7 Gy | 10 Gy | ||||
|
|
|
|
||||
| Time after irradiation | Mean ± SE | Change | Mean ± SE | Change | Mean ± SE | Change |
| Preirradiation | 1.00 ± 0.10 | N/A | 1.00 ± 0.10 | N/A | 1.00 ± 0.10 | N/A |
| 4h | 1.09 ± 0.06 | 8.7% | 1.80 ± 0.14 | 79.8%** | 1.26 ± 0.11 | 26.3% |
| Day 1 | 0.78 ± 0.10 | −21.7% | 0.58 ± 0.07 | −42.1% | 0.64 ± 0.16 | −35.6% |
| Day 4 | 0.40 ± 0.07 | −60.4%* | 0.58 ± 0.08 | −41.8% | 0.48 ± 0.14 | −52.1%# |
| Day 14 | 0.56 ± 0.02 | −44.2% | 0.47 ± 0.13 | −52.5%# | 0.64 ± 0.09 | −36.1% |
| Day 30 | 0.72 ± 0.16 | −27.9% | 0.53 ± 0.15 | −47.2% | 0.52 ± 0.12 | −47.8% |
| Day 90 | 0.89 ± 0.20 | −11.1% | 0.71 ± 0.15 | −29.3% | 0.52 ± 0.05 | −48.0% |
Note. Statistical significance for comparison with the pre-irradiation value by the Tukey test is indicated by symbols of
(P < 0.10),
P < 0.05) and
(P < 0.01), respectively.
FIG. 4.
Neutrophils in Yucatan minipigs after proton irradiation. The dose-response curve for each time point after irradiation is fitted using a linear-quadratic model.
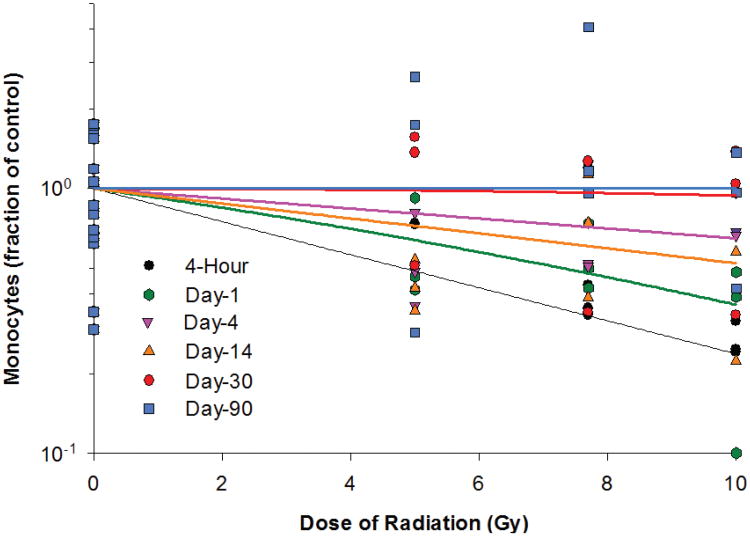
In the irradiated animals, the lowest level of monocyte counts was observed 4 h after irradiation and was 62.3, 62.7 and 73.2% below the baseline values for the 5, 7.7 and 10 Gy radiation groups, respectively (Table 5). Due to the relatively large variation within the treatment groups, the difference between different time points did not reach statistical significance for any of the three radiation dose groups. The monocyte count recovered slowly thereafter. On days 30 and 90, the monocyte count in the irradiated animals was not more than 8.2% below the baseline level. For the dose-response relationships at different time points after irradiation, significant radiation dose responses for monocytes were observed at 4 h and on day 1 after irradiation (Fig. 5). The dose response was marginally significant on day 14 postirradiation but was not statistically significant on days 4, 30 and 90 postirradiation. The linear component of the linear quadratic dose-response curve slope was 0.144 and 0.078, respectively, for 4 h and day 1 postirradiation. The steepest slope was observed at 4 h postirradiation. The quadratic component of the dose-response curve slope was 0.002 and 6.039 ×10−4 for days 1 and 30 after irradiation and was negligible (≤ 1.428 ×10−18) for all other time points investigated.
TABLE 5. Monocyte Counts in Animals Irradiated with Protons.
| Monocyte counts (fraction of control) in pigs irradiated at dose shown below | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 5 Gy | 7.7 Gy | 10 Gy | ||||
|
|
|
|
||||
| Time after irradiation | Mean ± SE | Change | Mean ± SE | Change | Mean ± SE | Change |
| Preirradiation | 1.00 ± 0.13 | N/A | 1.00 ± 0.13 | N/A | 1.00 ± 0.13 | N/A |
| 4h | 0.38 ± 0.36 | −62.3% | 0.37 ± 0.03 | −62.7% | 0.27 ± 0.02 | −73.2% |
| Day 1 | 0.60 ± 0.16 | –40.0% | 0.55 ± 0.10 | −44.7% | 0.32 ± 0.12 | −67.6% |
| Day 4 | 0.55 ± 0.14 | −44.7% | 0.75 ± 0.24 | −24.5% | 0.77 ± 0.10 | −22.7% |
| Day 14 | 0.44 ± 0.06 | −56.4% | 0.75 ± 0.22 | −24.6% | 0.59 ± 0.22 | –40.9% |
| Day 30 | 1.15 ± 0.32 | 14.7% | 0.93 ± 0.30 | −6.6% | 0.92 ± 0.31 | −8.2% |
| Day 90 | 1.55 ± 0.68 | 55.4% | 2.07 ± 1.00 | 106.5% | 0.92 ± 0.27 | −8.3% |
Notes. The one-way ANOVA P values for the 5 Gy, 7.7 Gy and 10 Gy radiation dose groups were 0.11, 0.08 and 0.07, respectively. No post-test comparison was performed for different time points in each radiation dose group since the one-way ANOVA P > 0.05.
FIG. 5.
Monocytes in Yucatan minipigs after proton irradiation. The dose-response curve for each time point after irradiation is fitted using a linear-quadratic model.
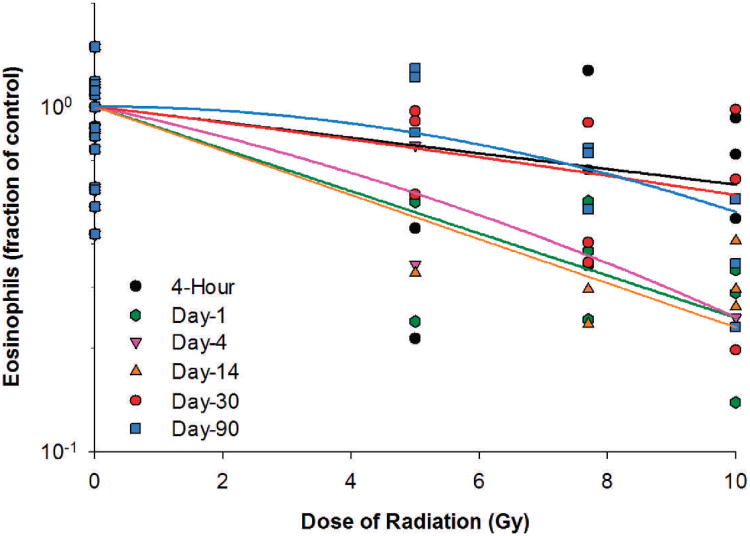
Eosinophil counts in the irradiated animals decreased ≥24.2% at 4 h postirradiation and reached the lowest levels between 4 h and day 14 postirradiation (Table 5), which were 67.2, 73.5 and 75.5% below the baseline levels for the 5, 7.5 and 10 Gy radiation dose groups, respectively. The eosinophil count recovered slowly after day 14, and by day 90, the eosinophil count was fully recovered for the 5 Gy radiation dose group but was still 33.5 and 62.6% below the baseline value for the 7.7 and 10 Gy radiation dose groups. For the dose-response relationships at different time points post-irradiation, significant or nearly significant radiation dose responses for eosinophils were observed in irradiated animals on days 1, 4, 14, 30 and 90 postirradiation (Fig. 6). The linear component of the linear quadratic dose-response curve slope was 0.141, 0.090, 0.147, 0.052 and 3.695 ×10−17, respectively, for days 1, 4, 14, 30 and 90 after irradiation. The steepest slope was reached on day 14 after irradiation. The quadratic component of the dose-response curve slope was 0.005, 6.843 ×10−4 and 0.007 for days 4, 30 and 90 after irradiation but was negligible (≤3.776 ×10−18) for all other time points investigated.
FIG. 6.
Eosinophils in Yucatan minipigs after proton irradiation. The dose-response curve for each time point after irradiation is fitted using a linear-quadratic model.
In the irradiated animals, RBC or platelet counts did not show a significant decrease at any of the time points investigated after proton-radiation exposure in the 5, 7.7 or 10 Gy radiation dose groups and the dose responses of RBCs and platelets were not statistically significant for any of the time points after irradiation (data not shown).
Discussion
In the present study, changes in the counts of WBCs, lymphocytes, neutrophils, monocytes, eosinophils, RBCs and platelets in response to proton SPE irradiation at doses of 5, 7.7 and 10 Gy were evaluated in Yucatan minipigs. Among the blood cell types evaluated, WBCs, lymphocytes, neutrophils, monocytes and eosinophils declined substantially in at least the two highest radiation dose groups within a day after irradiation. No significant decrease was observed for platelets and RBCs after the proton irradiation at any time points after irradiation in any of the three radiation dose groups. The lowest blood cell counts were observed at 4 h after irradiation for monocytes, on day 1 for lymphocytes, on day 4 for WBCs, between days 4–14 for neutrophils, and between 4 h and day 14 for eosinophils. At the lowest levels of the blood cell counts, the WBCs, lymphocytes, neutrophils, monocytes and eosinophils decreased by up to 59.1%, 89.5%, 60.4%, 73.2% and 75.5%, respectively, from the respective baseline control values (preirradiation values). After the initial decrease observed after irradiation, WBC, lymphocyte, neutrophil, monocyte and eosinophil counts recovered slowly to levels that were not more than 38.3%, 34.5%, 47.8%, 8.2% and 44.8% below the respective baseline control levels by day 30 postirradiation. By day 90 postirradiation, the WBCs, lymphocytes, neutrophils, monocytes and eosinophils in the 5 Gy radiation dose group had recovered to levels that were not more than 11.1% below their respective baseline levels. However, for the two higher radiation dose groups (7.7 and 10 Gy), the levels of WBCs, lymphocytes, neutrophils and eosinophils were still 18.7%, 15.8%, 29.3% and 33.5% below the respective baseline levels at day 90 postirradiation. Based on the magnitude of the decrease and the time required to reach the lowest blood cell counts after irradiation, lymphocytes appeared to be the most sensitive to SPE–like proton irradiation.
The neutrophil counts at the 4 h time point were increased compared to baseline values, followed by a reduction in counts by day 1 postirradiation. This observed increase is believed to be a phase of granulocytosis where mature and early precursor granulocytes are released from the bone marrow compartment (14). This process, called demargination of granulocytes, is thought to be provoked by endogenous (or exogenous) epinephrine, exercise or stress.
Due to technical limitations, the depth dose beam profiles for the simulated electron SPE and proton SPE could not be perfectly matched and there were slight variations in size between electron and proton SPE exposed animals. Previous results for hematopoietic cell loss after irradiation have used homogeneous doses, so knowing the exact target (i.e., marrow, circulating blood/lymphatic volume, etc.) was perhaps not as relevant. This is a major issue in interpreting the results of experiments in which inhomogeneous irradiation is delivered. We have previously described the development and testing of a GEANT4 based Monte Carlo dose modeling software tool that allows us to provide more accurate estimates for the dose to specific organs.2 Estimates of the median dose, mean dose and percentage of organ volume receiving 2 Gy for an animal with a nominal 5 Gy skin dose are shown in Table 1. We have included organs of skin, body (representing integral body dose), total lymphovascular volume (TLV, total body without skin) and bone marrow (marrow cavities of skull, spine, ribs, pelvis and proximal femur/humerus). This analysis demonstrates that the dose parameters for these volumes are highly similar with a median difference of electron or proton dose across all volumes of 5.6%. This is very close to the allowable variation across the radiation field as delivered in clinical radiotherapy and is approximately the allowable variation for human patients in prescribed vs. actual dose (typically a 5% difference) and therefore is highly unlikely to be the sole contributing factor behind any potential differences in hematopoietic responses that might be observed between electron and proton SPE exposure in animals.
The effects of proton radiation on blood cell counts have been evaluated previously in Rhesus monkeys exposed to 200 MeV protons at absorbed doses ranging from 175 to 500 rads (11) or exposed to 146.8 MeV protons at doses ranging from 275 to 900 rads (10). At the first time point (day 3) after irradiation with 200 MeV protons, the blood cell counts decreased by approximately 70% for WBCs and by approximately 80% or more for lymphocytes, neutrophils and eosinophils with the greatest initial depression in lymphocytes and the maximum level of depression observed during the second and third weeks after irradiation. In addition, the WBCs, lymphocytes, neutrophils, eosinophils and monocytes all exhibited a similar general pattern of depression and recovery after proton irradiation (11). The observations of the monkey studies showing the radiation-induced decrease in WBC, lymphocyte and neutrophil counts shortly after radiation exposure and the lymphocytes as the most sensitive blood cell type are similar findings to our observations in the present study utilizing the Yucatan minipig model. In Rhesus monkeys irradiated with 146.8 MeV protons, WBC and lymphocyte counts exhibited a dose-dependent decrease at the first time point (day 3) after irradiation and reached the lowest level on day 8 postirradiation for lymphocytes, between days 8–11 for WBCs, and on day 11 for neutrophils (10). The radiation induced reduction occurred earlier for lymphocytes than for WBCs in the Rhesus monkey, which corresponds to the results reported here.
The radiation dose-responses were determined for each blood cell type at each time point after radiation exposure. From the data represented in the dose-response curves, the steepest slope of the dose-response curve was observed 4 h postirradiation for monocytes, on day 1 postirradiation for lymphocytes, on day 4 postirradiation for WBCs and neutrophils and on day 14 postirradiation for eosinophils, which coincide with the timing of the lowest blood cell counts for the respective cell types. There are several mathematical models that can be used to establish the dose-response relationship between radiation dose and cell survival, with the linear-quadratic model being the most widely used. The linear-quadratic model assumes that there are two components to cell killing by radiation, with one proportional to radiation dose and the other proportional to the square of the radiation dose (15). A survival curve derived from the linear-quadratic model is not constrained as a straight log-linear line and therefore the slope can vary with the radiation dose, providing final slopes that differ even at the low-radiation dose range, in which a shoulder is prominent in standard survival curves. The shape of a linear quadratic survival curve is determined by a linear component and a quadratic component, which determines the extent of curvature of the survival curve on a log-scale. In the present studies, the survival curves were fitted using the linear quadratic equation. The quadratic component of the survival curves was negligible in most cases, with only a few exceptions. These results indicate that the dose-response relationship for blood cell counts in the Yucatan minipig after proton irradiation is essentially linear on a log scale and the linear-quadratic model is suitable in most situations for accurate description of the dose-response relationship for the blood cell counts in the proton irradiated Yucatan minipig.
Taken together, proton SPE radiation exposure in the Yucatan minipig results in a dose-response reduction in peripheral blood cell counts, contributing to the hematopoietic injury observed during the ARS. The data presented here indicate significant acute effects of proton radiation to the hematopoietic compartment, as well as potential late-radiation effects since blood cell counts did not recover by day 90 postirradiation in the 7.7 and 10 Gy dose groups, as they were observed to do in the 5 Gy dose group. Lastly, the Yucatan minipig serves as a suitable porcine model alternative to the nonhuman primate model when evaluating the hematopoietic effects of space radiation.
TABLE 6. Eosinophil Counts in Animals Irradiated with Protons.
| Eosinophil counts (fraction of control) in pigs irradiated at dose shown below | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 5 Gy | 7.7 Gy | 10 Gy | |||||
|
|
|
|
|||||
| Time after irradiation | Mean ± SE | Change | Mean ± SE | Change | Mean ± SE | Change | |
| Preirradiation | 1.00 ± 0.16 | N/A | 1.00 ± 0.16 | N/A | 1.00 ± 0.16 | N/A | |
| 4h | 0.33 ± 0.12 | −67.2% | 0.76 ± 0.27 | –24.2% | 0.71 ± 0.13 | −29.1% | |
| Day 1 | 0.43 ± 0.10 | −56.6% | 0.39 ± 0.08 | −61.4% | 0.25 ± 0.06 | −74.5% | |
| Day 4 | 0.56 ± 0.21 | −43.9% | a | a | 0.25a | −75.5% | |
| Day 14 | 0.33a | −67.1% | 0.27 ± 0.03 | −73.5% | 0.32 ± 0.04 | −67.8% | |
| Day 30 | 0.81 ± 0.12 | −18.8% | 0.55 ± 0.17 | −44.8% | 0.60 ± 0.23 | −40.2% | |
| Day 90 | 1.12 ± 0.14 | 11.6% | 0.67 ± 0.08 | −33.5% | 0.37 ± 0.09 | −62.6% | |
Notes. The one-way ANOVA P values for the 5, 7.7 and 10 Gy radiation dose groups were 0.11, 0.08 and 0.07, respectively. No post-test comparison was performed for different time points in each radiation dose group since the one-way ANOVA P > 0.05.
Mean and/or standard error were not calculated because valid eosinophil counts were not obtained from a sufficient number of animals.
Acknowledgments
This work was supported by the Center of Acute Radiation Research (CARR) grant from the National Space Biomedical Research Institute (NSBRI) through NASA NCC 9–58 and NIH Training Grant 2T32CA009677. We would like to thank Dr. Casey Maks for her involvement during the early planning stages of these experiments and her assistance with the animal procedures. We are grateful to Ms. Molly Peterlin for her contribution to the animal procedures and Mr. Celso Perez, Mr. Pete Koss and the Accelerator Team at LLUMC for their assistance with the animal irradiations.
References
- 1.Wilson JW, Cucinotta FA, Shinn JL, Simonsen LC, Dubey RR, Jordan WR, et al. Shielding from solar particle event exposures in deep space. Radiat Res. 1999;30:361–82. doi: 10.1016/s1350-4487(99)00063-3. [DOI] [PubMed] [Google Scholar]
- 2.Smart DF, Shea MA. The local time dependence of the anisotropic solar cosmic ray flux. Adv Space Res. 2003;32:109–14. doi: 10.1016/s0273-1177(03)90377-2. [DOI] [PubMed] [Google Scholar]
- 3.Hellweg CE, Baumstark-Khan C. Getting ready for the manned mission to Mars: the astronaut' risk from space radiation. Naturwissenschaften. 2007;94:517–26. doi: 10.1007/s00114-006-0204-0. [DOI] [PubMed] [Google Scholar]
- 4.Cengel KA, Diffenderfer ES, Avery S, Kennedy AR, Mcdonough J. Using electron beam radiation to simulate the dose distribution for whole body solar particle event proton exposure. Radiat Environ Biophys. 2010;49:715–21. doi: 10.1007/s00411-010-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu S, Kim MH, Mcclellan GE, Cucinotta FA. Modeling the acute health effects of astronauts from exposure to large solar particle events. Health Phys. 2009;96:465–76. doi: 10.1097/01.HP.0000339020.92837.61. [DOI] [PubMed] [Google Scholar]
- 6.Whelan HT, Smits RL, Jr, Buchman EV, Whelan NT, Turner SG, Margolis DA, et al. Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg. 2001;19:305–14. doi: 10.1089/104454701753342758. [DOI] [PubMed] [Google Scholar]
- 7.Dalrymple GV, Lindsay IR, Mitchell JC, Hardy KA. A review of the USAF/NASA proton bioeffects project: rationale and acute effects. Radiat Res. 1991;126:117–9. [PubMed] [Google Scholar]
- 8.Siegal AM, Casey HW, Bowman RW, Traynor JE. Leukemia in a rhesus monkey (Macaca mulatta) following exposure to whole-body proton irradiation. Blood. 1968;32:989–96. [PubMed] [Google Scholar]
- 9.Fanton JW, Golden JG. Radiation-induced endometriosis in Macaca mulatta. Radiat Res. 1991;126:141–6. [PubMed] [Google Scholar]
- 10.Traynor JE, Siegal AM. Early effects of 150-MEV proton irradiation in rhesus monkeys. SAM-TR-68-87. Tech Rep SAM-TR. 1968:1–7. [PubMed] [Google Scholar]
- 11.Taketa ST, Castle BL, Howard WH, Conley CC, Haymaker W, Sondhaus CA. Effects of acute exposure to high-energy protons on primates. Radiat Res (Suppl) 1967;7:336–59. [PubMed] [Google Scholar]
- 12.Wilson JM, Sanzari JK, Diffenderfer ES, Yee SS, Seykora JT, Maks C, et al. Acute biological effects of simulating the whole-body radiation dose distribution from a solar particle event using a porcine model. Radiat Res. 2011;176:649–59. doi: 10.1667/rr2541.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero-Weaver AL, Kennedy AR. Comparison of two methods for the determination of the effects of ionizing radiation on blood cell counts in mice. Int J Biomed Sci. 2012;8:7–15. [PMC free article] [PubMed] [Google Scholar]
- 14.Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513–28. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- 15.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]