Abstract
Objectives
To investigate how distressing participating in medical research is perceived to be, compared to everyday events.
Design
Anonymous questionnaire.
Setting
Scotland and New Zealand.
Participants
One hundred members of the Scottish general public, 94 University of Auckland students, 22 New Zealand Ministry of Health ethics committee members.
Main outcome measures
Distress ratings made on a 0–10 scale for everyday events and common medical research procedures.
Results
Both general population and student samples generally rated the distress caused by participating in various medical research procedures as low or very low. Most research procedures were rated less than the distress caused by not being able to find a car park at a supermarket. In contrast, the ethics committee members rated the distress caused by most of the medical research procedures at a significantly higher level than the ratings of the student and general population samples. Ethics committee members overestimated the distress caused by interview or questionnaire assessments (M = 203.31%, SE = 11.42, 95% CI [179.79, 226.83]) more than medical testing for research (M = 158.06%, SE = 12.33, 95% CI [132.66, 183.46], p = 0.04) and everyday events (M = 133.10%, SE = 7.80, 95% CI [117.03, 149.16], p < 0.001).
Conclusions
Common medical research procedures are not rated as particularly distressing by the general public, and ethics committees may be adopting an over-protective role when evaluating research applications that involve the use of questionnaire or survey methodology.
Introduction
The ethical review of studies is a necessary part of health and medical research. Investigators often ask participants to complete written questionnaires on their health or personal behaviour, undergo scans, as well as have blood, urine or other samples taken. Ethics committees are concerned about protecting participants from research procedures that could cause undue stress or distress. As part of this process, investigators are often questioned about whether their research protocol could cause distress or adverse effects in participants.
Unfortunately for researchers, there is little evidence to help guide informed responses to these questions, especially for common medical research measures. Negative effects appear to be rare following participation in medical studies, and some studies report that people often feel better about themselves after taking part in research, feeling that their involvement may help others in the future.1–3 There is also a reassuring review of psychiatric research, which indicates that only a small proportion of participants become distressed following participation in such research, and positive effects are more likely.4 However, there is very little information available on how individuals perceive the distress caused by routine medical research procedures, such as providing bodily samples or answering sensitive questions. It would be useful for both researchers and ethics committees to know how members of the general population and university students, two groups commonly used in research studies, assess completing common research procedures in comparison with common everyday events and hassles. A related question is how accurately members of ethics committees perceive the distress caused by medical research procedures in comparison to research participants.
This study had two aims; the first was to find out how distressing people rated participating in various medical research procedures in comparison to everyday events in their lives. The second aim was to compare these public perceptions with the perceptions of health and medical research ethics committee members who were asked how distressing they thought participants would find taking part in various research procedures.
Methods
Participants
The participants consisted of three samples. The first sample comprised 100 members of the general public. In an attempt to recruit a representative general population sample, participants were recruited from outside three Scottish supermarkets in Stirling (n = 38, 114 asked, 48 males and 28 females refused), Bathgate (n = 30, 84 asked, 26 males and 28 females refused) and Peebles (n = 32, 100 asked, 43 males and 25 females refused). This sample comprised 49 males and 51 females who were distributed evenly among five age groups ranging from 18–25 to over 60 years. The second sample comprised 94 students enrolled in a health sciences introductory course at the University of Auckland (153 approached, 59 refused). There were 65 females and 28 males in this sample (one participant did not provide this information), with a mean age of 19.5 years. The third sample was recruited from members of the six regional New Zealand Ministry of Health ethics committees. We received responses from 22 of 81 members (27% response rate).
Procedure and measures
The general population and student participants were invited to complete an anonymous questionnaire assessing their perceptions about the distress of participating in different types of medical research in comparison to everyday life events. The ethics committee members were invited to complete an anonymous questionnaire which asked them to rate their perceptions of how distressing members of the public would find taking part in various medical research procedures and everyday life events. The student and general population sample questionnaire was made up of 50 items comprising 35 everyday events with varying levels of stress randomly interspersed with 15 medical research procedures. Participants were asked to rate each item for the level of distress they would be likely to experience as a result of the event. Ratings were made on an 11-point scale, ranging from 0 ‘not at all distressed’ to 10 ‘extremely distressed’. Ethics committee members were asked to complete an abbreviated list of items comprising 15 of the same stressful everyday events and 13 of the medical research procedures. They were asked to rate how much distress they believed individuals were likely to experience from each item on the same 11-point scale.
Statistical analysis
Independent-samples t-tests were used to compare the ratings of the general population and student distress ratings for each of the 50 items. Independent-samples t-tests were also used to compare the average distress ratings of the combined general population and student samples with ethics committee distress ratings for the common 28 items. To analyse the degree to which ethics committee members’ ratings overestimated or underestimated the level of distress that participants would experience, ratio scores were calculated by dividing the ethics committee ratings by the combined general population and student mean ratings. These scores were multiplied by 100 to form a percentage that indicates the degree to which ethics committee members overestimated (>100%) or underestimated (<100%) the level of distress that participants would experience in the various circumstances. Pearson’s correlations were carried out between the percentage scores and the combined student and general population distress ratings in order to investigate whether ethics committee members systematically overestimated or underestimated items with higher or lower distress ratings. Finally, an analysis of variance was used to investigate whether the distress caused by questionnaire or medical research methods was over or underestimated by ethics committees when compared to everyday events with Bonferroni correction for post hoc tests. All tests were two-tailed, p < 0.05 was considered significant.
Results
We first examined the levels of distress reported for each of the 50 items from the general population and student samples. Most items were rated similarly between the two samples. Of the research items, only ‘answering a questionnaire about your income’ and ‘an in depth telephone interview about your health’ were rated significantly differently by the two samples, with the general population sample rating those items as more distressing than students. Nine of the ratings of everyday items differed between the student and general population samples. These differences were likely due to the nature of the two samples, and differences tended to occur in questions assessing perceived distress around money issues and daily tasks, typically less relevant to students.
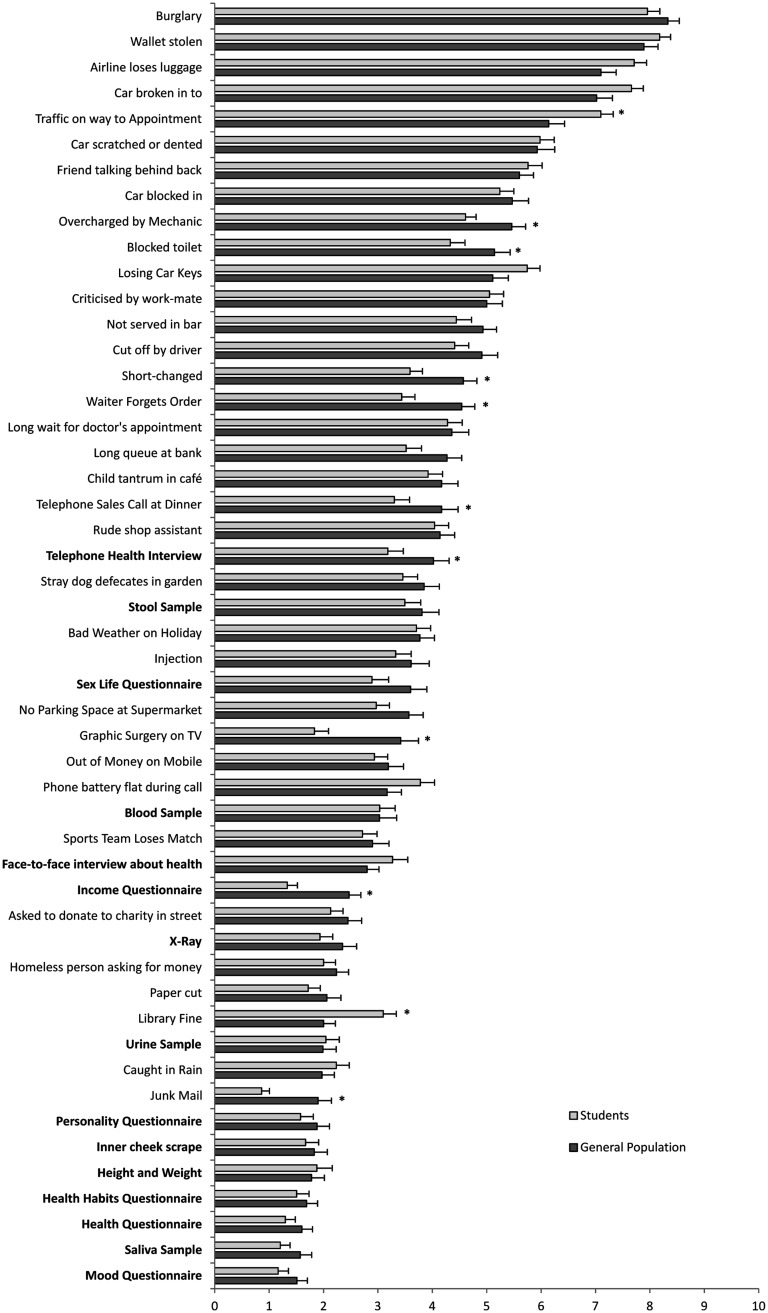
The items ‘having a burglary at your house’ and ‘having your wallet stolen’ received the highest distress ratings in both the general population and student samples (see Figure 1). The medical research items tended to be rated towards the lower end of the scale with the items ‘giving a stool sample for a research study’ and ‘taking part in an in-depth telephone interview about your health’ being the highest rated items, yet both items were rated as less distressing than having bad weather while on holiday. The items ‘giving a saliva sample for a medical research study’ and ‘answering a questionnaire about your mood’ were ranked lowest in terms of distress, rating lower than receiving junk mail. None of the medical research items had a mean rating above 4 on the 0–10 scale.
Figure 1.
Level of distress perceived to be caused by daily life events and medical research participation (in bold) in general population and student samples.
*p < 0.05.
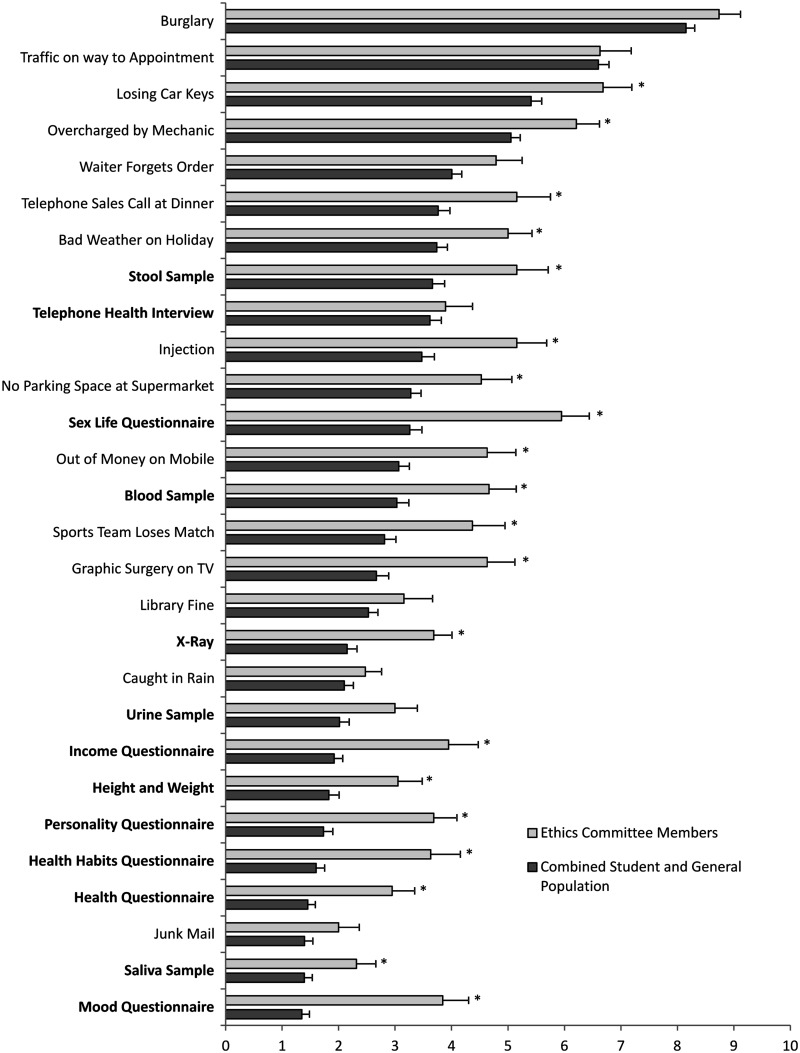
We next compared medical ethics committee members’ perceptions concerning the level of distress they believed participants in research studies would experience to the ratings of the combined general population and student samples. As can be seen in Figure 2, ethics committee members tended to rate both the distress caused by everyday events and medical research procedures as higher than the general population and student samples. Ethics committee members rated 20 of the 28 items, including 11 of the 13 research items, significantly higher than the combined general population and student samples. To ensure that the results were not due to underlying differences between the student and Scottish samples, ethics committee member ratings of medical research items were also compared to the other two samples separately. The pattern of results was identical to that seen in the comparison with the combined sample.
Figure 2.
A comparison between the level of distress perceived to be caused by daily life events and medical research participation (in bold) as rated by health ethics committee members and the combined general population and student samples.
*p < 0.05.
We also found a systematic bias in the ratings, with the items rated as less distressing by the general population and student samples being overestimated by the ethics committee members in comparison with the more distressing items. This is evidenced by a significant negative correlation between the average student and general population distress ratings and the percentage of ethics committee members’ overestimation of distress (r = −0.65, p < 0.001).
We further examined whether there were any significant differences in the ethics committee members’ overestimation of distress between different types of research procedures. We divided the items into interview or questionnaire assessments (e.g. sex life questionnaire, face-to-face interview about health), medical tests (blood sample, X-ray and urine sample) and everyday life events. The degree of difference between the ratings of potential research participants (student and general population samples) and ethics committee members differed significantly across item types (F (2, 25) = 12.91, p < 0.001). Compared to the distress ratings of the student and general population samples, ethics committee members overestimated the distress caused by interview or questionnaire assessments (M = 203.31%, SE = 11.42, 95% CI [179.79, 226.83]) more than medical testing for research (M = 158.06%, SE = 12.33, 95% CI [132.66, 183.46], p = 0.04) and everyday events (M = 133.10%, SE = 7.80, 95% CI [117.03, 149.16], p < 0.001). There was no significant difference between the ethics committee members’ overestimation of medical tests and everyday events (p = 0.30).
Discussion
The results of this study showed that both general population and student samples rated the distress caused by participating in various medical research procedures as low or very low. The medical research procedures did vary, with the more intrusive items being rated at a higher level. Importantly, none of the medical research items had a mean distress rating above 4 on the 0–10 scale. Furthermore, when the medical research items are calibrated against everyday events, participants rated everyday events such as a long queue at a bank, or a telephone sales call at dinner as more distressing than any of the medical research items and most were less than not being able to find a car park at a supermarket. In other words, participating in medical research was generally viewed as a temporary inconvenience that was not seen as causing significant distress.
In contrast, the ethics committee members rated the distress caused by most of the medical research procedures at a significantly higher level than the ratings of the student and general population samples. While it could be argued that ethics committee members were more cautious so as to protect research participants from more distressing research procedures, we found a bias in the opposite direction. Ethics committee members systematically overestimated the distress caused by minor research procedures in comparison to items rated by possible participants as more distressing. This is most clearly seen in the significant overestimation of the distress caused by questionnaire or interview research.
Strengths and weaknesses
It should be noted that the findings of the study may be limited by the research method which asked participants to estimate their level of distress rather than measuring actual distress following specific research procedures or life events. However, many of the medical and psychological research methods included in the questionnaire are also commonly experienced as part of everyday life and medical care, and many participants will have had first-hand experience of medical tests and questionnaire completion. The study is also limited by the low response rate of ethics committee members. Bearing these limitations in mind, the study does suggest that common medical research procedures are not rated as particularly distressing by the general public, and medical ethics committees may be adopting a well-intentioned but over-protective role when evaluating research applications that involve the use of questionnaire or survey methodology.
While there is little previous research available on the distress caused to participants as a result of participating in medical and clinical research, the studies that have been conducted indicate that negative effects are rare, and that positive effects of participation may be more likely.1–4 The strength of these studies is that they assessed distress ratings and positive effects in participants following research participation, rather than in hypothetical situations as in the current research. The strength of the current study is that it provides a comparison between research-related distress and other commonly experienced everyday scenarios.
Implications and future research
The study findings suggest that ethics committees may be adopting a more over-protective view of participation in questionnaire or interview-based research than is necessary. This can result in ethics committees being more reluctant to approve questionnaire or survey-based research which the committee members erroneously believe may cause distress to participants. The role of an ethics committee is to protect research participants, balancing the potential benefit of the research with the potential harm to those who take part. The overestimation of likely distress associated with more benign research methods, and particularly questionnaires and interviews, may hamper the completion of medical research studies using these procedures.
Researchers should consider collecting more data from research participants about their experience after participation in various research procedures in order to provide more data on actual participant experiences of both distress and benefits related to research participation. Future research is critical to provide ethics committee members, who are charged with making decisions about likely harm and benefit to research participants, with more information with which to judge the likely effects of the proposed research on participants.
DECLARATIONS
Competing interests
None declared
Funding
None declared
Ethical approval
This study was granted ethics approval by the University of Auckland Human Participants Ethics Committee (2009/273)
Guarantor
KP
Contributorship
KP had the original idea and wrote the draft of the manuscript with critical input from KF, RO and TAN. KP, KF and RO designed the study and analysed the data. KF and TAN recruited participants and collected the data. All authors approved the final version of the manuscript
Acknowledgements
We thank Julia O'Carroll and Liz Broadbent for their suggestions of general distress items
Provenance
Submitted; peer reviewed by Peter White
References
- 1. Scott DA, Valery PC, Boyle FM, Bain CJ. Does research into sensitive areas do harm? Experience of research participation after diagnosis with Ewing’s sarcoma. Med J Aust 2002; 177: 507–10 [DOI] [PubMed] [Google Scholar]
- 2. Daugherty TK, Lawrence JW. Short-term effects of research participation on college men. J Psychol 1996; 130: 71–7 [DOI] [PubMed] [Google Scholar]
- 3. Cook AS, Bosley G. The experience of participating in bereavement research: stressful or therapeutic? Death Stud 1995; 19: 157–70 [DOI] [PubMed] [Google Scholar]
- 4. Jorm AF, Kelly CM, Morgan AJ. Participant distress in psychiatric research: a systematic review. Psychol Med 2007; 37: 917–26 [DOI] [PubMed] [Google Scholar]