Abstract
Objective and design
We designed a study to detect downstream phosphorylation targets of PKCβ in MCP-1-induced human monocytes.
Methods
2-dimensional gel electrophoresis was performed for monocytes treated with MCP-1 in the presence or absence of PKCβ antisense oligodeoxyribonucleotides (AS-ODN) or a PKCβ inhibitor peptide, followed by phospho- and total protein staining. Proteins that stained less intensely with the phospho-stain, when normalized to the total protein stain, in the presence of PKCβ AS-ODN or the PKC β inhibitor peptide were sequenced.
Results
Of the proteins identified, vimentin was consistently identified using both experimental approaches. Upon 32P-labeling and vimentin immunoprecipitation, increased phosphorylation of vimentin was observed in MCP-1 treated monocytes as compared to the untreated monocytes. Both PKCβ AS-ODN and the PKCβ inhibitor reduced MCP-1-induced vimentin phosphorylation. IP of monocytes with anti-vimentin antibody and immunoblotting with a PKCβ antibody revealed that increased PKCβ becomes associated with vimentin upon MCP-1 activation. Upon MCP-1 treatment, monocytes were shown to secrete vimentin and secretion depended on PKCβ expression and activity.
Conclusions
We conclude that vimentin, a major intermediate filament protein, is a phosphorylation target of PKCβ in MCP-1-treated monocytes and that PKCβ phosphorylation is essential for vimentin secretion. Our recently published studies have implicated vimentin as a potent stimulator of the innate immune receptor Dectin-1 [1]. Taken together our findings suggest that inhibition of PKCβ regulates vimentin secretion and thereby, its interaction with Dectin-1 and downstream stimulation of superoxide anion production. Thus PKCβ phosphorylation of vimentin likely plays an important role in propagating inflammatory responses.
Keywords: Chemotaxis, Inflammation, Monocytes, MCP-1, PKCβ, Vimentin
Background
Directed migration of inflammatory macrophages under the influence of chemoattractant cytokines, termed chemotaxis, is one of the key events in the pathogenesis of atherosclerosis. A group of small peptides known as chemokines, primarily responsible for this chemotaxis, are critically involved in directing activation and trafficking of leukocytes in both acute and chronic inflammation [2–5]. Monocyte chemotactic protein-1 (MCP-1), a β-chemotactic cytokine secreted by the different types of arterial wall cells including endothelial cells, smooth muscle cells, macrophages and fibroblasts, plays an important role in atherogenesis by recruiting monocytes into the subendothelial cell layer[6–11]. Strong evidence shows the critical role of MCP-1 in the subendothelial recruitment of monocytes and the subsequent progression of atherosclerotic lesion development5,6. Upon extravasation into the subendothelial intimal space, monocytes differentiate into macrophages and acquire new functions [12, 13]. Accumulation of macrophages within plaques is a hallmark of this disease[14]. MCP-1-deficient mice showed minimal lipid deposition and fewer macrophages within the artery walls [15]. Mice deficient in the receptor for MCP-1, Chemokine receptor 2 (CCR2), display a phenotype similar to MCP-1-deficient mice with a pronounced defect in MCP-1-induced leukocyte strong adhesion to endothelium and decreased leukocyte extravasation suggesting MCP-1 signaling via the CCR2 receptor [16]. Signaling pathways regulating the process by which chemokines dynamically attract monocytes, modulate adhesion and transmigration are not yet well defined. Our lab has been investigating the signaling pathways involved in primary human monocyte chemotaxis, predominantly those regulating MCP-1 chemotaxis including serine/ threonine protein kinases [15, 17–20].
Using pharmacologic inhibitors and isoform specific PKCα and β AS-ODN, we have shown previously that PKCβ, not PKCα, mediates MCP-1-activated human monocyte chemotaxis [5]. In chronic stressful states like atherosclerosis and restenosis, induction of PKCβ and other key molecules has been observed to mediate inflammation, migration and proliferation leading to injury and dysfunction of the vascular tissues[21, 22]. During hyperlipidemic conditions, PKCβ activation has been shown to be a regulator of initiation and augment mechanisms involved in the progression of atherosclerosis. Assessment of the extent of activation of the PKCβ isoform in apoE−/ − mice implicates its contribution to the regulation of the pathogenesis in atherosclerosis. PKCβ accompanies intimal expansion as a vascular response to arterial injury during atherosclerosis as well as controlling MCP-1-induced chemotaxis [5, 23]. Studies showed that mice lacking both apoE and PKCβ displayed significantly decreased atherosclerosis compared with apoE-null mice. And also apoE-null mice, fed chow containing the PKCβ inhibitor ruboxistaurin, displayed significantly decreased atherosclerosis compared with the mice fed chow containing vehicle as a control [24, 25].
The aim of our study was to identify the downstream phosphorylation targets of PKCβ in MCP-1-activated human monocytes to further our understanding of the mechanisms involved in PKCβ regulation of inflammation. In this study, we have used the myristoylated PKCβ inhibitor peptide and our previously characterized PKCβ S- and AS-ODN as tools to identify PKCβ substrates [5].
Materials and Methods
Materials
PKCβ inhibitor peptide was purchased from Promega (Madison, WI). PKCβ S- and AS-ODN were custom ordered from Invitrogen based on our previously published effective sequences (Carlsbad, CA). MCP-1 was purchased from BD Biosciences and solubilized in 0.1% BSA in DMEM (San Jose, CA). [32P]-orthophosphate radionuclide (with specific activity 314–337 TBq/mMole) was purchased from Perkin Elmer (Waltham, MA). Primary antibodies used were V9 monoclonal antibodies from Sigma (St.Louis, MO), anti-vimentin antibody and phosphor (Ser) PKC substrate antibody from Cell Signaling (Danvers, MA), and rabbit anti-PKCβ antibody (NBP2-12572) from Novus Biologicals (Littleton, CO). Human MCP-1 was purchased from BD Biosciences and diluted to 50 μg/ mL with PBS containing 1 mg/ mL BSA as a 1,000-fold stock solution and stored at −80°C.
Isolation of primary human monocytes and treatment of cells with the oligodeoxyribonucleotides and PKCβ inhibitor peptide
Human monocytes were isolated from EDTA (3–4 mM) anticoagulated whole blood by sequential centrifugation over a Ficoll-Paque density solution to obtain mononuclear cells followed by platelet removal and adherence to tissue culture flasks precoated with bovine calf serum as previously described [26][27]. The non-adherent cells were discarded and only the adherent cells were released with EDTA and washed twice with PBS and then used in experiments. This procedure yields > 95% monocytes as determined by FACS analysis. The efficacies of the PKCβ isoenzyme-specific S- and AS-ODN used in our experiments were demonstrated in our previously published work [5]. The PKCβ isoenzyme-specific antisense ODN sequence was 5′-AGC GCA CGG TGC TCT CCT CG-3′. Phosphorothioate-modified ODN were used for these experiments to prevent ODN degradation and all ODN were HPLC purified [5]. Either S- or AS-ODNs (10μM) were added to the isolated human monocytes (2.5 × 106 cells/ mL) suspended in polypropylene tubes DMEM with 10% Bovine calf serum (BCS/DMEM) and incubated at 37°C in 10% CO2 for 24 h. The PKCβ inhibitor peptide (10μM) was added 30 min prior to the addition of MCP-1 (50 ng/ mL). MCP-1 was added during the last 30 min of incubation.
Preparation of cell lysates
After PKCβ AS- or S-ODN treatment for 24 h, MCP-1 (50 ng/ mL) was added to each tube and incubated for 30 min at 37°C. Cells were then treated with 1 mM sodium orthovanadate (Ipswich, MA) for 15 min to inhibit phosphatases, harvested, centrifuged and washed three times with PBS. The cells were then resuspended in lysis buffer (1% Triton X-100 (Sigma, St. Louis, MO), and 1:100 diluted phosphatase inhibitor and protease inhibitor mixture (Sigma, St. Louis, MO). After 30 min on ice, the extracts were centrifuged at 9300 x g for 15 min at 4°C and the supernatants were collected as cell lysates.
Two-dimensional gel electrophoresis of PKCβ sense/ anti-sense ODN-treated primary human monocytes
To obtain good protein separation for identification, 2-dimensional gel electrophoresis (DIGE) of primary human monocyte lysates was performed as previously described [28]. Cells were treated with MCP-1 in the presence or absence of PKCβ AS-ODN. Protein concentrations of the cell lysates were determined by the BCA Protein Assay Kit (Pierce, Rockford, IL) and the 2-D Clean-Up Kit (Amersham Biosciences, Piscataway, NJ) was used to reduce non-protein impurities and to improve the quality of 2-DIGE results. The gels were stained with Pro-Q Diamond phosphoprotein gel stain (Invitrogen, Carlsbad, CA) for 90 min with gentle agitation in the dark and then destained for 30 min three times and washed with distilled water for another 10 min. After imaging the gels, they were placed directly into SYPRO Ruby protein gel stain Invitrogen (Carlsbad, CA) for total protein staining in the dark for overnight. The gels were then washed twice for 30 min. Gel imaging was performed after rinsing the gels with distilled water. Finally, the gels were stained with a visible non-fluorescent Coomassie blue (GelCode Blue) stain (Pierce, Rockford, IL) to aid in locating the proteins that are identified by comparison of the two gels stained with Pro-Q Diamond. Molecular masses were determined by simultaneously running standard protein markers. Phosphoproteins that stained with more intensity in the MCP-1 treated group as compared to the PKCβ inhibitor peptide/ PKCβ AS-ODN treated group were cut from the gel, trypsinized, digested and analyzed by LC-mass spectrometry as described below. Immunoblots were probed with phosphor (Ser) PKC substrate antibody.
Liquid chromatography mass spectrometry (LC-MS)
To identify the proteins on gel pieces, LC-MS was performed as described[29]. For the protein digestion, the bands were cut from the gel and then dehydrated in acetonitrile, dried in a Speed-vac and digested with trypsin incubating overnight at room temperature. The peptides that were formed were extracted from the polyacrylamide in two aliquots of 30 μL 50% acetonitrile with 5% formic acid. The LC-MS system was a Finnigan LTQ linear ion trap mass spectrometer system. The digest was analyzed using the data dependent multitask capability of the instrument acquiring full scan mass spectra to determine peptide molecular weights and product ion spectra to determine amino acid sequence in successive instrument scans. This mode of analysis produces approximately 2500 collisionally-induced dissociation (CID) spectra of ions ranging in abundance over several orders of magnitude. The data were analyzed by using all CID spectra collected in the experiment to search the NCBI non-redundant database with the search program Mascot using a human taxonomy filter. All matching spectra were verified by manual interpretation. The interpretation process was aided by additional searches using the programs Sequest and Blast as needed.
Metabolic labeling and vimentin immunoprecipitation
Isolated primary human monocytes (5 × 106 cells/ 2mL/ well) were incubated in 10% BCS/ DMEM in the presence or absence of PKCβ S- or AS-ODN for 20 hr at 37°C in 10%CO2. Cells were then preincubated in the phosphate-free DMEM for 1 hr at 37°C in 10%CO2. Cells were labeled with [32P]-orthophosphate 100 μCi/mL for 3 hr. MCP-1 (50 ng/mL) was added to respective groups for 30 min, sodium orthovanadate (1 mM) was added for the last 15 min incubation. Cell lysates were immunoprecipitated with vimentin V9 antibody for 2 hr and protein G agarose beads (Roche diagnostics, Indianapolis, IN) were added overnight at 4°C, both with constant rotation. Beads were washed and sample buffer was added prior to boiling for 5 min, followed by electrophoresis on 10% SDS-polyacrylamide gel and transferred onto a polyvinylidene fluoride membrane [30]. Incorporation of [32P] was determined by analysis with a phosphorimager before vimentin loading was verified by immunoblotting using anti-vimentin antibody and detected by enhanced chemiluminescence.
Vimentin immunoprecipitation
Human monocytes (5 × 106 cells/ 2 mL/ well) were incubated in the presence or absence of the PKCβ inhibitor peptide for 30 min followed by treatment with MCP-1 for 30 min and sodium orthovanadate (1 mM) was added for the final 15 min of incubation. The cell lysates were immunoprecipitated with anti-vimentin antibody followed by SDS-PAGE and transferred onto a polyvinylidene fluoride membrane [30]. Anti-PKCβ antibody was used to detect the presence of PKCβ. The membrane was stripped using a 20 mL stripping solution (100 mM 2-mercaptoethanol, 2% (w/v) SDS, 62.5 mM Tris-HCl, pH 6.8) and reprobed with anti-vimentin antibody.
Detection of vimentin secretion
To determine whether MCP-1 promotes vimentin release from primary human monocytes, monocytes were treated with and without MCP-1, or with MCP-1 in the presence of PKCβ S-ODN or AS-ODN or the myristoylated PKCβ inhibitor peptide. Monocytes were then plated on a 6-well plate at a concentration of 5 × 106 cells/ 2 mL in Opti-MEM solution and incubated at 37 °C with 10% CO2 for 48 hours. This was followed by the treatment of PKCβ S- and AS-ODN (5 μM) to the respective groups for the last 24 hours. An hour before the end of the incubation, the inhibitor peptide (10 μM) was added to the corresponding group 30 minutes before the addition of MCP-1. MCP-1 (50ng/mL) and sodium orthovanadate were added 30 minutes and 15 minutes to the respective groups before the end of the incubation, respectively. Supernatants were collected from each well, centrifuged at 1000 g for 10 minutes to remove cell debris and the supernatants were concentrated in a centrifugal device (Amicon Ultracel 30 kDa) in the presence of protease inhibitors. The final concentrates were run on an SDS-PAGE, transferred onto a PVDF membrane and immunoblotted using anti-vimentin antibody. Recombinant human vimentin was used as a positive control.
Results
Vimentin is a potential substrate for PKCβ phosphorylation in MCP-1-activated human monocyte chemotaxis
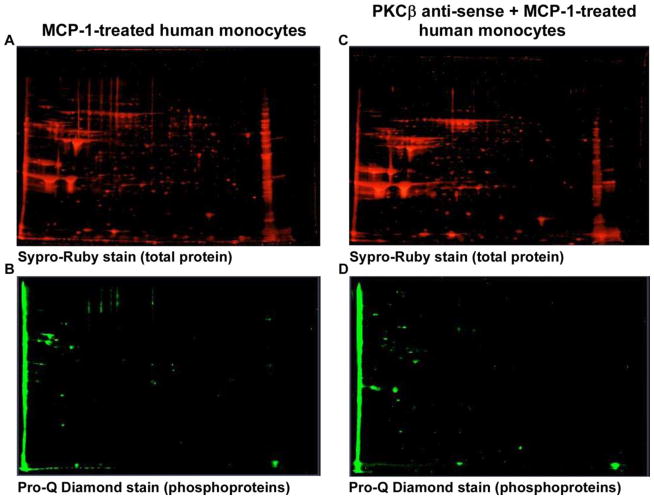
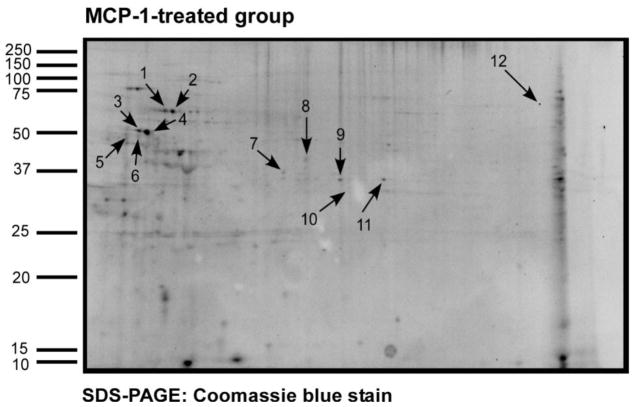
Prior studies in our lab showed that PKCβ is required for human monocyte chemotaxis to MCP-1 [5]. To identify potential substrates for PKCβ phosphorylation we performed 2-DIGE on lysates of monocytes that were treated with MCP-1 in the presence or absence specific antisense ODN to PKCβ [5]. Monocytes were treated with MCP-1 in the presence and absence of PKCβ AS-ODN. Figure 1 shows the SYPRORuby total protein and Pro-Q Diamond phosphoprotein stained gels. Figures 1A and 1B show the MCP-1 treated monocytes and Figures 1C and 1D show the PKCβ AS-ODN treated group. Figure 2 shows the same gel from Figure 1A/C stained with Coomassie blue. The arrows point to proteins that stained with less intensity on phosphoprotein staining in the PKCβ AS-ODN treated group. These proteins were cut from the gel, processed according to Methods and sequenced using mass spectrometry. Twelve potential PKCβ substrate proteins were located and identified (Table 1). Among the twelve proteins, four of them included vimentin, an intermediate filament protein, migrating in the area outlined by the oval in Figure 1. Vimentin was consistently detected on sequencing in several repeat experiments. The varied migration of vimentin is likely due to alternative post-translational modification since vimentin is highly phosphorylated. Two of the proteins (spot number 5 and 6) were identified as the capping protein gelsolin and two of the others were identified as biliverdin reductase, transaldolase, lasp-1 protein, annexin 1, lamin B1, L-plastin. The ovals on Figure 1 indicate the area of the gel where vimentin was detected and phosphoprotein staining was remarkably decreased in the presence of PKCβ antisense ODN.
Figure 1. Detection of potential PKCβ substrates in MCP-1-treated monocytes compared to PKCβ AS-ODN treated monocytes.
Figures 1A and 1C show SYPRORuby total protein stained gels of MCP-1-treated and MCP-1 and PKCβ-ODN-treated monocytes respectively run on 2-DIGE. Figures 1B and 1D show Pro-Q Diamond phosphoprotein stained gels of MCP-1-treated and MCP-1 and PKCβ AS-ODN-treated monocytes respectively. The ovals encircle areas where vimentin was detected.
Figure 2. Identification of potential PKCβ substrates in MCP-1-treated monocytes compared to the PKCβ AS-ODN treated monocytes.
The gel from Figure 1A/C was stained with Coomassie blue. The arrows point to the potential PKCβ substrate proteins that showed decreased intensity on phosphoprotein staining in monocytes treated with PKCβ antisense ODN as compared to the MCP-1 treated group. These proteins were sequenced using liquid chromatography mass spectrometry and identified proteins are listed in Table 1.
TABLE 1.
Identification of potential PKCβ substrates in MCP-1-treated monocytes compared to PKCβ-specific antisense ODN treated monocytes.
| Spot number | Protein name | NCBI accession number | Mol. mass (kDa) | Isoelectric point (pI) | Number of peptides (%sequence coverage) |
|---|---|---|---|---|---|
| 1 | Vimentin Tubulin, alpha |
37582 13436317 |
54 50 |
5.0 4.9 |
33 (73%) 3 (11%) |
| 2 | Vimentin ATP synthase, H+ transporting, mitochondrial F1 complex, beta peptide |
37582 16741373 |
54 56 |
5.0 5.2 |
28 (54%) 18 (49%) |
| 3 | Vimentin NF-M protein |
37582 35046 |
54 102 |
5.0 4.9 |
29 (55%) 4 (4%) |
| 4 | Vimentin Tubulin, alpha |
7576229 13436317 |
54 50 |
5.0 4.9 |
46 (73%) 11 (31%) |
| 5 | Lamin B1 | 576840 | 66.6 | 5.1 | 18 (29%) |
| 6 | Lymphocyte cytosolic protein 1 (L-plastin) | 8217500 | 71 | 5.3 | 13 (26%) |
| 7 | Capping protein gelsolin- like | 60655417 | 39 | 5.8 | 14 (34%) |
| 8 | Capping protein gelsolin- like | 60655417 | 39 | 5.8 | 14 (34%) |
| 9 | Annexin I Aflatoxin aldehyde reductase AFAR |
55959292 2736256 |
39 37 |
6.5 6.2 |
17 (47%) 2 (8%) |
| 10 | ENO1 (Enolase 1 variant) | 62896593 | 47 | 7.0 | 25 (53%) |
| 11 | ENO1 (Enolase 1 variant) Lasp-1 protein |
29792061 2135552 |
47 31 |
7.0 6.1 |
6 (18%) 5 (18%) |
| 12 | Bilverdin reductase A Transaldolase 1 |
13543489 14603290 |
34 38 |
6.0 6.3 |
6 (21%) 2 (5%) |
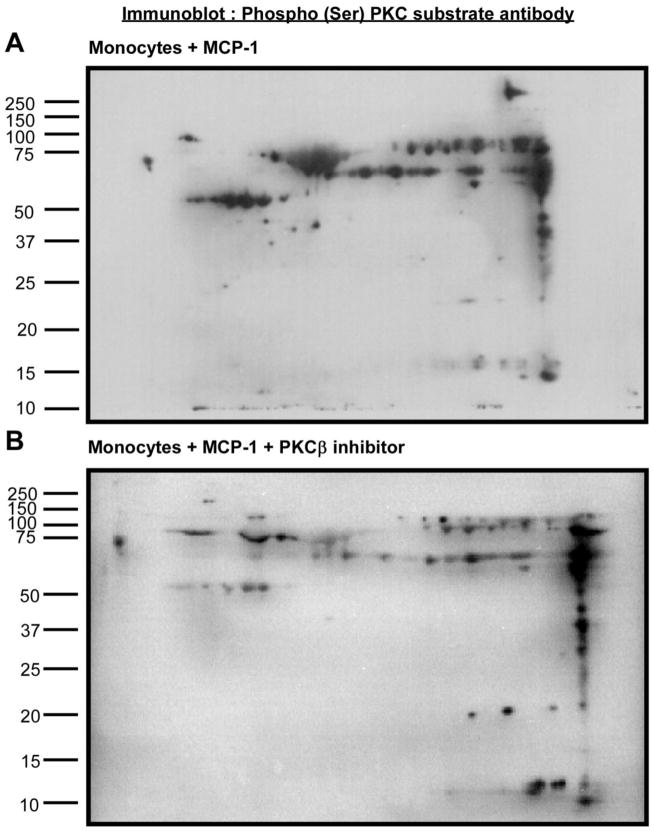
Although antisense-ODN provide a rather specific inhibition of PKCβ expression, we used a complementary approach to identify potential PKCβ substrates in MCP-1 activated monocytes. For these studies we used the PKCβ inhibitor peptide (Promega) that blocks enzymatic activity. After 2-DIGE, immunoblots were probed with Phospho (Ser) PKC antibody as shown in Figure 3A and 3B. Numerous differences were noted in the phosphorylation pattern. Pro-Q Diamond and SYPRO Ruby stains were used to stain phosphorylated proteins and all the proteins, respectively in duplicate gels. Phosphoproteins that stained with less intensity in the presence of the PKCβ inhibitor peptide when normalized for the total protein stain were visually located after Coomassie Blue staining of the gels, cut from the gel and processed as described in Methods. Figure 4 shows the Coomassie blue stained two-dimensional gel after electrophoresis with protein extracts prepared from monocytes treated with MCP-1. The arrows point to the potential PKCβ substrates. Thirteen proteins were identified by LC-MS and are listed in Table 2. MASCOT was used to analyze the data. As in the antisense ODN experiments, vimentin was again identified as a potential substrate for PKCβ phosphorylation.
Figure 3. Detection of phosphorylated proteins in MCP-1-treated monocytes in the presence/ absence of PKCβ inhibitor peptide.
Human monocyte lysates were fractionated by 2-DIGE and immunoblots were probed with phosphor (Ser) PKC substrate antibody. Figure 3A shows the monocyte group treated with MCP-1 and Figure 3B shows the monocyte group treated with MCP-1+PKCβ inhibitor peptide.
Figure 4. Detection of phosphorylated proteins in MCP-1-treated monocytes in the presence/ absence of PKCβ inhibitor peptide.
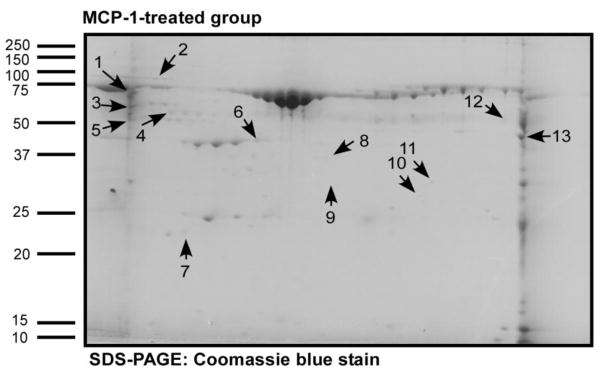
Human monocyte lysates were fractionated by 2-DIGE and stained with ProQ Diamond phosphoprotein stain followed by SYPRO Ruby total protein stain and the ratios of phosphostaining to total protein in each gel spot were evaluated. Figure 4 shows the Coomassie blue stained two-dimensional gel after electrophoresis with lysates prepared from monocytes treated with MCP-1. The arrows point to the potential PKCβ substrate proteins identified as described in Materials & Methods. These proteins showed decreased intensity on phosphoprotein staining as compared to total protein staining in the PKCβ inhibitor peptide-treated group as compared to the MCP-1 treated group. The proteins were sequenced using liquid chromatography mass spectrometry and identified proteins are listed in Table 2.
TABLE 2.
Identification of potential PKCβ substrates in MCP-1-treated monocytes compared to a PKCβ inhibitor peptide treated monocytes.
| Spot number | Protein name | NCBI accession number | Mol. mass (kDa) | Isoelectric point (pI) | Number of peptides (%sequence. coverage) |
|---|---|---|---|---|---|
| 1 | L-plastin | 4504965 | 70 | 5.2 | 5(8%) |
| 2 | Glucose-regulated protein | 16507237 | 72 | 5.0 | 9(19%) |
| 3 | Prolyl 4-hyodroxylase, beta subunit Coronin, actin binding protein, 1A |
20070125 5902134 |
57 51 |
4.7 6.2 |
21(36%) 2(4%) |
| 4 | Vimentin Tubulin alpha 6 |
62414289 14389309 |
53 50 |
5.0 4.9 |
2(5%) 2(6%) |
| 5 | Calreticulin precursor | 4757900 | 48 | 4.2 | 3(6%) |
| 6 | Beta actin Albumin precursor |
4501885 4502027 |
42 71 |
5.2 5.9 |
12(35%) 3(4%) |
| 7 | Rho GDP dissociation inhibitor (GDI) beta | 56676393 | 23 | 5.1 | 3(13%) |
| 8 | Gelsolin-like capping protein | 63252913 | 38 | 5.8 | 4(16%) |
| 9 | Protease activator subunit 1 isoform 1 | 5453990 | 28 | 5.7 | 2(8%) |
| 10 | Peroxisomal enoyl- coenzyme A hydratase- like protein | 70995211 | 36 | 8.1 | 4(14%) |
| 11 | Annexin I | 4502101 | 38 | 6.5 | 10(37%) |
| 12 | Coronin, actin binding protein, 1A | 5902134 | 51 | 6.2 | 1(2%) |
| 13 | Enolase I | 4503571 | 47 | 7.0 | 23(64%) |
We chose to further investigate vimentin as it was identified consistently in numerous repeat experiments, showed very marked inhibition and displayed decreased phosphorylation in the presence of either the PKCβ inhibitor peptide or AS-ODN specific for PKCβ.
PKCβ induces vimentin phosphorylation in MCP-1-activated human monocytes
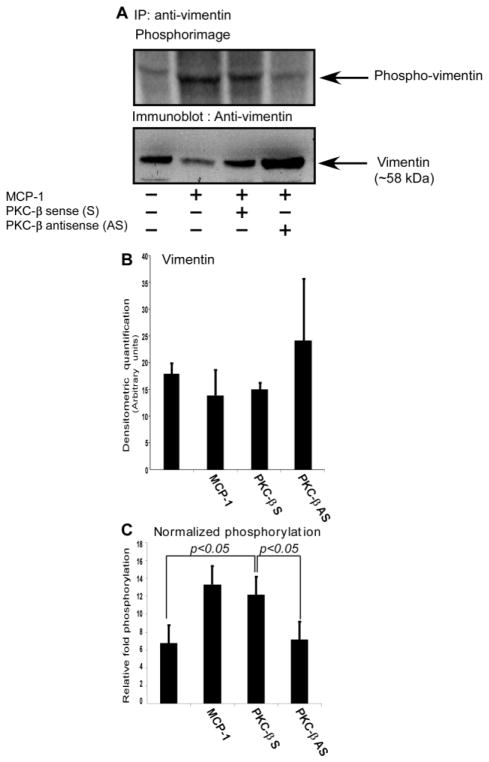
To validate whether vimentin phosphorylation is indeed regulated by PKCβ in MCP-1-activated human monocytes, the effect of PKCβ on vimentin phosphorylation was examined in primary human monocytes labeled with [32P]-orthophosphate. Monocytes were incubated with PKCβ S- or AS-ODN followed by [32P] labeling and subsequent MCP-1 activation. In the upper panel of Figure 5A, phosphorylation of vimentin was detected using phosphorimage analysis of immunoprecipitated vimentin. In the lower panel of Figure 5A, the vimentin content was analyzed by immunoblotting as a loading control. Although total vimentin appears to be elevated in Lane 4 of this blot, this was not seen in other experiments. To illustrate this Figure 5B shows the densitometric quantification of vimentin in the four different treatment groups of monocytes as the mean± standard deviation for three similar experiments Figure 5C shows quantitative results of phosphorylation of vimentin in primary human monocytes upon activation with MCP-1 as compared with non-activated monocytes as the mean ± standard deviation for three similar experiments. Vimentin phosphorylation normalized to total vimentin was increased by treatment of human monocytes with MCP-1. This increase was ablated in monocytes that were deficient in PKCβ expression due to specific AS-ODN treatment..
Figure 5. PKCβ antisense ODN inhibit vimentin phosphorylation in MCP-1 activated human monocytes.
Monocytes were labeled with [32P] orthophosphate as described in the Methods. The cell lysates were immunoprecipitated with vimentin antibody followed by SDS-PAGE and transferred onto a PVDF membrane. In the upper panel of 5A, phosphorylation of vimentin was detected using phosphorimage analysis. In the lower panel of 5A, vimentin loading was analyzed by Western blotting. The figure shows 4 lanes: Lane 1, monocytes; lane 2, monocytes treated with MCP-1; lane 3, monocytes treated with MCP-1 and PKCβ-sense (S) ODN; lane 4, monocytes treated with MCP-1 and PKCβ-anti-sense (AS) ODN. The vimentin phosphorylation levels in lane 2 and lane 3, wherein the monocytes were treated with MCP-1 and MCP-1 in the presence of PKCβ-sense ODN, respectively, showed a marked comparable increase compared to lanes 1 and 4. Figure 5B shows the densitometric quantification of total vimentin in the four treatment groups of monocytes. Figure 5C shows quantitative results of relative vimentin phosphorylation of the 4 treatment groups of monocytes. Both the data in Figure 5B and Figure 5C are the averages of three similar experiments and the error bars indicate standard deviation values. The data were derived from band densitometry of the phosphorylated protein signal and were normalized for the amount of vimentin detected by immunoblotting.
PKCβ associates with vimentin upon treatment with MCP-1 in primary human monocytes
Upon confirming that vimentin is a phosphorylation target of PKCβ in MCP-1 treated primary human monocytes, we wanted to examine whether vimentin associates with PKCβ upon MCP-1 treatment in monocytes. To investigate this, monocytes were left untreated, treated with MCP-1 or treated with MCP-1 and the PKCβ inhibitor peptide. The lysates were immunoprecipitated with anti-vimentin antibody followed by immunoblotting with anti-PKCβ antibody (Figure 6A). The blot was then stripped and reprobed with anti-vimentin antibody as shown in (Figure 6B). No association between PKCβ and vimentin was observed in untreated monocytes yet MCP-1 treatment induced association (Figure 6A) thereby indicating the essential role of MCP-1 in inducing PKCβ binding with vimentin. Treatment with the PKCβ inhibitor peptide had no effect on the association between these two proteins therefore, PKCβ enzymatic activity is not required for association.
Figure 6. MCP-1 induces the association of vimentin with PKCβ in primary human monocytes.
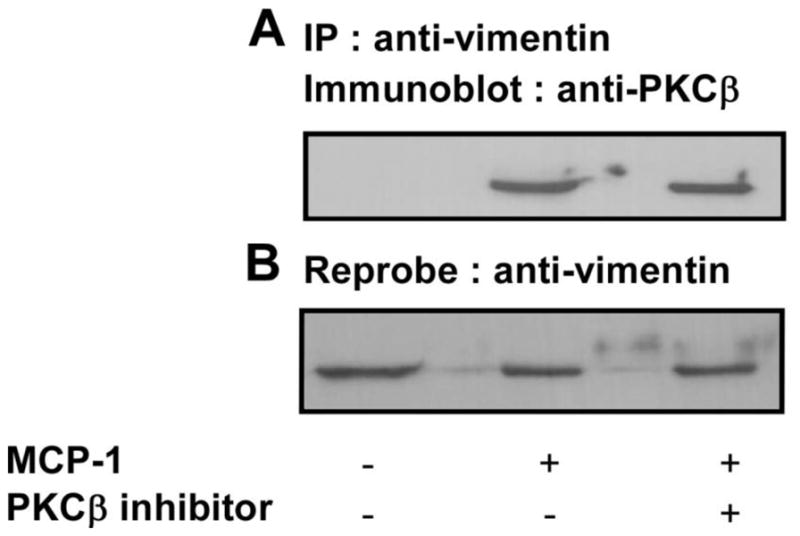
Human monocytes were incubated in the presence or absence of the myristoylated PKCβ inhibitor peptide for 30 min followed by treatment with MCP-1 for 30 min as described in the Methods. The cell lysates were immunoprecipitated with anti-vimentin antibody followed by immunoblotting with anti-PKCβ antibody. Figure 6A shows that upon MCP-1 treatment, increased association between PKCβ and vimentin occurs. No association of PKCβ with vimentin was observed in untreated monocytes. Upon PKCβ inhibitor treatment, the association between PKCβ and vimentin was not altered. The membrane was stripped and reprobed with anti-vimentin antibody to check loading as shown in 6B.
Detection of vimentin secretion by MCP-1 treated human monocytes
Vimentin, most commonly known for its functions as an intermediate filament protein, has recently been shown to be secreted by activated monocytes. Active secretion of vimentin has been observed to be upregulated in proinflammatory conditions and downregulated in anti-inflammatory conditions [31]. Vimentin secretion appears to depend on its phosphorylation since secretion of vimentin was increased by the phosphatase inhibitor okadaic acid and inhibited by the PKC inhibitor GO6983. We therefore investigated whether MCP-1 induces vimentin secretion from monocytes and further whether PKCβ is required for vimentin release. Upon treatment of isolated human monocytes with MCP-1, vimentin was secreted as shown in Figure 7. The MCP-1-induced secretion of vimentin by human monocytes was found to depend on PKCβ expression and PKCβ activity since both specific antisense ODN and the PKCβ inhibitor peptide blocked secretion.
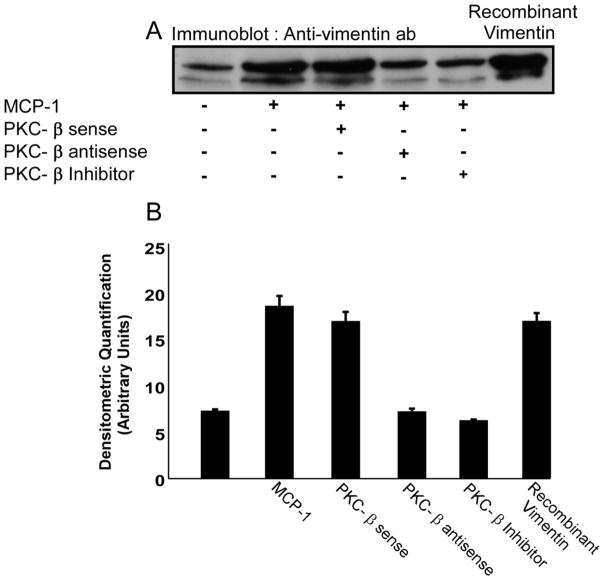
Figure 7. PKCβ induces vimentin phosphorylation and its extracellular release by primary human monocytes upon MCP-1 treatment.
MCP-1 treatment of primary human monocytes induced both vimentin phosphorylation (data not shown) and its release into the extracellular space. Extracellular release of vimentin was induced by MCP-1 and inhibited by incubation with PKCβ AS-ODN. Induction of vimentin release by PKCβ S-ODN treatment was comparable to the release induced by MCP-1 treated monocytes. The inhibition of vimentin release by PKCβ AS-ODN and PKC inhibitor peptide was comparable to the vimentin release of the untreated monocytes indicating that PKCβ phosphorylates vimentin upon MCP-1 treatment thereby inducing its release outside the cell. Recombinant human vimentin was used as a positive control. These data are representative of 3 identical experiments with different monocyte donors. Figure 7B shows quantitative analysis of vimentin secretion of the 5 treatments of monocytes from three experiments. The data were derived from band densitometry of the protein signal.
Discussion
MCP-1 plays a key role in monocyte recruitment by promoting migration to the vessel wall but the signal transduction pathways leading to migration and chemotaxis have not been fully elucidated. A newly developed fluorescent phosphosensor technology, the ProQ Diamond post-staining method that detects phosphoproteins in gels, to identify potential substrates for PKCβ phosphorylation in MCP-1-activated primary human monocytes was employed. For normalization we used SYPRO-Ruby, a total protein stain shown to be compatible with ProQ Diamond staining [32]. Of the proteins identified that stained with lesser intensity with the phosphostain in the presence of PKCβ antisense ODN or a PKCβ inhibitor peptide, vimentin was consistently present. Our study shows that vimentin phosphorylation is induced by MCP-1 activation of human monocytes.
Vimentin, a type III intermediate filament protein, is the most widely expressed intermediate filament protein with a rich filamentous network in monocytes/ macrophages. Vimentin retains a high level of sequence homology throughout all vertebrates from fish and Xenopus to humans strongly suggesting the physiological importance of vimentin [33]. During developmental stages, vimentin shows dynamically altered expression patterns and recent studies show involvement of vimentin in cell adhesion, migration, signaling and wound healing with distinct localization at the leading edge of keratinocytes migrating sheet [34–37]. Vimentin has been reported to co-localize with transient actin-rich adhesion sites that participate in cell migration [38]. Vimentin also regulates integrin functions in endothelial cell adhesion and serves as a major contributor to leukocyte transmigration [39–41]. Although genetic knockout of vimentin in animal models showed no gross phenotypic abnormalities [42], defects were observed in special physiological and pathological conditions including the observation that peripheral blood mononuclear cells showed reduced in vivo migration and diapedesis across the endothelium [35, 36]. Vimentin has additionally been shown to contribute to tumor cell invasiveness, metastasis and poor prognosis [43–46].
Organization of intermediate filament networks is observed to be primarily regulated and modulated by phosphorylation. The phosphorylation pattern of vimentin is highly complex involving different sites and kinases specific for unique cellular processes like differentiation, stress and mitosis [47]. Chemotactic factors such as formyl-peptides, have been shown to promote vimentin phosphorylation in vitro [48] and vimentin in neutrophils is phosphorylated upon stimulation with phorbol myristate acetate, strongly suggesting that it can be a substrate for PKC [49]. Indeed, vimentin has been reported to serve as a substrate for, and colocalize with several isoforms of PKC in varying cell types under certain conditions [50–53].
After identifying vimentin as a potential substrate for PKCβ in MCP-1-activated human monocytes, our further studies, using [32P] labeling and vimentin immunoprecipitation in the presence of specific antisense ODN to PKCβ, validated our initial results (Fig. 5). Furthermore, immunoprecipitation of monocyte lystates with an antibody to vimentin and immunoblotting with a PKCβ antibody revealed that increased PKCβ becomes associated with vimentin upon MCP-1 activation and this binding is independent of PKCβ functional activity (Fig. 6). Although we are the first to report PKCβ association with vimentin upon monocyte activation, others have previously observed a propensity for PKCβ, among other PKC isoforms to colocalize with vimentin filaments. Taking all of these data together we conclude that vimentin associates with PKCβ and is a target of PKCβ phosphorylation in MCP-1-activated human monocytes and likely controls the mechanics of monocyte chemotaxis to MCP-1.
As mentioned in Results, vimentin can also be secreted and secretion has been observed upon exposure to various activating stimuli in macrophages, platelets, neutrophils, T lymphocytes and endothelial cells [31, 54–56]. The physiological significance of released or secreted vimentin remains unknown. Secreted vimentin has been reported to induce the oxidative burst of macrophages since anti-vimentin antibody added to mature monocyte-derived macrophages reduced the superoxide anion production by these cells [31]. We have shown recently that soluble vimentin serves as a potent, endogenous ligand for the monocyte innate immune receptor, Dectin-1. We have also shown that binding of vimentin to Dectin-1 induces NADPH oxidase activity generating O2− in primary human monocytes[1].
Conclusions
There is renewed interest in finding novel approaches for the treatment of chronic inflammatory diseases and understanding the role of chemokines in the recruitment of leukocytes to sites of inflammation and it is therefore a focus of considerable research. Chemokine antagonists may stabilize established atherosclerotic plaques or cause them to regress in experimental animals. The PKCβ inhibitor ruboxistaurine is currently being tested as a potential therapeutic target for chronic vascular stress and diabetes in ongoing pre-clinical and clinical trials yet the scope of PKCβ contributions in atherogenesis have not been fully elucidated. These trials may provide a clearer picture as to whether this drug is a good option for PKCβ inhibition and whether this is an effective approach for treating cardiovascular disease, and particularly atherosclerosis [57] [58]. Alternatively antisense or RNA based therapies may prove effective. Recently, PKCβ was found to correlate with increased NADPH oxidase activation exacerbating oxidative stress [59]. Our findings suggest that in addition to our findings that PKCβ controls monocyte chemotaxis [5], PKCβ inhibition may prevent vimentin phosphorylation and its subsequent secretion by MCP-1 activated monocytes. This inhibition of extracellular vimentin may thereby interfere with the ability of extracellular vimentin to trigger superoxide anion production of monocyte/macrophages and limit oxidative stress in inflammatory sites. PKCβ activation and phosphorylation of vimentin thus plays a pivotal role in two major pro-inflammatory functions of human monocytes.
Acknowledgments
We would like to thank Meenakshi Shukla for isolating primary human monocytes for our study.
Funding Sources
Our study was sponsored by NIH grants HL051068, HL61971 and HL087018 to M.K.C and National Center for Research resources, CTSA 1UL1RR024989.
Footnotes
Competing Interests
The author(s) declare that they have no competing interests.
References
- 1.Thiagarajan PS, Yakubenko VP, Elsori DH, Yadav SP, Willard B, Tan CD, et al. Vimentin is an endogenous ligand for the pattern recognition receptor Dectin-1. Cardiovasc Res. 2013;99:494–504. doi: 10.1093/cvr/cvt117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 3.Osterud B, Bjorklid E. Role of monocytes in atherogenesis. Physiol Rev. 2003;83:1069–112. doi: 10.1152/physrev.00005.2003. [DOI] [PubMed] [Google Scholar]
- 4.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 5.Carnevale KA, Cathcart MK. Protein kinase C beta is required for human monocyte chemotaxis to MCP-1. J Biol Chem. 2003;278:25317–22. doi: 10.1074/jbc.M304182200. [DOI] [PubMed] [Google Scholar]
- 6.Liebler JM, Kunkel SL, Allen RM, Burdick MD, Strieter RM. Interferon-gamma Stimulates Monocyte Chemotactic Protein-1 Expression by Monocytes. Mediators Inflamm. 1994;3:27–31. doi: 10.1155/S0962935194000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rollins BJ, Stier P, Ernst T, Wong GG. The human homolog of the JE gene encodes a monocyte secretory protein. Mol Cell Biol. 1989;9:4687–95. doi: 10.1128/mcb.9.11.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu X, Dluz S, Graves DT, Zhang L, Antoniades HN, Hollander W, et al. Elevated expression of monocyte chemoattractant protein 1 by vascular smooth muscle cells in hypercholesterolemic primates. Proc Natl Acad Sci U S A. 1992;89:6953–7. doi: 10.1073/pnas.89.15.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marmur JD, Poon M, Rossikhina M, Taubman MB. Induction of PDGF-responsive genes in vascular smooth muscle. Implications for the early response to vessel injury. Circulation. 1992;86:III53–60. [PubMed] [Google Scholar]
- 10.Rollins BJ, Yoshimura T, Leonard EJ, Pober JS. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990;136:1229–33. [PMC free article] [PubMed] [Google Scholar]
- 11.Strieter RM, Wiggins R, Phan SH, Wharram BL, Showell HJ, Remick DG, et al. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989;162:694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz CJ, Valente AJ, Sprague EA, Kelley JL, Nerem RM. The pathogenesis of atherosclerosis: an overview. Clin Cardiol. 1991;14:I1–16. doi: 10.1002/clc.4960141302. [DOI] [PubMed] [Google Scholar]
- 13.Sozzani S, Locati M, Zhou D, Rieppi M, Luini W, Lamorte G, et al. Receptors, signal transduction, and spectrum of action of monocyte chemotactic protein-1 and related chemokines. J Leukoc Biol. 1995;57:788–94. doi: 10.1002/jlb.57.5.788. [DOI] [PubMed] [Google Scholar]
- 14.Wilson HM, Barker RN, Erwig LP. Macrophages: promising targets for the treatment of atherosclerosis. Curr Vasc Pharmacol. 2009;7:234–43. doi: 10.2174/157016109787455635. [DOI] [PubMed] [Google Scholar]
- 15.Sozzani S, Luini W, Molino M, Jilek P, Bottazzi B, Cerletti C, et al. The signal transduction pathway involved in the migration induced by a monocyte chemotactic cytokine. J Immunol. 1991;147:2215–21. [PubMed] [Google Scholar]
- 16.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci U S A. 1997;94:12053–8. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita T, Asai T, Andrassy M, Stern DM, Pinsky DJ, Zou YS, et al. PKCbeta regulates ischemia/reperfusion injury in the lung. J Clin Invest. 2004;113:1615–23. doi: 10.1172/JCI19225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aragay AM, Mellado M, Frade JM, Martin AM, Jimenez-Sainz MC, Martinez AC, et al. Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2. Proc Natl Acad Sci U S A. 1998;95:2985–90. doi: 10.1073/pnas.95.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penton-Rol G, Polentarutti N, Luini W, Borsatti A, Mancinelli R, Sica A, et al. Selective inhibition of expression of the chemokine receptor CCR2 in human monocytes by IFN-gamma. J Immunol. 1998;160:3869–73. [PubMed] [Google Scholar]
- 20.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–76. [PubMed] [Google Scholar]
- 21.Shyy YJ, Hsieh HJ, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci U S A. 1994;91:4678–82. doi: 10.1073/pnas.91.11.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berliner JA, Territo MC, Sevanian A, Ramin S, Kim JA, Bamshad B, et al. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990;85:1260–6. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan SF, Harja E, Andrassy M, Fujita T, Schmidt AM. Protein kinase C beta/early growth response-1 pathway: a key player in ischemia, atherosclerosis, and restenosis. J Am Coll Cardiol. 2006;48:A47–55. doi: 10.1016/j.jacc.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 24.Harja E, Bucciarelli LG, Lu Y, Stern DM, Zou YS, Schmidt AM, et al. Early growth response-1 promotes atherogenesis: mice deficient in early growth response-1 and apolipoprotein E display decreased atherosclerosis and vascular inflammation. Circ Res. 2004;94:333–9. doi: 10.1161/01.RES.0000112405.61577.95. [DOI] [PubMed] [Google Scholar]
- 25.Harja E, Chang JS, Lu Y, Leitges M, Zou YS, Schmidt AM, et al. Mice deficient in PKCbeta and apolipoprotein E display decreased atherosclerosis. FASEB J. 2009;23:1081–91. doi: 10.1096/fj.08-120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumagai K, Itoh K, Hinuma S, Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29:17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- 27.Cathcart MK, Morel DW, Chisolm GM., 3rd Monocytes and neutrophils oxidize low density lipoprotein making it cytotoxic. J Leukoc Biol. 1985;38:341–50. doi: 10.1002/jlb.38.2.341. [DOI] [PubMed] [Google Scholar]
- 28.Keightley JA, Shang L, Kinter M. Proteomic analysis of oxidative stress-resistant cells: a specific role for aldose reductase overexpression in cytoprotection. Mol Cell Proteomics. 2004;3:167–75. doi: 10.1074/mcp.M300119-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Kinter M, Sherman NE. Protein sequencing and identification using tandem mass spectrometry. New York: John Wiley; 2000. p. xvi.p. 301. [Google Scholar]
- 30.Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–12. [PubMed] [Google Scholar]
- 31.Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5:59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 32.Steinberg TH, Agnew BJ, Gee KR, Leung WY, Goodman T, Schulenberg B, et al. Global quantitative phosphoprotein analysis using Multiplexed Proteomics technology. Proteomics. 2003;3:1128–44. doi: 10.1002/pmic.200300434. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann H, Fouquet B, Franke WW. Expression of intermediate filament proteins during development of Xenopus laevis. I. cDNA clones encoding different forms of vimentin. Development. 1989;105:279–98. doi: 10.1242/dev.105.2.279. [DOI] [PubMed] [Google Scholar]
- 34.Biddle D, Spandau DF. Expression of vimentin in cultured human keratinocytes is associated with cell - extracellular matrix junctions. Arch Dermatol Res. 1996;288:621–4. doi: 10.1007/BF02505266. [DOI] [PubMed] [Google Scholar]
- 35.Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, et al. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci. 1998;111 (Pt 13):1897–907. doi: 10.1242/jcs.111.13.1897. [DOI] [PubMed] [Google Scholar]
- 36.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–62. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 37.Rius C, Aller P. Vimentin expression as a late event in the in vitro differentiation of human promonocytic cells. J Cell Sci. 1992;101 (Pt 2):395–401. doi: 10.1242/jcs.101.2.395. [DOI] [PubMed] [Google Scholar]
- 38.Correia I, Chu D, Chou YH, Goldman RD, Matsudaira P. Integrating the actin and vimentin cytoskeletons. adhesion-dependent formation of fimbrin-vimentin complexes in macrophages. J Cell Biol. 1999;146:831–42. doi: 10.1083/jcb.146.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barberis L, Pasquali C, Bertschy-Meier D, Cuccurullo A, Costa C, Ambrogio C, et al. Leukocyte transmigration is modulated by chemokine-mediated PI3Kgamma-dependent phosphorylation of vimentin. Eur J Immunol. 2009;39:1136–46. doi: 10.1002/eji.200838884. [DOI] [PubMed] [Google Scholar]
- 40.Brown MJ, Hallam JA, Colucci-Guyon E, Shaw S. Rigidity of circulating lymphocytes is primarily conferred by vimentin intermediate filaments. J Immunol. 2001;166:6640–6. doi: 10.4049/jimmunol.166.11.6640. [DOI] [PubMed] [Google Scholar]
- 41.Gonzales M, Weksler B, Tsuruta D, Goldman RD, Yoon KJ, Hopkinson SB, et al. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol Biol Cell. 2001;12:85–100. doi: 10.1091/mbc.12.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreuzer J, Denger S, Schmidts A, Jahn L, Merten M, von Hodenberg E. Fibrinogen promotes monocyte adhesion via a protein kinase C dependent mechanism. J Mol Med. 1996;74:161–5. doi: 10.1007/BF01575449. [DOI] [PubMed] [Google Scholar]
- 43.Chu YW, Runyan RB, Oshima RG, Hendrix MJ. Expression of complete keratin filaments in mouse L cells augments cell migration and invasion. Proc Natl Acad Sci U S A. 1993;90:4261–5. doi: 10.1073/pnas.90.9.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilles C, Polette M, Piette J, Delvigne AC, Thompson EW, Foidart JM, et al. Vimentin expression in cervical carcinomas: association with invasive and migratory potential. J Pathol. 1996;180:175–80. doi: 10.1002/(SICI)1096-9896(199610)180:2<175::AID-PATH630>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 45.Sommers CL, Heckford SE, Skerker JM, Worland P, Torri JA, Thompson EW, et al. Loss of epithelial markers and acquisition of vimentin expression in adriamycin- and vinblastine-resistant human breast cancer cell lines. Cancer Res. 1992;52:5190–7. [PubMed] [Google Scholar]
- 46.Hu L, Lau SH, Tzang CH, Wen JM, Wang W, Xie D, et al. Association of Vimentin overexpression and hepatocellular carcinoma metastasis. Oncogene. 2004;23:298–302. doi: 10.1038/sj.onc.1206483. [DOI] [PubMed] [Google Scholar]
- 47.Geisler N, Hatzfeld M, Weber K. Phosphorylation in vitro of vimentin by protein kinases A and C is restricted to the head domain. Identification of the phosphoserine sites and their influence on filament formation. Eur J Biochem. 1989;183:441–7. doi: 10.1111/j.1432-1033.1989.tb14947.x. [DOI] [PubMed] [Google Scholar]
- 48.O’Connor CM, Gard DL, Lazarides E. Phosphorylation of intermediate filament proteins by cAMP-dependent protein kinases. Cell. 1981;23:135–43. doi: 10.1016/0092-8674(81)90278-6. [DOI] [PubMed] [Google Scholar]
- 49.Huang CK, Devanney JF, Kennedy SP. Vimentin, a cytoskeletal substrate of protein kinase C. Biochem Biophys Res Commun. 1988;150:1006–11. doi: 10.1016/0006-291x(88)90728-0. [DOI] [PubMed] [Google Scholar]
- 50.Ivaska J, Vuoriluoto K, Huovinen T, Izawa I, Inagaki M, Parker PJ. PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility. Embo J. 2005;24:3834–45. doi: 10.1038/sj.emboj.7600847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murti KG, Kaur K, Goorha RM. Protein kinase C associates with intermediate filaments and stress fibers. Exp Cell Res. 1992;202:36–44. doi: 10.1016/0014-4827(92)90401-s. [DOI] [PubMed] [Google Scholar]
- 52.Owen PJ, Johnson GD, Lord JM. Protein kinase C-delta associates with vimentin intermediate filaments in differentiated HL60 cells. Exp Cell Res. 1996;225:366–73. doi: 10.1006/excr.1996.0187. [DOI] [PubMed] [Google Scholar]
- 53.Spudich A, Meyer T, Stryer L. Association of the beta isoform of protein kinase C with vimentin filaments. Cell Motil Cytoskeleton. 1992;22:250–6. doi: 10.1002/cm.970220405. [DOI] [PubMed] [Google Scholar]
- 54.Xu B, deWaal RM, Mor-Vaknin N, Hibbard C, Markovitz DM, Kahn ML. The endothelial cell-specific antibody PAL-E identifies a secreted form of vimentin in the blood vasculature. Mol Cell Biol. 2004;24:9198–206. doi: 10.1128/MCB.24.20.9198-9206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huet D, Bagot M, Loyaux D, Capdevielle J, Conraux L, Ferrara P, et al. SC5 mAb represents a unique tool for the detection of extracellular vimentin as a specific marker of Sezary cells. J Immunol. 2006;176:652–9. doi: 10.4049/jimmunol.176.1.652. [DOI] [PubMed] [Google Scholar]
- 56.Mahesh B, Leong HS, McCormack A, Sarathchandra P, Holder A, Rose ML. Autoantibodies to vimentin cause accelerated rejection of cardiac allografts. Am J Pathol. 2007;170:1415–27. doi: 10.2353/ajpath.2007.060728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bynagari-Settipalli YS, Chari R, Kilpatrick L, Kunapuli SP. Protein kinase C - possible therapeutic target to treat cardiovascular diseases. Cardiovasc Hematol Disord Drug Targets. 2010;10:292–308. doi: 10.2174/187152910793743869. [DOI] [PubMed] [Google Scholar]
- 58.Rask-Madsen C, King GL. Proatherosclerotic mechanisms involving protein kinase C in diabetes and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:487–96. doi: 10.1161/01.ATV.0000155325.41507.e0. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y, Lei S, Gao X, Mao X, Wang T, Wong GT, et al. PKCbeta inhibition with ruboxistaurin reduces oxidative stress and attenuates left ventricular hypertrophy and dysfunction in rats with streptozotocin-induced diabetes. Clin Sci (Lond) 2012;122:161–73. doi: 10.1042/CS20110176. [DOI] [PubMed] [Google Scholar]