Abstract
The brain melanocortin (MC) system is one of numerous overlapping systems regulating energy balance; it consists of peptides including α-MSH that act via MC receptors (MCR). Mutations and polymorphisms in MC3R and MC4R have been identified as one of the most common genetic contributors to obesity in human studies. Brain MC3R and MC4R are known to modulate energy expenditure (EE) and food intake, but much less is known regarding brain MC5R. To test the hypothesis that brain MC modulates physical activity (PA) and EE, we compared brain MCR profiles in rats that consistently show high vs. low levels of “spontaneous” daily PA. Compared to low-activity rats, high-activity rats show enhanced mRNA expression of MC receptors in the brain, specifically of MC3R in the paraventricular nucleus (PVN), and MC4R and MC5R in the perifornical lateral hypothalamus (PeFLH). Next, we microinjected the MCR agonist MTII into the PVN region and measured PA and EE. Intra-PVN MTII caused a dose-dependent increase in PA and this effect was greater in high-activity compared to low-activity rats. These results implicate region-specific brain MC receptor expression in the heightened PA seen in association with high endurance capacity and identify promising targets in the brain MC system that may contribute to inter-individual variability in energy balance.
Keywords: Physical activity, non-exercise activity thermogenesis, brain melanocortin system, MTII, obesity
Introduction
The increasing prevalence of obesity continues to be a cause of concern across the globe [1]. Recent work recognizes the importance of daily physical activity (PA) in both weight management and cardiovascular disease risk [1]. The energy expenditure (EE) of daily living, called non-exercise activity thermogenesis (NEAT), has emerged as a crucial factor that accounts for individual differences in body weight, interacting with genetic predisposition [2]. As in humans, animal models of obesity tend to have low levels of PA [2]. In both rats and humans, high intrinsic aerobic capacity is tightly linked with high levels of PA [3]; both of these traits could serve to identify the lean phenotype.
The brain melanocortin (MC) system is one of several overlapping systems regulating energy balance [4]. The prohormone pro-opiomelanocortin (POMC) is cleaved into several bioactive peptides, one of which is α-melanocyte stimulating hormone (α-MSH). These peptides act as ligands for MC receptor subtypes MCR1-5; three of these are found in the adult mammalian brain and mediate a multitude of physiological and behavioral effects [5]. Brain MC3R and MC4R have been shown to modulate EE and food intake, but much less is known about the role of MC5R in the brain [7, 8]. Mutations and deficiencies in POMC and MC receptors lead to obesity and there is evidence linking this system to PA in humans as well as animal models of obesity [9]. Thus, brain MC circuitry is relevant for a naturally occurring obese phenotype.
In 1996, Koch and Britton initiated development of rat lines that differ for intrinsic aerobic treadmill running capacity via two-way (divergent) artificial selection, establishing models for leanness (HCR) and obesity (LCR) [12]. HCR have consistently higher levels of daily “spontaneous” PA relative to LCR, independent of differences in body weight or lean mass [13, 14]. Our long-term goal is to understand the mechanisms causative of low and high PA as they relate to obesity. Here we tested the hypothesis that regional MC receptor expression within the hypothalamus contributes to individual differences in PA in the LCR-HCR model system.
Methods
Quantitative real-time (Q-PCR) analysis
9 of each HCR and LCR male rats (generation 21), obtained from the University of Michigan, were individually housed on a 24-hour light:dark cycle with ad lib access to standard chow (Lab Diet 5001) for 28 days [14]. A detailed protocol for obtaining brain micropunches has been described previously [13]. Briefly, brains were taken after rapid decapitation and sectioned using a Hatton apparatus using optic chiasm as the primary landmark. 100 μm and 200 μm sections were taken, and punches of the paraventricular nucleus (PVN) or the perifornical part of lateral hypothalamus (PeFLH) were extracted using a micropuncher, flash frozen with liquid nitrogen, and stored at -80°C for later processing. Using samples from the arcuate nucleus, PVN, and PeFLH, 20–40mg of tissue was homogenized for RNA isolation to be estimated by quantitative real-time PCR (Q-PCR). The samples were purified using an RNA purification kit (Ribopure from Ambion) and only samples with optimum RNA integrity numbers were used for further processing. The RNA concentration was measured using NANODROP (ND-1000, Nanodrop technologies) and ~20–100ng/μl of islolated RNA was used for cDNA synthesis. Purified total RNA was reversed transcribed using Applied Biosystems (ABI) Reverse Transcription reagents kit, using standard suggested protocol with random hexamers. Q-PCR was conducted using Taqman Universal Master Mix from ABI on the experimental samples using Taqman probes for the genes of interest, using approximately 20–100 ng of cDNA. The annealing temperature was 60 °C with 40 cycles. All samples were run in triplicates on Strategene Mx3005P™ Real-Time PCR System by Agilent. GAPDH was used as the control gene for all assays and relative expression was calculated using Comparative Ct method (ΔΔCt) method. HCR mean ΔΔCt values were used to define 100%, and each animal’s data were calculated as a percentage of the mean. Mean and variance values were calculated and unpaired 2-tailed t-tests were used for analyses. Differences of p<0.05 were considered significant.
Physical activity and EE measurements induced by MCR agonist Melanoton II (MTII)
6 HCR and 5 LCR female rats (generation 25), also obtained from the University of Michigan, were used for this study. After acclimation to our facility, stereotaxic surgeries were performed to implant guide cannulae aimed at PVN [13, 15]. Inhaled isoflurane was used for anesthesia and care was taken to avoid animals’ suffering at each stage of the experiment. Post-surgery, animals were allowed to recover for one week, after which a body composition measurement was taken using magnetic resonance spectroscopy with an EchoMRI-700 (Echo Medical Systems, Houston, TX) to determine fat and lean mass [16]. The rats were then placed in the calorimetry room, where they were allowed to acclimate in their testing cage for 48 hours before the start of any experiment. These rats were then microinjected with either a vehicle (aCSF) or the MCR 3,4,5 agonist (MTII, Phoenix Pharmaceuticals). Three different doses of MTII (5,10, and 20 pmol in 200 nl) were used to establish a dose-response curve, with each rat receiving each dose in random order, separated by 48 hours between subsequent injections. Using the Oxymax FAST system (Columbus Instruments, Columbus, OH), EE (kcal/hr) and PA (counts/min) were measured with a temporal resolution of 30 seconds for a total of 3 hours. The first 20 minutes of data post-injection were not included for analysis to account for handling-induced activity and to allow the air-exchange to settle.
Placement of the cannula and potential spread of microinjected compounds were determined at the end of the study by histologically examining the anatomic placement of an injection of India ink (200 nl). Only rats whose dye injection site corresponded to the stereotaxic coordinates (within 250μm of the PVN) were used for data analysis. One of the LCR rats died during the study due to an unrelated cause and we used simulation-based statistical software (NORM) for estimating the missing activity value based on multiple imputations for one of the doses [17]; the imputed data point was not included in post-hoc analyses, however. We used a 4×2 mixed ANOVA, with dose of MTII as the within-subjects independent variable, and high-activity HCR vs. low-activity LCR rats as the between-subjects independent variable; the dependent variables (PA and EE) were analyzed separately. We compared each rat’s activity with its vehicle value to account for individual differences in activity. In order to assess the dose range to use in HCR/LCR, the same experimental procedure was followed in 6 Sprague-Dawley (SD) rats targeting PVN or PeFLH. As a result, we were able to identify the optimal dose range and also proceed with fewer total microinjections in HCR/LCR rats by eliminating one dose in the higher range for further experiments.
All animal procedures followed were approved by the Institutional Animal Care and Use Committee of Kent State University and in accordance with the Guide for the Care and Use of Laboratory Animals.
Results
Lean rats have greater site-specific MCR mRNA expression
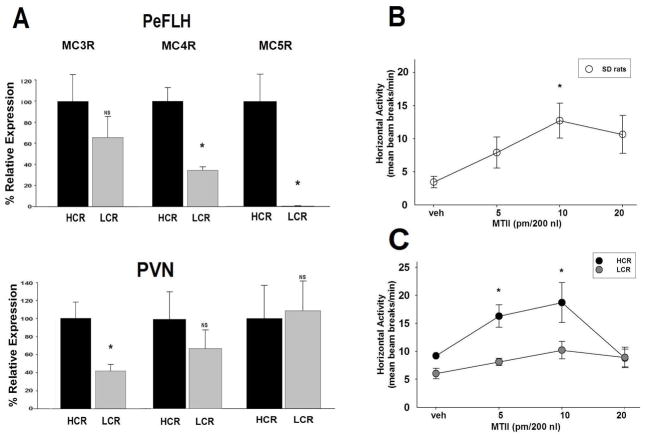
Q-PCR data are shown in Figure 1A and Table 1. We examined the basal mRNA expression of MC receptors MC3R, MC4R, and MC5R in two brain hypothalamic regions, the PVN and the PeFLH. Upon comparing HCR with LCR animals, MC3R mRNA expression level was significantly higher in HCR in the PVN (Figure 1A). No significant difference was seen in the PeFLH region for this receptor subtype. Both MC4R and MC5R mRNA, on the other hand, were significantly higher in HCR rats in the PeFLH only (Figure 1A), but not in the PVN. mRNA expression of MC precursor popypeptide POMC, and the processing enzymes prohormone convertase (PC) 1 and 2 in the arcuate nucleus, were not found to be significantly different between LCR and the lean HCR rats (Table 1). Of the other peptides examined (see Table 1), only the expression of BDNF in the PeFLH was found to significantly differ between groups (HCR>LCR).
Figure 1.
(A) Quantitative RT-PCR of receptor mRNA levels in target brain regions. Compared to low-activity rats (LCR), high-activity rats (HCR) have greater expression of MC 3, 4, and 5 receptors (MC3R, MC4R, MC5R) in the paraventricular nucleus (PVN) and perifornical lateral hypothalamus (PeFLH). MC3R in PeFLH: HCR (N=8), LCR (N=8), MC4R in PeFLH: HCR (N=7), LCR (N=8), MC5R in PeFLH: HCR (N=8), LCR (N=8), MC3R in PVN: HCR (N=8), LCR (N=8), MC4R in PVN: HCR (N=7), LCR (N=8), MC5R in PVN: HCR (N=5), LCR (N=8). (B) Intra-PVN microinjections of MCR agonist Melanotan II (MTII) induced physical activity in male Sprague-Dawley (SD) rats (N=4); no effect was found after intra-PeFLH injections (data not shown). (C) Intra-PVN MTII-induced activity was significantly greater in high-activity rats (HCR, N=4) compared to low-activity rats (LCR, N=5). *p <0.05, NS: not significant.
Table 1.
Quantitative mRNA measurement of melanocortin (MC) receptors in hypothalamic nuclei.
| Brain Region Micropunch | Q-PCR Probe | p-value |
|---|---|---|
| Arcuate nucleus (ARC) | PC1 | 0.06 |
| PC2 | 0.53 | |
| POMC | 0.78 | |
| GHSR | 0.30 | |
| AgRP | 0.10 | |
| NHLH2 | 0.96 | |
| Perifornical lateral hypothalamus (PeFLH) | MC3R | 0.29 |
| MC4R | 0.02* | |
| MC5R | 0.02* | |
| BDNF | 0.03* | |
| TRH | 0.68 | |
| GHSR | 0.15 | |
| Paraventricular nucleus (PVN) | MC3R | <0.01* |
| MC4R | 0.55 | |
| MC5R | 0.87 | |
| BDNF | 0.13 | |
| TRH | 0.86 | |
| GHSR | 0.75 |
MCR 3, 4, and 5, pro-opio melanocortin (POMC), prohormone convertases (PC1, PC2), Agouti-related peptide (AgRP) brain derived neurotropic factor (BDNF), thyrotropin-releasing hormone (TRH), ghrelin receptors (GHSR), and nescient helix-loop-helix 2 (NHLH2) in target brain regions: arcuate nucleus, paraventricular nucleus (PVN), and perifornical lateral hypothalamic area (PeFLH);
p <0.05 (HCR>LCR).
MCR agonist in specific brain nuclei increases short-term activity and EE
We measured activity of SD rats for a 3-hour period after microinjecting the common MCR agonist MTII into the PVN and the PeFLH. We found a short-term increase in home-cage PA with PVN microinjections, which was significant compared to the vehicle aCSF (Figure 1B). We did not detect a similar increase in the PeFLH region (data not shown). Therefore, we next focused on targeting the PVN region to examine the effect of agonist MTII in HCR and LCR rats. When comparing HCR and LCR, we found that intra-PVN MTII-induced physical activity was significantly greater in HCR than LCR (Figure 1C). EE analysis revealed significant effects of dose, group, and body weight; follow-up analyses of EE (using analysis of covariance) at the 5pmol/200nl dose showed greater MTII-induced EE for HCR than LCR.
Discussion
The brain MC system is known to play an important role in satiety and obesity propensity. Moreover, evidence that POMC, α-MSH, and MCRs are important in satiety and EE in animal studies has been corroborated by genetic studies in humans that consistently identify MCR point mutations and polymorphisms in human obesity. How these genetic differences may possibly manifest to change energetics remains poorly understood, however. Here, we show for the first time that regional differences in brain MC responses are associated with individual differences in PA, which are linked to obesity resistance or propensity [7, 8, 10, 11]. These findings may give an insight into how individuals differ in their brain MC responses, which could in turn make them more or less physically active.
While POMC expression in the brain is mainly limited to the arcuate nucleus and area postrema, it is cleaved post-translationally to yield different bioactive compounds that act on MC receptors in numerous brain regions to alter several components of energy balance [7, 8, 10, 11]. Heightened POMC results in decreased MC receptors and upregulation of α-MSH [4, 6], suggesting the possibility that the heightened MCR seen in the high-activity rats might be secondary to higher overall MC “tone” in these rats. Our data do not support this interpretation, however. First, it is not likely that differences in activity levels in the rats originate with altered arcuate MC as we found no significant difference in precursor POMC mRNA expression and only a trend in the processing enzyme PC1 (not significantly higher in HCRs, p= 0.06, Table 1). Second, heightened global MC release would be expected to alter MC receptors similarly regardless of subtype or brain region, while we found a region and subtype-specific expression. Altogether, our data suggest the profile of MC receptor expression may be intrinsic to the high-activity, high-endurance phenotype.
Moving downstream from the arcuate and area prostema, the action of MCs is limited to three receptors in adult mammalian brain. The receptors MC3R, MC4R, and MC5R are localized to the PVN, PeFLH, and dorsomedial hypothalamus (DMH), among other brain regions [6, 8]. Our data demonstrate that the PVN of the high-activity rats shows higher expression of MC3R, but not of MC4R or MC5R. Moreover, we found that intra-PVN microinjections of MTII increase physical activity and energy expenditure of activity (NEAT), and that increase in short-term, daytime NEAT was greater in high-activity HCR compared to low-activity LCR. We attribute the heightened EE after intra-PVN MTII to activity EE, but the contribution of intra-PVN MC to brown adipose tissue thermogenesis cannot be ruled out [18]. These results illustrate possible mechanisms through which individual differences in brain MC contribute to changes in energy balance—specifically EE—that predispose an individual to be lean or obese. This may also suggest that MC3R could contribute to the physical activity differences seen between HCR/LCR rats. Because MTII is not specific for MC3R, however, we cannot rule out the possibility that other MC receptors in the PVN contribute to physical activity and EE. Though a first step, these results can be further defined by specific localization of function using MCR subtype-specific agonists and antagonists [19, 20].
We then examined MC expression in other hypothalamic regions and systems known to be important in regulating PA. Previously, site-specific microinjections of orexin in the orexin cell-body containing area of the hypothalamus, PeFLH, was shown to increase physical activity and EE [13]. We identified no differences in the expression of orexin or its receptor that could underlie the differences in PA seen in these rats, however, which led us to examine related parallel brain systems that impact PA. Our data indicate that high-activity HCR rats show enhanced expression of MC4R and MC5R, but not MC3R in the PeFLH region. While several studies have demonstrated the importance of MC4R in obesity and appetite, very little is known about MC5R or its role in the brain in particular. Our results suggest that region-specific differences in MC5R, specifically within the PeFLH, could also contribute to individual differences in PA. These findings are a good example of the utility of examining the lean phenotype. It has been suggested that standard “non-obese” animal models might essentially have metabolic syndrome due to housing in a sedentary environment with unrestricted food supply and limited physical challenges [21]. It is conceivable that MC5R is low or undetectable in the PeFLH of standard rodent models, as it is in the obesity-prone, low-activity LCR (Figure 1A).
MC is known to interact with several other neuropeptide systems, including orexin [13]. Therefore, we also looked at other potential downstream neuropeptide mediators including brain derived neurotropic factor (BDNF) and thyrotropin-releasing hormone (TRH), as well as ghrelin receptor GHSR, all of which are important in these hypothalamic circuits [22]. In the regions examined, BDNF showed a significant difference, being significantly higher in high-activity HCR in the PeFLH (Table 1). BDNF has been previously described as an important component of the hypothalamic pathway that regulates body weight and energy homeostasis [22]. Our results suggest that BDNF may be a critical component in the melnaocortinergic brain system regulating spontaneous physical activity and this system may differ between lean and obesity-prone individuals. Agouti-related peptide (AgRP) is an inverse antagonist of the MCRs and is known to alter food intake as well [23]. On examining AgRP mRNA levels in the arcuate nucleus, we did not detect a difference between HCR/LCR rats (Table 1). Here, we have focused on hypothalamic circuits, though forebrain reward systems and hindbrain MC are also known to affect energy balance, and may potentially differ between high- and low-activity individuals as well [24].
Several lines of evidence highlight the close association between high intrinsic aerobic capacity and high daily PA. In both rats and humans, those with high running endurance are also consistently more physically active [13]. The present study suggests that differences in the brain MC system in these rats, with regional distribution of MC receptors in particular, could underlie individual differences in the tendency to be more or less active. While MCRs are known to be associated with human obesity, here we highlight that this effect may partly manifest as differences in PA. Though others have demonstrated the relevance of MC3R and MC4R in the regulation of energy balance, satiety, and human obesity, the potential importance of brain MC5R is yet to be considered [8, 24, 25]. Here we found that highly specific, differential regional activation of brain MC receptors could affect different aspects of energy balance, particularly PA [18].
Acknowledgments
We are grateful to Minzhi Zhang for her help in sample collection. We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Gilligan. The LCR and HCR model can be made available for collaborative study (contact: brittons@umich.edu or lgkoch@umich.edu).
Source of funding: NIH R01NS055859 to CMN and AHA 11PRE7320029 to CS. The LCR-HCR rat model system was funded by the National Center for Research Resources grant R24 RR017718 and is currently supported by the Office of Research Infrastructure Programs/OD grant ROD012098A (to LGK and SLB) from the NIH. SLB was also supported by NIH RO1 DK077200.
Footnotes
Conflict of Interest: none declared.
References
- 1.Achike FI, et al. Obesity, metabolic syndrome, adipocytes and vascular function: A holistic viewpoint. Clin Exp Pharmacol Physiol. 2011;38(1):1–10. doi: 10.1111/j.1440-1681.2010.05460.x. [DOI] [PubMed] [Google Scholar]
- 2.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283(5399):212–4. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 3.Novak CM, et al. Endurance capacity, not body size, determines physical activity levels: role of skeletal muscle PEPCK. PLoS ONE. 2009;4(6):e5869. doi: 10.1371/journal.pone.0005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zemel MB, Shi H. Pro-opiomelanocortin (POMC) deficiency and peripheral melanocortins in obesity. Nutr Rev. 2000;58(6):177–80. doi: 10.1111/j.1753-4887.2000.tb01857.x. [DOI] [PubMed] [Google Scholar]
- 5.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31(4):506–43. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol. 2002;172(3):411–21. doi: 10.1677/joe.0.1720411. [DOI] [PubMed] [Google Scholar]
- 7.Chen AS, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26(1):97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 8.Mountjoy KG, Wild JM. Melanocortin-4 receptor mRNA expression in the developing autonomic and central nervous systems. Brain Res Dev Brain Res. 1998;107(2):309–14. doi: 10.1016/s0165-3806(98)00015-7. [DOI] [PubMed] [Google Scholar]
- 9.Farooqi IS, et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–95. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 10.Cauchi S, et al. Combined effects of MC4R and FTO common genetic variants on obesity in European general populations. J Mol Med (Berl) 2009;87(5):537–46. doi: 10.1007/s00109-009-0451-6. [DOI] [PubMed] [Google Scholar]
- 11.MacKenzie RG. Obesity-associated mutations in the human melanocortin-4 receptor gene. Peptides. 2006;27(2):395–403. doi: 10.1016/j.peptides.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 12.Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5(1):45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- 13.Novak CM, et al. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav. 2010;58(3):355–67. doi: 10.1016/j.yhbeh.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch LG, Britton SL. Divergent selection for aerobic capacity in rats as a model for complex disease. Integr Comp Biol. 2005;45(3):405–15. doi: 10.1093/icb/45.3.405. [DOI] [PubMed] [Google Scholar]
- 15.Novak CM, Zhang M, Levine JA. Neuromedin U in the paraventricular and arcuate hypothalamic nuclei increases non-exercise activity thermogenesis. J Neuroendocrinol. 2006;18(8):594–601. doi: 10.1111/j.1365-2826.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- 16.Nixon JP, et al. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity (Silver Spring) 2010;18(8):1652–9. doi: 10.1038/oby.2009.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schafer JL. NORM: Multiple imputation of incomplete multivariate data under a normal model. 1999. Vol. version 2. [Google Scholar]
- 18.Song CK, et al. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1467–76. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 19.Grieco P, et al. D-Amino acid scan of gamma-melanocyte-stimulating hormone: importance of Trp(8) on human MC3 receptor selectivity. J Med Chem. 2000;43(26):4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- 20.Grieco P, et al. Design and microwave-assisted synthesis of novel macrocyclic peptides active at melanocortin receptors: discovery of potent and selective hMC5R receptor antagonists. J Med Chem. 2008;51(9):2701–7. doi: 10.1021/jm701181n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin B, et al. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci U S A. 2010;107(14):6127–33. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C, et al. Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure. Brain Res. 2010;1336:66–77. doi: 10.1016/j.brainres.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stutz AM, Morrison CD, Argyropoulos G. The agouti-related protein and its role in energy homeostasis. Peptides. 2005;26(10):1771–81. doi: 10.1016/j.peptides.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Bromberg-Martin ES, Hikosaka O. Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron. 2009;63(1):119–26. doi: 10.1016/j.neuron.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler AA, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141(9):3518–21. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]