Abstract
These are exciting times for bioinformaticians, computational biologists and drug designers with the genome and proteome sequences and related structural databases growing at an accelerated pace. The post-genomic era has triggered high expectations for a rapid and successful treatment of diseases. However, in this biological information rich and functional knowledge poor scenario, the challenges are indeed grand, no less than the assembly of the genome of the whole organism. These include functional annotation of genes, identification of druggable targets, prediction of three-dimensional structures of protein targets from their amino acid sequences, arriving at lead compounds for these targets followed by a transition from bench to bedside. We propose here a “Genome to Hits In Silico“ strategy (called Dhanvantari) and illustrate it on Chikungunya virus (CHIKV). “Genome to hits” is a novel pathway incorporating a series of steps such as gene prediction, protein tertiary structure determination, active site identification, hit molecule generation, docking and scoring of hits to arrive at lead compounds. The current state of the art for each of the steps in the pathway is high-lighted and the feasibility of creating an automated genome to hits assembly line is discussed.
Keywords: Genome annotation, protein folding, docking and scoring, lead molecule, CHIKV.
1. INTRODUCTION
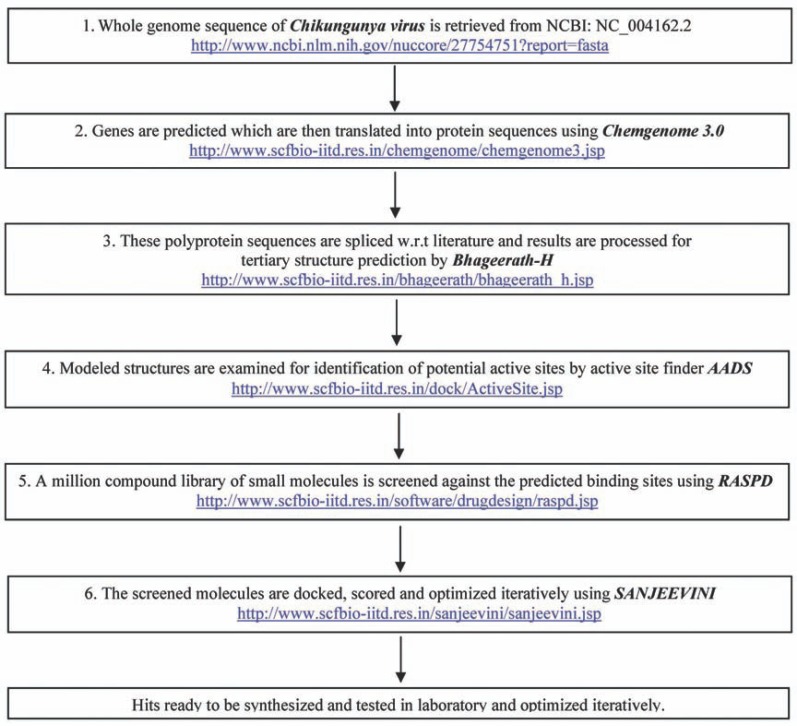
The automation of genomes to hit molecules pathway poses several challenges. It involves, inter alia, (i) accurate genome annotation, (ii) identification of druggable target proteins, (iii) determination of 3-dimensional structures of protein targets, (iv) identification of hits for the target, (v) optimization of hits to lead molecules to realize high levels of affinity and selectivity to the target and low toxicity. Here, we describe the progresses achieved in each of the above areas, the conceivability of a “Genome to hits” assembly line in silico (Fig. 1) and illustrate the approach with chikungunya virus (CHIKV).
Fig. (1).
Flow diagram illustrating the steps involved in Dhanvantari pathway to achieve hit molecules from genomic information.
2. BACKGROUND
We describe here the science and the software behind “Genome to Hits” assembly line which comprises six steps (Fig. 1), classifiable into three major areas of research viz. (a) genome annotation (steps 1 and 2), (b) protein tertiary structure prediction (step 3) and (c) structure based drug design (steps 4 to 6). Information available on chikungunya virus, which is taken up as an illustrative case in this study is summarized in the subsection (d).
a. Genome Annotation.
The computational genome annotation can play a vital role in finding potential therapeutic target molecules for pathogens. In the present research scenario, it is a big challenge to carry out the structural and functional annotation of the whole genome sequence or the translated ORFs (open reading frames). These annotations can be used in comparative genomics, pathway reconstruction and particularly in drug design.
Genome annotation is the process of exploring biological/functional information from sequences (Table 1). It is done by following two main steps: (i) identification of distinct, potentially functional elements on the genome, a process called gene prediction in the context of identification of protein coding regions and (ii) assignment of biological function to these elements (genes or proteins).
Table 1.
Some Typical Features Considered During Genome Annotation
| Genome Annotation | |
|---|---|
| Structural annotation: identification of genomic elements | Functional annotation: assigning biological information to genomic elements |
|
|
Automated annotation tools provide a faster computational annotation as compared to manual annotation (curation) which involves human expertise. Ideally, these approaches coexist and complement each other in the same annotation pipeline. The basic level of annotation involves finding genes and isolating the protein coding sequences from non-coding sequences. A variety of computational approaches have been developed to permit scientists to view and share genome annotations (Table 2). Most of the available computational methods are knowledge-based and adopt techniques like Hidden Markov Models or machine learning methods. The accuracies of these models are limited by the availability of data on experimentally validated genes, and as typically seen in newly sequenced genomes, can lead to suboptimal levels of prediction. Ab initio methods originating in physico-chemical properties of DNA can help overcome the limitations of knowledge-based methods.
Table 2.
List of Tools Available for Gene Prediction
Generally for annotation purposes, homologous sequences in protein sequence databases are searched. The state of the art tool for such database searches is PSI-BLAST (Position Specific Iterated Basic Local Alignment Search Tool) [1, 2]. The performance of PSI-BLAST and other database search tools to identify homologs of a given query in a sequence database has been measured by others [3]. However these benchmarks do not suffice the requirements in genome annotation. Our efforts are aimed at eliminating the limitations of PSI-BLAST in correctly annotating protein coding sequences in genomes by using ab initio approach. Physico-chemical properties such as hydrogen bonding, stacking, solvation etc. show clear signatures of the functional destiny of DNA sequences [4-8], which has formed the basis of Chemgenome. In the present study, we have used Chemgenome, the SCFBio tool (http://www.scfbio-iitd.res.in/chemgenome/chemgenome3.jsp) to produce and interpret structural annotations for the viral genome of Chikungunya virus.
b. Protein tertiary structure prediction.
The genome annotation is followed by protein annotation at structural, functional and at genomic scale which is essential for routine work in biology and for any systematic approach to the modeling of biological systems. To bridge the expanding sequence-structure gap, many computational approaches are becoming available which assign structure to a novel protein from its amino acid sequence. A plethora of automated methods to predict protein structure have been developed based on a variety of approaches. These include (a) homology modeling, (b) fold recognition or threading, (c) ab initio or de novo methods. Homology modeling and fold recognition methods utilize the information derived from structures solved previously via x-ray and NMR methods. This method is effective, popular, reliable and fast for protein tertiary structure prediction when a close sequence homolog exists in the structural repositories. Several protein structure prediction tools are available in the public domain (Table 3). To make biological sense out of large volumes of sequence data, it is necessary to compare the protein sequences with those proteins that have been already characterized biochemically. To design drug molecules, structural annotation plays an important role. Structural genomics (SG) efforts facilitate such comparisons by determining the structures for a large number of protein sequences, but most SG targets have not been functionally characterized. It is already known that accurate functional details of a protein can neither be inferred from its sequence alone nor from sequence comparisons with other proteins whose structures and functions are known but only from its own native structure [9-11].
Table 3.
List of Tools Available for Protein Tertiary Structure Prediction
| Sl. No | Softwares | URLs | Description |
|---|---|---|---|
| 1. | CPHModels3.0 | http://www.cbs.dtu.dk/services/CPHmodels/ | Protein homology modeling server |
| 2. | SWISS-MODEL | http://swissmodel.expasy.org/SWISS-MODEL.html | A fully automated protein structure homology-modeling server |
| 3. | Modeller | http://salilab.org/modeller/ | Program for protein structure modeling by satisfaction of spatial restraints |
| 5. | 3D-JIGSAW | http://3djigsaw.com/ | Server to build three-dimensional models for proteins based on homologues of known structure |
| 6. | PSIPRED | http://bioinf.cs.ucl.ac.uk/psipred/ | A combination of methods such as sequence alignment with structure based scoring functions and neural network based jury system to calculate final score for the alignment |
| 7. | 3D-PSSM | http://www.sbg.bio.ic.ac.uk/~3dpssm/index2.html | Threading approach using 1D and 3D profiles coupled with secondary structure and solvation potential |
| 8. | ROBETTA | http://robetta.bakerlab.org | De novo Automated structure prediction analysis tool used to infer protein structural information from protein sequence data |
| 9. | PROTINFO | http://protinfo.compbio.washington.edu/ | De novo protein structure prediction web server utilizing simulated annealing for generation and different scoring functions for selection of final five conformers |
| 10. | SCRATCH | http://scratch.proteomics.ics.uci.edu/ | Protein structure and structural features prediction server which utilizes recursive neural networks, evolutionary information, fragment libraries and energy |
| 11. | I-TASSER | http://zhanglab.ccmb.med.umich.edu/I-TASSER/ | Predicts protein 3D structures based on threading approach |
| 12. | BHAGEERATH | http://www.scfbio-iitd.res.in/bhageerath/index.jsp | Energy based methodology for narrowing down the search space of small globular proteins |
| 13. | BHAGEERATH-H | http://www.scfbio-iitd.res.in/bhageerath/bhageerath_h.jsp | A Homology ab-initio Hybrid Web server for Protein Tertiary Structure Prediction |
Several efforts are being made to unravel the physico-chemical basis of protein structures and to establish some fundamental rules of protein folding. Despite the successes, protein tertiary structure prediction still remains a grand challenge - an unsolved problem in computational biochemistry [11, 12-26].
Ab initio or de novo methods are frequently employed for predicting tertiary structures of proteins by incorporating the basic physical principles, irrespective of the availability of structural homologs. In this study, Bhageerath and Bhageerath-H servers are employed for protein structure prediction. Bhageerath is an energy based software suite for predicting tertiary structures of small globular proteins, available at http://www.scfbio-iitd.res.in/bhageerath/index.jsp [12, 27]. It predicts five candidates for the native, from the input query sequence. Bhageerath-H [28] is a hybrid (homology + ab initio) server for protein tertiary structure prediction [29, 30]. It identifies regions which show local sequence similarity in respect to sequences in RCSB (protein data bank) to generate 3D fragments which are patched with ab initio modeled fragments to generate complete structures of the proteins. This server again predicts the best five energetically favorable structures, which are expected to be close to the native. The knowledge of tertiary structures of proteins serves as a basis for structure-based drug design.
c. Structure based drug design.
Design of small molecules in structure based drug discovery requires knowledge of the binding pocket on the protein which upon blockade results in loss of function. Experimental information on protein active sites and function loss are useful. In the absence of any experimental information, one could identify all potential binding sites on the protein from the structural information (Table 4). In this study we use, AADS (http://www.scfbio-iitd.res.in/dock/ActiveSite_new.jsp) methodology for an automated identification of ten potential binding pockets which are expected to bracket the true “active site” (binding pocket). AADS requires the 3D structure of the target protein and detects the top 10 potential binding sites with 100% accuracy in capturing the actual binding (active) site.
Table 4.
List of Software Available for Active Site Prediction
Once the binding pockets on proteins are identified, libraries of small molecules are screened against these sites to identify a few hit molecules using software such as RASPD (http://www.scfbio-iitd.res.in/software/drugdesign/raspd.jsp). RASPD protocol is designed in the spirit of structure-based drug design approach but with a rapid turnover rate. RASPD screens small molecule databases against the active sites based on physiochemical descriptors or in general the set of Lipinski parameters such as hydrogen bond donors, hydrogen bond acceptors, molar refractivity, Wiener index and volume for the protein and drug and also the functional groups [31-33]. The most interesting feature of RASPD is that it generates a set of hit molecules based on the complementarities of the afore-mentioned properties with a certain cutoff binding affinity bypassing the conventional docking and scoring strategies, which reduces the search time significantly. The libraries incorporated in RASPD are a million compound library of small molecules and a natural product library. The users can also sketch molecules of their choice or use a non-redundant dataset of small molecules NRDBSM [34] (http://www.scfbio-iitd.res.in/software/nrdbsm/index.jsp) and submit them for RASPD screening.
The screening is followed by atomic level docking and scoring strategies (Table 5) such as Sanjeevini (http://www.scfbio-iitd.res.in/sanjeevini/sanjeevini.jsp) to identify a few candidates which could be pursued as leads for experimental synthesis and validation [35, 36]. ParDOCK module of Sanjeevini is an all-atom energy based Monte Carlo algorithm for protein-ligand docking. It involves the positioning of ligands optimally with best configuration in the protein binding site and scores them based on their interaction energies. This utility is freely accessible at http://www.scfbio-iitd.res.in/dock/pardock.jsp [37]. ParDOCK uses BAPPL scoring function [38] for atomic level scoring of non-metallo protein ligand complexes and in ranking them accurately with their estimated free energies. BAPPL is again freely accessible at http://www.scfbio-iitd.res.in/software/drugdesign/bappl.jsp. The accuracy of this scoring function in predicting binding free energy is high with ±1.02 kcal/mol average error and a correlation coefficient of 0.92 between the predicted and experimental binding energies for 161 protein-ligand complexes. An extended version of BAPPL, i.e. BAPPL-Z can be used for the prediction of binding energies of the complexes having zinc metal ion in their active sites. BAPPL-Z utility is accessible at http://www.scfbio-iitd.res.in/software/drugdesign/bapplz.jsp [39]. All these tools are collectively gathered in Sanjeevini software, which is a complete drug design software suite, freely accessible at (http://www.scfbio-iitd.res.in/sanjeevini/sanjeevini.jsp) [34, 40-47]. Thus, the assessment of candidate molecules is done based on their binding energies and the molecules identified as good binders to the target are considered further for synthesis and testing.
Table 5.
A list of Softwares for Drug Design
| Sl. No. | Softwares | URL | Description |
|---|---|---|---|
| 1 | Discovery studio | http://accelrys.com/products/discovery-studio/structure-based-design.html | Molecular modeling and de novo drug design |
| 2 | Sybyl | http://www.tripos.com/ | Computational software for drug discovery |
| 3 | Bio-Suite | http://www.staff.ncl.ac.uk/p.dean/Biosuite/body_biosuite.html | Tool for Drug Design, structural analysis and simulations |
| 4 | Molecular Operating Environment (MOE) | http://www.chemcomp.com/ | Structure-based drug design, molecular modeling and simulations |
| 5 | Glide | https://www.schrodinger.com/products/14/5 | Ligand-receptor docking |
| 6 | Autodock | http://autodock.scripps.edu/ | Protein-ligand docking |
| 7 | DOCK | http://dock.compbio.ucsf.edu/ | Protein-ligand docking |
| 8 | Sanjeevini | http://www.scfbio-iitd.res.in/sanjeevini/sanjeevini.jsp | A complete software suite for structure-based drug design |
| 9 | ArgusLab | http://www.arguslab.com/arguslab.com/ArgusLab.html | Ligand-receptor docking |
| 10 | eHITS | http://www.simbiosys.ca/ehits/index.html | Ligand-receptor docking |
| 11 | FlexX | http://www.biosolveit.de/FlexX/ | Ligand-receptor docking |
| 12 | FLIPDock | http://flipdock.scripps.edu/ | Ligand-receptor docking |
| 13 | FRED | http://www.eyesopen.com/oedocking | Ligand-receptor docking |
| 14 | GOLD | http://www.ccdc.cam.ac.uk/products/life_sciences/gold/ | Protein-ligand docking |
| 15 | ICM-Docking | http://www.molsoft.com/docking.html | Protein-ligand docking |
| 16 | PLANTS | http://www.tcd.uni-konstanz.de/research/plants.php | Protein-ligand docking |
| 17 | Surflex | http://www.biopharmics.com/ | Protein-ligand docking |
d. Chikungunya Virus.
Chikungunya fever (CHIK) is a mosquito (Aedes aegypti) borne devastating disease caused by Chikungunya virus (CHIKV), an alphavirus belonging to the family Togaviridae. It is one of the most important re-emerging infectious diseases in Africa and Asia with sporadic intervals and is responsible for significant global impact on public health problems [48-62]. CHIKV is listed as a category C pathogen in 2008 by National Institute of Allergy and Infectious Diseases (NIAID) and as a biosafety level 3 (BSL3) pathogen [50, 63-66]. CHIKV causes debilitating and prolonged arthralgic syndrome incapacitating the affected population for longer periods. CHIKV is usually found in tropics but has widespread across the globe in recent years due to a range of transmission vectors, globalization and climatic changes [67-111]. The ‘Chikungunya’ word has originated from the Makonde root verb kungunyala, meaning “that which bends up” [112, 113] which is in reference to drying up or becoming contorted and signifies the cause of stooped posture developed due to the excruciating joint and muscle pain and other rheumatologic manifestations [114, 115]. The disease etiology consists of sudden onset of fever with arthalgia, which generally resolves within a few days [116, 117].
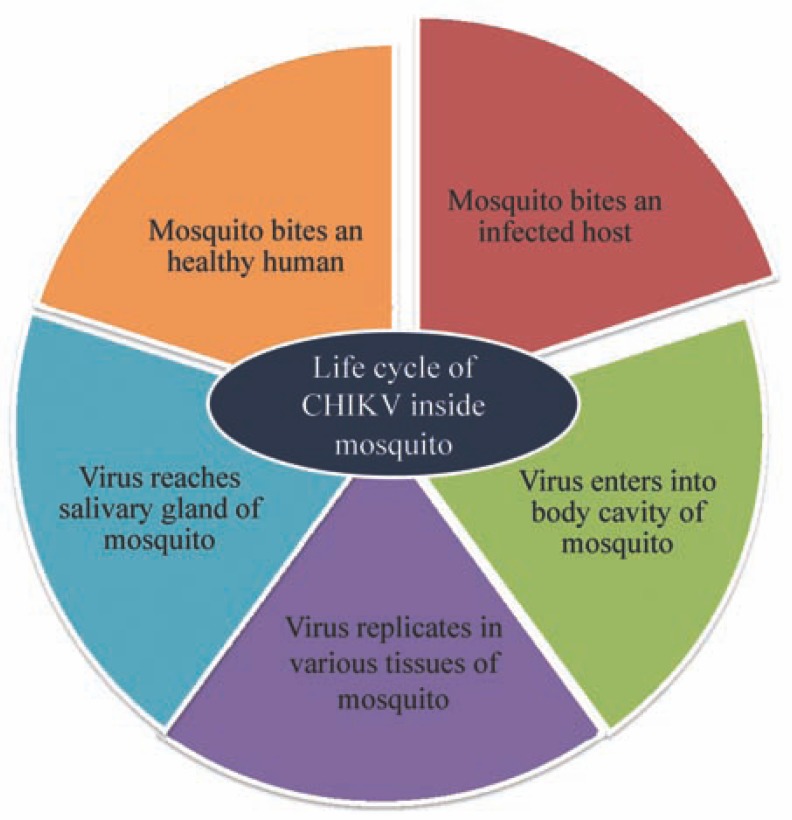
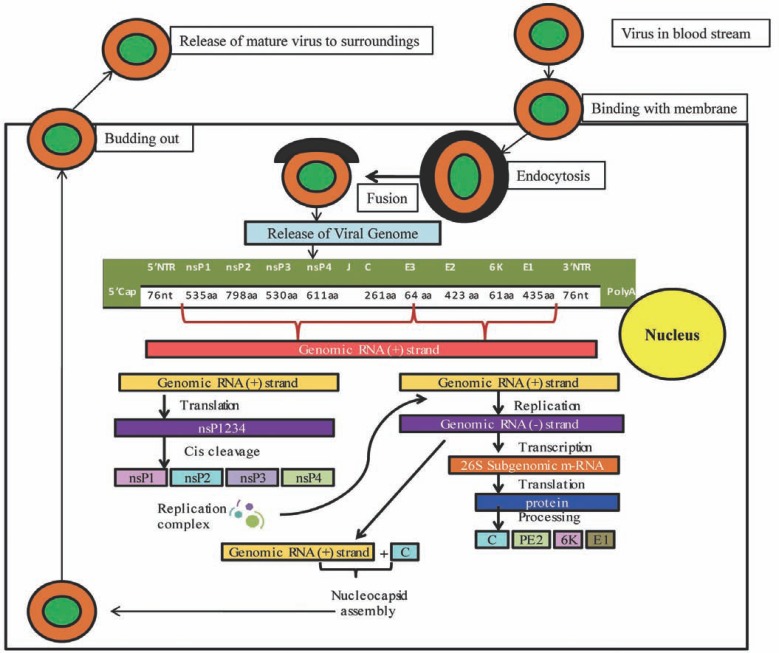
Female mosquitoes acquire the virus by taking blood from viremic vertebrate hosts (Fig. 2). The virus elicits a persistent infection and replicates at a high pace, especially in the salivary glands of the insects [118, 119]. In addition to salivary glands, it replicates in various other organs inside body cavity including gut, ovary, neural tissue, body fat etc. [120]. When this CHIKV loaded mosquito infects a healthy human, it transfers the virus into its blood stream. These virions through interaction with the receptors reach the target cells by endocytosis. The acidic environment of the endosome triggers conformational changes in the viral envelope that expose the E1 peptide [121-125], which mediates virus-host cell membrane fusion. This allows cytoplasmic delivery of the core and release of the viral genome in cytoplasm. The site of mRNA transcription is in the cell cytoplasm.
Fig. (2).
Flow diagram depicting the movement of CHIKV from veremic host to mosquito and from mosquito to healthy host.
CHIKV is an enveloped, spherical bodied virus of about 70nm in diameter. The virion genome consists of a linear single-stranded (ss), positive-sense RNA molecule of approximately 11.8 kb length, where the 5’ end is capped with a 7-methylguanosine while the 3’ end is polyadenylated. The CHIKV genome is comprised of 30% A, 25% C, 25% G and 20% T (U) base pairs with two long open reading frames (ORF) that encode the non-structural (2474 amino acids) and structural polyproteins (1244 amino acids) [126-131].
The genomic organization of CHIKV is considered to be 5’cap-nsP1-nsP2-nsP3-nsP4-(junction)-C-E3-E2-6K-E1-Poly (A)3’ (Fig. 3). The non-structural polyproteins (nsP1-4) located in an ORF of 7425 nucleotides get initiated by a start codon at position 77-79 and terminated by a stop codon at position 7499-7501. This polyprotein is autocatalytically cleaved to produce nonstructural proteins nsP1, nsP2, nsP3 and nsP4. In contrast, the structural polyproteins are located on an ORF of 3735 nucleotides with a start codon at position 7567-7569 and a stop codon at position 11299-11313. Likewise, this polyprotein is cleaved to produce the structural proteins namely the capsid protein (C), the glycoproteins E1, E2 and E3 and 6K [126, 132-136]. The polypeptides are cleaved into active proteins by viral and cellular proteases [137-148]. The functional properties of the active cleaved proteins are summarized in (Table 6).
Fig. (3).
The lifecycle of CHIKV in the host cell.
Table 6.
Functional Properties of Structural and Non-structural Proteins Found in Chikungunya
| Protein Type | Proteins | Functions |
|---|---|---|
| NonStructural Proteins NP_690588.1 | nsP1 |
|
| nsP2 |
|
|
| nsP3 |
|
|
| nsP4 |
|
|
| Structural proteins NP_690589.2 | C |
|
| E3 |
|
|
| E2 |
|
|
| 6K |
|
|
| E1 |
|
Although no specific drugs are available, CHIK is usually treated with non-steroidal anti-inflammatory drugs (NSAIDs), with inconsistent success [149-172] (Table 7). Owing to the non-availability of a potential drug to cure the disease, there is an urgent need to adopt a skilled strategy to develop new therapeutics. We describe in the following section how computational approaches can help in reducing the time in arriving at potential lead molecules.
Table 7.
A list of Drugs Available for Treating Chikungunya Fever
| Drug | Category | Description |
|---|---|---|
| Chloroquine | Antirheumatic Agents / Antimalarials / Amebicides | It is believed to inhibit the heme polymerase activity |
| Aspirin | Anticoagulants / cyclooxygenase(COX) Inhibitors / PlateletAggregation Inhibitors | Irreversibly inhibits the activity of both types of cyclooxygenase (COX-1 and COX-2) |
| Ibuprofen | Anti-inflammatory Agents / COX Inhibitors / Analgesics / Nonsteroidal Anti-inflammatory Agents (NSAIAs) | A non-selective inhibitor of cyclooxygenase, an enzyme invovled in prostaglandin synthesis via the arachidonic acid pathway |
| Naproxen | COX Inhibitors / Gout Suppressants | It is believed to be associated with the inhibition of cyclooxygenase activity |
| Ribavirin | Antiviral Agents / Antimetabolites | A potent competitive inhibitor of inosine monophosphate (IMP) dehydrogenase, viral RNA polymerase and messenger RNA (mRNA) guanylyl trasferase (viral); may get incorporated into RNA in RNA viral species. |
| Prednisolone | Hormonal Glucocorticoids | The antiinflammatory actions of glucocorticoids are thought to involve phospholipase A2 inhibitory proteins, lipocortins |
| Acetaminophen | Analgesics, Non-Narcotic / Antipyretics | Inhibits various forms of cyclooxygenase, COX-1, COX-2, and COX-3 enzymes |
3. CALCULATIONS & RESULTS: APPLICATION OF THE G2H ASSEMBLY LINE TO CHIKV
The genome sequence of Chikungunya virus was retrieved from NCBI (http://www.ncbi.nlm.nih.gov/nuccore/NC_004162). For gene prediction, the sequence was processed using ChemGenome 3.0 (http://www.scfbio-iitd.res.in/chemgenome/chemgenome3.jsp) software [5, 6]. The results displayed the existence of two genes which were similar to the already published ones, essentially implying that in this case, 100% accuracy is achieved with ChemGenome 3.0. These nucleotide sequences were translated to protein sequences by ChemGenome 3.0. The proteins in CHIKV are polyproteins i.e. the sequence displayed in results contains sequences for all proteins coded by the gene. The individual proteins from polyprotein are cleaved during post translational processing. Till date no reliable computational approach is available to cleave the polyproteins, therefore the sequences were dissected manually for each protein, based on literature and experimental evidence to identify cleavage site. The ChemGenome 3.0 results are archived at http://www.scfbio-iitd.res.in/software/chemgenomeresult.jsp.
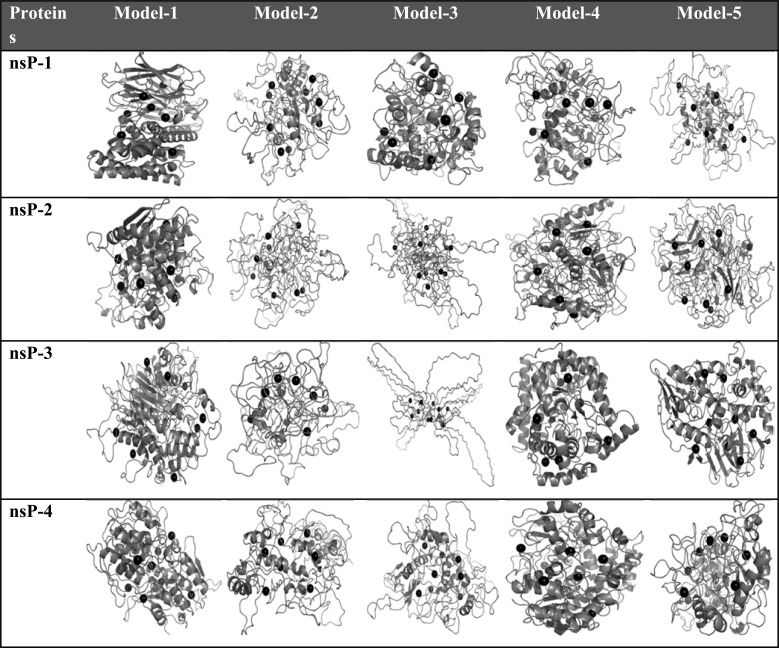
The sequences extracted from Chemgenome 3.0 served as inputs to Bhageerath-H (http://www.scfbio-iitd.res.in/bhageerath/bhageerath_h.jsp), a tertiary structure prediction server [28]. For each submitted sequence, five structures were returned by the server. The results received from Bhageerath-H are shown in (Fig. 4). As no homolog information is available to give strength to these structural models, all the five structures are considered as plausible candidates for the native, and considered for further studies. It may be noted that tertiary structure prediction of structural proteins associated with membranes is a nascent area with low success rate at this stage and hence the focus here has been on nonstructural proteins which can fold autonomously.
Fig. (4).
An illustration of the protein structures of CHIKV predicted by Bhageerath-H shown along with their binding pockets.
Most of the experimentally determined structures have some information of ligand binding domain/site but in the present scenario, CHIKV proteins lack the structural information, thus necessitating detection of ligand binding sites (active sites). In order to facilitate active site detection, an automated version of active site finder i.e. AADS (Automated active site docking and scoring) (http://www.scfbio-iitd.res.in/dock/ActiveSite_new.jsp) is utilized which predicts the potential binding site(s) and further performs the docking of the selected molecule to the top ten cavities in an automated mode [40]. Binding sites on each of the five structural models of each nonstructural protein are identified. Not all cavities determined by the active site identifier may be true binding sites with functional implication but one among them is very likely to be such a site. The additional cavities can be checked for their ability to act as allosteric sites. The predicted top 10 binding sites are shown as black dots in the protein structures (Fig. 4).
In search of probable hits, the 10 cavities per structure identified by AADS are further subjected to RASPD (Rapid screening of preliminary drugs) (http://www.scfbio-iitd.res.in/software/drugdesign/raspd.jsp) software [41]. The RASPD returned more than 500 molecules against the predicted cavities of CHIKV proteins with -8.00 kcal/mol as the binding energy cutoff.
The in silico drug design beyond this stage involves rigorous docking and scoring [173, 174]. The hits identified from screening via RASPD above are further docked with their respective target site using Sanjeevini software (http://www.scfbio-iitd.res.in/sanjeevini/sanjeevini.jsp) which utilizes ParDOCK as a docking tool. For all the modeled structures, one molecule for each cavity has been proposed on SCFBio’s CHIKV webpage which is accessible at http://www.scfbio-iitd.res.in/software/chikv.jsp. This webpage contains information on the genome annotation, protein tertiary structure prediction, and hit molecule identification and docking and scoring results of the complete genome to hit protocol.
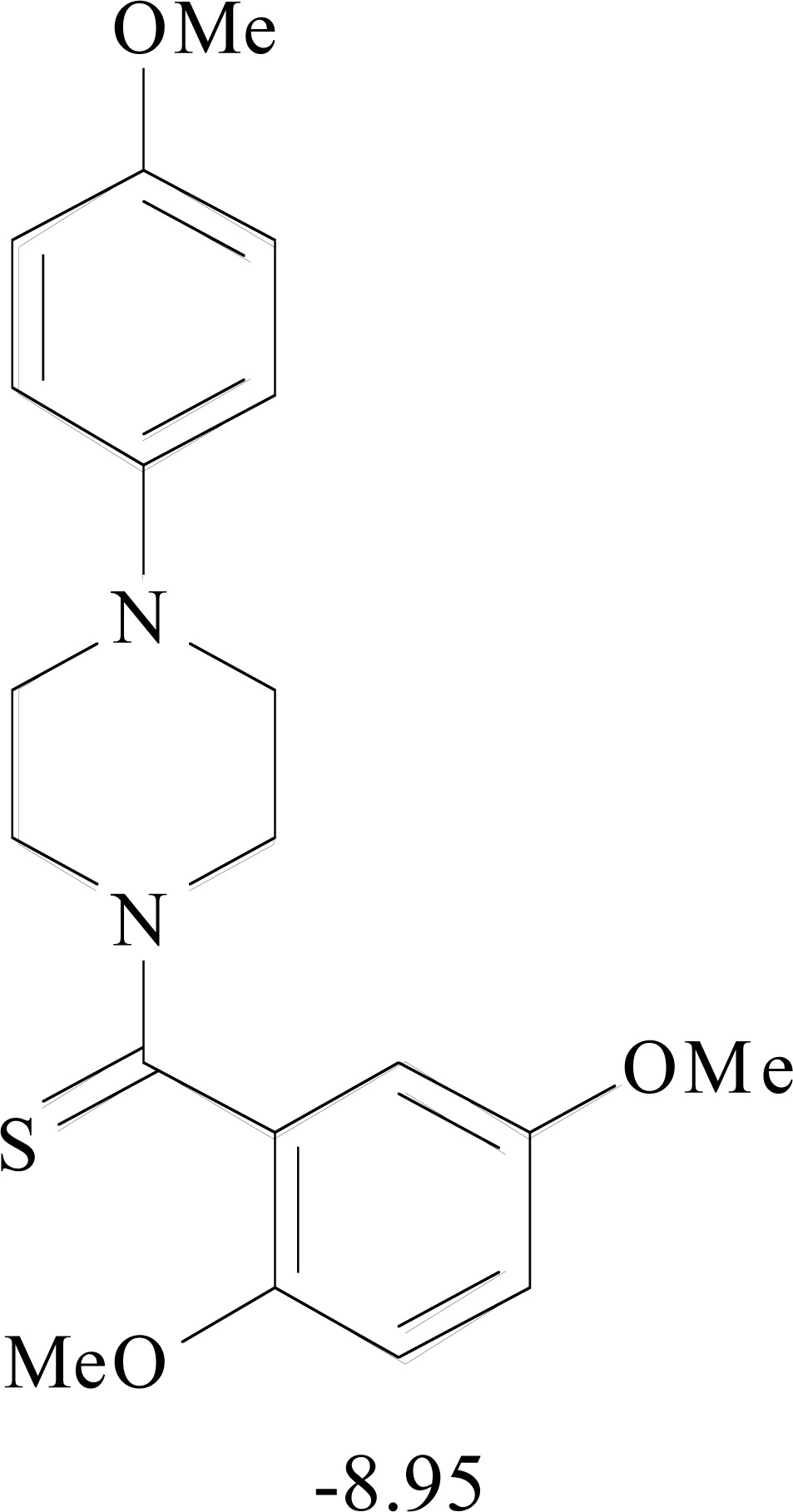
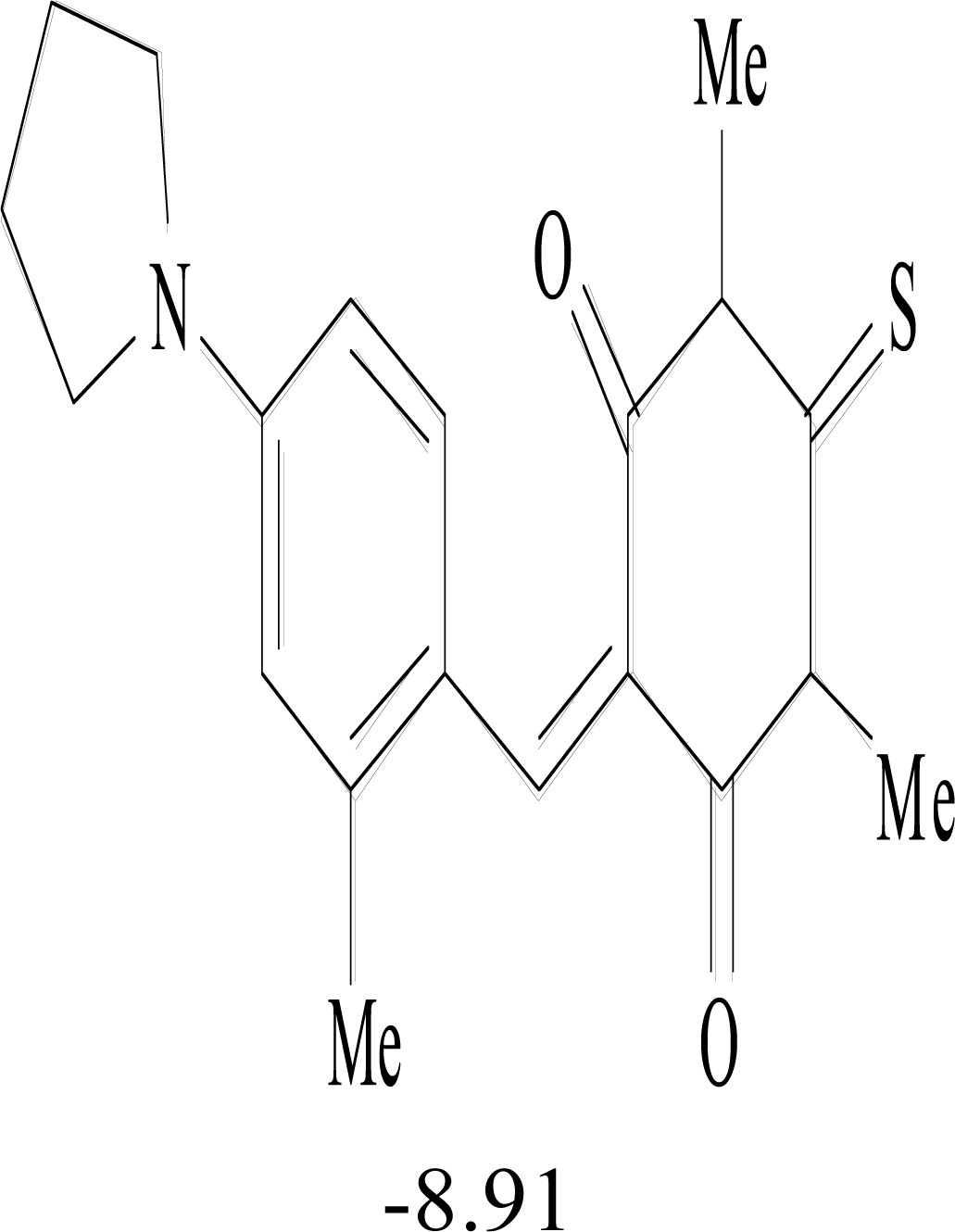
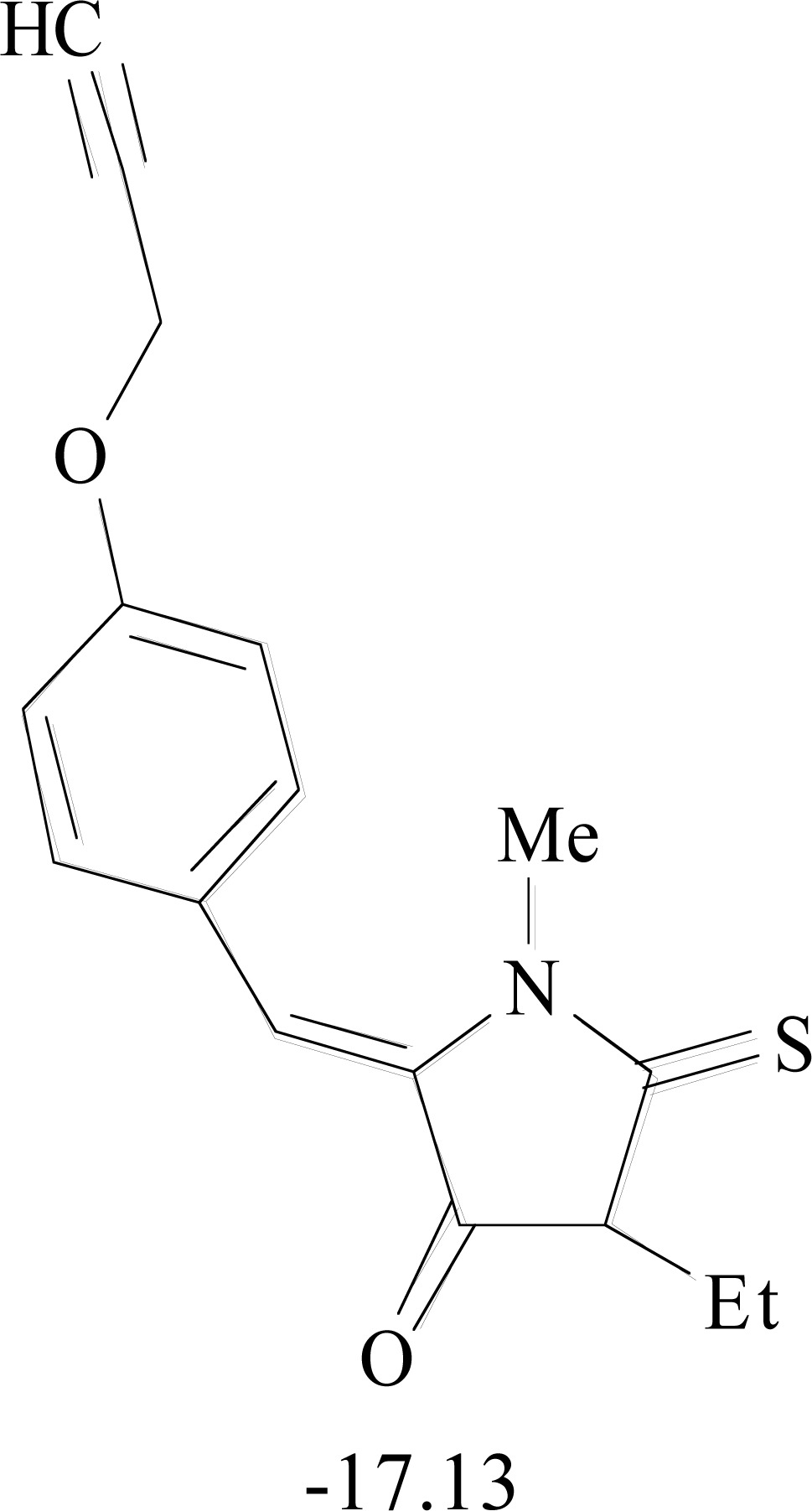
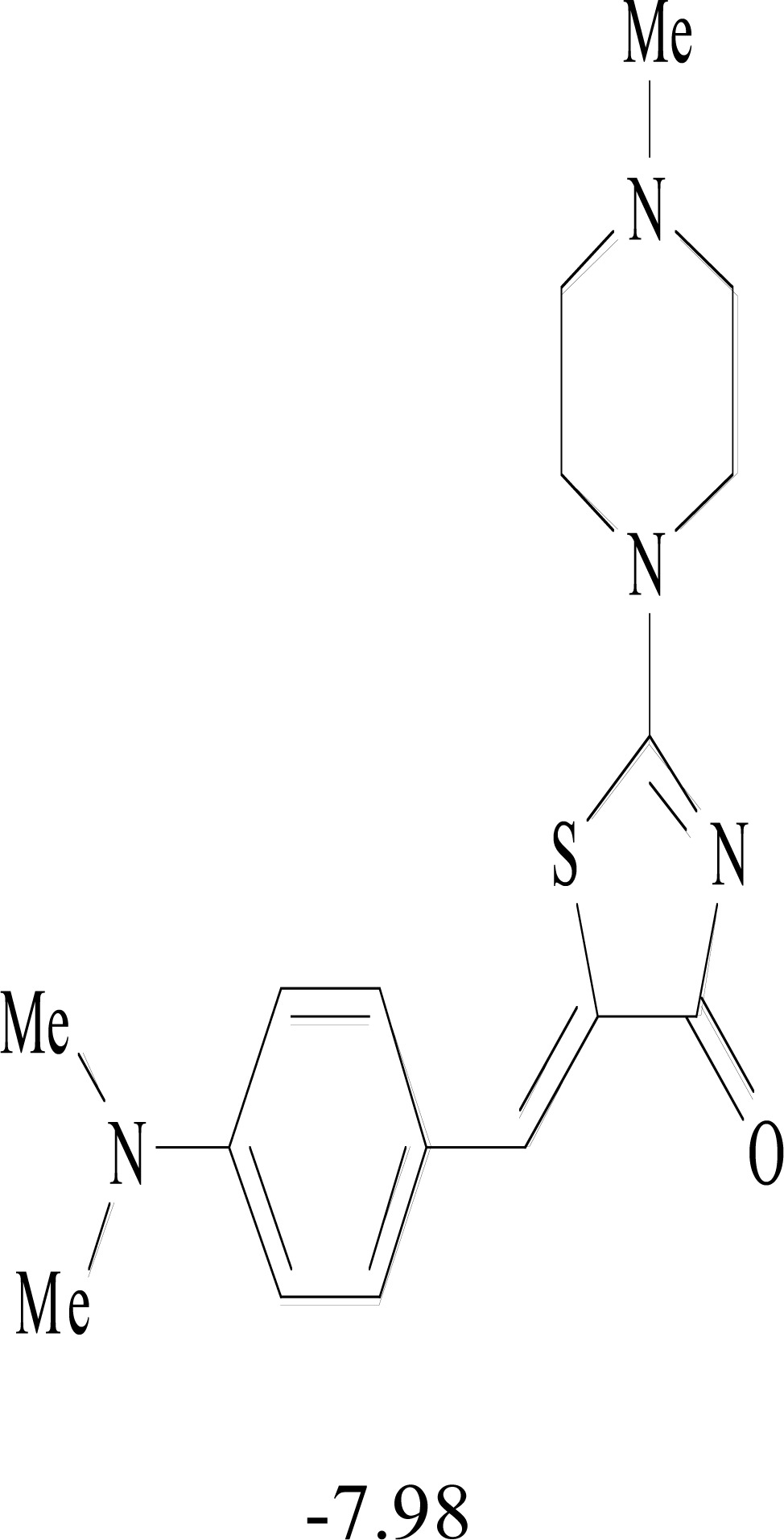
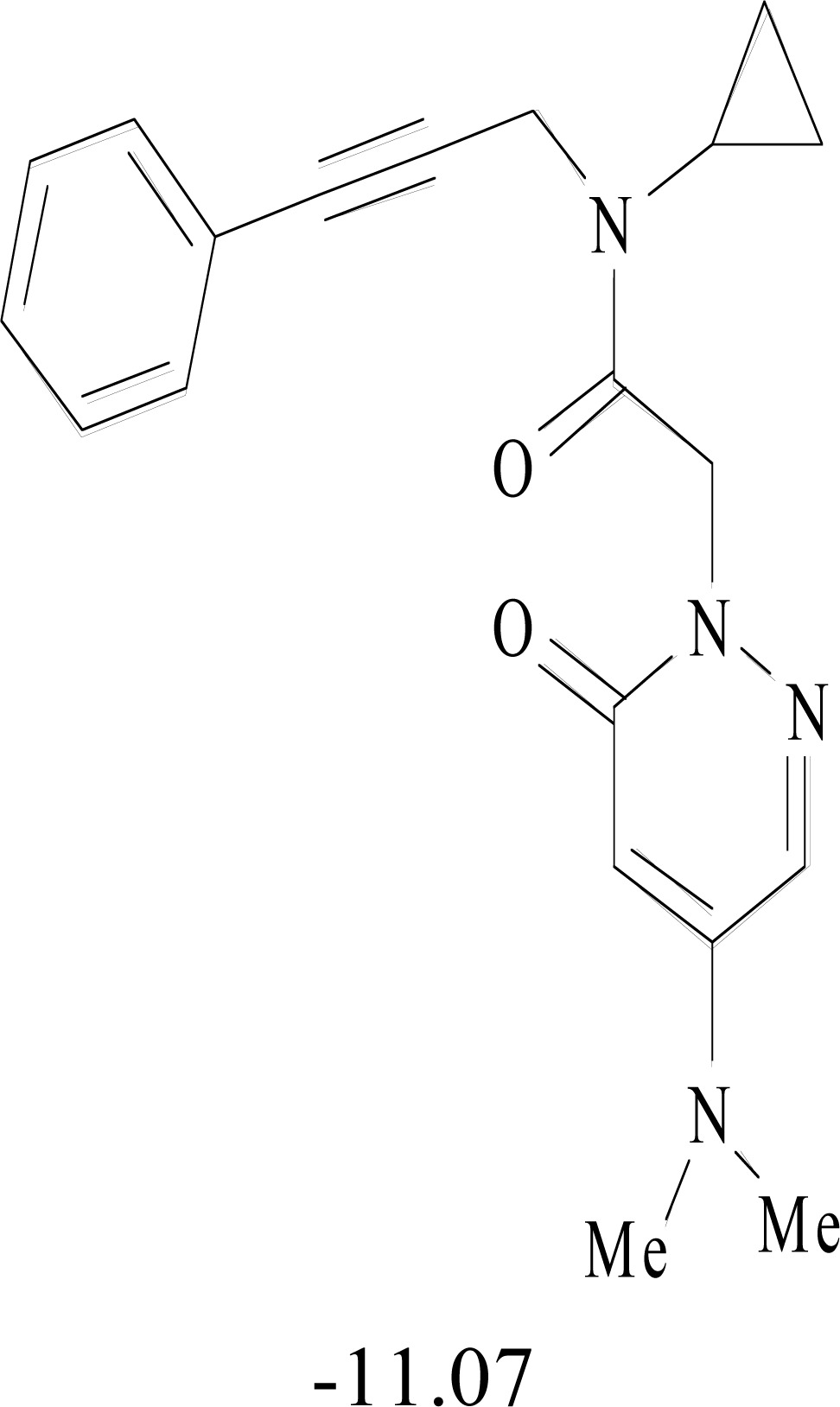
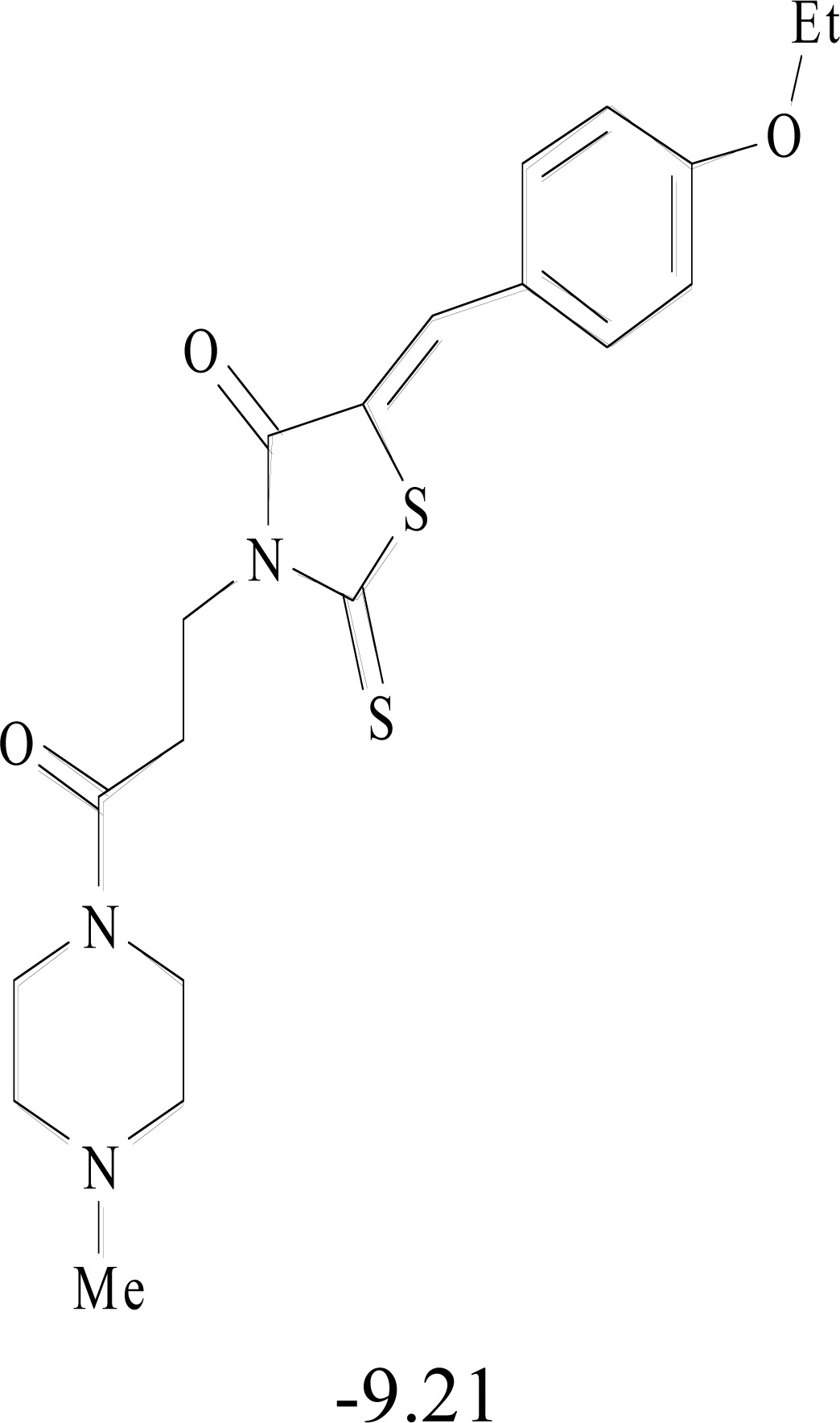
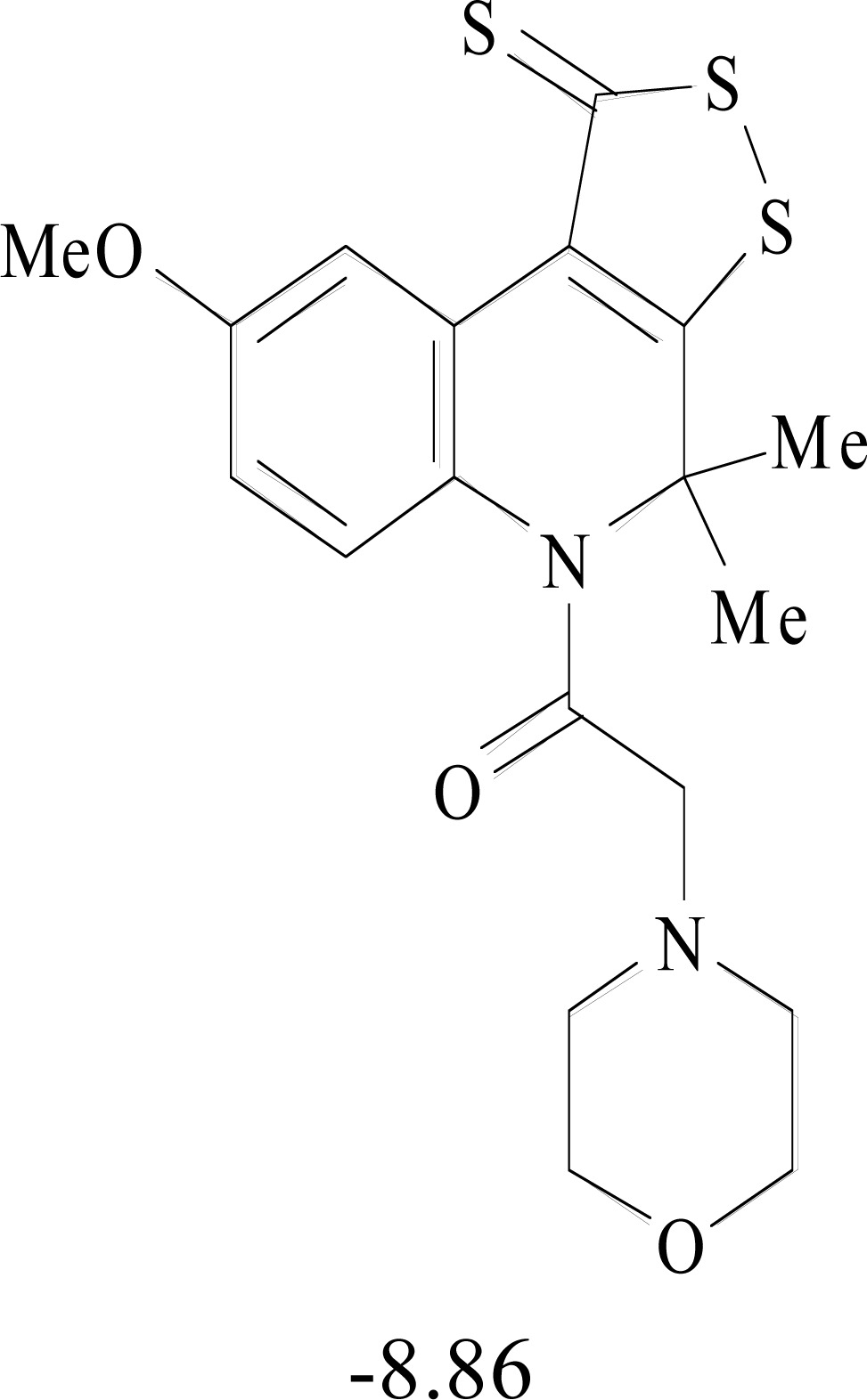
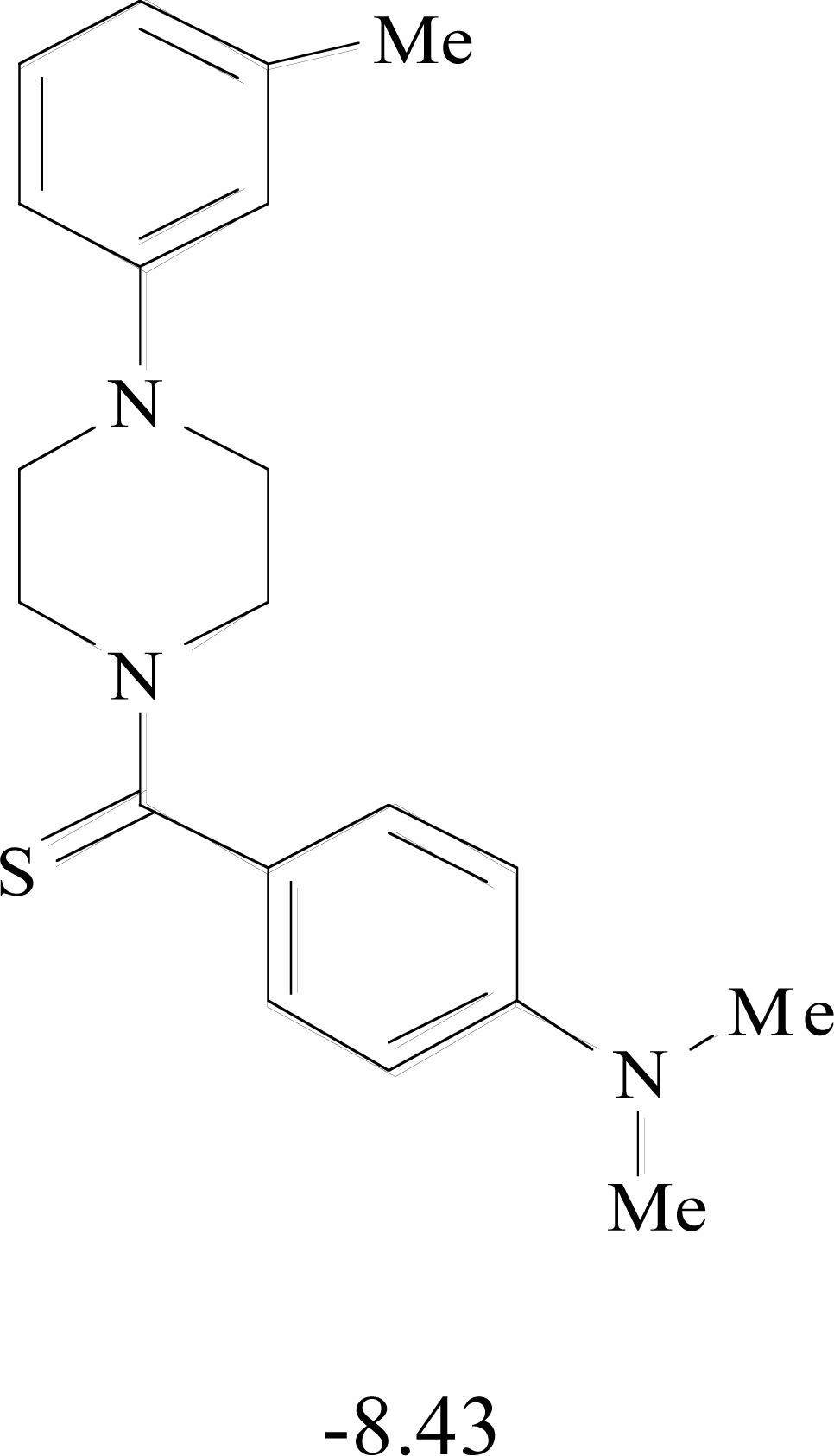
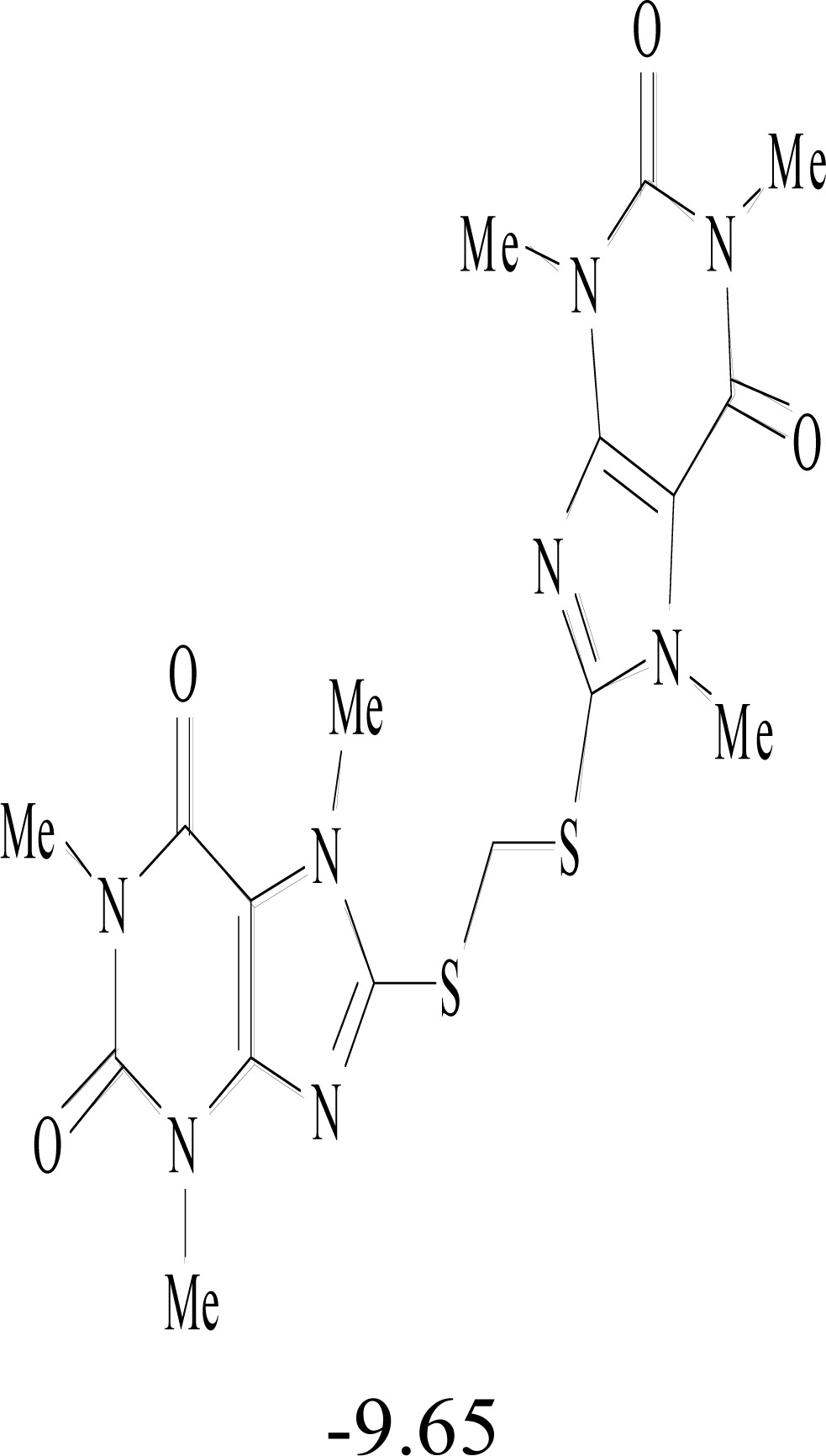
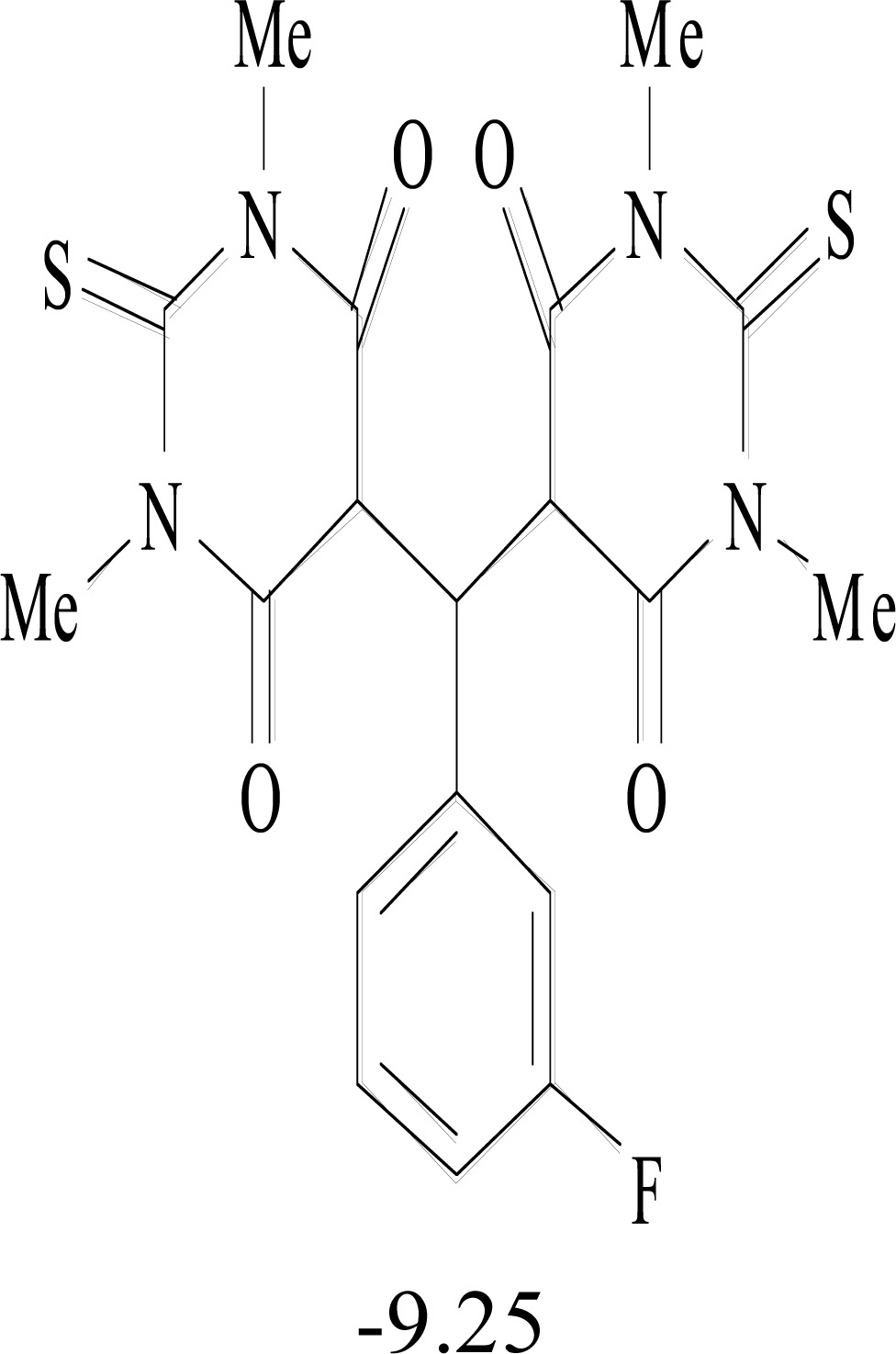
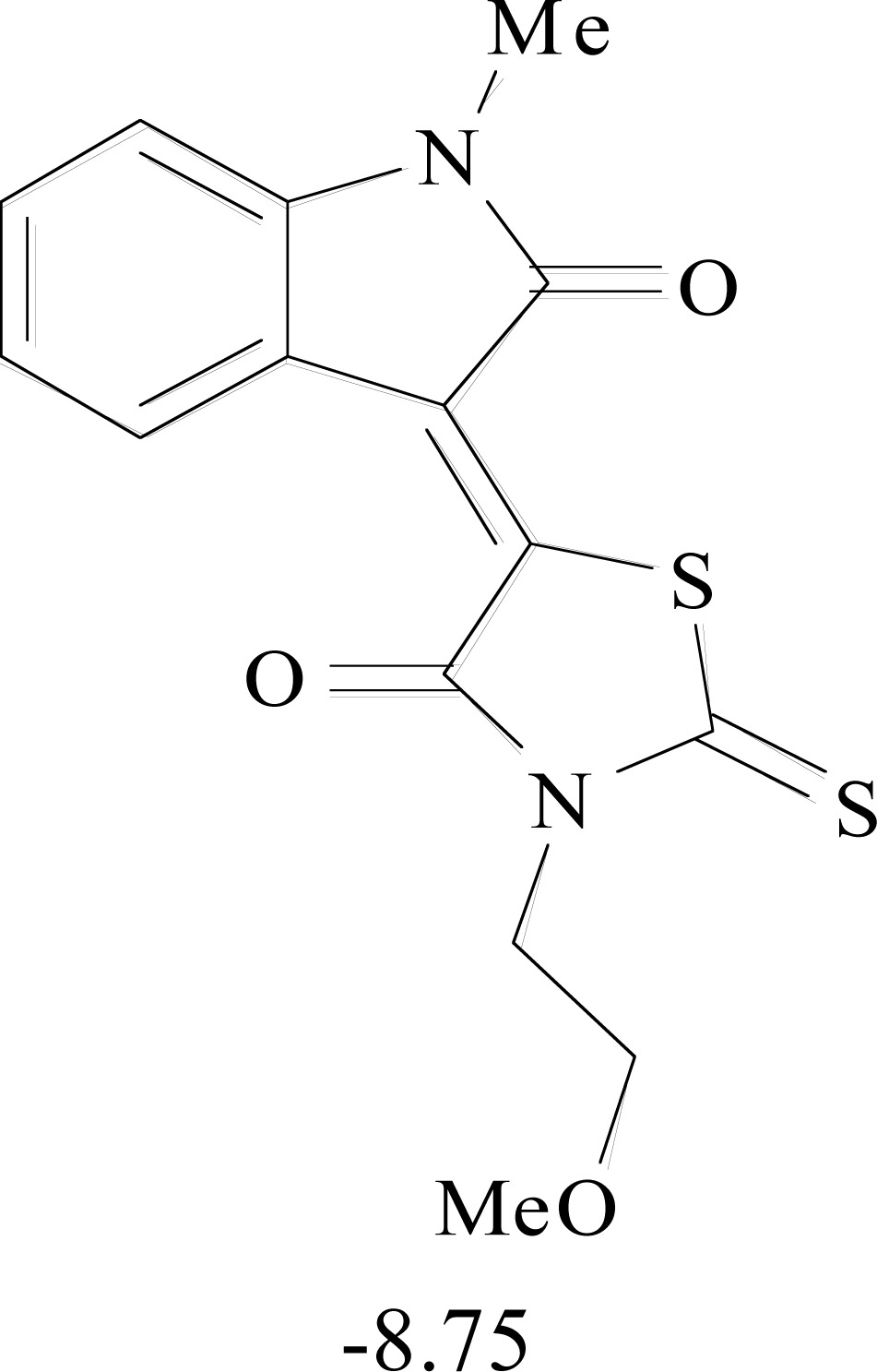
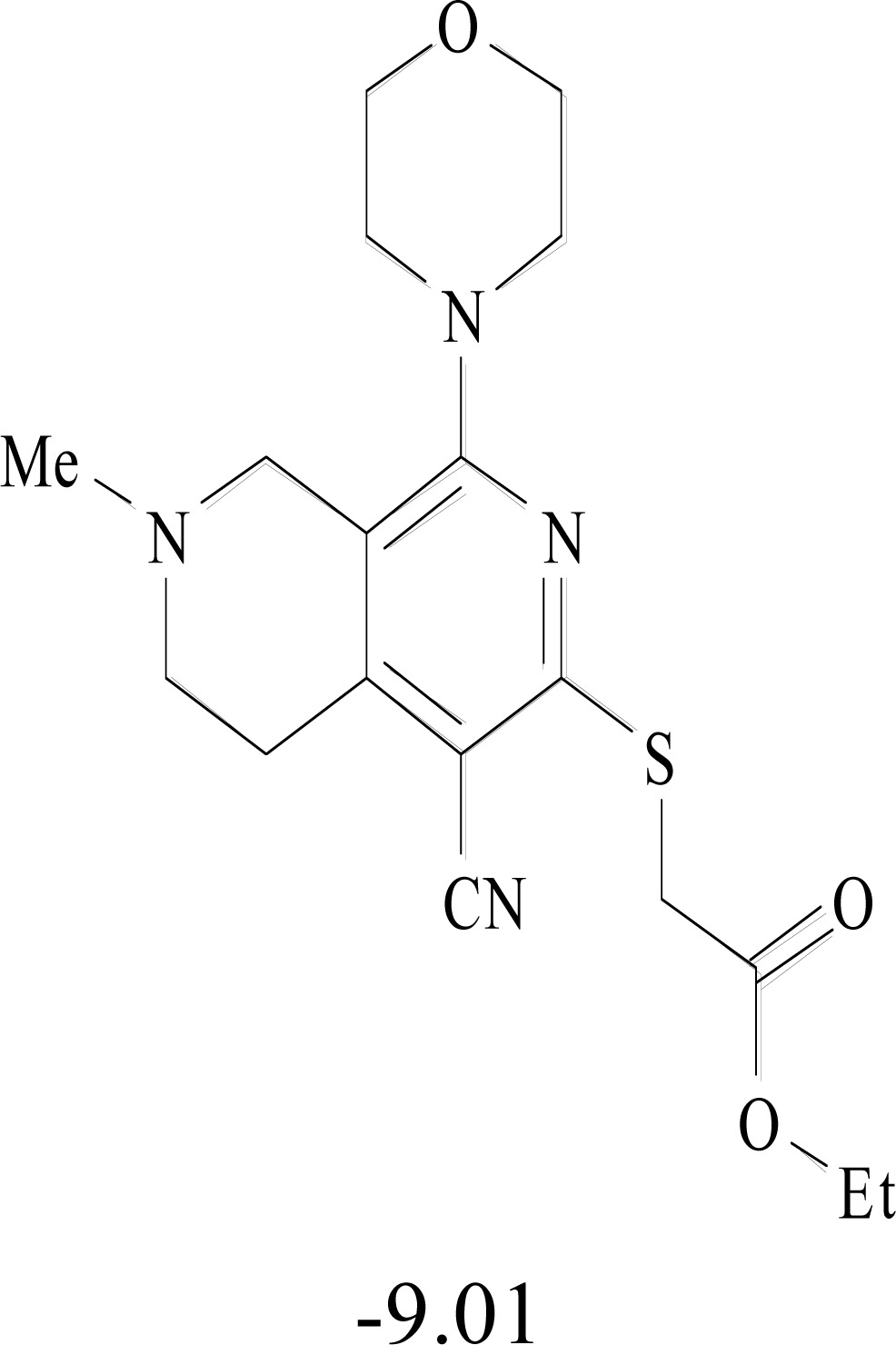
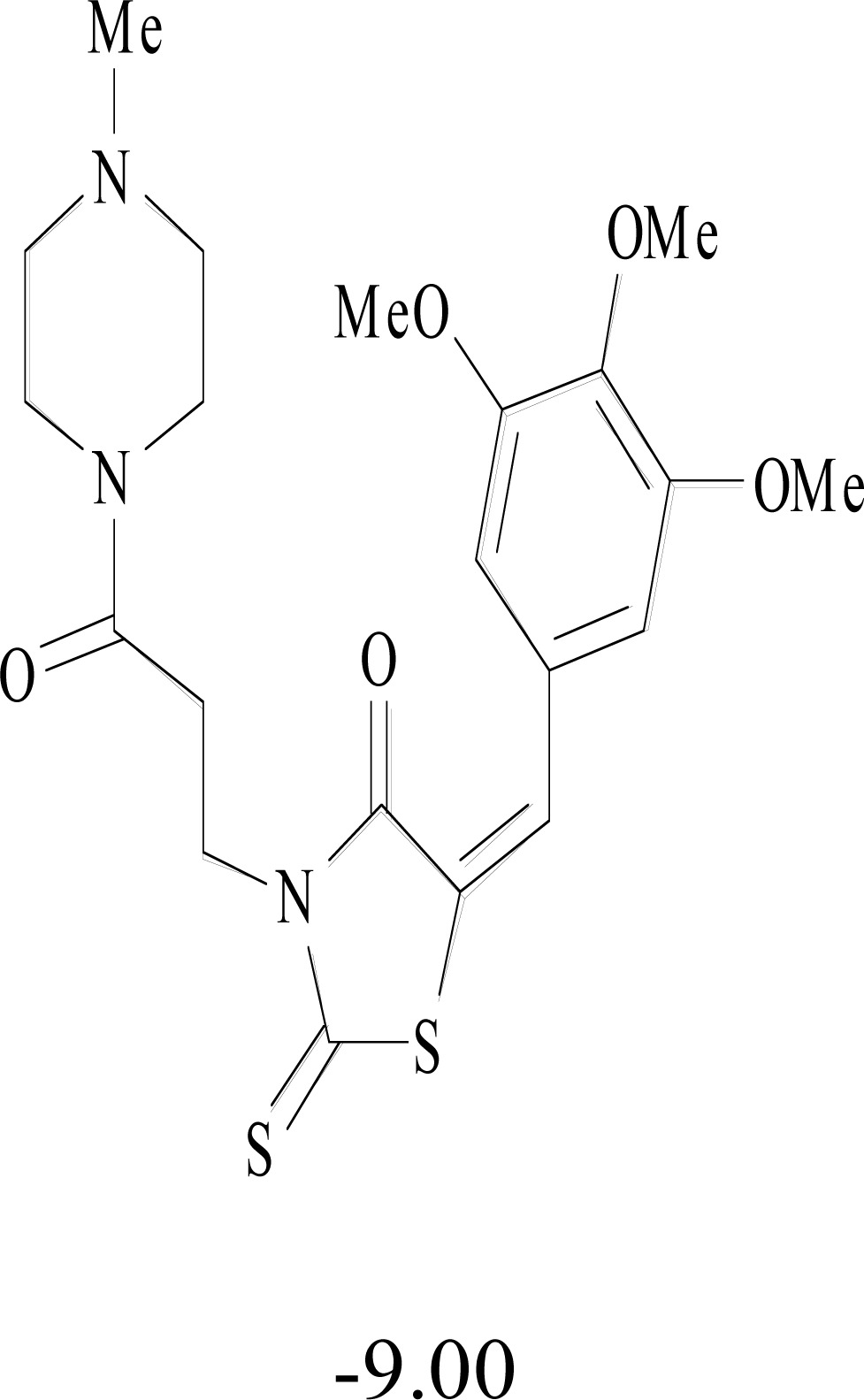
The best 20 molecules selected against the nonstructural proteins of CHIKV are displayed in (Table 8). From here on, the in silico strategies go hand-in-hand with experimentation. In an iterative process of synthesis, testing, modification, docking and scoring, these molecules can be further improved to yield candidate drugs while taking care of the ADMET profiles [175-180].
Table 8.
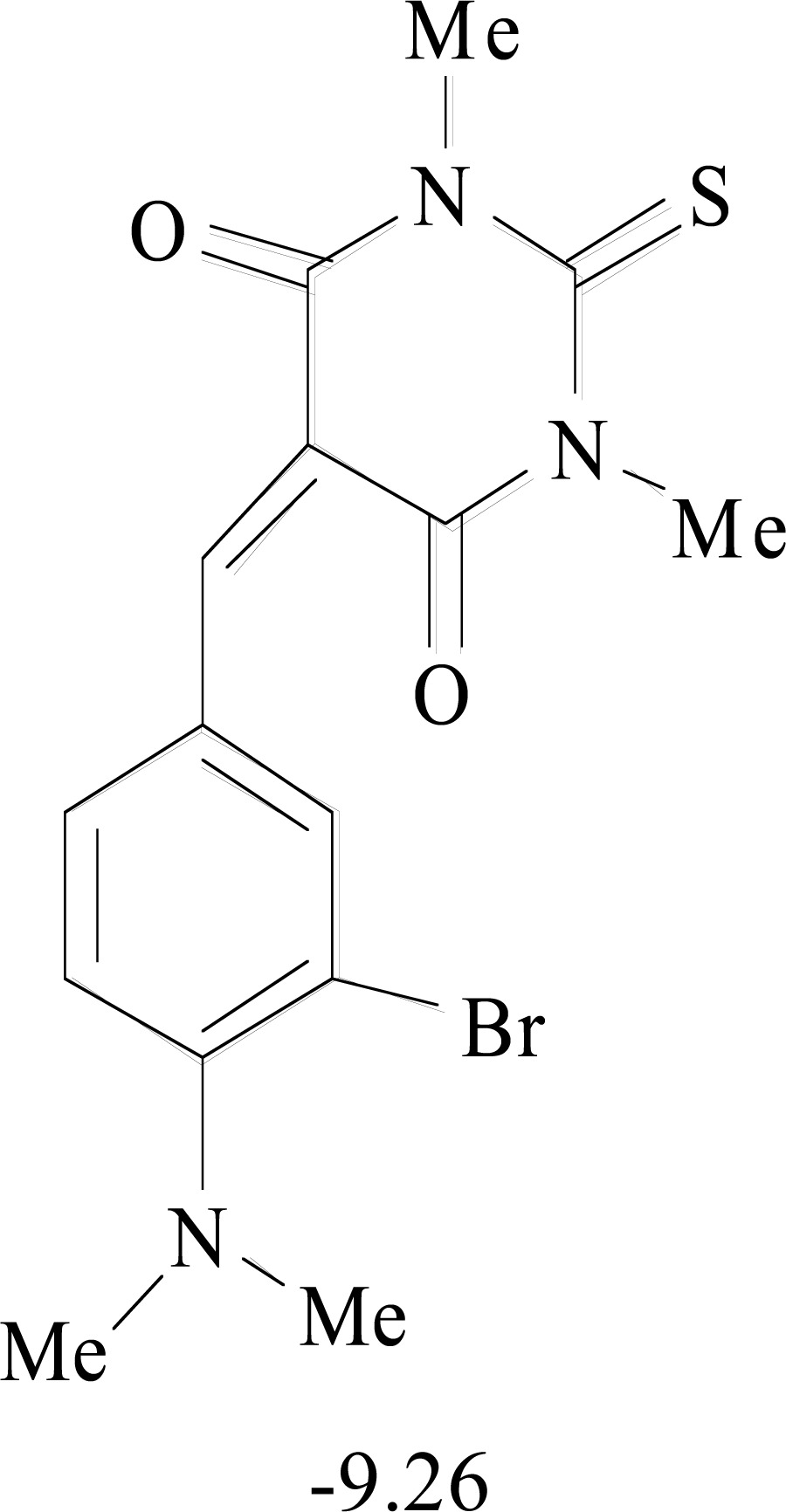
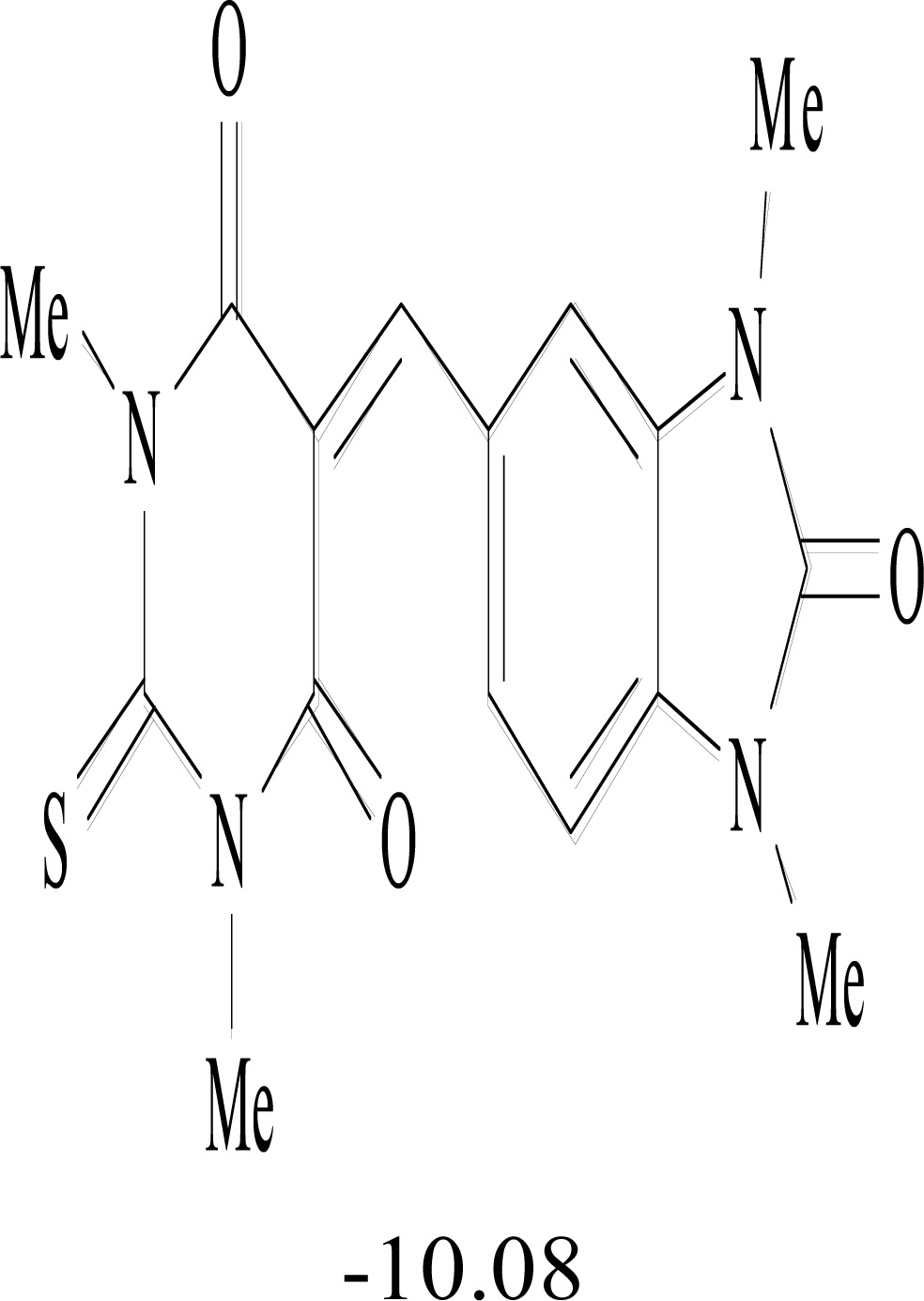
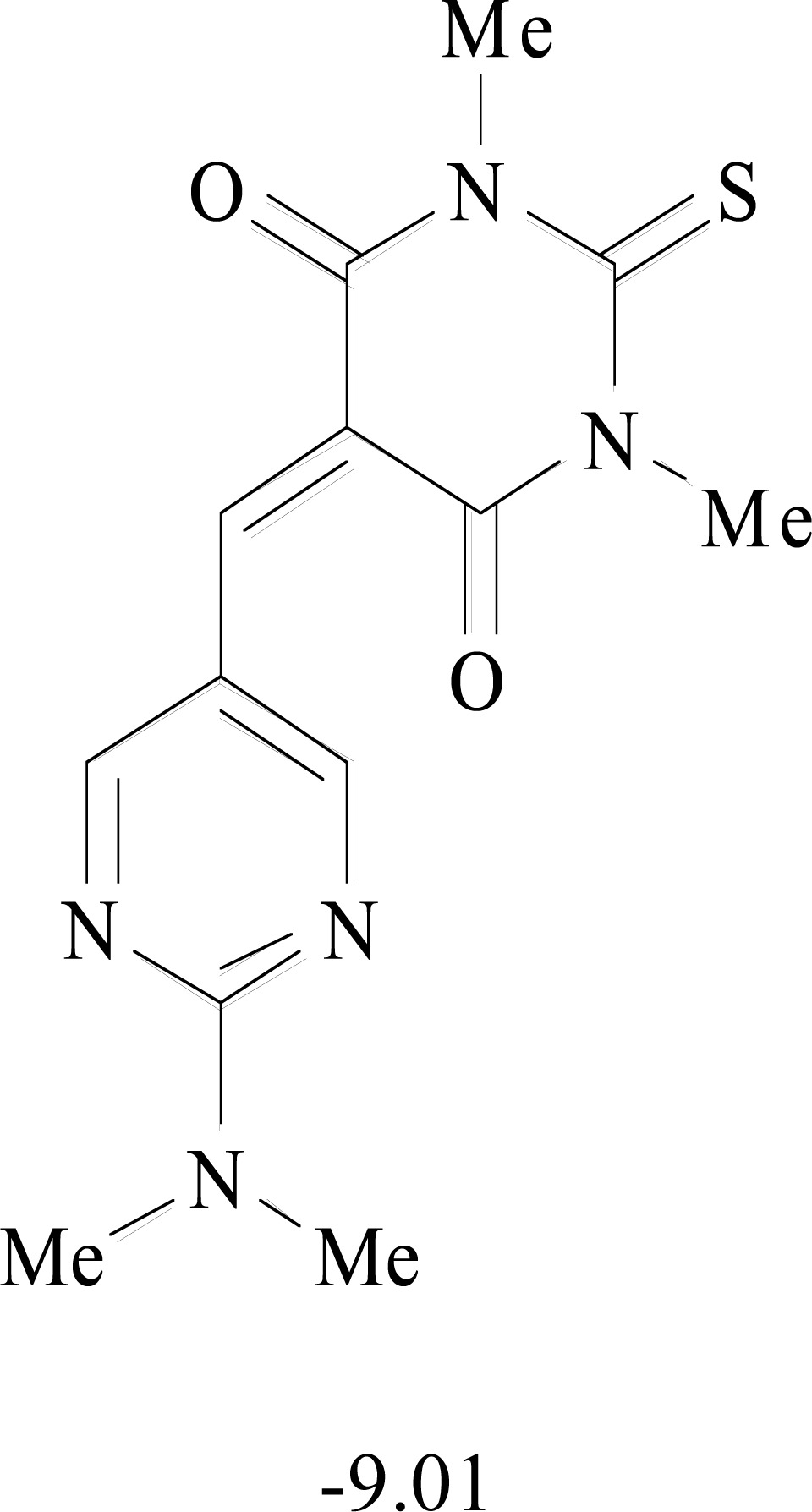
Structural Representations of 20 Molecules Showing high Affinity to the Nonstructural Proteins of CHIKV. (Computed Binding Energies are also Shown in kcal/mol Underneath Each Molecule)
| Protein | Model-1 | Model-2 | Model-3 | Model-4 | Model-5 |
|---|---|---|---|---|---|
| nsP1 | 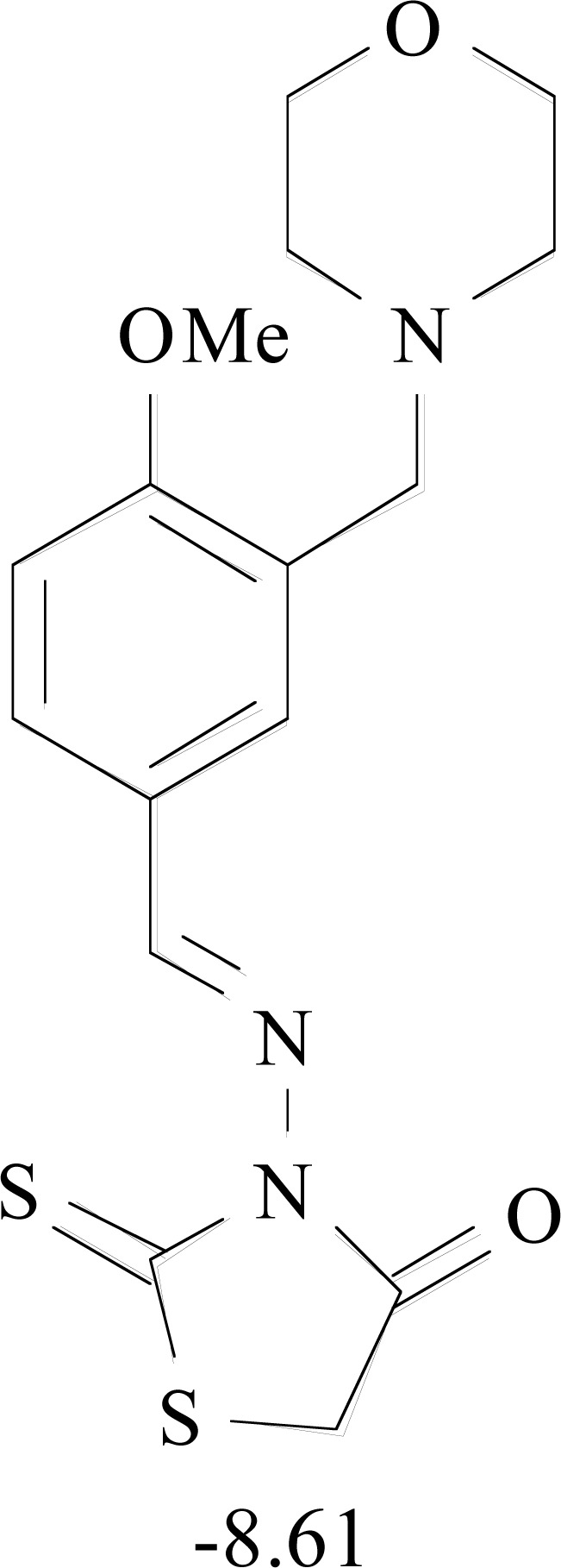 |
 |
 |
 |
 |
| nsP2 | 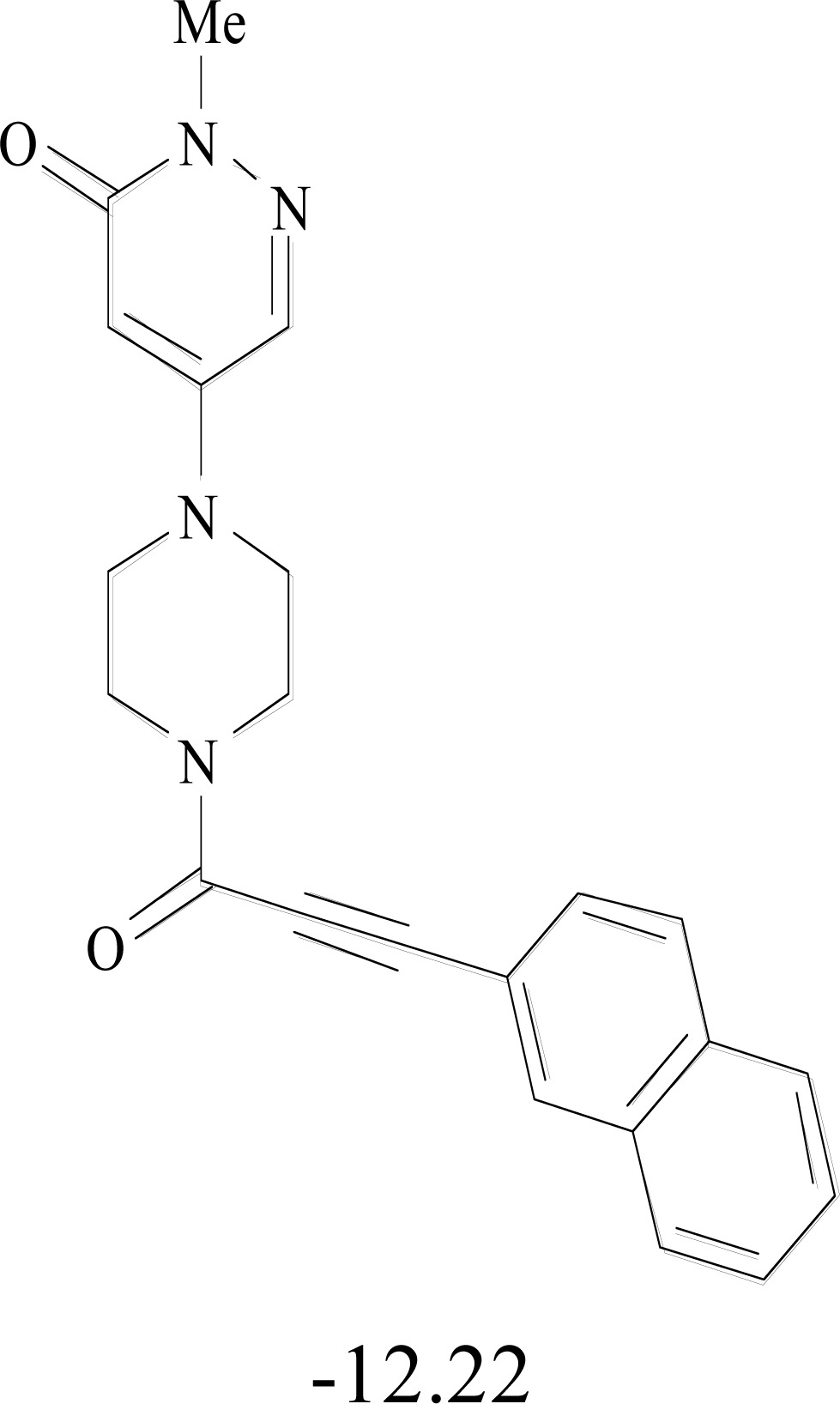 |
 |
 |
 |
 |
| nsP3 | 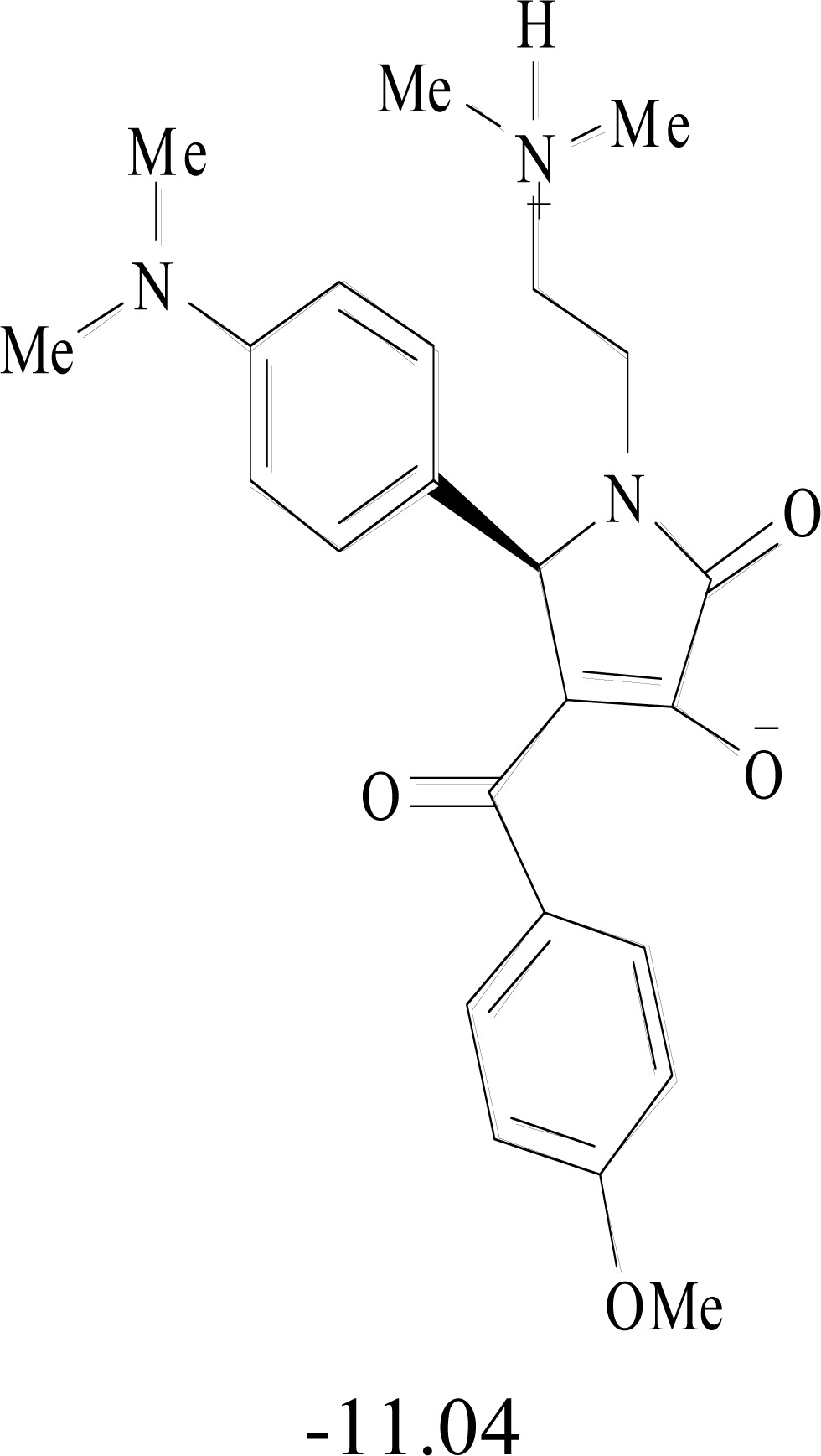 |
 |
 |
 |
 |
| nsP4 | 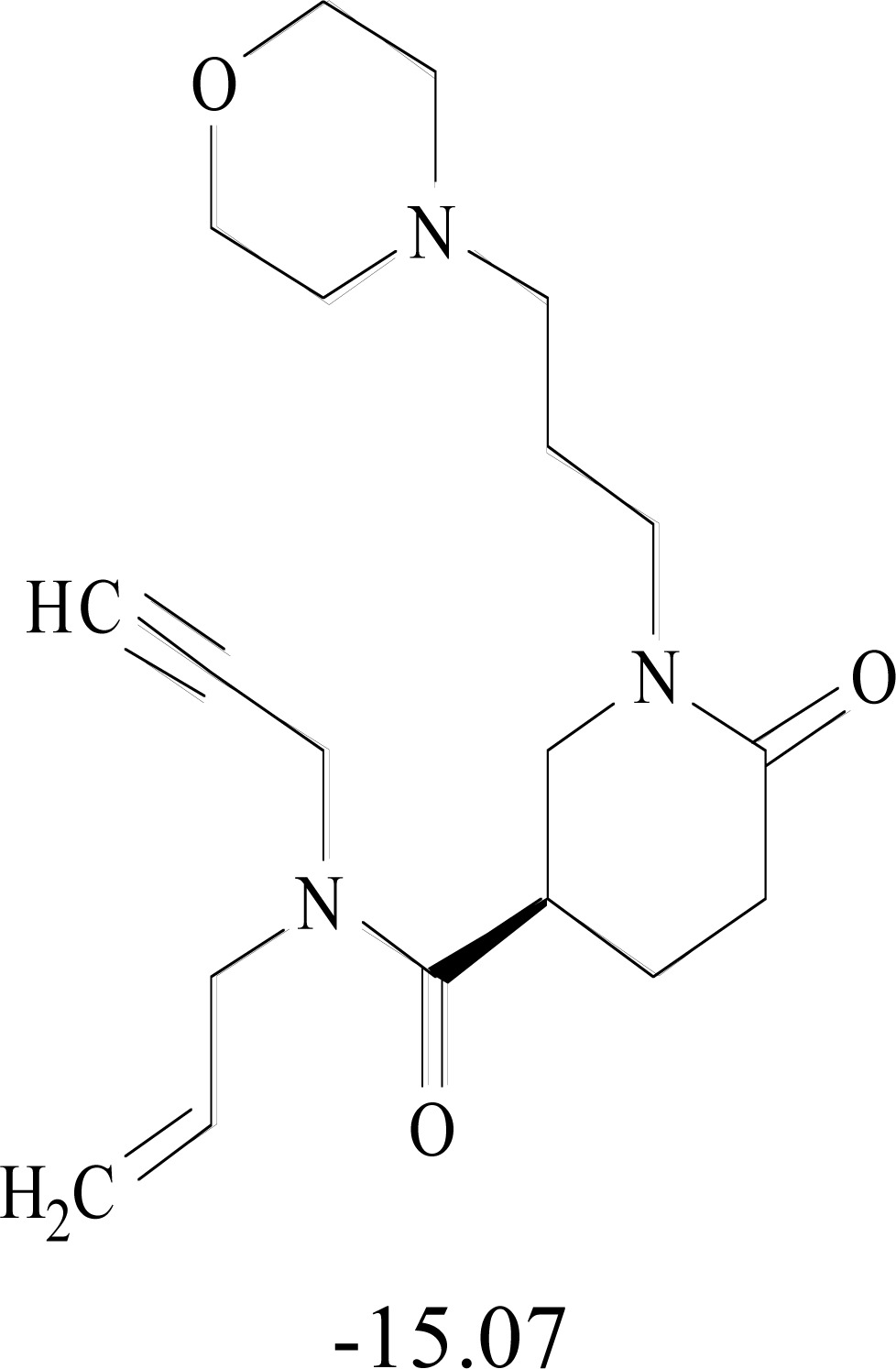 |
 |
 |
 |
 |
4. DISCUSSION ON THE G2H ASSEMBLY LINE
The wealth of information available from experimental host-pathogen interaction studies invites computational biologists to develop databases and newer computational methods to advance further focused experimentation. Consequently, bioinformatics is rapidly evolving into independent fields addressing specific problems in interpreting (i) genomic sequences, (ii) protein sequences and 3D-structures, as well as (iii) transcriptome and macromolecular interaction data. It is thus increasingly difficult for the biologist to choose the computational approaches that perform best in inhibiting the growth of pathogen in the host.
A basic overview of the G2H technology is given in this review with an application to Chikungunya virus. G2H assembly line is a culmination of several recent advances in computational chemistry and computational biology implemented in a high performance computing environment. At least three areas for further improvement can be immediately identified: (i) development of algorithms for cleavage of polyproteins, (ii) algorithms for identification of druggable protein targets, (iii) improved accuracies in tertiary structure prediction of nonstructural proteins, (iv) development of methods for determining tertiary structures of structural proteins and (v) identification of hit molecules with reduced toxicities. This protocol should ultimately result in an accelerated emergence of new methods for treating infectious diseases. Similarly, metabolic disorders can also be accessed via the “Genome to Hit” pathway.
5. CONCLUSION & PERSPECTIVES
Post-genomic research era encompasses many diverse aspects of modern science. The “Genome to hits” pathway described here symbolizes the emergence of an integrated technology to address specific health issues, and more specifically provides a novel and rapid approach to identifying new and potent hit molecules from genomic information.
SUPPLEMENTARY INFORMATION ON CHIKUNGUNYA AT SCFBIO WEBSITE
Details of the results on genes and protein tertiary structures predicted, binding pockets, hit molecules identified and lead molecules proposed for synthesis are available for free download from the SCFBio website (http://www.scfbio-iitd.res.in/software/chikv.jsp). These results will be updated periodically with improvements in protocols for protein structure prediction and ADMET evaluations.
ACKNOWLEDGEMENTS
This project is funded by the programme support to SCFBio from the Department of Biotechnology, Govt. of India. The authors gratefully acknowledge the help received from Ms. Garima Khandelwal, Ms. Priyanka Dhingra, Ms. Tanya Singh, Mr. Goutam Mukherjee, Mr. Avinash Mishra, Mr. Shashank Shekhar and Ms. Vandana Shekhar. The authors thank Dr. Aditya Mittal for useful discussions and a critical reading of the manuscript.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Altschul SF, Madden TL, Schäffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Chia N, Lauria M, Bundschuh R. A performance enhanced PSI-BLAST based on hybrid alignment. Bioinformatics. 2011;27:31–7. doi: 10.1093/bioinformatics/btq621. [DOI] [PubMed] [Google Scholar]
- 3.Standley DM, Toh H, Nakamura H. Functional annotation by sequence-weighted structure alignments: statistical analysis and case studies from the Protein 3000 structural genomics project in Japan. Proteins. 2008;72:1333–51. doi: 10.1002/prot.22015. [DOI] [PubMed] [Google Scholar]
- 4.Khandelwal G, Jayaram B. A phenomenological model for predicting melting temperatures of DNA sequences. PLoS ONE. 2010;5:e12433. doi: 10.1371/journal.pone.0012433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutta S, Singhal P, Agrawal P, et al. A physicochemical model for analyzing DNA sequences. J Chem Inf Model. 2006;46:78–85. doi: 10.1021/ci050119x. [DOI] [PubMed] [Google Scholar]
- 6.Singhal P, Jayaram B, Dixit SB, Beveridge DL. Prokaryotic gene finding based on physicochemical characteristics of codons calculated from molecular dynamics simulations. Biophys J. 2008;94:4173–83. doi: 10.1529/biophysj.107.116392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khandelwal G, Jayaram B. DNA-water interactions distinguish messenger RNA genes from transfer RNA genes. J. Am. Chem. Soc. 2012;134:8814–16. doi: 10.1021/ja3020956. [DOI] [PubMed] [Google Scholar]
- 8.Khandelwal G, Gupta J, Jayaram B. DNA energetics based analyses suggest additional genes in prokaryotes. J Bio Sc. 2012;37:433–44. doi: 10.1007/s12038-012-9221-7. [DOI] [PubMed] [Google Scholar]
- 9.Shenoy SR, Jayaram B. Proteins: Sequence to Structure and Function-Current Status. Curr Protein Pept Sci. 2010;11:498–514. doi: 10.2174/138920310794109094. [DOI] [PubMed] [Google Scholar]
- 10.Jayaram B, Dhingra P. Towards creating complete proteomic structural databases of whole organisms. Curr Bioinform. 2012;7:424–35. [Google Scholar]
- 11.Leopold PE, Montal M, Onuchic JN. Protein Folding Funnels - a Kinetic Approach to the Sequence Structure Relationship. Proc Natl Acad Sci USA. 1992;89:8721–25. doi: 10.1073/pnas.89.18.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayaram B, Dhingra P, Lakhani B, Shekhar S. Bhageerath-targeting the near impossible: pushing the frontiers of atomic models for protein tertiary structure prediction. J Chem Sci. 2012;124:83–91. doi:10.1007/s12039-011-0189-x. [Google Scholar]
- 13.Narang P, Bhushan K, Bose S, Jayaram B. A computational pathway for bracketing native-like structures for small alpha helical globular proteins. Phys Chem Chem Phys. 2005;7:2364–75. doi: 10.1039/b502226f. [DOI] [PubMed] [Google Scholar]
- 14.Narang P, Bhushan K, Bose S, Jayaram B. Protein structure evaluation using an all-atom energy based empirical scoring function. J Biomol Struct Dyn. 2006;23:385–406. doi: 10.1080/07391102.2006.10531234. [DOI] [PubMed] [Google Scholar]
- 15.Arora N, Jayaram B. The Strength of Hydrogen Bonds in Alpha Helices. J Comput Chem. 1997;18:1245–52. [Google Scholar]
- 16.Surjit B Dixit, Bhasin R, Rajasekaran E, and Jayaram B. Solvation Thermodynamics of Amino Acids: Assessment of the Electrostatic Contribution and Force Field Dependence. J. Chem. Soc. Farad. Trans. 1997;93:1105–13. [Google Scholar]
- 17.Mittal A, Jayaram B, Shenoy S, Bawa TS. A Stoichiometry driven universal spatial organization of backbones of folded proteins: Are there Chargaff's rules for protein folding? J Biomol Struct Dyn. 2010;28:133–42. doi: 10.1080/07391102.2010.10507349. [DOI] [PubMed] [Google Scholar]
- 18.Mittal A, Jayaram B. Backbones of folded proteins reveal novel invariant amino-acid neighborhoods. J Biomol Struct Dyn. 2011;28:443–54. doi: 10.1080/073911011010524954. [DOI] [PubMed] [Google Scholar]
- 19.Mittal A, Jayaram B. The Newest View on Protein Folding: Stoichiometric and Spatial Unity in Structural and Functional Diversity. J Biomol Struct Dyn. 2011;28:669–74. [Google Scholar]
- 20.Dill KA, Ozkan SB, Weikl TR, Chodera JD, Voelz VA. The protein folding problem: when will it be solved? Curr Opin Struct Biol. 2007;17:342–6. doi: 10.1016/j.sbi.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Kim DE, Blum B, Bradley P, Baker D. Sampling Bottlenecks in De novo Protein Structure Prediction. J Mol Bio. 2009;393:249–60. doi: 10.1016/j.jmb.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dill KA, Chan HS. From Levinthal to pathways to funnels. Nat Struct Biol. 1997;4:10–19. doi: 10.1038/nsb0197-10. [DOI] [PubMed] [Google Scholar]
- 23.Lindorff-Larsen K, Piana S, Palmo K, et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins. 2010;78:1950–8. doi: 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berka K, Laskowski RA, Hobza P, Vondrasek J. Energy Matrix of Structurally Important Side-Chain/Side-Chain Interactions in Proteins. J. Chem. Theory Comput. 2010;6:2191–203. doi: 10.1021/ct100007y. [DOI] [PubMed] [Google Scholar]
- 25.Cooper S, Khatib F, Treuille A, et al. Predicting protein structures with a multiplayer online game. Nature. 2010;466:756–60. doi: 10.1038/nature09304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faver JC, Benson ML, He X, et al. The energy computation paradox and ab initio protein folding. PLoS One. 2011;6:e18868. doi: 10.1371/journal.pone.0018868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayaram B, Bhushan K, Shenoy SR, et al. Bhageerath: an energy based web enabled computer software suite for limiting the search space of tertiary structures of small globular proteins. Nucleic Acids Res. 2006;34:6195–204. doi: 10.1093/nar/gkl789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhingra P, Mishra A, Rao S, Jayaram B. Bhageerath-H: A Homology ab initio Hybrid Webserver for Protein Tertiary Structure Prediction. (Manuscript in preparation) [Google Scholar]
- 29.Mishra A, Rao S, Mittal A, Jayaram B. Capturing native protein structures with a physico-chemical metric (pcSM), BBA-Proteins & proteomics, 2013, under revision. [DOI] [PubMed]
- 30.Dhingra P, Lakhani B, Jayaram B. Genarating native-like structures of soluble monomeric proteins via Bhageerath-H - a homology/ab initio hybrid method. J Comput Chem. 2013 Under revision. [Google Scholar]
- 31.Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today: Tech. 2004;1:337–41. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 33.Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44:235–49. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 34.(a) Shaikh SA, Jain T, Sandhu G, Latha N, Jayaram B. From drug target to leads-sketching a physicochemical pathway for lead molecule design in Silico. Curr Pharm Des. 2007;13:3454–70. doi: 10.2174/138161207782794220. [DOI] [PubMed] [Google Scholar]; (b) Shaikh SA, Jain T, Sandhu G, Soni A, Jayaram B. From drug target to leads-sketching a physicochemical pathway for lead molecule design in Silico. Frontiers in Medicinal Chemistry. 2012;6:324–360. doi: 10.2174/138161207782794220. Eds: Atta ur Rahman, Allen B. Reitz and M. Iqbal Choudhary, Bentham Publishers. doi:10.2174/97816080546401120601. [DOI] [PubMed] [Google Scholar]
- 35.Jayaram B, Dhingra P, Mukherjee G, Perumal V. “Genomes to Hits: The Emerging Assembly Line In Silico", Proceedings of the Ranbaxy Science Foundation 17th Annual Symposium on "New Frontiers in Drug Design, Discovery and Development", 2012, Chapter 3. pp. 13–35.
- 36.Das A, Kalra P, Latha N, Jayaram B. In Silico Trends in Thermodynamics and Kinetics of Binding - New Tools for De Novo Drug Design. In: Dr Srivastava MM, Shalini Srivastava, editors. Recent Trends in Chemistry. 2002. pp. 218–234. Chapter-15. [Google Scholar]
- 37.Gupta A, Gandhimathi A, Sharma P, Jayaram B. ParDOCK: An All Atom Energy Based Monte Carlo Docking Protocol for Protein- Ligand Complexes. Protein Pept Lett. 2007;14:632–46. doi: 10.2174/092986607781483831. [DOI] [PubMed] [Google Scholar]
- 38.Jain T, Jayaram B. An all atom energy based computational protocol for predicting binding affinities of protein.ligand complexes. FEBS Lett. 2005;579:6659–66. doi: 10.1016/j.febslet.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 39.Jain T, Jayaram B. Computational Protocol for Predicting the Binding Affinities of Zinc Containing Metalloprotein-Ligand Complexes. Proteins. 2007;67:1167–78. doi: 10.1002/prot.21332. [DOI] [PubMed] [Google Scholar]
- 40.Singh T, Biswas D, Jayaram B. AADS-an automated active site identification, docking and scoring protocol for protein targets based on physico-chemical descriptors. J Chem Inf Model. 2011;51:2515–27. doi: 10.1021/ci200193z. [DOI] [PubMed] [Google Scholar]
- 41.Mukherjee G, Jayaram B. A rapid scoring methodology based on physico-chemical descriptors of small molecules (RASPD) for identifying hits against a protein target. Phys Chem Chem Phys. 2013 doi: 10.1039/c3cp44697b. DOI:10.1039/C3CP44697B. [DOI] [PubMed] [Google Scholar]
- 42.Shaikh SA, Ahmed SR, Jayaram B. A molecular thermodynamic view of DNA-drug interaction: A case study of 25 minor groove binders. Arch Biochem Biophys. 2004;429:81–99. doi: 10.1016/j.abb.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Shaikh SA, Jayaram B. A Swift All-Atom Energy-Based Computational Protocol to Predict DNA-Ligand Binding Affinity and ΔTm. J Med Chem. 2007;50:2240–4. doi: 10.1021/jm060542c. [DOI] [PubMed] [Google Scholar]
- 44.Jayaram B, Latha N, Jain T, Sharma P, Gandhimathi A, Pandey VS. Sanjeevini: A comprehensive active site directed lead design software. Indian J Chem A. 2006;45:1834–37. [Google Scholar]
- 45.Jayaram B, Singh T, Mukherjee G, Mathur A, Shekhar S, and Shekhar V. Sanjeevini: A freely accessible web-server for target directed lead molecule discovery. BMC Bioinformatics. 2012;13:S7. doi: 10.1186/1471-2105-13-S17-S7. doi:10.1186/1471-2105-13-S17-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalra P, Reddy TV, Jayaram B. Free Energy Component Analysis for Drug Design: A Case Study of HIV-1 Protease.Inhibitor Binding. J Med Chem. 2001;44:4325–38. doi: 10.1021/jm010175z. [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee G, Patra N, Barua P, Jayaram B. A Fast Empirical GAFF Compatible Partial Atomic Charge Assignment Scheme for Modeling Interactions of Small Molecules with Biomolecular Targets. J Comput Chem. 2011;32:893–907. doi: 10.1002/jcc.21671. [DOI] [PubMed] [Google Scholar]
- 48.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–77. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 49.Gubler DJ. Human arbovirus infections worldwide. Ann N Y Acad Sci. 2001;951:13–24. doi: 10.1111/j.1749-6632.2001.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 50.Thiboutot MM, Kannan S, Kawalekar OU, et al. Chikungunya: A Potentially Emerging Epidemic? PLoS Negl Trop Dis. 2010;4:e623. doi: 10.1371/journal.pntd.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson MC. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952.53. I. Clinical features. Trans R Soc Trop Med Hyg. 1955;49:28–32. doi: 10.1016/0035-9203(55)90080-8. [DOI] [PubMed] [Google Scholar]
- 52.Sumathy K, Ella KM. Genetic diversity of chikungunya virus, India 2006-2010: Evolutionary dynamics and serotype analyses. J Med Virol. 2012;84:462–70. doi: 10.1002/jmv.23187. [DOI] [PubMed] [Google Scholar]
- 53.Anyamba A, Linthicum KJ, Small JL, et al. Climate teleconnections and recent patterns of human and animal disease outbreaks. PLoS Negl Trop Dis. 2012;6:e1465. doi: 10.1371/journal.pntd.0001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ross RW. The Newala epidemic: III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg. 1956;54:177–91. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lumsden WH. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952.53. II. General description and epidemiology. Trans R Soc Trop Med Hyg. 1955;49:33–57. doi: 10.1016/0035-9203(55)90081-x. [DOI] [PubMed] [Google Scholar]
- 56.Powers AM, Brault AC, Tesh RB, Weaver SC. Re-emergence of chikungunya and o.nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81:471–9. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 57.Arankalle VA, Shrivastava S, Cherian S, et al. Genetic divergence of Chikungunya viruses in India (1963-2006) with special reference to the 2005-2006 explosive epidemic. J Gen Virol. 2007;88:1967–76. doi: 10.1099/vir.0.82714-0. [DOI] [PubMed] [Google Scholar]
- 58.Yergolkar PN, Tandale BV, Arankalle VA, et al. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12:1580–3. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ravi V. Re-emergence of chikungunya virus in India. Indian J Med Microbiol. 2006;24:83–4. doi: 10.4103/0255-0857.25175. [DOI] [PubMed] [Google Scholar]
- 60.Lahariya C, Pradhan SK. Emergence of chikungunya virus in Indian subcontinent after 32 years: A review. J Vector Borne Dis. 2006;43:151–60. [PubMed] [Google Scholar]
- 61.Tsetsarkin KA, McGee CE, Volk SM, Vanlandingham DL, Weaver SC, Higgs S. Epistatic roles of E2 glycoprotein mutations in adaption of chikungunya virus to Aedes albopictus and Ae. aegypti mosquitoes. PLoS One. 2009;4:e6835. doi: 10.1371/journal.pone.0006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sam IC, Chan YF, Chan SY, et al. Chikungunya virus of Asian and Central/East African genotypes in Malaysia. J Clin Virol. 2009;46:180–3. doi: 10.1016/j.jcv.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 63.Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49:942–8. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- 64.Vassil St. Georgiev. Emerging and Re-emerging Infectious Diseases. National Institute of Allergy and Infectious Diseases, NIH Infectious Disease. 2009;Part I:23–28. DOI: 10.1007/978-1-60327-297-1_4. [Google Scholar]
- 65.Department of Health and Human Services; Centers for Disease Control and Prevention; National Institutes of Health. Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition. Washington DC: US Government Printing Office; 2007. Arboviruses and Related Zoonotic Viruses. [Google Scholar]
- 66.World Health Organization. Chikungunya fever, laboratory diagnosis of Chikungunya fevers. Geneva: World Health Organization; 2007. Communicable Diseases Branch. [Google Scholar]
- 67.Rezza G. Re-emergence of Chikungunya and other scourges: the role of globalization and climate change. Ann Ist Super Sanita. 2008;44:315–8. [PubMed] [Google Scholar]
- 68.Epstein PR. Chikungunya fever resurgence and global warming. Am J Trop Med Hyg. 2007;76:403–4. [PubMed] [Google Scholar]
- 69.Tabachnick WJ. Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. J Exp Biol. 2010;213:946–54. doi: 10.1242/jeb.037564. [DOI] [PubMed] [Google Scholar]
- 70.Stock I. Chikungunya fever--expanded distribution of a reemerging tropical infectious disease. Med Monatsschr Pharm. 2009;32:17–26. [PubMed] [Google Scholar]
- 71.Gould EA, Gallian P, De Lamballerie X, Charrel RN. First cases of autochthonous dengue fever and chikungunya fever in France: from bad dream to reality! Clin Microbiol Infect. 2010;16:1702–4. doi: 10.1111/j.1469-0691.2010.03386.x. [DOI] [PubMed] [Google Scholar]
- 72.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85:328–45. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moulay D, Aziz-Alaoui MA, Cadivel M. The chikungunya disease: modeling, vector and transmission global dynamics. Math Biosci. 2011;229:50–63. doi: 10.1016/j.mbs.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 74.Lam SK, Chua KB, Hooi PS, et al. Chikungunya infection - emerging disease in Malaysia. Southeast Asian J Trop Med Public Health. 2001;32:447–51. [PubMed] [Google Scholar]
- 75.Laras K, Sukri NC, Larasati RP, et al. Tracking the re-emergence of epidemic chikungunya virus in Indonesia. Trans R Soc Trop Med Hyg. 2005;99:128–41. doi: 10.1016/j.trstmh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 76.Mathew T, Tiruvengadam KV. Further studies on the isolate of Chikungunya from the Indian repatriates of Burma. Indian J Med Res. 1973;61:517–20. [PubMed] [Google Scholar]
- 77.Pastorino B, Muyembe-Tamfum JJ, Bessaud M, et al. Epidemic resurgence of Chikungunya virus in Democratic Republic of the Congo: identification of a new central African strain. J Med Virol. 2004;74:277–82. doi: 10.1002/jmv.20168. [DOI] [PubMed] [Google Scholar]
- 78.Rezza G, Nicoletti L, Angelini K, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–6. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 79.Borgherini G, Poubeau P, Staikowsky F, et al. Outbreak of chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin Infect Dis. 2007;44:1401–7. doi: 10.1086/517537. [DOI] [PubMed] [Google Scholar]
- 80.Lemant J, Boisson V, Winer A, et al. Serious acute chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005.2006. Crit Care Med. 2008;36:2536–41. doi: 10.1097/CCM.0b013e318183f2d2. [DOI] [PubMed] [Google Scholar]
- 81.Selvavinayagam TS. Chikungunya fever outbreak in Vellore, South India. Indian J Community Med. 2007;32:286–7. [Google Scholar]
- 82.Dwibedi B, Sabat J, Mahapatra N, Kar SK, Kerketta AS, et al. Rapid spread of chikungunya virus infection in Orissa: India. Indian J Med Res. 2011;133:316–21. [PMC free article] [PubMed] [Google Scholar]
- 83.Dutta P, Khan SA, Khan AM, Borah J, Chowdhury P, Mahanta J. First evidence of chikungunya virus infection in Assam, Northeast India. Trans R Soc Trop Med Hyg. 2011;105:355–7. doi: 10.1016/j.trstmh.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 84.Reiter P, Fontenille D, Paupy C. Aedes albopictus as an epidemic vector of chikungunya virus: another emerging problem? Lancet Infect Dis. 2006;6:463–4. doi: 10.1016/S1473-3099(06)70531-X. [DOI] [PubMed] [Google Scholar]
- 85.Townson H, Nathan MB. Resurgence of Chikungunya. Trans R Soc Trop Med Hyg. 2008;102:308–9. doi: 10.1016/j.trstmh.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 86.Srikanth P, Sarangan G, Mallilankaraman K, et al. Molecular characterization of Chikungunya virus during an outbreak in South India. Indian J Med Microbiol. 2010;28:299–302. doi: 10.4103/0255-0857.71812. [DOI] [PubMed] [Google Scholar]
- 87.Santhosh SR, Dash PK, Parida M, Khan M, Rao PV. Appearance of E1: A226V mutant Chikungunya virus in coastal Karnataka, India during 2008 outbreak. Virol J. 2009;6:172. doi: 10.1186/1743-422X-6-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kannan M, Rajendran R, Sunish IP, et al. A study on Chikungunya outbreak during 2007 in Kerala, South India. Indian J Med Res. 2009;129:311–5. [PubMed] [Google Scholar]
- 89.Schuffenecker I, Iteman I, Michault A, et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manimunda SP, Sugunan AP, Rai SK, et al. Outbreak of Chikungunya Fever, Dakshina Kannada District, South India. Am J Trop Med Hyg. 2010;83:751–54. doi: 10.4269/ajtmh.2010.09-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ray P, Ratagiri VH, Kabra SK, et al. Chikungunya Infection in India: Results of a prospective hospital based multi-centric study. PLoS ONE. 2012;7(2):e30025. doi: 10.1371/journal.pone.0030025. doi:10.1371/journal.pone.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chem YK, Zainah S, Berendam SJ, Rogayah TA, Khairul AH, Chua KB. Molecular epidemiology of chikungunya virus in Malaysia since its first emergence in 1998. Med J Malaysia. 2010;65(1):31–5. [PubMed] [Google Scholar]
- 93.M Naresh Kumar CV, Anthony Johnson AM, R Sai Gopal DV. Molecular characterization of chikungunya virus from Andhra Pradesh, India & phylogenetic relationship with Central African isolates. Indian J Med Res. 2007;126:534–40. [PubMed] [Google Scholar]
- 94.Dash PK, Parida MM, Santhosh SR, et al. East Central South African genotype as the causative agent in reemergence of Chikungunya outbreak in India. Vector Borne Zoonotic Dis. 2007;7:519–27. doi: 10.1089/vbz.2007.7272. [DOI] [PubMed] [Google Scholar]
- 95.Diallo M, Thonnon J, Traore-Lamizana M, Fontenille D. Vectors of Chikungunya virus in Senegal: current data and transmission cycles. Am J Trop Med Hyg. 1999;60:281–6. doi: 10.4269/ajtmh.1999.60.281. [DOI] [PubMed] [Google Scholar]
- 96.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vazeille M, Moutailler S, Coudrier D, et al. Two chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS One. 2007;2:e1168. doi: 10.1371/journal.pone.0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mourya DT, Yadav P. Vector biology of dengue & chikungunya viruses. Indian J Med Res. 2006;124:475–80. [PubMed] [Google Scholar]
- 99.Pages F, Peyrefitte CN, Mve MT, et al. Aedes albopictus mosquito: the main vector of the 2007 Chikungunya outbreak in Gabon. PLoS One. 2009;4:e4691. doi: 10.1371/journal.pone.0004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vazeille M, Martin E, Mousson L, Failloux AB. Chikungunya, a new threat propagated by the cosmopolite Aedes albopictus. BMC Proceedings. 2011;5:O8. [Google Scholar]
- 101.Pardigon N. The biology of Chikungunya: a brief review of what we still do not know. Pathol Biol. 2009;57:127–32. doi: 10.1016/j.patbio.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 102.Martin E, Moutailler S, Madec Y, Failloux A-B. Differential responses of the mosquito Aedes albopictus from the Indian Ocean region to two chikungunya isolates. BMC Ecology. 2010;10:8. doi: 10.1186/1472-6785-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ng LC, Tan LK, Tan CH, et al. Entomologic and virologic investigation of Chikungunya, Singapore. Emerg Infect Dis. 2009;15:1243–9. doi: 10.3201/eid1508.081486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsetsarkin KA, McGee CE, Higgs S. Chikungunya virus adaptation to Aedes albopictus mosquitoes does not correlate with acquisition of cholesterol dependence or decreased pH threshold for fusion reaction. Virology. 2011;8:376. doi: 10.1186/1743-422X-8-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsetsarkin KA, Chen R, Leal G, et al. Chikungunya virus emergence is constrained in Asia by lineage-specific adaptive landscapes. Proc Natl Acad Sci USA. 2011;108:7872–7. doi: 10.1073/pnas.1018344108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hapuarachchi HC, Bandara KB, Sumanadasa SD, Hapugoda MD, Lai YL, Lee KS. Re-emergence of chikungunya virus in Southeast Asia: virological evidence from Sri Lanka and Singapore. J Gen Virol. 2010;91:1067–76. doi: 10.1099/vir.0.015743-0. [DOI] [PubMed] [Google Scholar]
- 107.Volk SM, Chen R, Tsetsarkin KA, Adams AP, Garcia TI, Sall AA. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J Virol. 2010;84:6497–504. doi: 10.1128/JVI.01603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chretien JP, Anyamba A, Bedno SA, et al. Drought-associated Chikungunya emergence along coastal East Africa. Am J Trop Med Hyg. 2007;76:405–7. [PubMed] [Google Scholar]
- 109.Anish TS, Vijayakumar K, Leela Itty Amma KR. Domestic and environmental factors of chikungunya-affected families in Thiruvananthapuram (Rural) district of Kerala, India. J Global Infect Dis. 2011;3:32–6. doi: 10.4103/0974-777X.77293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chakkaravarthy VM, Vincent S, Ambrose T. Novel Approach of Geographic Information Systems on Recent Out-Breaks of Chikungunya in Tamil Nadu, India. J Environ Sci Technol. 2011;4:387–94. [Google Scholar]
- 111.Lakshmi V, Neeraja M, Subbalaxmi MVS, et al. Clinical Features and Molecular Diagnosis of Chikungunya Fever from South India. Clin Infect Dis. 2008;46:1436–42. doi: 10.1086/529444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Her Z, Kam YW, Lin RT, Ng LF. Chikungunya: a bending reality. Microbes Infect. 2009;11:1165–76. doi: 10.1016/j.micinf.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 113.Kondekar S, Gogtay NJ. Why Chikungunya is called chikungunya. J Postgrad Med. 2006;52:307. [Google Scholar]
- 114.Sudeep AB, and Parashar D. Chikungunya: an overview. J Biosci. 2008;33:443–9. doi: 10.1007/s12038-008-0063-2. [DOI] [PubMed] [Google Scholar]
- 115.Yazdani R, Kaushik VV. Chikungunya fever. Rheumatology. 2007;46:1214–15. doi: 10.1093/rheumatology/kem059. [DOI] [PubMed] [Google Scholar]
- 116.De Andrade DC, Jean S, Clavelou P, Dallel R, Bouhassira D. Chronic pain associated with the Chikungunya Fever: long lasting burden of an acute illness. BMC Infect Dis. 2010;10:31–7. doi: 10.1186/1471-2334-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Suhrbier A, La Linn M. Clinical and pathologic aspects of arthritis due to Ross River virus and other alphaviruses. Curr Opin Rheumatol. 2004;16:374–9. doi: 10.1097/01.bor.0000130537.76808.26. [DOI] [PubMed] [Google Scholar]
- 118.Dubrulle M, Mousson L, Moutailler S, Vazeille M, Failloux A-B. Chikungunya Virus and Aedes Mosquitoes: Saliva Is Infectious as soon as Two Days after Oral Infection. PLoS ONE. 2009;4:e5895. doi: 10.1371/journal.pone.0005895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chamberlain RW. Epidemiology of arthropod-borne togaviruses: The role of arthropods as hosts and vectors and of vertebrate hosts in natural transmission cycles. In: Schlesinger RW, editor. The Togaviruses: Biology, structure, replication. Orlando: Academic; 1980. pp. 175–227. [Google Scholar]
- 120.Mourya DT, Ranadive SN, Gokhale MD, Barde PV, Padbidri VS, Banerjee K. Putative Chikungunya virus-specific receptor proteins on the midgut brush border membrane of Aedes aegypti mosquito. Indian J Med Res. 1998;107:10–4. [PubMed] [Google Scholar]
- 121.Kielian M, Rey FA. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat Rev Microbiol. 2006;4:67–76. doi: 10.1038/nrmicro1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–40. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lu YE, Cassese T, Kielian M. The cholesterol requirement for Sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J Virol. 1999;73:4272–8. doi: 10.1128/jvi.73.5.4272-4278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahn A, Schoepp RJ, Sternberg D, Kielian M. Growth and stability of a cholesterol-independent Semliki Forest virus mutant in mosquitoes. Virology. 1999;262:452–6. doi: 10.1006/viro.1999.9932. [DOI] [PubMed] [Google Scholar]
- 125.Solignat M, Gay B, Higgs S, Briant L, Devaux C. Replication cycle of chikungunya: a re-emerging arbovirus. Virology. 393:183–97. doi: 10.1016/j.virol.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khan AH, Morita K, Parquet Md Mdel C, Hasebe F, Mathenge EG, Igarashi A. Complete nucleotide sequence of chikungunya virus and evidence for an internal polyadenylation site. J Gen Virol. 2002;83:3075–84. doi: 10.1099/0022-1317-83-12-3075. [DOI] [PubMed] [Google Scholar]
- 127.Ou JH, Strauss EG, Strauss JH. Comparative studies of the 3.- terminal sequences of several alphavirus RNAs. Virology. 1981;109:281–9. doi: 10.1016/0042-6822(81)90499-2. [DOI] [PubMed] [Google Scholar]
- 128.Ou JH, Trent DW, Strauss JH. The 3.-noncoding regions of alphavirus RNAs contain repeating sequences. J Mol Biol. 1982;156:719–30. doi: 10.1016/0022-2836(82)90138-3. [DOI] [PubMed] [Google Scholar]
- 129.Ou JH, Strauss EG, Strauss JH. The 5.-terminal sequences of the genomic RNAs of several alphaviruses. J Mol Biol. 1983;168:1–15. doi: 10.1016/s0022-2836(83)80319-2. [DOI] [PubMed] [Google Scholar]
- 130.Pfeffer M, Kinney RM, Kaaden OR. The alphavirus 3.- nontranslated region: size heterogeneity and arrangement of repeated sequence elements. Virology. 1998;240:100–8. doi: 10.1006/viro.1997.8907. [DOI] [PubMed] [Google Scholar]
- 131.Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol. 2010;8:491–500. doi: 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]
- 132.Simizu B, Yamamoto K, Hashimoto K, Ogata T. Structural proteins of Chikungunya virus. J Virol. 1984;51:254–58. doi: 10.1128/jvi.51.1.254-258.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weaver S C, Frey T K, Huang H V, Kinney R M, Rice C M, Roehrig J T, Shope R E, & Strauss E G. Togaviridae. In: Fauquet C M, Mayo M A, Maniloff J, Desselberger U, & Ball L A, editors. Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. Amsterdam: Elsevier Academic Press; 2005. pp. 999–1008. [Google Scholar]
- 134.Schlesinger M, & Schlesinger S. Formation and assembly of alphavirus glycoproteins. In: Schlesinger S, & Schlesinger M J, editors. The Togaviridae and Flaviviridae. New York: Plenum Publishing Corp; 1986. pp. 121–148. [Google Scholar]
- 135.Strauss EG, Strauss JH. Structure and replication of the alphavirus genome. In: Schlesinger S, & Schlesinger M J, editors. The Togaviridae and Flaviviridae. New York: Plenum Press; 1986. pp. 35–90. [Google Scholar]
- 136.Kääriäinen L, Takkinen K, Keränen S, Söderlund H. Replication of the genome of alphaviruses. J Cell Sci Suppl. 1987;7:231–50. [PubMed] [Google Scholar]
- 137.Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ten Dam E, Flint M, Ryan MD. Virus-coded proteinases of the Togaviridae. J Gen Virol. 1999;80:1879–88. doi: 10.1099/0022-1317-80-8-1879. [DOI] [PubMed] [Google Scholar]
- 139.Lemm JA, Rümenapf T, Strauss EG, Strauss JH, Rice CM. Polypeptide requirements for assembly of functional Sindbis virus replication complexes: a model for the temporal regulation of minus- and plus-strand RNA synthesis. EMBO J. 1994;13:2925–34. doi: 10.1002/j.1460-2075.1994.tb06587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Voss JE, Vaney MC, Duquerroy S, et al. Glycoprotein organization of Chikungunya virusparticles revealed by X-ray crystallography. Nature. 2010;468:709–12. doi: 10.1038/nature09555. [DOI] [PubMed] [Google Scholar]
- 141.Söderlund H, Ulmanen I. Transient association of Semliki Forest virus capsid protein with ribosomes. J Virol. 1977;24:907–9. doi: 10.1128/jvi.24.3.907-909.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choi H-K, Tong L, Minor W, et al. Structure of Sindbis virus core protein reveals a chymotrypsin-like serine proteinase and the organization of the virion. Nature. 1991;354:37–43. doi: 10.1038/354037a0. [DOI] [PubMed] [Google Scholar]
- 143.Ulmanen I, Söderlund H, and Kääriäinen L. Semliki Forest virus capsid protein associates with the 60 S ribosomal subunit in infected cells. J Virol. 1976;20:203–10. doi: 10.1128/jvi.20.1.203-210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garoff H, Simons K, Dobberstein B. Assembly of the Semliki Forest virus membrane glycoproteins in the membrane of the endoplasmic reticulum in vitro. J Mol Biol. 1978;124:587–600. doi: 10.1016/0022-2836(78)90173-0. [DOI] [PubMed] [Google Scholar]
- 145.Sariola M, Saraste J, Kuismanen E. Communication of post-Golgi elements with early endosytic pathway:regulation of endoproteolytic cleavage of Semliki Forest virus p62 precursor. J Cell Sci. 1995;108:2465–75. doi: 10.1242/jcs.108.6.2465. [DOI] [PubMed] [Google Scholar]
- 146.Wahlberg JM, Boere WAM, Garoff H. The heterodimeric association between the membrane proteins of Semliki Forest virus changes its sensitivity to low pH during virus maturation. J Virol. 1989;63:4991–7. doi: 10.1128/jvi.63.12.4991-4997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao H, Garoff H. Role of cell surface spikes in alphavirus budding. J Virol. 1992;66:7089–95. doi: 10.1128/jvi.66.12.7089-7095.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Suomalainen M, Liljeström P, Garoff H. Spike protein-nucleocapsid interactions drive the budding of alphaviruses. J Virol. 1992;66:4737–47. doi: 10.1128/jvi.66.8.4737-4747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Queyriaux B, Simon F, Grandadam M, Michel R, Tolou H, Boutin JP. Clinical burden of chikungunya virus infection. Lancet Infect Dis. 2008;8:2–3. doi: 10.1016/S1473-3099(07)70294-3. [DOI] [PubMed] [Google Scholar]
- 150.Mourya DT, Mishra AC. Chikungunya fever. Lancet. 2006;368:186–7. doi: 10.1016/S0140-6736(06)69017-X. [DOI] [PubMed] [Google Scholar]
- 151.Mohan A, Kiran DHN, Manohar IC, Kumar DP. Epidemology, clinical manifestations and diagnosis of chikungunya fever: lessons learned from the re-emerging epidemic. Indian J Dermatol. 2010;55:54–63. doi: 10.4103/0019-5154.60355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ali Ou Alla S, Combe B. Arthritis after infection with Chikungunya virus. Best Pract Res Clin Rheumatol. 2011;25:337–46. doi: 10.1016/j.berh.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 153.Brighton SW, Prozesky OW, De La Harpe AL. Chikungunya virus infection: a retrospective study of 107 cases. S Afr Med J. 1982;63:313–15. [PubMed] [Google Scholar]
- 154.Vijayakumar KP, Anish TS, George B, Lawrence T, Muthukkutty SC, Ramachandran R. Clinical profile of chikungunya patients during the epidemic of 2007 in Kerala, India. J Global Infect Dis. 2011;3:221–6. doi: 10.4103/0974-777X.83526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Couderc T, Lecuit M. Focus on Chikungunya pathophysiology in human and animal models. Microbes Infect. 2009;11:1197–1205. doi: 10.1016/j.micinf.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 156.Robin S, Ramful D, Le Seach' F, Jaffar-Bandjee MC, Rigou G, Alessandri JL. Neurologic manifestations of pediatric chikungunya infection. J Child Neurol. 2008;23:1028–35. doi: 10.1177/0883073808314151. [DOI] [PubMed] [Google Scholar]
- 157.Pakran J, George M, Riyaz N, et al. Purpuric macules with vesiculobullous lesions: a novel manifestation of chikungunya. Int J Dermatol. 2011;50:61–9. doi: 10.1111/j.1365-4632.2010.04644.x. [DOI] [PubMed] [Google Scholar]
- 158.Parida MM, Santhosh SR, Dash PK, et al. Rapid and real-time detection of Chikungunya virus by reverse transcription loop-mediated isothermal amplification assay. J Clin Microbiol. 2007;45:351–7. doi: 10.1128/JCM.01734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yap G, Pok KY, Lai YL, et al. Evaluation of chikungunya diagnostic assays: differences in sensitivity of serology assays in two independent outbreaks. PLoS Negl Trop Dis. 2010;4:e753. doi: 10.1371/journal.pntd.0000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Edwards CJ, Welch SR, Chamberlain J, et al. Molecular diagnosis and analysis of Chikungunya virus. J Clin Virol. 2007;39:271–5. doi: 10.1016/j.jcv.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 161.Mishra B, Sharma M, Pujhari SK, et al. Utility of multiplex reverse transcriptase-polymerase chain reaction for diagnosis and serotypic characterization of dengue and chikungunya viruses in clinical samples. Diagn Microbiol Infect Dis. 2011;71:118–25. doi: 10.1016/j.diagmicrobio.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 162.Alphey L, Benedict M, Bellini R, et al. Sterile-Insect Methods for Control of Mosquito-Borne Diseases: An Analysis. Vector Borne Zoonotic Dis. 2010;10:295–311. doi: 10.1089/vbz.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE, Jr, Lupton HW. Development of an attenuated strain of Chikungunya virus for use in vaccine production. Vaccine. 1986;4:157–62. doi: 10.1016/0264-410x(86)90003-4. [DOI] [PubMed] [Google Scholar]
- 164.Harrison VR, Eckels KH, Bartelloni PJ, Hampton C. Production and valuation of a formalin killed chikungunya vaccine. J Immunol. 1971;107:643–7. [PubMed] [Google Scholar]
- 165.Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, and Mangiafico JA. Phase II safety and immunogenicity study of live chikungunya virus Vaccine TSI-GSD-218. Am J Trop Med Hyg. 2000;62:681–5. doi: 10.4269/ajtmh.2000.62.681. [DOI] [PubMed] [Google Scholar]
- 166.Akahata W, Yang ZY, Andersen H, et al. A VLP vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16:334–8. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Anbarasu K, Manisenthil KK, Ramachandran S. Antipyretic, anti-inflammatory and analgesic properties of nilavembu kudineer choornam: a classical preparation used in the treatment of chikungunya fever. Asian Pac J Trop Med. 2011;4:819–23. doi: 10.1016/S1995-7645(11)60201-0. [DOI] [PubMed] [Google Scholar]
- 168.Delogu I, Pastorino B, Baronti C, Nougairède A, Bonnet E, de Lamballerie X. In vitro antiviral activity of arbidol against Chikungunya virus and characteristics of a selected resistant mutant. Antiviral Res. 2011;90:99–107. doi: 10.1016/j.antiviral.2011.03.182. [DOI] [PubMed] [Google Scholar]
- 169.Partidos CD, Weger J, Brewoo J, et al. Probing the attenuation and protective efficacy of a candidate chikungunya virus vaccine in mice with compromised interferon (IFN) signaling. Vaccine. 2011;29:3067–73. doi: 10.1016/j.vaccine.2011.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang D, Suhrbier A, Penn-Nicholson A, et al. A complex adenovirus vaccine against chikungunya virus provides complete protection against viraemia and arthritis. Vaccine. 2011;29:2803–9. doi: 10.1016/j.vaccine.2011.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.De Lamballerie X, Ninove L, Charrel RN. Antiviral treatment of chikungunya virus infection. Infect Disord Drug Targets. 2009;9:101–4. doi: 10.2174/187152609787847712. [DOI] [PubMed] [Google Scholar]
- 172.Khan M, Santhosh SR, Tiwari M, Lakshmana Rao PV, Parida M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against Chikungunya virus in vero cells. J Med Virol. 2010;82:817–24. doi: 10.1002/jmv.21663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shoichet BK, McGovern SL, Wei B, Irwin JJ. Lead Discovery Using Molecular Docking. Curr Opin Chem Biol. 2002;6:439–46. doi: 10.1016/s1367-5931(02)00339-3. [DOI] [PubMed] [Google Scholar]
- 174.Cross JB, Thompson DC, Rai BK, et al. Comparison of several molecular docking programs: Pose prediction and virtual screening accuracy. J Chem Inf Model. 2009;49:1455–74. doi: 10.1021/ci900056c. [DOI] [PubMed] [Google Scholar]
- 175.Di L, Kerns EH, Carter GT. Drug-like property concepts in pharmaceutical design. Curr Pharm Des. 2009;15:2184–94. doi: 10.2174/138161209788682479. [DOI] [PubMed] [Google Scholar]
- 176.Wang J. Comprehensive assessment of ADMET risks in drug discovery. Curr Pharm Des. 2009;15:2195–219. doi: 10.2174/138161209788682514. [DOI] [PubMed] [Google Scholar]
- 177.Raunio H. In silico toxicology - non-testing methods. Front Pharmacol. 2011;2:33. doi: 10.3389/fphar.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhu F, Qin C, Tao L, et al. Clustered patterns of species origins of nature-derived drugs and clues for future bioprospecting. Proc Natl Acad Sci USA. 2011;108:12943–8. doi: 10.1073/pnas.1107336108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tang W, Lu AY. Drug metabolism and pharmacokinetics in support of drug design. Curr Pharm Des. 2009;15:2170–83. doi: 10.2174/138161209788682451. [DOI] [PubMed] [Google Scholar]
- 180.Férriz JM, Vinsová J. Prodrug design of phenolic drugs. Curr Pharm Des. 2010;16:2033–52. doi: 10.2174/138161210791293042. [DOI] [PubMed] [Google Scholar]