Significance
When we see a person act (e.g., reach for a cup), we observe physical movements but infer underlying goals. Many have proposed that this ability depends on having produced motor actions oneself. We investigated the relationship between action understanding and production by giving young infants novel experience producing actions. Consistent with prior research, infants exhibited understanding of others’ actions only when they had produced similar actions themselves. However, infants exhibited expectations about action efficiency that went beyond what could be learned from the training, suggesting that some knowledge of goal-directed action precedes this first-person motoric experience.
Keywords: goal inference, action representation, motor cognition, conceptual development
Abstract
Do infants learn to interpret others’ actions through their own experience producing goal-directed action, or does some knowledge of others’ actions precede first-person experience? Several studies report that motor experience enhances action understanding, but the nature of this effect is not well understood. The present research investigates what is learned during early motoric production, and it tests whether knowledge of goal-directed actions, including an assumption that actors maximize efficiency given environmental constraints, exists before experience producing such actions. Three-month-old infants (who cannot yet effectively reach for and grasp objects) were given novel experience retrieving objects that rested on a surface with no barriers. They were then shown an actor reaching for an object over a barrier and tested for sensitivity to the efficiency of the action. These infants showed heightened attention when the agent reached inefficiently for a goal object; in contrast, infants who lacked successful reaching experience did not differentiate between direct and indirect reaches. Given that the infants could reach directly for objects during training and were given no opportunity to update their actions based on environmental constraints, the training experience itself is unlikely to have provided a basis for learning about action efficiency. We suggest that infants apply a general assumption of efficient action as soon as they have sufficient information (possibly derived from their own action experience) to identify an agent’s goal in a given instance.
A hallmark of goal-directed action is its flexibility (1). Intelligent agents draw on causal and functional knowledge to update their action plans based on the constraints and affordances of the current environment. As a result, the same goal can be achieved with various means depending on the situation, and the same movements can reflect diverse goals. This flexibility poses a nontrivial inference problem for the outside observer seeking to uncover agents’ intentions and predict their future behavior.
Humans nonetheless make sense of others’ behavior, and do so by recruiting a “theory of mind” that relates observable actions to subjective mental states (2). This intuitive theory relies on a key assumption that agents are rational, pursuing their goals with the most efficient means possible in a given context (3, 4). By assuming that agents pursue their goals efficiently, an observer can flexibly adapt expectations about others’ actions to the constraints of a given situation, and can interpret the same actions differently based on the context. This efficiency assumption thus constrains an otherwise underdetermined inference problem, providing a flexible schema for explaining and predicting action.
The Origins of Rational Action Knowledge
Studies with human infants suggest that this efficiency assumption is in place by the second half of the first year. When shown visual displays of an agent passing over an obstacle to approach a goal object, infants expect the agent to take a more direct approach once the obstacle is removed, exhibiting violation of expectation if the agent continues to perform a curvilinear trajectory (5, 6). Moreover, when infants have begun to imitate other people’s actions on objects, their patterns of imitation depend on the constraints on the other agent (ref. 7; see discussion in refs. 8, 9): for example, if 14-mo-old infants view an agent activating a light by pressing with her forehead, they tend to activate the light with a simple press of the hand when the observed agent’s hands were occupied (and hence, this more efficient means of activating the light was unavailable to her), and use their foreheads when the observed agent’s hands were free (and hence, she chose the forehead action over the apparently more efficient means). These studies suggest that infants encode actions in terms of goals by analyzing them with respect to physical constraints, and expect agents to update actions as affordances of the environment change. It seems that a productive, efficiency-based action schema is available from early in life, supporting flexible, context-dependent interpretations of others’ actions (5, 10, 11).
In the present research, we aim to shed light on (i) the origins of these goal-based action representations and (ii) the specific mechanisms that support action understanding in early infancy. In particular, we explore the claim that our ability to understand others’ goals depends on first-person experience producing goal-directed action (12–15). On one version of this proposal—the ideomotor or motor resonance view (16)—online processing of others’ actions requires reactivation of motor representations (so-called mirror neurons; refs. 17, 18) that are derived from first-person production and correspond to the kinematics of specific actions in the observer’s motor repertoire (8, 15). By this hypothesis, visuomotor “mirror” representations—which could be innate (18, 19) or built from correlated sensory and motor action input (20, 21)—allow for the activation of a corresponding motor program following perception of another’s action. Spreading activation from the motor plan to a representation of the action’s salient visual or auditory effects then allows the observer to categorize the observed action as directed toward that effect or end state (22, 23). Neurons in macaque inferior parietal lobule that are tuned to the end goal of an observed action (i.e., grasp to eat vs. grasp to place; ref. 24) may be the last stage in a feed-forward chain of such action–effect associations (25).
If such a mechanism were the sole foundation for action comprehension, infants’ initial understanding of action goals should be limited to those for which they have built the relevant set of associations at this first-person motoric level (13, 15). There is ample evidence that humans develop alternative mechanisms, as even infants at the end of their first year are able to interpret (and reason about the efficiency of) unfamiliar actions performed by novel agents (5, 6), as well as actions that violate human biomechanical constraints (26). However, these more abstract action representations may be analogical extensions of a mechanism initially derived from and limited by first-person motor experience (27). The first aim of the present research was to test this possibility by investigating whether 3-mo-old infants (the youngest age in which goal-based action representations have been reported) can understand actions that are kinematically distinct from those they have produced.
Our second aim was to investigate a related proposal about the origins of goal-based action representations—specifically, the claim that the general concept of goal-directed action is itself constructed from active production (12, 13). On the strongest version of this view, the very notion that movements are directed toward goals may be learned from early first-person experience engaging in intentional action (27, 28). At approximately 4 to 5 mo of age, infants begin to retrieve objects by reaching and grasping (29), and at 6 to 7 mo, they begin to locomote independently (30). By performing such actions, infants may learn that their own movements are driven by internal goals or desires, that the physical environment imposes constraints on those movements, and that they tend to achieve goals efficiently given the constraints of the environment. Infants might observe, for example, that they reach toward objects via a direct path when possible, and learn through trial and error that indirect paths are necessary when physical barriers impede the direct route. On this view, infants’ initial representations of actions would be limited to perceptual properties of the moving body (e.g., visual kinematics), with first-person action experience providing a basis for building additional abstractions like goals or intentions, and for constructing a set of rules or principles for reasoning about actions directed toward goals. One alternative to this view is that infants construct these concepts based, in part, on visual analysis of actions performed by others (31). Another possibility is that action understanding relies on evolved, domain-specific mechanisms for interpreting and predicting the actions of others (32), and that certain abstract assumptions, including a principle of efficiency, are available as innate constraints on this action perception system (33). Thus, the present research aimed to investigate whether the full breadth of early goal knowledge can be explained in terms of information acquired during first-person motor production.
Probing the Role of Active Experience
The view that motor representations are necessary for online action processing and the view that goal concepts are built from first-person experience both make the prediction that infants will be unable to identify others’ goals before experience engaging in goal-directed action, and that the range of actions they can successfully interpret will expand with the development of their motor repertoires (27, 34). Broadly consistent with these predictions, correlational studies find that the ability to organize movements around goals is related to improvements in understanding others’ actions (35–38). However, correlational findings do not reveal whether action experience plays a causal role in the development of action understanding.
Training studies that experimentally alter infants’ early action experience provide an especially useful tool for addressing this question, and reveal striking and reliable effects of infants’ first-person action experience on subsequent perception of goal-directed actions (34, 39, 40). Sommerville et al. (39) report one particularly dramatic training effect at 3 mo, an age at which infants can neither locomote to objects nor reach for them (41). Earlier research found that, if habituated to an agent reaching for one of two objects, 6-mo-old infants dishabituate when the agent reaches for the previously ignored object, even when the reach direction is consistent with that of the previously demonstrated action (42). This tendency to encode the object of an observed reach suggests that infants represent the action in terms of its goal or end-state over other more superficial properties (e.g., reach trajectory). Because intentional grasping behavior tends to emerge between 4 and 5 mo (29, 41), the theories reviewed earlier predict that infants younger than 4 mo should not encode the end-goal in this task. Indeed, 3-mo-old infants generally fail to do so (39). However, infants given a brief training intervention in which they wore Velcro-covered mittens while interacting with Velcro-covered objects (allowing them to successfully manipulate and retrieve the objects) showed heightened attention to test displays in which an agent who had previously reached for one object began to reach for the other (29). This phenomenon appears specific to active engagement, as infants failed to encode the goal-object following a matched observational training condition (40).
These and related findings (34) are consistent with the hypothesis that experience producing actions plays a central role in the emergence of action understanding in infancy, and raise questions concerning the nature of this contribution. First, information acquired from action production could be represented at various levels of abstraction: the low-level kinematic properties of the executed movements, the causal or functional properties exploited in executing the action, and the abstract goal to which the actions are directed. If early action understanding relies on a motor resonance mechanism that binds sensory effects to actions represented at a kinematic level, infants should initially fail to generalize goals to actions with different motoric properties. The present studies test whether infants derive information from their own actions at a level of abstraction that supports generalization to kinematically distinct actions.
Second, Sommerville et al.’s results (39) suggest that motorically trained 3-mo-old infants encode goal-relevant properties of an action (i.e., its end-state), but these results do not distinguish the information infants gain from their own actions from the knowledge of goal-directed action they might possess before the training experience. Specifically, it is unknown whether their goal representations are already constrained by the efficiency-based schema that structures action representation later in development. Identifying an agent’s goal in a particular instance can rely on abstract knowledge of others’ goals and behavior (principles of rational action), as well as more specific information relevant to the particular goal context (e.g., knowledge of the kinds of goals people tend to have in a given situation and of the causal relations that link actions to their effects on the environment). Do infants derive one or more of these pieces of information from their own action production, or is this information available to infants independently of the training experience?
Importantly, intervention on infants’ action experiences can provide two distinct windows into the origins of goal representation. First, with these interventions, we can identify developmental experiences that contribute to successful goal attribution by manipulating these experiences in a controlled manner. Second, by enabling infants to identify an agent’s goal much earlier than they otherwise could, these paradigms allow us to investigate whether infants, upon gaining the ability to identify simple reaching goals, exhibit more general expectations have about how those goals will be pursued. By probing infants’ knowledge about intentional action as they first become capable of inferring a goal in a particular instance, we can gain insights into the prior assumptions prereaching infants bring to the training experience. Thus, the second aim of the present studies was to investigate whether information derived from actions produced during training phase interacts with more general principles that are in place independent of that experience, including the assumption that actions are efficient and subject to physical constraints.
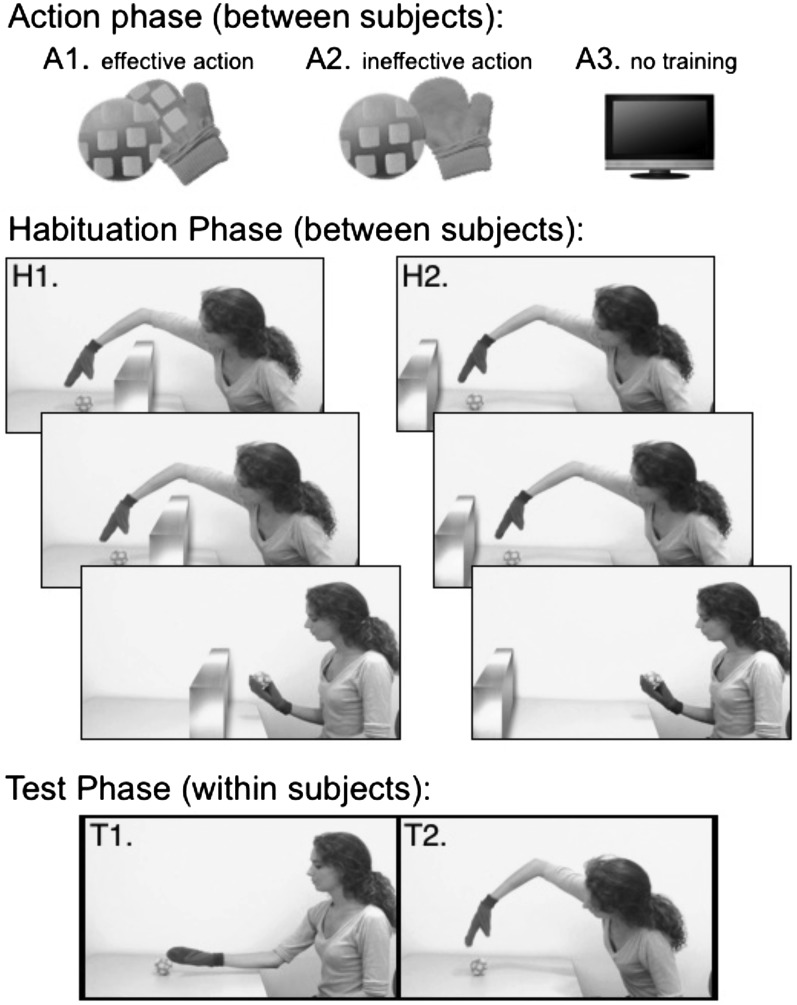
Here, we adopt the training manipulation used by Sommerville et al. (39) and test infants with a paradigm that requires flexibly updating action expectations based on the presence or position of physical obstacles (5). Across five experiments, we manipulate 3-mo-old infants’ experience producing simple object-retrieval actions and test their sensitivity to the efficiency of reaching actions as measured by looking time (Fig. 1). In experiments 1 to 3 (discussed first), infants were assigned to one of three training manipulations: an effective action condition (experiment 1) in which they interact with Velcro-covered objects while wearing Velcro-covered mittens, an ineffective action condition (experiment 2) in which they interact with the same objects while wearing mittens that lack Velcro, and a no-training condition (experiment 3) in which infants perform the looking time task without any prior exposure to the objects. Infants in all three experiments participated in the same violation-of-expectation task (43) in which they were habituated to an agent reaching in an arched path over a barrier to grasp and retrieve an object. If infants understand that hands cannot reach through solid barriers, the arched path can be seen as an efficient means of retrieving the object. The barrier was then removed and the agent pursued the goal with a familiar but now inefficient arched reach, or with a novel but efficient direct reach. In experiments 4 and 5 (discussed second), we aimed to replicate the results of experiment 1 and confirm that they depend on an analysis of the efficiency of the reaching action with respect to physical constraints of the environment.
Fig. 1.
In experiment 1, subjects participated in effective action training (A1) followed by a constrained action habituation phase (H1) and a test phase involving three efficient action displays (T1) and three inefficient action displays (T2), presented in alternation. In experiments 2 and 3, subjects viewed the same sequence of habituation and test displays, but preceded by the ineffective action training (A2) and no training (A3), respectively. Experiment 4 replicated experiment 1 (A1, H1). In experiment 5, subjects participated in the effective action training (A1) followed by an unconstrained action habituation phase (H2). All five experiments present the identical test displays (T1 and T2).
Results
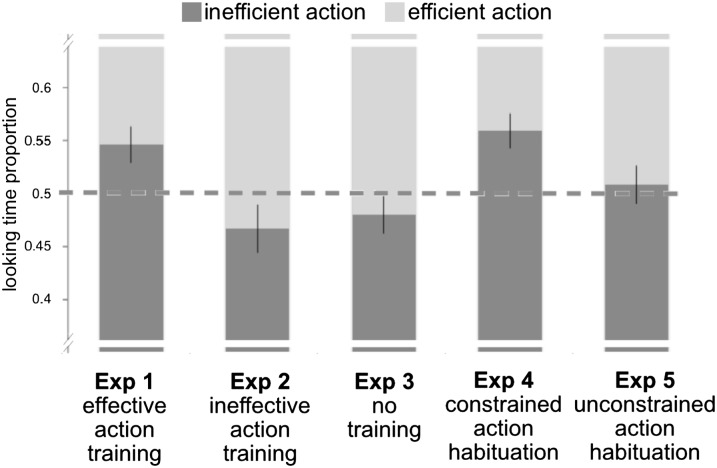
The proportion of looking to the inefficient action event relative to the efficient action event was calculated for each test pair and averaged across all three test pairs for each subject. Results are displayed in Fig. 2. Comparing experiments 1 through 3, we observed a significant difference in the proportion of looking to the inefficient action across the training groups [F(2, 57) = 4.794, P = 0.012]. One-sample t tests comparing to a chance level of 0.5 revealed a significantly greater proportion of looking to the inefficient action in the effective action training condition [t(19) = 2.669, P = 0.015], but not in the ineffective action training condition [t(19) = −1.477, P = 0.156], nor the no-training condition [t(19) = −1.122, P = 0.276]. The proportion of looking to the inefficient action was higher in the effective action condition than in the ineffective action condition [t (38) = 2.791, P = 0.008] or the no-training condition [t(38) = 2.658, P = 0.011]. Analysis of raw looking times yielded comparable results (SI Data, Section 1.2).
Fig. 2.
Proportion of looking time to inefficient vs. efficient action displays across experiments 1 to 5 (n = 112). Error bars reflect ±1 SEM.
Thus, following training in which they were able to manipulate and retrieve objects, infants expected an agent to take the shortest available path to the demonstrated goal, exhibiting heightened attention when an agent performed a perceptually familiar but newly inefficient reach. This effect was absent in infants given no experience, or experience in which their actions were ineffective, who tended to look longer at the kinematically novel straight reach. Reaching for and retrieving objects evidently provides infants with information needed to encode the goal of another agent’s action, even when that action is kinematically distinct from those performed during training. Having identified the agent’s goal, infants then expect it to be pursued with efficient means.
Previous research suggests that older, more motorically capable infants expect a direct path only when a previously demonstrated indirect path could be explained in terms of physical constraints on the action (5). If an agent previously reached on an indirect path in the absence of any barrier, infants did not represent the action as directed toward the goal object. In experiment 4, we replicate the procedure of experiment 1 and compare it to experiment 5, in which infants undergo effective action training followed by a habituation phase in which the arched path of the agent’s reach is not necessitated the physical constraints of the environment, and therefore cannot readily be interpreted as directed toward the object. If infants trained with effective actions look longer to the arching reach because it is inefficient with respect to a previously demonstrated goal, and understand that solid objects constrain action, then looking time to the very same test displays should differ depending on whether an obstacle was present (constrained action condition, experiment 4) or absent (unconstrained action condition, experiment 5) during the habituation phase. These experiments also address two alternative explanations of the effects in experiment 1. First, infants in the effective action condition may have performed more straight object-directed reaches than infants in the ineffective action condition, and then reacted to the low-level similarity between the actions they produced during the training phase and those viewed during the test events. Second, infants in the effective action condition may have become more attentive to objects in the agent’s environment. During both test displays, the barrier present during habituation is removed; if infants represented the inefficient test display as an otherwise familiar event in which an object has disappeared, they might find this event more interesting than the efficient test display in which the barrier has disappeared but the action is also different. If infants in the effective action condition showed heightened attention to an arching reach test event because this reach was perceptually dissimilar from the actions they produced during the training or because the disappearance of the barrier was more salient in this event, infants in experiment 4 and experiment 5 should show this looking preference.
Experiments 4 and 5 suggest that infants are indeed sensitive to the efficiency of the reaching actions rather than more superficial properties of the events, as their patterns of looking depended on the constraints on action during the initial goal familiarization. At test, there was a significant difference in the proportion of looking to the efficient reaching action across the two habituation groups [F(1, 50) = 4.241, P = 0.045], with infants in the constrained action condition (experiment 4) looking longer at the inefficient actions [t(25) = 3.539, P = 0.002], replicating the results of experiment 1, whereas those in the unconstrained action condition showed no looking preferences at test [t(25) = 0.440, P = 0.663; SI Data, Section 1.2, includes analysis of raw looking times]. Experiment 4 therefore replicated the results of experiment 1, and experiment 5 revealed that infants identified the object as the actor’s goal only when her actions were efficient with respect to that goal during the habituation phase.
These findings provide evidence that subjects encoded the reach as directed toward the object only when the indirect path observed in the habituation phase could be explained by the physical constraints posed by the obstacle. It is therefore unlikely that the pattern of looking times observed in experiments 1 through 3 was a result of the visual similarity between movements produced during training and those observed in the test events. In experiments 4 and 5, infants were presented with the same training and the same test displays, but exhibited increased looking to inefficient reaches only when a barrier constrained action during habituation. Thus, prereaching infants, given experience in the absence of barriers, interpret others’ actions in accord with assumptions of solidity (agents cannot pass through solid obstacles) and efficiency (agents will reach on the most direct unimpeded path).
Discussion
Together, these results accord with previous research in finding that 3-mo-old infants extract the goal of simple object-directed actions following active reaching experience. Motorically experienced infants not only encode goal-relevant properties of observed actions (39, 40), but are also sensitive to the directness of actions toward that goal. These expectations emerge only when the agent’s initial arched reach is necessitated by a physical barrier, suggesting that infants represent actions as subject to physical constraints of the environment.
These findings provide two important constraints on the nature of the relationship between action production and comprehension in infancy. First, infants themselves can reach directly for the objects in the training task, yet must generalize what is learned to an action in which an agent takes an arched path over a barrier. This generalization does not reflect a simple failure to distinguish between direct and indirect paths, as infants exhibit sensitivity to these differing trajectories in the test phase. Thus, the effects of first-person action experience do not require alignment between the trajectories of the observed action and the motor act performed by the infant, casting doubt on theories that explain early action processing in terms of a direct mapping of observed actions onto the motoric/kinematic properties of executed acts (15) or their associated bodily configurations (14).
Furthermore, these data constrain the scope of what could be learned during first-person action production. Infants in the present experiment could reach freely for objects and did not need to update their actions based on context. The training itself is therefore unlikely to have provided a basis for learning that goal-directed actions are efficient and limited by physical obstacles, making it implausible that an understanding of efficient goal-directed action was constructed de novo during the training phase. The observed efficiency expectations might instead reflect innate constraints on an evolved action perception system, or regularities learned from visual analysis of others’ actions over the first 3 mo of life. Of course, the present results can only directly speak to the role of the experience manipulated during the experiment: thus, we cannot exclude the possibility that an efficiency assumption is learned from motor production before the training. However, given the limited motor repertoire available at this age, we find it unlikely that such expectations could be derived solely from first-person motor experience.
Either way, the present findings suggest that effects of first-person experience observed in previous studies (39) may not reflect the initial construction of goal knowledge; instead, manipulations of first-person experience likely provide information necessary for the expression of this knowledge. Crucially, an observer can only evaluate the efficiency of an action with respect to a specific goal and specific environmental constraints—if infants lacked information relevant to identifying the goal, they would be unable to analyze the efficiency of the action even if solidity and efficiency assumptions were in place as abstract constraints on infants’ action interpretation. If experience producing simple reaching actions provides infants with information relevant to inferring the goal of a given object-directed reach, more general knowledge about solidity and efficiency could be applied as soon as the agent’s particular goal has been identified.
Relation to Motor Theories
Based on effects of first-person experience on processing of others’ actions (34, 39) and on overlap in the neural representations recruited during action production and perception (17, 18), it has been argued that action understanding depends on reactivation of motor representations derived from first-person experience. Motor resonance and “direct matching” theories propose that action understanding requires linking visual input to motor representations in premotor and parietal cortices and perhaps running forward simulations on these motor chains (18, 25, 44). Although advocates of these theories grant that alternative mechanisms (e.g., nonmotoric regions that show heightened response to inefficient actions; ref. 45) may exist (46), they argue that the ability to map observed actions directly onto first-person motor representations provides a privileged and developmentally foundational route to understanding to others’ intentions (13, 20).
Importantly, the leanest direct matching version of the motor resonance model (18, 20) cannot account for goal identification in situations that require integrating nonmotoric situational information (in particular, information about the constraints on the agent) in interpreting an action. Context-specificity can be incorporated in an ideomotor model by assuming that action-effect relationships are learned in a state-dependent manner (22). However, although a store of such contingencies could eventually support context-sensitive action interpretation, this learning would require large corpora of action statistics wherein the action–effect pairings are learned separately under each possible environmental state. If infants adapt their expectations to physical constraints on an agent only by learning separate action–effect associations under different environmental conditions, producing actions under a fixed set of constraints should fail to provide a benefit in the looking time task used here. In the present experiment, subjects were sensitive to the context in which the action was executed (i.e., whether the barrier constrained the action or not) even though no obstacles were present in the training phase. Infants could not solve this task by learning specific associations between particular actions and effects under particular environmental conditions, but rather must have integrated more general assumptions of efficiency and solidity to analyze the observed actions. These results suggest that infants possess a general schema for relating actions to effects across constraint variation that they themselves have not experienced. Thus, even the very earliest evidence for goal-based action processing suggests representations more abstract than those assumed by dominant motor resonance theories. Others have adopted a broader definition of “motor” simulation that includes representations abstracted away from the kinematic properties of actions, encompassing functional or causal information about how physical movements generate effects on the environment (47, 48). These accounts are broadly consistent with the perspective provided here in that they aim to explain how top-down constraints enable the identification of abstract goals from visual representations of kinematics.
What Are Infants Learning?
We find it unlikely that infants learn a general principle of efficiency on the basis of information provided during the training phase, as they themselves did not face varying environmental constraints. Why, then, do infants exhibit sensitivity to efficiency only following action training? One possibility is that successfully reaching for and displacing objects may heighten infants’ attention to events in which others engage in similar actions. Having attended to the events, infants may then apply an assumption of efficient action to identify the agent’s goal. Alternatively, infants may learn about specific properties of action from their own action experience. In this case, we must distinguish between learning the concept of a goal itself, and acquiring information relevant to identifying a particular goal in a given instance. Recent computational models that characterize goal inference as “inverse planning” (49, 50) help to make this distinction explicit. According to these models, an observer can predict an agent’s movements based on knowledge of what the agent wants (i.e., the value associated with particular states of the world), causal knowledge of which action sequences can generate desired states, and knowledge of the costs associated with particular actions (by assuming that agents will maximize expected utility). Goals can be inferred from observed movements by inverting this forward planning schema and integrating it with prior knowledge about what goals are likely in a given context.
According to this inverse planning framework, goal attribution depends on the general inferential schema that represents actions as directed toward goal states and assumes efficient pursuit of those states (i.e., general principles of rational action), but also on information specific to a given action and context. First, the observer must have knowledge of the relationships between particular actions and their effects. In the model of Baker et al. (49), forward planning computations operate over a hard-coded state transition distribution, which specifies the probability that taking a particular action A in a particular state S will generate state S′. This knowledge of how actions relate to states of the world can be thought of as a simple representation of the relevant causal structure of the environment, which is needed to implement forward and inverse planning in a given action context.
With respect to the present results, it is possible that active training provides infants with causal knowledge relevant to understanding the structure of grasping actions. Perhaps infants learn, for example, that a hand making contact with an object can successfully entrain the object and displace it toward the body. When infants understand the basic causal relations that structure reaching actions, they can interpret the observed actions of other agents as a means of bringing about the goal state of retrieval. This view is consistent with the results of a recent study (51) in which 12-mo-old infants’ sensitivity to the functional affordances of different grasp configurations was related to infants’ own grasp production. Only infants who produced precision grasps themselves appeared to detect when another agent initiated a goal-directed action with a causally inappropriate grasp configuration (i.e., a precision grasp for lifting an inverted bowl; see also ref. 52). Another prerequisite knowledge structure in the model of Baker et al. (49) is a probability distribution over possible goals. For the inversion of forward planning to generate a unique solution, the observer needs some constraints on the goals to be entertained. One possibility is that infants initially lack certain knowledge of which goals are likely in a given context and that first-person experience raises infants’ representation of the probability of object-directed goals, constraining the set of possible goals they consider and evaluate against the observed action.
Alternatively, infants might already possess the information needed to identify the actor’s goal, but be unable to analyze the efficiency of the action toward that goal state. Infants might learn, for example, about the cost associated with different reaches (e.g., via variability in their own reaching, learn that longer paths are more effortful). Infants would then need to generalize cost information from these executed actions to the actions observed in the looking time task, which raises the more general question of how observers identify the costs of actions outside of their motor repertoire (discussed in refs. 11, 26). Another possibility is that the training heightens infants’ sensitivity to environmental constraints: in the test trials, the barrier present during the habituation phase was removed. If training leads infants to be more attentive to the environment surrounding theirs and others’ actions, it is possible that only the trained infants notice the disappearance of the barrier from an otherwise familiar arching reach event (and represent the direct reach as a novel event altogether). However, because the effect is observed only following constrained-action habituation, we would have to assume that the trained infants are uniquely attentive to objects that stand between an agent and her goal. Such selective attention to objects that function as barriers would imply that infants represent objects as constraints on possible actions (where the notion of constraint itself depends on an assumption that actions are efficient).
Although the present results do not bear on these alternatives directly, the training experiences available in experiments 1 and 2 mostly differ in the extent to which infants successfully entrained and retrieved the objects. The fact that infants in effective and ineffective action conditions were exposed to the objects, and presumably experienced the goal of contacting them, lends credence to the hypothesis that infants are learning about the causal affordances of the newly effective hand. However, further research is needed to directly test these and other possibilities. The present experiments nonetheless cast doubt on the proposal that manipulations of first-person action experience (39, 40) facilitate the construction of the very concept of a goal or the principle of rational action. Instead, we suggest that these interventions provide infants with more specific knowledge that enables them to identify the agent’s goal, and that infants can then reason about this goal in accordance with more general assumptions that exist independently of the manipulated action experience.
Materials and Methods
Experiments 1 to 3.
Participants.
A total of 60 infants (Meanage = 3.60 mo; range, 91–122 d) were randomly assigned to one of three training conditions (effective action condition, n = 20; ineffective action condition, n = 20; or no-training condition, n = 20; SI Procedures, Section 2.1, includes recruitment and inclusion information).
Materials and procedure.
In the training phase of experiments 1 and 2, subjects were seated on a caregiver’s lap at an 86 × 58-cm table. Hands were fitted with gray mittens that were or were not covered with Velcro (effective and ineffective action conditions, respectively). Subjects were then given 3 min to freely interact with a Velcro-covered ball and bear (SI Procedures, Section 2.2). Infants in the no-training condition were not exposed to the objects and proceeded immediately to the looking time assessment.
In the violation-of-expectation task, video stimuli were presented with Keynote software on a 62 × 38-cm LCD screen. Each trial ended when subjects looked away from the stimulus for 2 s consecutively, or if they looked for a cumulative 45 s (SI Procedures, Section 2.3, provides details of presentation and coding procedures). During the habituation phase, each video clip (∼5 s duration) began with a person wearing a gray mitten seated at a table facing a barrier that stood between the person and a Velcro-covered ball (the same ball used during training). The person then reached in an arching manner over the barrier to retrieve the object, and the video froze on the final frame of the person holding the ball in front of her torso. The barrier varied in height over the course of habituation trials (SI Data, Section 1.1, provides analysis of habituation data), and the height of the person’s reach aligned with that of the barrier. Infants then viewed a transition trial in which the barrier was removed and only the table and the object remained. During the test phase, the barrier remained absent and the person reached with the same arching path viewed during habituation (no longer an efficient means) or with a perceptually novel straight path (now efficient). Subjects viewed three efficient reaches in alternation with three inefficient reaches, with order of efficient and inefficient trials counterbalanced across subjects.
Experiments 4 and 5.
Participants.
A total of 52 infants (Meanage = 3.56 mo; range, 93–122 d) were randomly assigned to one of two conditions (constrained action, n = 26; unconstrained action, n = 26; SI Procedures, Section 2.1).
Materials and procedure.
All subjects participated in the effective action training condition, replicating the training procedure described earlier (SI Procedures, Section 2.4, provides a description of minor changes to the procedure). The displays used in experiment 4 (constrained action during habituation) were identical to those in experiment 1. The displays in experiment 5 (unconstrained action during habituation) were identical except that the barrier was placed on the opposite side of the goal object during the habituation events such that it did not constrain the person’s reaching action. Test events were as in experiments 1 to 3.
Supplementary Material
Acknowledgments
The authors thank R. Saxe, J. Koster-Hale, L. Powell, and H. Richardson, M. Cohen, T. Ullman, and J. Tenenbaum for helpful comments and discussion. Research was supported by National Institutes of Health Grant 5R01 HD23103 (to E.S.S.) and a National Science Foundation Graduate Research Fellowship Program Grant (to A.E.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312322110/-/DCSupplemental.
References
- 1.Dickinson A, Balleine B. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18. [Google Scholar]
- 2.Gopnik A, Wellman HM. Why the child’s theory of mind really is a theory. Mind Lang. 1992;7(1‐2):145–171. [Google Scholar]
- 3.Dennett DC. The Intentional Stance. Cambridge, MA: MIT Press; 1987. [Google Scholar]
- 4.Gergely G, Csibra G. Teleological reasoning in infancy: The naive theory of rational action. Trends Cogn Sci. 2003;7(7):287–292. doi: 10.1016/s1364-6613(03)00128-1. [DOI] [PubMed] [Google Scholar]
- 5.Gergely G, Nádasdy Z, Csibra G, Bíró S. Taking the intentional stance at 12 months of age. Cognition. 1995;56(2):165–193. doi: 10.1016/0010-0277(95)00661-h. [DOI] [PubMed] [Google Scholar]
- 6.Csibra G. Goal attribution to inanimate agents by 6.5-month-old infants. Cognition. 2008;107(2):705–717. doi: 10.1016/j.cognition.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Gergely G, Bekkering H, Király I. Rational imitation in preverbal infants. Nature. 2002;415(6873):755. doi: 10.1038/415755a. [DOI] [PubMed] [Google Scholar]
- 8.Paulus M, Hunnius S, Vissers M, Bekkering H. Imitation in infancy: Rational or motor resonance? Child Dev. 2011;82(4):1047–1057. doi: 10.1111/j.1467-8624.2011.01610.x. [DOI] [PubMed] [Google Scholar]
- 9.Király I, Csibra G, Gergely G. Beyond rational imitation: Learning arbitrary means actions from communicative demonstrations. J Exp Child Psychol. 2013;116(2):471–486. doi: 10.1016/j.jecp.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott RM, Baillargeon R. Do infants really expect agents to act efficiently? A critical test of the rationality principle. Psychol Sci. 2013;24(4):466–474. doi: 10.1177/0956797612457395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csibra G, Gergely G. Teleological action understanding in infancy. In: Banaji M, Gelman S, editors. Navigating the Social World: What Infants, Children, and Other Species Can Teach Us. New York: Oxford Univ Press; 2013. pp. 38–43. [Google Scholar]
- 12.Woodward AL. Infant foundations of intentional understanding. In: Banaji M, Gelman S, editors. Navigating the Social World: What Infants, Children, and Other Species Can Teach Us. New York: Oxford Univ Press; 2013. pp. 75–80. [Google Scholar]
- 13.Gallese V, Rochat M, Cossu G, Sinigaglia C. Motor cognition and its role in the phylogeny and ontogeny of action understanding. Dev Psychol. 2009;45(1):103–113. doi: 10.1037/a0014436. [DOI] [PubMed] [Google Scholar]
- 14.Meltzoff AN. Origins of social cognition: Bidirectional self-other mapping and the “Like-Me” hypothesis. In: Banaji M, Gelman S, editors. Navigating the Social World: What Infants, Children, and Other Species Can Teach Us. New York: Oxford Univ Press; 2013. pp. 139–144. [Google Scholar]
- 15.van Elk M, van Schie HT, Hunnius S, Vesper C, Bekkering H. You’ll never crawl alone: Neurophysiological evidence for experience-dependent motor resonance in infancy. Neuroimage. 2008;43(4):808–814. doi: 10.1016/j.neuroimage.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 16.Paulus M. Action mirroring and action understanding: an ideomotor and attentional account. Psychol Res. 2012;76(6):760–767. doi: 10.1007/s00426-011-0385-9. [DOI] [PubMed] [Google Scholar]
- 17.Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res. 1996;3(2):131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 18.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 19.Bonini L, Ferrari PF. Evolution of mirror systems: A simple mechanism for complex cognitive functions. Ann N Y Acad Sci. 2011;1225:166–175. doi: 10.1111/j.1749-6632.2011.06002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Giudice M, Manera V, Keysers C. Programmed to learn? The ontogeny of mirror neurons. Dev Sci. 2009;12(2):350–363. doi: 10.1111/j.1467-7687.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 21.Press C, Heyes C, Kilner JM. Learning to understand others’ actions. Biol Lett. 2011;7(3):457–460. doi: 10.1098/rsbl.2010.0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulus M, Hunnius S, van Elk M, Bekkering H. How learning to shake a rattle affects 8-month-old infants’ perception of the rattle’s sound: Electrophysiological evidence for action-effect binding in infancy. Dev Cogn Neurosci. 2012;2(1):90–96. doi: 10.1016/j.dcn.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hommel B, Müsseler J, Aschersleben G, Prinz W. The Theory of Event Coding (TEC): A framework for perception and action planning. Behav Brain Sci. 2001;24(5):849–878. doi: 10.1017/s0140525x01000103. [DOI] [PubMed] [Google Scholar]
- 24.Fogassi L, et al. Parietal lobe: From action organization to intention understanding. Science. 2005;308(5722):662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- 25.Chersi F, Ferrari PF, Fogassi L. Neuronal chains for actions in the parietal lobe: A computational model. PLoS ONE. 2011;6(11):e27652. doi: 10.1371/journal.pone.0027652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Southgate V, Johnson MH, Csibra G. Infants attribute goals even to biomechanically impossible actions. Cognition. 2008;107(3):1059–1069. doi: 10.1016/j.cognition.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Gerson S, Woodward AL. Building intentional action knowledge with one’s hands. In: Johnson SP, editor. Neo-Constructivism. New York: Oxford Univ Press; 2010. [Google Scholar]
- 28.Woodward AL. Infants’ grasp of others’ intentions. Curr Dir Psychol Sci. 2009;18(1):53–57. doi: 10.1111/j.1467-8721.2009.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Hofsten C. Predictive reaching for moving objects by human infants. J Exp Child Psychol. 1980;30(3):369–382. doi: 10.1016/0022-0965(80)90043-0. [DOI] [PubMed] [Google Scholar]
- 30.Adolph KE, Vereijken B, Denny MA. Learning to crawl. Child Dev. 1998;69(5):1299–1312. [PubMed] [Google Scholar]
- 31.Cicchino JB, Aslin RN, Rakison DH. Correspondences between what infants see and know about causal and self-propelled motion. Cognition. 2011;118(2):171–192. doi: 10.1016/j.cognition.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carey S. The Origin of Concepts. New York: Oxford Univ Press; 2009. [Google Scholar]
- 33.Wood JN, Glynn DD, Phillips BC, Hauser MD. The perception of rational, goal-directed action in nonhuman primates. Science. 2007;317(5843):1402–1405. doi: 10.1126/science.1144663. [DOI] [PubMed] [Google Scholar]
- 34.Sommerville JA, Hildebrand EA, Crane CC. Experience matters: The impact of doing versus watching on infants’ subsequent perception of tool-use events. Dev Psychol. 2008;44(5):1249–1256. doi: 10.1037/a0012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sommerville JA, Woodward AL. Pulling out the intentional structure of action: The relation between action processing and action production in infancy. Cognition. 2005;95(1):1–30. doi: 10.1016/j.cognition.2003.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodward AL, Guajardo JJ. Infants’ understanding of the point gesture as an object-directed action. Cogn Dev. 2002;17:1061–1084. [Google Scholar]
- 37.Cannon EN, Woodward AL, Gredebäck G, von Hofsten C, Turek C. Action production influences 12-month-old infants’ attention to others’ actions. Dev Sci. 2012;15(1):35–42. doi: 10.1111/j.1467-7687.2011.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gredebäck G, Kochukhova O. Goal anticipation during action observation is influenced by synonymous action capabilities, a puzzling developmental study. Exp Brain Res. 2010;202(2):493–497. doi: 10.1007/s00221-009-2138-1. [DOI] [PubMed] [Google Scholar]
- 39.Sommerville JA, Woodward AL, Needham A. Action experience alters 3-month-old infants’ perception of others’ actions. Cognition. 2005;96(1):B1–B11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerson SA, Woodward AL. Learning from their own actions: the Unique effect of producing actions on infants’ action understanding. Child Dev. 2013 doi: 10.1111/cdev.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rochat P. Object manipulation and exploration in 2- to 5-month-old infants. Dev Psychol. 1989;25:871–884. [Google Scholar]
- 42.Woodward AL. Infants selectively encode the goal object of an actor’s reach. Cognition. 1998;69(1):1–34. doi: 10.1016/s0010-0277(98)00058-4. [DOI] [PubMed] [Google Scholar]
- 43.Phillips AT, Wellman HM. Infants’ understanding of object-directed action. Cognition. 2005;98(2):137–155. doi: 10.1016/j.cognition.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 45.Brass M, Schmitt RM, Spengler S, Gergely G. Investigating action understanding: Inferential processes versus action simulation. Curr Biol. 2007;17(24):2117–2121. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 46.Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nat Rev Neurosci. 2010;11(4):264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- 47.Kilner JM, Friston KJ, Frith CD. The mirror-neuron system: A Bayesian perspective. Neuroreport. 2007;18(6):619–623. doi: 10.1097/WNR.0b013e3281139ed0. [DOI] [PubMed] [Google Scholar]
- 48.Grafton ST, Hamilton AF. Evidence for a distributed hierarchy of action representation in the brain. Hum Mov Sci. 2007;26(4):590–616. doi: 10.1016/j.humov.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker CL, Saxe R, Tenenbaum JB. Action understanding as inverse planning. Cognition. 2009;113(3):329–349. doi: 10.1016/j.cognition.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Ng AY, Russell S. ICML ‘00 Proceedings of the Seventeenth International Conference on Machine Learning. San Francisco: Morgan Kaufmann; 2000. Algorithms for inverse reinforcement learning; pp. 663–670. [Google Scholar]
- 51.Loucks J, Sommerville JA. The role of motor experience in understanding action function: The case of the precision grasp. Child Dev. 2012;83(3):801–809. doi: 10.1111/j.1467-8624.2012.01735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rakison DH, Krogh L. Does causal action facilitate causal perception in infants younger than 6 months of age? Dev Sci. 2012;15(1):43–53. doi: 10.1111/j.1467-7687.2011.01096.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.