Significance
This study discloses a role for Numb in the activation and proliferation of adult muscle satellite cells and a unique function in the regulation of the muscle mass determinant Myostatin. Using two different genetic approaches to ablate Numb, one that ablated Numb in the myogenic lineage developmentally leading to reduced muscle mass. We determined that, in Numb-deficient muscle, regeneration was impaired, there was reduced stem cell proliferation, and there was an up-regulation of Myostatin. Overexpression of Numb suppressed Myostatin expression, and Myostatin-specific siRNA rescued the proliferation defect. These studies increase our knowledge of the signaling pathways involved in stem cell function and raise the possibility of regulating the Numb/Myostatin balance as a therapeutic approach to enhance muscle regeneration.
Keywords: myogenesis, stem cell, skeletal muscle, conditional mutation
Abstract
The adaptor protein Numb has been implicated in the switch between cell proliferation and differentiation made by satellite cells during muscle repair. Using two genetic approaches to ablate Numb, we determined that, in its absence, muscle regeneration in response to injury was impaired. Single myofiber cultures demonstrated a lack of satellite cell proliferation in the absence of Numb, and the proliferation defect was confirmed in satellite cell cultures. Quantitative RT-PCR from Numb-deficient satellite cells demonstrated highly up-regulated expression of p21 and Myostatin, both inhibitors of myoblast proliferation. Transfection with Myostatin-specific siRNA rescued the proliferation defect of Numb-deficient satellite cells. Furthermore, overexpression of Numb in satellite cells inhibited Myostatin expression. These data indicate a unique function for Numb during the initial activation and proliferation of satellite cells in response to muscle injury.
Satellite cells represent a muscle-specific stem cell population that allows for muscle growth postnatally and is necessary for muscle repair (1). In response to muscle-fiber damage, quiescent satellite cells that lie along the myofibers under the plasmalemma are activated and proliferate. Proliferating satellite cells have a binary fate decision to make—they can differentiate into myoblasts and intercalate into myofibers by fusion to repair the damaged muscle or they can renew the satellite cell population and return to a quiescent state (2–4). Quiescent satellite cells express paired box 7 (Pax7), but low or undetectable levels of the myogenic regulatory factors Myf5 and MyoD (5, 6). Activated satellite cells robustly express Pax7 and MyoD/Myf5, but a subset will subsequently down-regulate the myogenic regulatory factors in the process of satellite cell self-renewal (7). Recent studies have demonstrated that, in vivo, Pax7-positive cells are necessary for muscle repair (8, 9).
Notch signaling is an important regulator of satellite cell function; it is implicated in satellite cell activation, proliferation (2, 10, 11), and maintenance of quiescence (12, 13). Expression of constitutively active Notch1 results in maintenance of Pax7 expression and down-regulation of Myod/Myf5 whereas inhibition of Notch signaling leads to myogenic differentiation (10, 14). In fact, conditional ablation of Rbpj embryonically results in hypotrophic muscle (15), and, if ablated in the adult, satellite cells undergo spontaneous activation and precocious differentiation with a failure of self-renewal (12, 13). In adult muscle, the Notch ligand, Delta-like1 (Dll1), is expressed on satellite cells, myofibers, and newly differentiating myoblasts and is necessary for repair (10, 11, 16). In aged muscle, impairment of regeneration is due, in part, to a failure of Dll1 expression (17).
Numb en`s four proteins with molecular masses of 65, 66, 71, and 72 kDa by alternative splicing of two exons (18, 19). The Numb proteins are cytoplasmic adaptors that direct ubiquitination and degradation of Notch1 by recruiting the E3 ubiquitin ligase Itch to the receptor (18–22). Numb is a cell-fate determinant that mediates asymmetric cell division, leading to selective Notch inhibition in one daughter cell and its subsequent differentiation whereas the other daughter has active Notch signaling and remains proliferative (10). Embryonically, Numb is expressed in the myotome whereas Notch1 is limited to the dermomyotome (23, 24). This pattern suggests that the expression of Numb in one daughter cell allows entry into the myogenic lineage. Indeed, overexpression of Numb embryonically increases the number of myogenic progenitors in the somite (25, 26).
Numb expression increases during the activation and proliferative expansion of satellite cells, becoming asymmetrically segregated in transit-amplifying cells and leading to asymmetric cell divisions (10, 27). These observations led to a model in which Numb inhibits Notch signaling in one daughter satellite cell, allowing it to undergo myogenic differentiation. The molecular switch that controls the decision of satellite cell progeny to continue proliferating or to differentiate is not well understood. This process seems to be controlled by a decrease of Notch signaling due to increased expression of Numb and an increase in Wnt signaling (10–14, 17, 28). In these studies, we examined the role of Numb in satellite cell function by genetic deletion of Numb from myogenic progenitors and satellite cells. Our observations reveal that Numb is necessary for satellite cell-mediated repair. Furthermore, Numb-deficient satellite cells have an unexpected proliferation defect due to an up-regulation of Myostatin. These data indicate a unique role for Numb in regulating the activation and proliferation of satellite cells.
Results
To determine whether Numb plays a critical role in muscle regeneration, it was genetically excised in the myogenic lineage using mice with Cre recombinase inserted in the Pax7 locus (Pax7ICNm) (29) interbred with mice with a floxed Numb allele that deletes exons 4 and 5 (30) and a Numblike (Nbl) null mutation (31, 32) (Fig. S1) (we will refer to the Pax7 ICNm /+ Numbfl/fl Numblike−/− mice as Pax7-Numbfl/fl Nbl−/−). When bred into the R26RYFP line (33), the recombination frequency in single-fiber culture was ∼100% of Pax7-positive (Pax7+ve) cells, based on YFP expression (Fig. S2 A and B). The high level of recombination was confirmed by FACS analysis detecting YFP+ve cells in the Vcam+ve/Cd45-ve /Cd31-ve /Sca1-ve population isolated from muscle pre- and post-BaCl2 injury (Fig. S2 C and D). The Pax7-Numbfl/fl Nbl−/− mice weighed significantly less than Numbfl/fl Nbl−/− or Pax7-Numbfl/+ Nbl+/− mice at 1 mo of age (Fig. S3). Also, the tibialis anterior (TA) muscles weighed significantly less, and their myofibers had a smaller average cross-sectional area (CSA) than controls (Fig. S3 C–F), indicating a potential role for Numb in the growth of muscle.
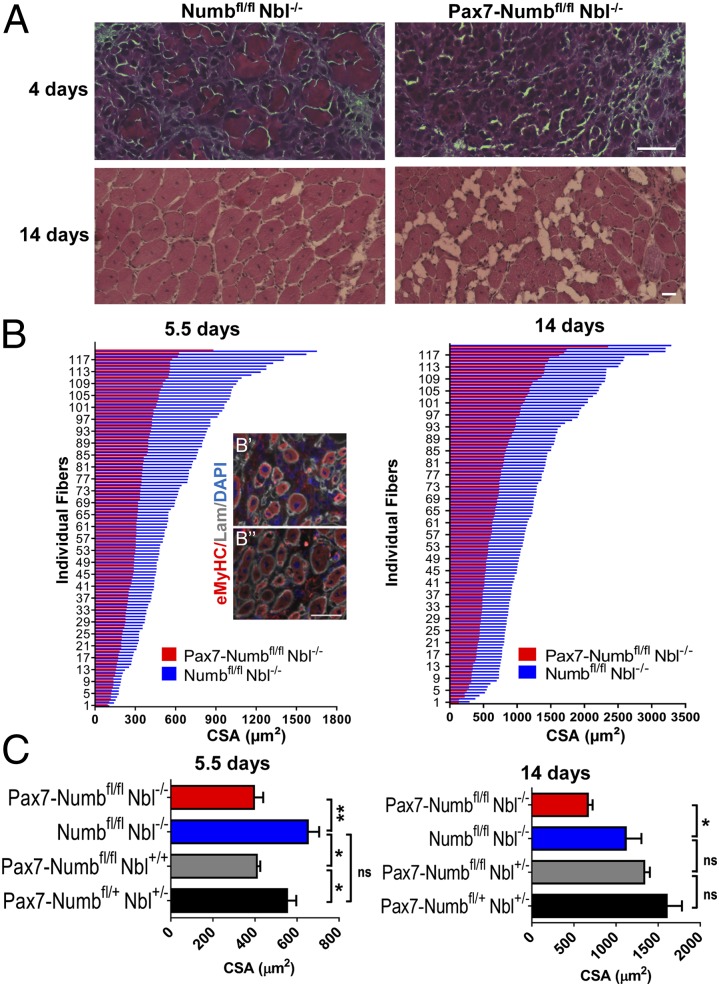
TA muscles of Pax7-Numbfl/fl Nbl−/− and Numbfl/fl Nbl−/− mice were injured with BaCl2 and harvested at different time points. At 5.5 d postinjury (dpi), there were significantly fewer embryonic myosin heavy chain (eMyHC) positive fibers detected in the Pax7-Numbfl/fl Nbl−/− muscle than in Numbfl/fl Nbl−/− controls (Fig. 1B). At 14 dpi, Numb/Nbl-deficient muscle demonstrated fatty deposition, increased endomysial connective tissue, and infiltration of fibrotic cells, but controls healed with no visible fibrotic or degenerative changes (Fig. 1A). Consistently, the CSA of regenerated fibers was significantly smaller in Pax7-Numbfl/fl Nbl−/− TA muscles than in Numbfl/fl Nbl−/− muscles (Fig. 1C). These differences persisted 2 mo postinjury (Fig. S4). These data indicate that the loss of Numb in the Pax7-lineage, including satellite cells, results in a defective repair response in adult muscle.
Fig. 1.
Reduced muscle mass in mice lacking Numb in the Pax7-lineage after injury. (A) TA muscles from Pax7-Numbfl/fl Nbl−/− and Numbfl/fl Nbl−/− adult mice isolated 4 or 14 d after BaCl2 injury and H&E stained. (Scale bars: 50 μm.) (B) CSA of regenerating fibers at 5.5 and 14 d post BaCl2 injury in muscles from Pax7-Numbfl/fl Nbl−/− and Numbfl/fl Nbl−/− littermates. Representative sections of Pax7-Numbfl/fl Nbl−/− and Numbfl/fl Nbl−/− TA muscles at 5.5 dpi stained with antibodies against eMyHC and Laminin (Lam) respectively in B′ and B′′. Nuclei were DAPI stained. (Scale bars: 50 μm.) (C) The mean of the average CSA calculated (n > 3) at 5.5 and 14 dpi. Pax7-Numbfl/fl Nbl−/− showed a statistically significant reduction in regenerating fiber size at both time points. When Numb, but not Nbl, was ablated, a significant reduction in fiber size was observed at 5.5 dpi but not 14 dpi. Data are the mean ± SEM. *P < 0.05; **P < 0.01; ns, not significant.
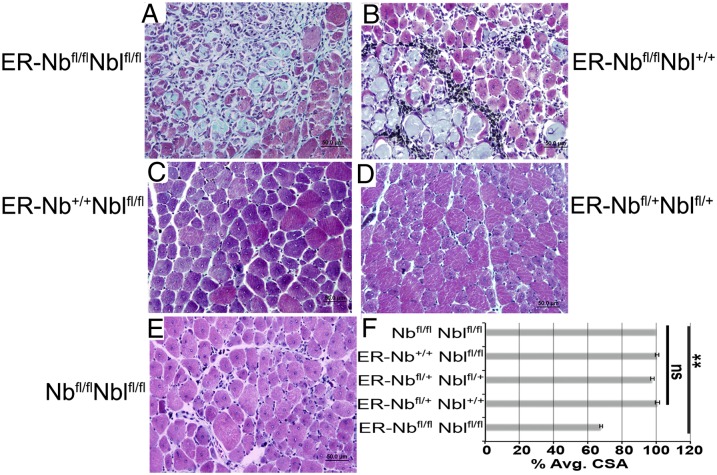
The observed deficits in myofiber size, muscle, and body weight may be due to the fact that Numb regulates the size of the pool of myogenic progenitor cells during development (25, 26). It was possible that a developmental deficit affected postnatal repair; therefore, mice with a ubiquitously expressed, tamoxifen (TMX)-inducible Cre recombinase transgene (CAGG ER-Cre, referred to as ER) (34, 35) were bred to mice with floxed alleles of Numb, deleting exon 1, and Numblike, deleting exons 3–5, Numbtm1zili/tm1zili Nbltm1zili/tm1zili, referred to as Nbfl/fl Nblfl/fl (Fig. S1) (36, 37). In these mice, Numb is expressed normally during growth, thus avoiding potential developmental effects. Adult mice were treated with TMX, and the quadriceps femoris (QF) muscles were injured with cardiotoxin (CTX) and were examined histologically at 10 dpi. In regenerating muscle from ER-Nbfl/fl Nblfl/fl and ER-Nbfl/fl Nbl+/+ mice, there were degenerative changes, including collagen deposition, myofiber degradation, and infiltration of fibrotic cells, that were not evident in ER-Nb+/+ Nblfl/fl, double heterozygous, or no Cre controls (Fig. 2 A–E). The CSA of regenerating myofibers was significantly smaller in ER-Nbfl/fl Nblfl/fl muscle than controls, consistent with that observed with Pax7-Numbfl/fl Nbl−/− mice (Fig. 2F). Our data indicate that, regardless of the method of injury, genetic lesion, Cre driver, or muscle examined, Numb-deficient muscle had a defective repair response. Although we cannot completely rule out a role for Numb in other cell types in regenerating muscle, the phenocopy of the defects noted using the ubiquitous and Pax7-specific Cre alleles indicates that Numb is intrinsically necessary for satellite cell function in regenerative myogenesis.
Fig. 2.
Impaired regeneration in muscle lacking Numb post-TMX induction of ER-Cre recombinase. (A–E) Representative sections show aberrant repair in Numb-deficient muscle including increased collagen deposition, fibrotic cells, and necrotic myofibers. TMX-treated mice had the left QF injected with CTX, and muscles were harvested 10 dpi and trichrome stained. Genotypes are as indicated (n = 5 per genotype). (Scale bars: 50 μm.) (F) The average CSA as a percentage of control muscle from a minimum of 200 fibers per muscle (n > 5 mice per genotype) at 10 dpi. ER-Nbfl/fl Nblfl/fl muscle had a significant decrease in fiber size compared with controls (**P < 0.0001).
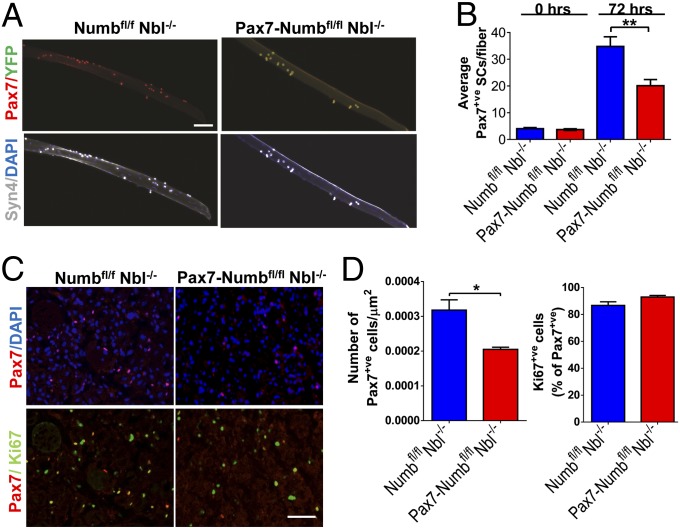
Numb, by virtue of its ability to regulate Notch signaling, has been implicated as part of the molecular switch that controls the decision of satellite cells to continue proliferating or to differentiate (10, 24–26, 28, 38–41). The current model posits that a loss of Numb should result in increased proliferation of the progenitor cells. A lack of differentiating satellite cells would result in aberrant repair. To examine proliferation, single muscle fibers were isolated from Pax7-Numbfl/fl Nbl−/− and Numbfl/fl Nbl−/− mice that were bred with the R26RYFP line (Fig. 3A). There was no significant difference in the average number of satellite cells per fiber in Numb-deficient muscles compared with controls at time 0. After 72 h in culture, Pax7-Numbfl/fl Nbl−/− fibers unexpectedly had significantly fewer satellite cells than controls (Fig. 3 A and B and Fig. S5). When muscles were examined at 4 dpi, there were significantly fewer Pax7+ve cells in sections of Pax7-Numbfl/fl Nbl−/− than in Numbfl/fl Nbl−/− mice (Fig. 3 C and D). However, the proportion of Pax7+ve cells that were also Ki67+ve was the same (Fig. 3D).
Fig. 3.
Decreased numbers of satellite cells in single-fiber cultures and injured muscles from mice lacking Numb in the Pax7-lineage. (A) Single fibers and associated satellite cells from EDL muscles of Numbfl/fl Nbl−/− and Pax7-Numbfl/fl Nbl−/− mice bred with R26RYFP mice were cultured for 66 h and stained with antibodies recognizing Syn4, Pax7, and GFP. Nuclei were DAPI stained. (Scale bar: 100 μm.) Note that ∼98% of the Syn4+ve cells also express Pax7. A majority of Pax7+ve satellite cells from Pax7-Numbfl/fl Nbl−/− are YFP+ve, but YFP was not detected in Numbfl/fl Nbl−/− mice. (B) Pax7+ve cells were quantified immediately (0 h) or after 72 h of culture (50 fibers per time point). Significantly fewer satellite cell progeny, P < 0.001, were found on the fibers from the Pax7-Numbfl/fl Nbl−/− muscle after 72 h. (C) Pax7 and Ki67 IF of TA muscles from Numbfl/fl Nbl−/− and Pax7-Numbfl/fl Nbl−/− mice 4 d after BaCl2 injury. Nuclei were stained with DAPI. (Scale bar: 50 μm.) (D) The average number of Pax7+ve cells per μm2 and the percentage of Pax7+ve cells that are Ki67+ve were quantified 4 d after BaCl2 injury. At least four fields were evaluated for each genotype (n = 3 experiments). There are fewer Pax7+ve cells in Pax7-Numbfl/fl Nbl−/− muscle, but a similar proportion are proliferating (Ki67+ve), in both Pax7-Numbfl/f l Nbl−/− and control muscle at this time point.
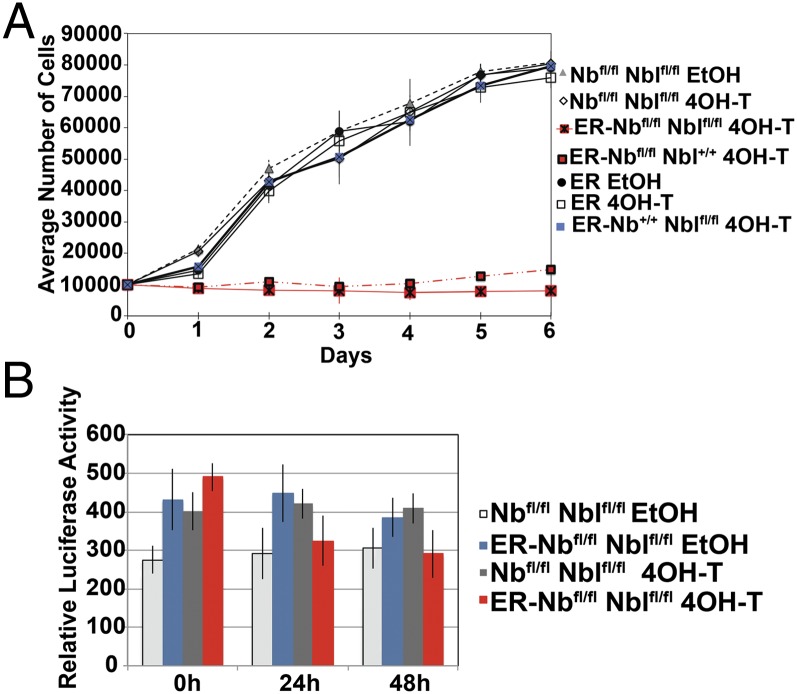
The cause of the intriguing loss of satellite cells was tested directly in vitro; mononucleated satellite cells were isolated from QF muscles of ER-Nbfl/fl Nblfl/fl mice and were treated with 4-hydroxytamoxifen (4OH-T) or ethanol, in vitro, then assayed by genomic qPCR to determine the level of Numb excision. Only cells that demonstrated recombination levels >90% and were >90% Pax7+ve (Fig. S6) were used for analysis. In culture, control cells proliferated regardless of treatment, but Numb-deficient cells demonstrated a dramatic decrease in cell number (Fig. 4A and Fig. S7). Daily inspection revealed no obvious cell death, and there was no increase in apoptosis in 4OH-T–treated ER-Nbfl/fl Nblfl/fl cells, as determined by Caspase 3/7 activity (Fig. 4B). In the absence of niche support, Numb-deficient cells did not proliferate. These data reveal an unexpected unique function for Numb in the regulation of satellite cell proliferation.
Fig. 4.
Numb-deficient satellite cells have a proliferation deficit. (A) The loss of Numb resulted in a static cell number. Satellite cells were plated in growth medium after treatment with 4OH-T or ethanol; genotypes are as indicated. Triplicate wells were trypsinized daily, stained with 0.4% Trypan Blue, and counted. Data are mean number of cells ± SD (n = 3). (B) Caspase 3/7activity showed that Numb-deficient satellite cells had no increase in apoptosis in culture. ER-Nbfl/fl Nblfl/fl and Nbfl/fl Nblfl/fl satellite cells after 4OH-T or ethanol treatment; data are mean Luciferase levels ± SD (n = 4).
To determine the molecular basis of the proliferation defect, we compared gene expression in ER-Nbfl/fl Nblfl/fl satellite cells treated with vehicle or 4OH-T. We were specifically interested in the expression of Notch signaling pathway genes given the well-known role of Numb in Notch regulation. Our analysis showed that there was no significant difference in the expression of Notch1, or its ligand Dll1. Also, there was only a minor increase in the expression of the Notch target genes Hes1 and Hes6 (Table S1). There was no significant difference in the expression levels of Myod and Pax7. Intriguingly, the cell-cycle regulator p21 and Myostatin (Mstn), both of which inhibit myoblast proliferation (4, 41–50), were significantly up-regulated in 4OH-T–treated cells (Table S1).
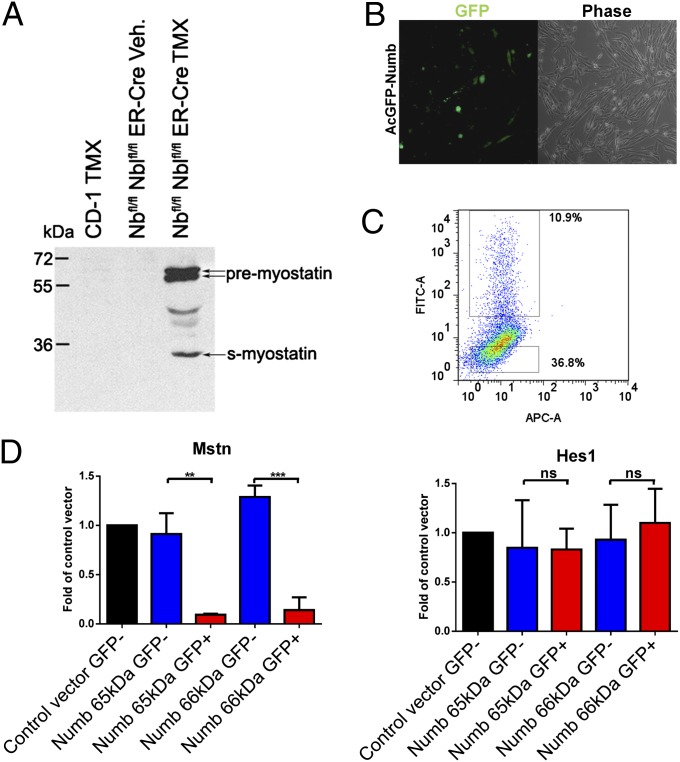
Myostatin is necessary for the balance between proliferation and differentiation of muscle progenitors embryonically and postnatally (41–50). Myostatin signaling activates expression of p21, inhibiting myoblast proliferation (41–43, 48–50). Based on the high level of Mstn mRNA in 4OH-T–treated ER-Nbfl/fl Nblfl/fl satellite cells, we next determined whether it was also elevated in vivo. QF muscles of TMX or vehicle-treated mice were injected with CTX, and satellite cells were harvested 5 dpi and analyzed by Western blot. The Numb-deficient satellite cells had high levels of Myostatin, which was undetectable in controls (Fig. 5A).
Fig. 5.
Numb affects Myostatin expression in myoblasts. (A) Western blot of satellite cells harvested 5 d post-CTX injury shows that only Numb-deficient satellite cells expressed detectable levels of Myostatin. Total protein lysates were analyzed using an anti-Myostatin antibody; genotypes are as indicated (n = 3). (B) Micrograph of primary myoblasts 1 d after transfection with AcGFP-Numb (66-kDa isoform). (C) FACS plot 2 d after transfection with AcGFP-Numb (65-kDa isoform). The APC channel was used to evaluate autofluorescence. (D) qRT-PCR of Mstn and Hes1 expression in the GFP+ve and GFP-ve fractions of myoblasts transfected with AcGFP-Numb. Expression is reported as average fold induction over control vector-transfected cells. A similar trend was observed with two different Numb isoforms (n = 3). **P < 0.01; ***P < 0.001; ns, not significant.
The ability of Numb to affect Mstn expression in cells was further examined by overexpression of Numb in primary myoblasts. Cells were transfected with plasmids that express Numb-GFP fusion proteins (AcGFP-Numb) (Fig. S8), and then FACS sorted 2 d posttransfection for GFP (Fig. 5 B and C). The expression of Mstn and Hes1 was determined by qRT-PCR comparing GFP+ve and GFP-ve fractions. Two different isoforms of Numb (65 and 66 kDa) were evaluated; in both cases, GFP+ve cells demonstrated significantly less Mstn mRNA than control cells (Fig. 5D). These data indicate that Numb can regulate Mstn levels. Interestingly, Hes1 expression was not significantly decreased by increased Numb (Fig. 5D), suggesting that Notch signaling is not the primary target of Numb in proliferating satellite cells. We next determined whether knockdown of Mstn would rescue the proliferation defect. Muscle-derived ER-Nbfl/fl Nblfl/fl cells treated in vitro with either 4OH-T or vehicle and transfected with three different Mstn-specific siRNAs demonstrated > 85% knockdown, but the scramble siRNA had no effect on Mstn levels (Fig. 6A). Treatment of satellite cells with Mstn-specific siRNA resulted in increased proliferation of Numb-deficient and control cells (Fig. 6B). These data indicate a previously unrecognized function for Numb in the regulation of Mstn expression and, as a result, in the control of myogenic progenitor proliferation.
Fig. 6.
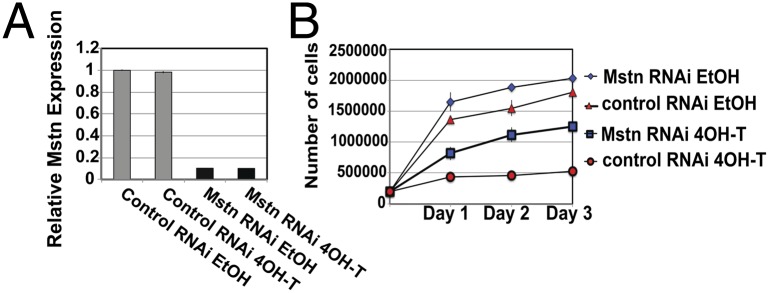
Mstn knock-down rescued the proliferation defect in Numb-deficient satellite cells. (A) ER-Nbfl/fl Nblfl/fl satellite cells that were 4OH-T or ethanol treated were transfected with Mstn-specific or scramble siRNAs. Mstn expression was determined by qRT-PCR and Mstn-specific siRNAs knockdown expression by 85%. Data are relative gene expression ± SD (n = 3). (B) Proliferation assays demonstrate that Mstn-specific siRNA rescues the proliferation defect. ER-Nbfl/fl Nblfl/fl satellite cells were 4OH-T or ethanol treated and transfected with Mstn or scramble siRNA. Daily, triplicate wells were counted; data are average cell number ± SD, (n = 3).
Discussion
Skeletal muscle repair is dependent on the proliferative expansion of satellite cells and the balance between the proliferation of myogenic progenitors and their differentiation into muscle fibers. Numb participates in protein ubiquitination (18–22) and is a regulator of binary cell fate (51). The Numb expression pattern in activated satellite cells predicted a role for this gene in regulating skeletal muscle repair. We directly tested this role by conditionally deleting Numb to examine the impact that a lack of Numb has on satellite cell function and muscle regeneration. Numb-deficient muscles demonstrated defective myofiber repair (Figs. 1 and 2), significantly fewer satellite cells were found on single fiber cultures (Fig. 3 and Fig. S5), and isolated satellite cells that had Numb deleted in vitro had a proliferation defect (Fig. 4 and Fig. S7). The loss of only Nbl did not result in aberrant repair or proliferation. The current model of Numb function in adult muscle is that it regulates asymmetric division of satellite cell progeny and the decision point between differentiation or continued proliferation (10, 16, 40, 51, 52). Our studies show that Numb also has a role much earlier than this cell-fate decision: controlling activation-associated proliferative expansion of satellite cell progeny.
Numb can negatively regulate Notch signaling (20–22, 32, 36, 53–56). However, in keeping with the observation that Notch signaling was not significantly affected in the somite of Numb-overexpressing transgenic mice (25), we found very limited changes in the expression of Notch genes in the myogenic cells isolated from Numb KO mice, leading us to screen for alternative mediators. Our data suggest that Mstn is regulated by Numb. After injury, Numb-deficient muscle demonstrated high levels of Mstn compared with control muscle. Overexpression of two Numb isoforms, p65 and p66, decreased Mstn levels. Mstn-specific siRNA rescued the proliferation deficit in Numb-deficient satellite cells. These data demonstrate that the increased expression of Mstn is responsible for the lack of proliferative expansion of Numb-deficient satellite cells. Mstn is an important regulator of postnatal myogenesis; it is expressed in quiescent satellite cells and inhibits their activation (41, 43). It also inhibits myoblast proliferation, inducing differentiation (42, 44, 48–50). Mstn loss-of-function mutations in mice, cattle, and sheep result in dramatically increased muscle mass (57–62). Mstn−/− mice demonstrate enhanced muscle repair, even in senescent animals (63, 64).
Recent work has demonstrated that Mstn regulates myoblast differentiation through the Notch pathway (43), and it was proposed that a balance between Notch and Smad3, the downstream effector of Mstn, regulated regenerative competence of satellite cells (43). Furthermore, in aging satellite cells, this balance is changed, leading to impaired regeneration (38). This interaction is likely altered in the absence of Numb, but, as there was no significant change in the expression levels of the canonical Notch genes, it is difficult to conclude that Notch is the target that results in the proliferation defect. It is possible that Mstn governs satellite cell proliferation through still undefined mechanisms not relying on changes in the Smad3/Notch balance. The recent observation that the atrophy `n in Smad3−/− muscles could be due to altered Mstn levels supports this idea (65). Mstn may signal independently of Smad3 via either Smad2 or other pathways such as the phosphatidylinositol 3-kinase Wnt and c-Jun N-terminal kinase pathways (66–68).
The molecular mechanisms used by Numb to regulate Mstn expression remain to be defined. In mammals Numb regulates several signaling pathways with different modalities, mainly affecting the ubiquitin and endocytosis network (54). For example, Numb regulates differentiation of cerebellar granule cell progenitors (GCPs) by targeting Gli1, an effector of Hedgehog signaling, to the proteasome, allowing GCPs to differentiate (69). Numb interacts with Mdm2 to hamper ubiquitination of p53 (70). Numb interacts with the endocytic machinery to regulate EGF, transferrin (71), and integrin (72) trafficking. Numb binds to activated TrkB, a receptor for BDNF, and causes endocytic recycling of this receptor (73). Numb also acts as a scaffold for aPKC in the cytoplasm, promoting both BDNF-dependent activation of aPKC and neural precursor cell migration (73). Numb interacts with E-cadherin and regulates its localization. Similarly, knockdown of Numb in MDCK cells destabilizes E-cadherin–based cell adhesion and potentiates sensitivity to hepatocyte growth factor (HGF) (74, 75). Intriguingly, we recently reported that high concentrations of HGF inhibit satellite cell proliferation by inducing Mstn (76). This observation opens the possibility that HGF-dependent signaling could link Numb to Mstn expression.
Androgens and anabolic steroids reduce muscle loss caused by immobilization and spinal-cord injury. Interestingly, recent reports show that the steroid Nandrolone, which counteracts denervation-dependent atrophy, induces increased Numb expression in denervated muscles (77). Mstn up-regulation has been observed in gastrocnemius muscles after denervation and is therefore implicated in the associated atrophy (78, 79). This finding, along with the observation reported here that Numb overexpression reduces Mstn levels, opens the possibility that the beneficial effects of Nandrolone are mediated by a change in the Numb/Mstn balance. Increased levels of Mstn (80, 81) and decreased expression of Numb (82) have been observed during aging, suggesting that this balance could play a role in age-dependent sarcopenia. These findings suggest that a link between Numb and Mstn could control biological events beyond the regulation of the regenerative capacity of satellite cells after injury.
Materials and Methods
Mice.
Numblike (Nbl−/−) (31, 32) and conditional Numb mutant (Numbfl/fl) (30) mice were provided by W. Zhong (Yale University, New Haven, CT). Pax7-Cre mice (Pax7ICNm) (29) were provided by C. Keller (Oregon Health and Science University, Portland, OR). R26RYFP (33), Numbtm1Zili/tm1Zili/J, Numbltm1Zili/tm1Zili/J (36), and the TMX-inducible Cre recombinase line CAGG ER-Cre (34, 35) were obtained from The Jackson Laboratory. Mice were housed and experiments were done, under protocols approved by the Institutional Animal Care and Use Committees at the Veterinary Medical Unit at the VA Palo Alto Health Care Systems and Arizona State University and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Treatment with BaCl2, TMX, and CTX.
Adult (≥60 d old) mice were injected intraperitoneally with 0.4 mg of TMX (Sigma-Aldrich) dissolved in corn oil twice a day for 5 d. After 28 d, QF muscles were injected with 10 μM CTX (Calbiochem) with 0.02% India ink suspension to mark the injection site. For BaCl2 injury (Sigma-Aldrich), adults were anesthetized by isoflurane inhalation. TA muscles were injected with 50 μL of BaCl2 solution (1.2% in sterile 0.9% NaCl). Muscles were harvested from age- and sex-matched control and mutant animals.
Morphometry, Histology, and Immunofluorescence.
Muscles were flash frozen in isopentane, and midbelly cryostat sections (8 μM) were stained with hematoxylin/eosin (H&E) or with antibodies against Laminin (Sigma-Aldrich), eMyHC (DHSB), GFP (Aves), MyoD (Becton Dickson), Pax7 (DHSB), and Ki67 (Becton Dickson), as described (83). Nuclei were DAPI stained. Technical details are in SI Materials and Methods. The area of 120 fibers in four stochastically chosen microscopic fields for each TA/QF was measured using ImagePro Plus software (Media Cybernetics). At least three muscles were analyzed per genotype and time point. Gomorri’s trichrome stain was done on paraffin-embedded muscle (5 μm) sections at 10 dpi. Details are in SI Materials and Methods.
Single-Fiber Isolation and Culture.
Single fibers were isolated from EDL muscles as described (28) and fixed immediately or cultured for 72 h in Ham’s F-10 with 20% (vol/vol) FBS and 5 ng/mL FGF (Atlanta Biological). Fibers were fixed in 2% paraformaldehyde for immunofluorescence (IF) with antibodies against Syn4 (gift of B. Olwin, University of Colorado, Boulder CO), Pax7, and GFP. Pax7/YFP+ve cells associated with single fibers in two experiments were quantified for each condition.
Satellite Cell Preparation.
Satellite cells were isolated as described (84). Details are in SI Materials and Methods. To activate Cre recombinase, cells were treated with 1 μM 4OH-T (Sigma-Aldrich) dissolved in ethanol for 48 h.
FACS Analysis.
Satellite cells were isolated from the hindlimb muscles, and FACS was done as described (12), using a FACSAria III (BD Biosciences). Antibodies used to identify the satellite cells recognized CD31, CD45, Sca1, and CD106. Details are in SI Materials and Methods. Physical parameters (SSC and FSC) and DAPI dilactate (250 µg/µl; Invitrogen) excluded dead cells.
Proliferation and Caspase 3/7 Assays.
Satellite cells were plated at 1 × 105 cells per mL on Matrigel-coated 96-well plates in triplicate for both assays. Daily, cells were trypsinized, stained with 0.4% Trypan Blue (CellGro), and counted using a hemocytometer (n = 3). A Caspase-Glo 3/7 assay (Promega) was used to quantify apoptosis per the manufacturer (n = 3).
Western Blots.
Muscles of TMX- or vehicle-treated mice were injected with CTX, satellite cells were isolated 5 dpi, and total protein lysates were made. Proteins were detected with mouse anti-Mstn (1:1,000; Abcam) and visualized with anti-mouse-AP antibody using ECL substrate (GE Healthcare)(n = 3). Technical details are in SI Materials and Methods.
Overexpression Studies.
Primary myoblasts were isolated from Sv129 mice (Charles River) as described (83). Myoblasts and HEK 293T [American Type Culture Collection (ATCC)] cells were plated on laminin/ collagen-coated wells and transfected with Lipofectamine 2000 (Invitrogen) per the manufacturer. Two days posttransfection, cells were fixed in 4% (wt/vol) paraformaldehyde for IF with antibodies against Numb (1:100, Abcam) and GFP (1:500) or processed for FACS. Mouse Numb cDNAs (65- and 66-kDa isoforms) from C2C12 cells (ATCC) were cloned into pAcGFP-C1 vector (Clontech). The AcGFP-Numb fusion proteins were verified by sequencing.
qRT-PCR.
Satellite cell RNA was isolated using TRIzol (Invitrogen). RNA was treated with DNase I before cDNA synthesis using SuperScriptIII (Invitrogen). cDNA was quantified using transcript-specific, intron-spanning primers and Sybergreen I (Eurogentec) on an ABI7900HT thermocycler (Applied Biosystems). Samples were normalized to Gapdh using ΔΔCt analysis. Data are mean relative expression ± SD.
siRNA.
Satellite cells were seeded at 1 × 105 cells per mL and transfected 24 h later with 50 nM Mstn specific or scramble siRNA in 1.5 mL of Lipofectamine RNAiMax (Invitrogen). Total RNA was isolated 3 d posttransfection, and qRT-PCR was done as described.
Statistical Analysis.
For morphometry, two-tailed t tests were used to calculate the significance of observed differences. For qRT-PCR, body/muscle weight, and number of satellite cells on single fibers, data were analyzed using two-tailed t tests with significance of P < 0.05 at the 95% confidence level. For caspase and proliferation assays, significance was determined using one-way ANOVA and the post hoc Tukey test.
Supplementary Material
Acknowledgments
We thank Drs. W. Zhong, C. Keller, and B. Olwin for mice and reagents; Drs. M. Quarta, C. Bjornson, and P. Carlig for help and suggestions; and L. Geiger for technical help. The Pax7 and eMyHC monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biology at the University of Iowa. This work was supported by grants from the Glenn Foundation for Medical Research, by National Institutes of Health (NIH) Grants P01 AG036695, R37 AG023806, R01 AR056849, R01 AR062185, and DP1 OD000392 (NIH Director's Pioneer Award), and the Department of Veterans Affairs (Merit Review) (to T.A.R.), and by Muscular Dystrophy Association Grant MDA 114214 and a grant from the American Heart Association (to J.W.R., A.R., and R.E.A.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311628110/-/DCSupplemental.
References
- 1.Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: Lessons from muscle. Cell Cycle. 2005;4(3):407–410. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- 2.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: Molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15(12):666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: The stem cell that came in from the cold. J Histochem Cytochem. 2006;54(11):1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 4.Chargé SBP, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 5.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435(7044):948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 6.Kuang S, Chargé SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172(1):103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zammit PS, et al. Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J Cell Biol. 2004;166(3):347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138(17):3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambasivan R, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138(17):3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 10.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3(3):397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 11.Schuster-Gossler K, Cordes R, Gossler A. Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. Proc Natl Acad Sci USA. 2007;104(2):537–542. doi: 10.1073/pnas.0608281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjornson CRR, et al. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30(2):232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mourikis P, et al. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012;30(2):243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- 14.Wen Y, et al. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol Cell Biol. 2012;32(12):2300–2311. doi: 10.1128/MCB.06753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasyutina E, et al. RBP-J (Rbpsuh) is essential to maintain muscle progenitor cells and to generate satellite cells. Proc Natl Acad Sci USA. 2007;104(11):4443–4448. doi: 10.1073/pnas.0610647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun D, Li H, Zolkiewska A. The role of Delta-like 1 shedding in muscle cell self-renewal and differentiation. J Cell Sci. 2008;121(Pt 22):3815–3823. doi: 10.1242/jcs.035493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302(5650):1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 18.Dho SE, French MB, Woods SA, McGlade CJ. Characterization of four mammalian numb protein isoforms: Identification of cytoplasmic and membrane-associated variants of the phosphotyrosine binding domain. J Biol Chem. 1999;274(46):33097–33104. doi: 10.1074/jbc.274.46.33097. [DOI] [PubMed] [Google Scholar]
- 19.Verdi JM, et al. Distinct human NUMB isoforms regulate differentiation vs. proliferation in the neuronal lineage. Proc Natl Acad Sci USA. 1999;96(18):10472–10476. doi: 10.1073/pnas.96.18.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beres BJ, et al. Numb regulates Notch1, but not Notch3, during myogenesis. Mech Dev. 2011;128(5-6):247–257. doi: 10.1016/j.mod.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 21.McGill MA, McGlade CJ. Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J Biol Chem. 2003;278(25):23196–23203. doi: 10.1074/jbc.M302827200. [DOI] [PubMed] [Google Scholar]
- 22.McGill MA, Dho SE, Weinmaster G, McGlade CJ. Numb regulates post-endocytic trafficking and degradation of Notch1. J Biol Chem. 2009;284(39):26427–26438. doi: 10.1074/jbc.M109.014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venters SJ, Ordahl CP. Asymmetric cell divisions are concentrated in the dermomyotome dorsomedial lip during epaxial primary myotome morphogenesis. Anat Embryol (Berl) 2005;209(6):449–460. doi: 10.1007/s00429-005-0461-2. [DOI] [PubMed] [Google Scholar]
- 24.Holowacz T, Zeng L, Lassar AB. Asymmetric localization of numb in the chick somite and the influence of myogenic signals. Dev Dyn. 2006;235(3):633–645. doi: 10.1002/dvdy.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jory A, et al. Numb promotes an increase in skeletal muscle progenitor cells in the embryonic somite. Stem Cells. 2009;27(11):2769–2780. doi: 10.1002/stem.220. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, et al. Bmp signaling at the tips of skeletal muscles regulates the number of fetal muscle progenitors and satellite cells during development. Dev Cell. 2010;18(4):643–654. doi: 10.1016/j.devcel.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129(5):999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2(1):50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Keller C, Hansen MS, Coffin CM, Capecchi MR. Pax3:Fkhr interferes with embryonic Pax3 and Pax7 function: Implications for alveolar rhabdomyosarcoma cell of origin. Genes Dev. 2004;18(21):2608–2613. doi: 10.1101/gad.1243904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong W, et al. Mouse numb is an essential gene involved in cortical neurogenesis. Proc Natl Acad Sci USA. 2000;97(12):6844–6849. doi: 10.1073/pnas.97.12.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419(6910):929–934. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- 32.Petersen PH, Zou K, Krauss S, Zhong W. Continuing role for mouse Numb and Numbl in maintaining progenitor cells during cortical neurogenesis. Nat Neurosci. 2004;7(8):803–811. doi: 10.1038/nn1289. [DOI] [PubMed] [Google Scholar]
- 33.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8(24):1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244(2):305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 36.Zilian O, et al. Multiple roles of mouse Numb in tuning developmental cell fates. Curr Biol. 2001;11(7):494–501. doi: 10.1016/s0960-9822(01)00149-x. [DOI] [PubMed] [Google Scholar]
- 37.Wilson A, et al. Normal hemopoiesis and lymphopoiesis in the combined absence of numb and numblike. J Immunol. 2007;178(11):6746–6751. doi: 10.4049/jimmunol.178.11.6746. [DOI] [PubMed] [Google Scholar]
- 38.Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454(7203):528–532. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins CA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122(2):289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Sun H, et al. Stra13 regulates satellite cell activation by antagonizing Notch signaling. J Cell Biol. 2007;177(4):647–657. doi: 10.1083/jcb.200609007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162(6):1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manceau M, et al. Myostatin promotes the terminal differentiation of embryonic muscle progenitors. Genes Dev. 2008;22(5):668–681. doi: 10.1101/gad.454408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McFarlane C, et al. Human myostatin negatively regulates human myoblast growth and differentiation. Am J Physiol Cell Physiol. 2011;301(1):C195–C203. doi: 10.1152/ajpcell.00012.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joulia D, et al. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res. 2003;286(2):263–275. doi: 10.1016/s0014-4827(03)00074-0. [DOI] [PubMed] [Google Scholar]
- 45.Langley B, et al. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem. 2002;277(51):49831–49840. doi: 10.1074/jbc.M204291200. [DOI] [PubMed] [Google Scholar]
- 46.Parker SB, et al. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995;267(5200):1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- 47.Zhang P, et al. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 1999;13(2):213–224. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas M, et al. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275(51):40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- 49.Ríos R, Carneiro I, Arce VM, Devesa J. Myostatin regulates cell survival during C2C12 myogenesis. Biochem Biophys Res Commun. 2001;280(2):561–566. doi: 10.1006/bbrc.2000.4159. [DOI] [PubMed] [Google Scholar]
- 50.Zimmers TA, et al. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296(5572):1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 51.Cayouette M, Raff M. Asymmetric segregation of Numb: A mechanism for neural specification from Drosophila to mammals. Nat Neurosci. 2002;5(12):1265–1269. doi: 10.1038/nn1202-1265. [DOI] [PubMed] [Google Scholar]
- 52.Shinin V, Gayraud-Morel B, Gomès D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8(7):677–687. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- 53.Pece S, et al. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J Cell Biol. 2004;167(2):215–221. doi: 10.1083/jcb.200406140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pece S, Confalonieri S, R Romano P, Di Fiore PP (2011) NUMB-ing down cancer by more than just a NOTCH. Biochim Biophys Acta 1815(1):26–43. [DOI] [PubMed]
- 55.Luo D, Renault VM, Rando TA. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin Cell Dev Biol. 2005;16(4-5):612–622. doi: 10.1016/j.semcdb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Spana EP, Doe CQ. Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron. 1996;17(1):21–26. doi: 10.1016/s0896-6273(00)80277-9. [DOI] [PubMed] [Google Scholar]
- 57. Grobet L, et al. (2003) Modulating skeletal muscle mass by postnatal, muscle-specific inactivation of the myostatin gene. Genesis 35(4):227–238. [DOI] [PubMed]
- 58.Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7(9):910–916. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- 59.Lee S-J, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98(16):9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 61.Schuelke M, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350(26):2682–2688. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 62.Clop A, et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet. 2006;38(7):813–818. doi: 10.1038/ng1810. [DOI] [PubMed] [Google Scholar]
- 63.McCroskery S, et al. Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci. 2005;118(Pt 15):3531–3541. doi: 10.1242/jcs.02482. [DOI] [PubMed] [Google Scholar]
- 64.Wagner KR, Liu X, Chang X, Allen RE. Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci USA. 2005;102(7):2519–2524. doi: 10.1073/pnas.0408729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ge X, et al. Smad3 signaling is required for satellite cell function and myogenic differentiation of myoblasts. Cell Res. 2011;21(11):1591–1604. doi: 10.1038/cr.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang W, Zhang Y, Li Y, Wu Z, Zhu D. Myostatin induces cyclin D1 degradation to cause cell cycle arrest through a phosphatidylinositol 3-kinase/AKT/GSK-3 beta pathway and is antagonized by insulin-like growth factor 1. J Biol Chem. 2007;282(6):3799–3808. doi: 10.1074/jbc.M610185200. [DOI] [PubMed] [Google Scholar]
- 67.Steelman CA, Recknor JC, Nettleton D, Reecy JM. Transcriptional profiling of myostatin-knockout mice implicates Wnt signaling in postnatal skeletal muscle growth and hypertrophy. FASEB J. 2006;20(3):580–582. doi: 10.1096/fj.05-5125fje. [DOI] [PubMed] [Google Scholar]
- 68.Huang Z, et al. Regulation of myostatin signaling by c-Jun N-terminal kinase in C2C12 cells. Cell Signal. 2007;19(11):2286–2295. doi: 10.1016/j.cellsig.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Di Marcotullio L, et al. Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nat Cell Biol. 2006;8(12):1415–1423. doi: 10.1038/ncb1510. [DOI] [PubMed] [Google Scholar]
- 70.Colaluca IN, et al. NUMB controls p53 tumour suppressor activity. Nature. 2008;451(7174):76–80. doi: 10.1038/nature06412. [DOI] [PubMed] [Google Scholar]
- 71.Santolini E, et al. Numb is an endocytic protein. J Cell Biol. 2000;151(6):1345–1352. doi: 10.1083/jcb.151.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13(1):15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 73.Zhou P, et al. Numb links extracellular cues to intracellular polarity machinery to promote chemotaxis. Dev Cell. 2011;20(5):610–622. doi: 10.1016/j.devcel.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Z, Sandiford S, Wu C, Li SS-C. Numb regulates cell-cell adhesion and polarity in response to tyrosine kinase signalling. EMBO J. 2009;28(16):2360–2373. doi: 10.1038/emboj.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lau KM, McGlade CJ. Numb is a negative regulator of HGF dependent cell scattering and Rac1 activation. Exp Cell Res. 2011;317(4):539–551. doi: 10.1016/j.yexcr.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 76.Yamada M, et al. High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: A possible mechanism for reestablishing satellite cell quiescence in vivo. Am J Physiol Cell Physiol. 2010;298(3):C465–C476. doi: 10.1152/ajpcell.00449.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu X-H, et al. Nandrolone reduces activation of Notch signaling in denervated muscle associated with increased Numb expression. Biochem Biophys Res Commun. 2011;414(1):165–169. doi: 10.1016/j.bbrc.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 78.Baumann AP, Ibebunjo C, Grasser WA, Paralkar VM. Myostatin expression in age and denervation-induced skeletal muscle atrophy. J Musculoskelet Neuronal Interact. 2003;3(1):8–16. [PubMed] [Google Scholar]
- 79.Zhang D, Liu M, Ding F, Gu X. Expression of myostatin RNA transcript and protein in gastrocnemius muscle of rats after sciatic nerve resection. J Muscle Res Cell Motil. 2006;27(1):37–44. doi: 10.1007/s10974-005-9050-5. [DOI] [PubMed] [Google Scholar]
- 80.Léger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008;11(1):163–175B. doi: 10.1089/rej.2007.0588. [DOI] [PubMed] [Google Scholar]
- 81.Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid NF. Serum myostatin-immunoreactive protein is increased in 60-92 year old women and men with muscle wasting. J Nutr Health Aging. 2002;6(5):343–348. [PubMed] [Google Scholar]
- 82.Carey KA, Farnfield MM, Tarquinio SD, Cameron-Smith D. Impaired expression of Notch signaling genes in aged human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2007;62(1):9–17. doi: 10.1093/gerona/62.1.9. [DOI] [PubMed] [Google Scholar]
- 83.Quach NL, Biressi S, Reichardt LF, Keller C, Rando TA. Focal adhesion kinase signaling regulates the expression of caveolin 3 and beta1 integrin, genes essential for normal myoblast fusion. Mol Biol Cell. 2009;20(14):3422–3435. doi: 10.1091/mbc.E09-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tatsumi R, et al. Low-pH preparation of skeletal muscle satellite cells can be used to study activation in vitro. Int J Biochem Cell Biol. 2006;38(10):1678–1685. doi: 10.1016/j.biocel.2006.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.