Significance
DIM (3,3′-diindolylmethane) is a small molecule compound under investigation as a cancer preventive agent. This research addresses a potential usage of DIM as a medical countermeasure to prevent or mitigate acute radiation syndrome due to whole body exposure. In this regard, DIM can be administered safely to humans and animals by oral or subcutaneous routes. DIM may also be useful in preventing or mitigating late normal tissue damage due to partial body radiation exposure during cancer treatment. DIM works, in part, by a mechanism distinct from other radioprotectors and mitigators involving stimulation of the DNA damage response, including DNA repair, and activation of cell survival signaling through the transcription factor NF-κB.
Abstract
DIM (3,3′-diindolylmethane), a small molecule compound, is a proposed cancer preventive agent that can be safely administered to humans in repeated doses. We report that administration of DIM in a multidose schedule protected rodents against lethal doses of total body irradiation up to 13 Gy, whether DIM dosing was initiated before or up to 24 h after radiation. Physiologic submicromolar concentrations of DIM protected cultured cells against radiation by a unique mechanism: DIM caused rapid activation of ataxia-telangiectasia mutated (ATM), a nuclear kinase that regulates responses to DNA damage (DDR) and oxidative stress. Subsequently, multiple ATM substrates were phosphorylated, suggesting that DIM induces an ATM-dependent DDR-like response, and DIM enhanced radiation-induced ATM signaling and NF-κB activation. DIM also caused activation of ATM in rodent tissues. Activation of ATM by DIM may be due, in part, to inhibition of protein phosphatase 2A, an upstream regulator of ATM. In contrast, DIM did not protect human breast cancer xenograft tumors against radiation under the conditions tested. In tumors, ATM was constitutively phosphorylated and was not further stimulated by radiation and/or DIM. Our findings suggest that DIM is a potent radioprotector and mitigator that functions by stimulating an ATM-driven DDR-like response and NF-κB survival signaling.
A diet rich in cruciferous vegetables (e.g., cabbage, broccoli, cauliflower) is linked to a reduced risk of several human cancers (1, 2), and dietary supplementation with indole-3-carbinol (I3C), a phytochemical from cruciferous vegetables, prevents tumors in animals (3–5). I3C is hydrolyzed to various products in the stomach, including DIM (3,3′-diindolylmethane), which is acid stable and is a major bioactive metabolite (6). I3C and DIM are proposed cancer preventive agents and each can be given safely in oral form in repeated doses to rodents and humans (7–12). In humans, oral I3C or DIM at nontoxic doses yielded peak plasma levels of 0.25–2.5 µM (9–12).
The mechanism by which DIM prevents cancer is unknown. Most studies have used supraphysiological concentrations of DIM (10–30 μM) and indicate that DIM can inhibit invasion, angiogenesis, and proliferation and induce apoptosis in tumor cells by modulating signaling pathways involving AKT, NF-κB, and FOXO3 (13–17). It can also inhibit estrogen-inducible gene expression and cause an endoplasmic reticulum stress response (17–22). DIM alters estrogen metabolism by shifting metabolism from carcinogenic 16α-hydroxy to inert 2-hydroxy derivatives, and it antagonizes estrogen and androgen receptor activity (17, 20–24).
Low concentrations of DIM that can be achieved safely in humans (≤1 μM) protect cells against oxidative stress (25). Protection required the tumor suppressor BRCA1 and, in particular, its ATM (S1387 and S1524). ATM is activated via autophosphorylation in response to DNA double-strand breaks (DSBs), and phospho-ATM then phosphorylates multiple substrates involved in the DNA damage response (DDR), resulting in activation of DNA repair mechanisms, cell cycle checkpoints, antioxidant pathways, and survival pathways (e.g., NF-κB signaling) (26, 27).
Here, we describe an activity for DIM as a radioprotector and mitigator; and we establish a unique mechanism, i.e., stimulation of ATM signaling without causing DNA damage.
Results
In Vivo Radioprotection and Mitigation by DIM.
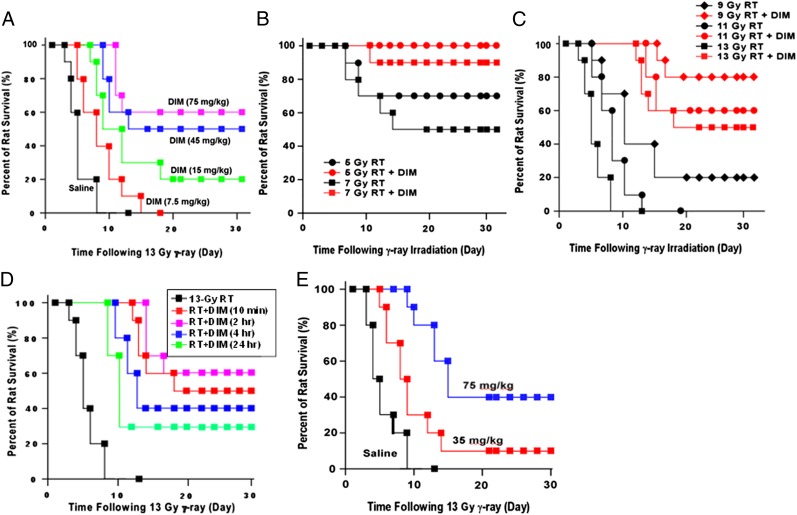
DIM can be given to mice by gavage at 250 mg/kg with no toxicity and wide tissue distribution (7). We usually gave DIM by i.p. injection for convenience, because preliminary studies showed DIM was most effective against total body irradiation (TBI) when given in multiple once-daily doses. Fig. 1A shows dose-dependent protection of Sprague–Dawley (SD) rats given daily injections of DIM for 14-d starting 10 min after TBI (13 Gy). Although control animals died by day 10, the 30-d survival rates were 60% (P < 0.001 vs. vehicle control, log-rank test), 50% (P < 0.001), 20%, and 0% for 75, 45, 15, and 7.5 mg/kg DIM, respectively. When the first DIM dose was given 24-h before TBI (13 Gy), a lower daily dose of DIM (7.5 mg/kg) yielded 55% 30-d survival (P < 0.001), suggesting that if one DIM dose is given before exposure, radioprotection is achieved with a much lower dose. DIM similarly protected C57BL/6 mice against TBI, indicating that protection is not species specific. In C57BL/6 mice, five treatments with DIM significantly attenuated the reductions in red blood cells, white blood cells, and platelets due to lower doses of TBI (2–6 Gy) (Table S1).
Fig. 1.
DIM protects rats against total body irradiation (RT) when the first dose is administered after RT. (A) SD rats (n = 20 per group) were exposed to 13 Gy of 60Co γ rays and given once-daily doses of DIM for 14 d, with the first dose 10 min after RT. (B and C) Rats (n = 20 per group) were exposed to different doses of RT and given once-daily doses of DIM (75 mg/kg) for 14 d starting 10 min after RT. (D) Rats (n = 20 per group) were given 13 Gy, and DIM (75 mg/kg) was administered starting at different times after RT. In A–D, DIM was administered by i.p. injection. Control animals were irradiated but received injections of vehicle. (E) Rats (20 per group) were given 13 Gy of RT, and starting 10 min after RT, daily doses of DIM were given s.c. for 14 d. Survival was plotted by Kaplan–Meier method.
When different TBI doses were given followed by daily DIM injections (75 mg/kg for 14 d starting 10 min after TBI), DIM conferred large increases in survival at each radiation dose (Fig. 1 B and C). From the data, we estimate the dose-modifying factor (DMF) for DIM (ratio of LD50/30 values ± DIM) administered shortly after TBI is 1.9. Using a TBI dose of 13 Gy, 30-d survival was 50%, 60%, 40%, and 30%, respectively, when the first DIM dose was given 10 min, 2 h, 4 h, or 24 h after TBI (P < 0.01, DIM vs. no DIM) (Fig. 1D). Thus, delaying DIM treatment for 2 h caused no loss of survival, and when DIM was delayed for 24 h, survival was still appreciable (30%), suggesting that DIM can mitigate radiation injury.
We tested another route for DIM delivery. SD rats were given TBI (13 Gy), and starting 10 min after TBI, rats were given daily s.c. DIM injections for 14 d. This route was chosen because after 13 Gy, it was expected that the oral route is compromised because of damage to the gastrointestinal (GI) system. At 75 mg/kg DIM, 30-d survival was 40% (Fig. 1E), compared with 50–60% for i.p. DIM. The 40% survival rate (P < 0.001, compared with control) is still an impressive result, because most radioprotectors do not yield 30-d survivors at doses higher than 9–11 Gy.
In further studies, DIM (75 mg/kg) was given i.p. for 14 d starting 24 h after TBI. The 30-d survival rates were 60%, 40%, and 20% for vehicle-treated animals at 5, 7, and 9 Gy, respectively, and 90%, 70%, and 50% for DIM-treated rats (Fig. S1A). Together with the 13 Gy data, the DMF for DIM started 24 h after TBI was 1.6.
Finally, in several experiments, (DIM+TBI)-treated rats that survived 30 d were carried for longer time intervals. There was little or no falloff in survival after 30 d and up to 90 d, and the animals appeared healthy and regained their original weight (Fig. S1B).
DIM Does Not Alter Growth or Radiosensitivity of MDA-MB-231 Breast Cancer Xenografts.
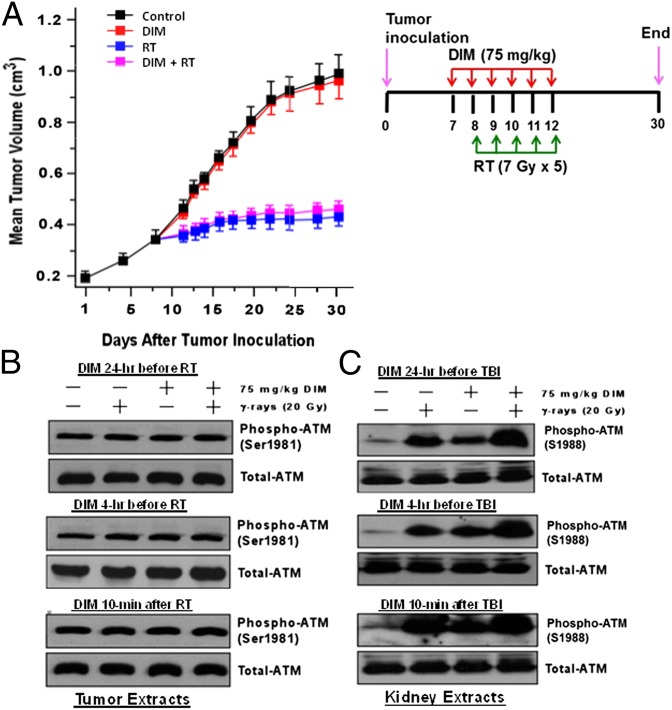
Here, MDA-MB-231 cells grown as xenograft tumors in the flanks of nude mice were sham treated or irradiated by using a fractionated regimen of five treatments of 7 Gy (one per day). Starting 1 d before irradiation, the mice were given once-daily injections of DIM (75 mg/kg) or vehicle for 6 d. DIM had no effect on the growth of unirradiated or irradiated tumors (Fig. 2A). Similar results were observed in a second experiment by using four 5-Gy treatments.
Fig. 2.
DIM does not protect MDA-MB-231 xenografts against fractionated radiation. (A) Tumor cells (4 × 106) were injected into each flank of 40 nude mice, and mice were given the indicated treatments. Fractionated irradiation (RT) was carried out so that one flank was exposed while the other flank and the rest of the mouse were shielded. Tumor sizes are means ± SEMs of n = 20 tumors per group. (B) Nude mice containing MDA-MB-231 xenografts were treated without or with one dose of DIM (75 mg/kg) at different times before or after irradiation (20 Gy). Mice were killed at 24 h after irradiation, and tumor extracts were Western blotted for phosphor-ATM or total ATM. (C) Mice were treated ± DIM (75 mg/kg) at the indicated time before or after TBI (20 Gy). Twenty-four hours after TBI, the animals were killed and extracts of kidney tissue were subjected to Western blotting. S1988 in rat ATM corresponds to S1981 in human ATM.
DIM Activates ATM in Normal Tissues.
We tested the ability of DIM to activate ATM in vivo, using phospho-ATM (S1981) as a marker of activation (26, 27). ATM was constitutively phosphorylated in MDA-MB-231 tumors and was not further activated by DIM and/or radiation (20 Gy) (Fig. 2B). No increases in phospho-ATM were observed whether DIM was given 4 or 24 h before or 10 min after radiation. In contrast, in normal mouse kidney tissue, basal phospho-ATM levels were low, and the levels were increased by DIM and/or radiation (Fig. 2C). Phospho-ATM levels 24 h after irradiation and appeared to be greater after treatment with DIM+radiation than either agent alone. Under different conditions, DIM caused ATM phosphorylation and enhanced radiation-induced phosphorylation in normal rat kidney, liver, and brain (Fig. S2 A–C). Similar results were observed for DIM administered 24 h before, 1 h before, or 10 min after irradiation. Phospho-ATM levels were elevated at 24 h after TBI, consistent with the idea that radiation causes ongoing oxidative stress in normal tissues (28, 29), because most DSBs in irradiated cells are repaired in 2–3 h (30, 31).
Although ATM was constitutively activated (at least phosphorylated) in MDA-MB-231 tumors, downstream signaling was defective, in the sense that there was little phosphorylation of several substrates (e.g., p53, CHK2, and SMC1) in the tumors, whereas phosphorylation of p53, CHK1, and SMC1 was increased in kidney tissue in (DIM+radiation)-treated animals (Fig. S2 D and E). Our results suggest that DIM can enhance radiation-induced ATM signaling activation in vivo. BRCA1 and CHK2 antibodies cross-reactive in mouse tissue were not available.
Radioprotection of Cultured Cells.
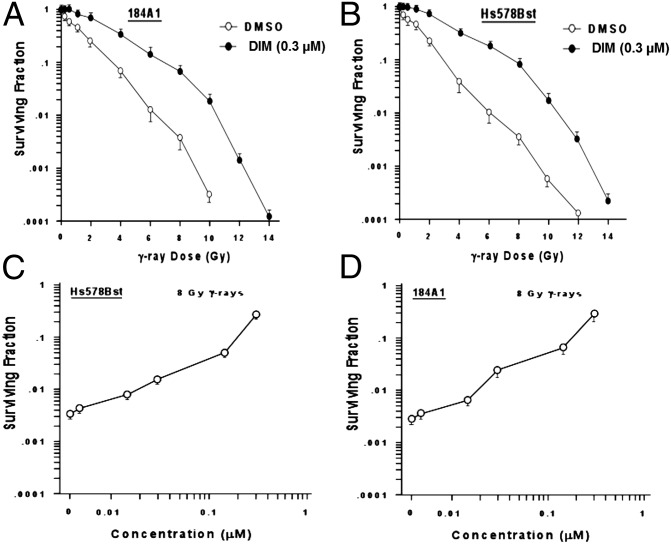
Cells were exposed to DIM (0.3 μM) for 24 h, irradiated, and harvested for clonogenic survival assays (32). Fig 3 A and B illustrate DIM protection of two nontumorigenic human mammary epithelial cell lines, 184A1 and Hs578Bst (P < 0.001 for all but the lowest radiation doses, two-tailed t tests). show the effect of DIM concentration on protection, Using a single radiation dose (8 Gy), concentration-dependent protection was observed up to 0.3 µM of DIM (Fig. 3 C and D).
Fig. 3.
Radioprotection of cultured cells by DIM. (A and B) The 184A1 (A) and Hs578Bst (B) cells were pretreated with DIM (0.3 μM) or vehicle for 24 h, irradiated by using different doses of 137Cs γ rays, harvested, plated at different densities, incubated for 14 d, and counted for colony formation. (C–D) Clonogenic survival of 184A1 (C) and Hs578Bst (D) cells was determined by using one dose of radiation (8 Gy) and different concentrations of DIM. Values are means ± SEMs of three replicate dishes.
DIM Stimulates ATM Signaling.
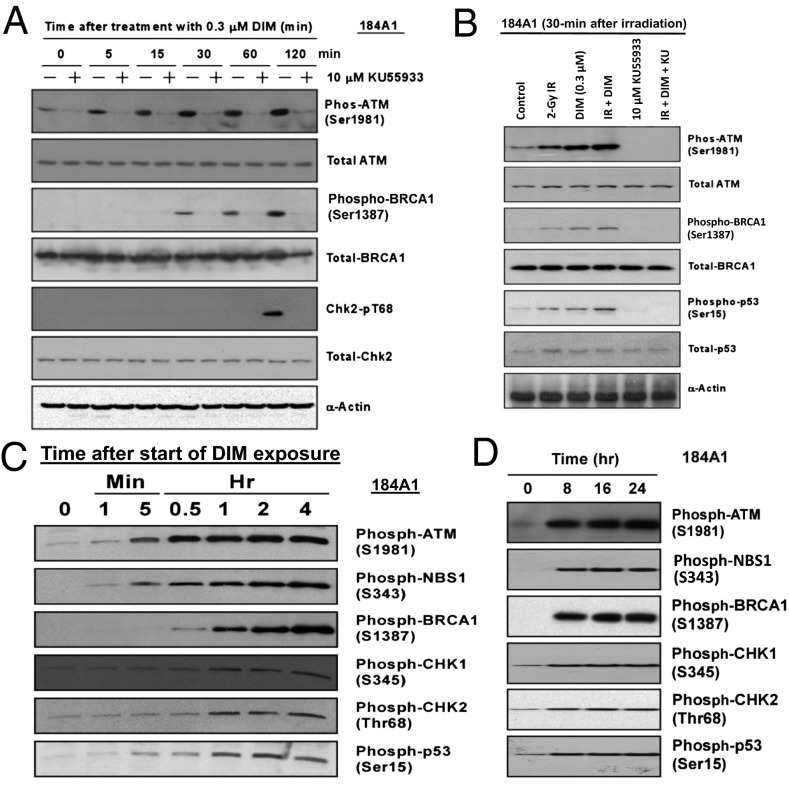
DIM alone caused rapid activation of ATM, indicated by phosphorylation on S1981, in 184A1 cells (Fig. 4A) and other cell types. Subsequently, phosphorylation was observed on two ATM substrates (BRCA1 and CHK2) at their ATM sites. These events were blocked by a selective ATM inhibitor (KU55933) (33), suggesting the pathway is ATM driven. With combined DIM+radiation, at 30 min after radiation, the combination gave greater phosphorylation of ATM and several substrates than either agent alone (Fig. 4B), suggesting DIM can “hyperactivate” ATM. Increased phosphorylation of ATM and NBS1, a key substrate, was observed after a 1-min exposure to DIM, followed by phosphorylation of p53, BRCA1, and CHK2 (Fig. 4C). DIM also stimulated phosphorylation of CHK1 on S345. CHK1 is usually phosphorylated by ATR rather than ATM (34), but some studies indicate a role for ATM in CHK1 phosphorylation. High levels of the six phosphoproteins were maintained for ≥24 h (Fig. 4D). Total protein levels remain unchanged (Fig. S3). These findings suggest DIM stimulates an ATM-driven DDR-like response.
Fig. 4.
DIM activates ATM signaling. (A) The 184A1 cells were exposed to DIM for different times and Western blotted to detect phosphorylated or total levels of ATM and substrates. As a control, cells were treated with an ATM kinase inhibitor (KU55933). (B) Cells were treated ± radiation (2 Gy) and ± DIM and harvested for Western blotting 30 min after irradiation. (C and D) The 184A1 cells were treated with DIM for various times and Western blotted for phospho-ATM and five substrates. Blots showing the total protein levels for each protein are provided in Fig. S3.
ATM, BRCA1, and MRE11 Are Required for Radioprotection.
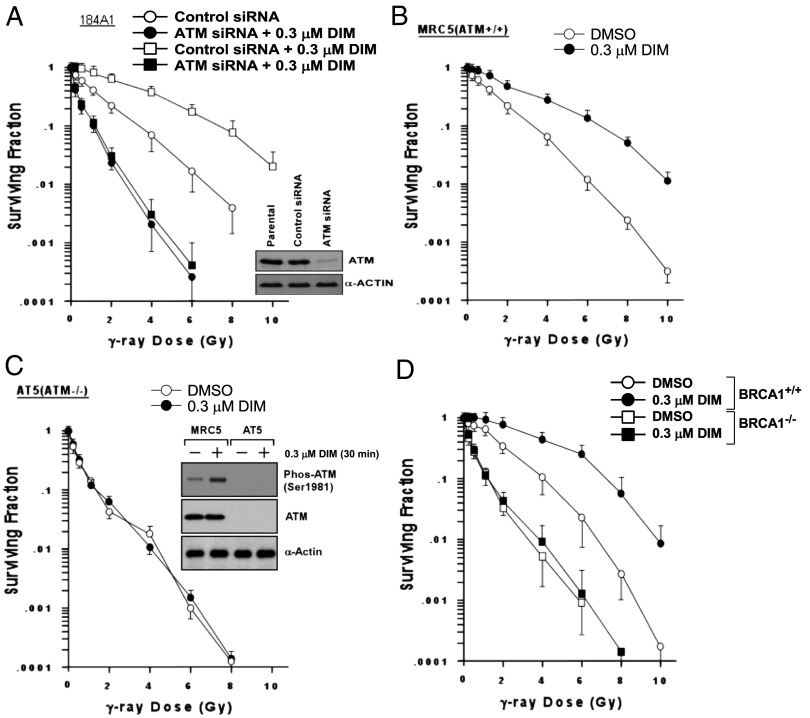
Knockdown of ATM using siRNA radiosensitized 184A1 cells and abolished radioprotection by DIM, whereas cells treated with control-siRNA were protected (Fig. 5A). Using a genetic approach, ATM−/− fibroblasts (AT5) were more radiosensitive than ATM+/+ human fibroblasts (MRC5) and were not protected by DIM (Fig. 5 B and C). Brca1−/− mouse embryo fibroblasts (MEFs) were also more sensitive to radiation than Brca1+/+ MEFs and were not protected by DIM (Fig. 5D). MRE11 is a component of the MRN (MRE11-RAD50-NBS1) complex, which acts as a DNA damage sensor and upstream activator of ATM (35). MRE11 mutations cause an ataxia-telangiectasia–like disorder (ATLD) (36). Whereas wild-type human dermal fibroblasts (CWAT) were protected by DIM (0.3 μM), MRE11-deficient cells (ATLD2 and ATLD3) were more radiosensitive than CWAT and were not protected by DIM (Fig. S4 A–C). MRE11-deficient cells showed no phosphorylation of ATM or BRCA1 after a 30-min exposure to DIM (Fig. S4D). These findings suggest that MRE11, ATM, and BRCA1 are required for DIM radioprotection.
Fig. 5.
ATM and BRCA1 are required for DIM radioprotection. (A) The 184A1 cells were pretreated with control-siRNA or ATM-siRNA, treated ± DIM for 24 h, irradiated, and assayed for clonogenic survival. Knockdown of ATM is shown in the inset Western blot. (B and C) ATM-competent (+/+) MRC5 (B) or ATM mutant (−/−) AT5 (C) fibroblasts were pretreated ± DIM for 24 h, irradiated, and assayed for survival. The Western blot inset in C shows ATM protein levels. (D) Brca1-competent (Brca1+/+) or deficient (Brca1−/−) MEFs were pretreated ± DIM for 24 h, irradiated, and assayed for survival.
DIM Stimulates DNA Repair and Inhibits Apoptosis.
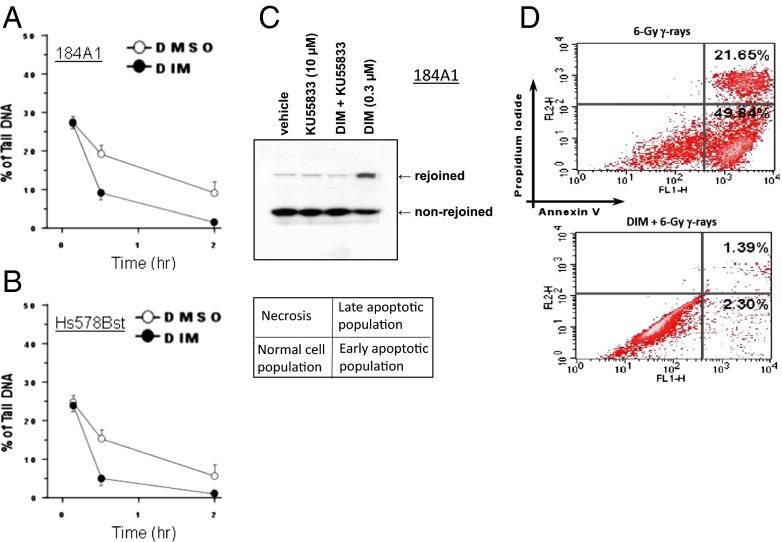
We tested whether DIM could stimulate DNA repair. The neutral comet assay is an electrophoretic method to measure DNA damage that reflects DSBs (37). The percent of tail DNA reflects the number of DSBs. Pretreatment with DIM (0.3 µM) for 24 h reduced the percent comet tail DNA at 30 min and 2 h after radiation (3 Gy), suggesting more rapid DNA repair (Fig. 6 A and B). The data in Fig. 6 A and B are representative of three independent experiments per each cell line. We also performed a DNA strand-rejoining assay based on the ability of cell nuclei to rejoin a linearized plasmid, detected by Southern blotting. This assay reflects DSB repair by nonhomologous end joining. Vehicle-treated 184A1 cells showed a modest ability to rejoin DNA strands that was greatly enhanced by pretreatment with DIM (Fig. 6C). KU55933 blocked DIM-induced strand rejoining, suggesting it is ATM dependent. Based on three independent experiments per cell line, quantification revealed the following strand rejoining: 184A1; vehicle, 1.2 ± 2.3%; KU55933, 1.9 ± 2.1%; DIM, 32.2 ± 6.3%; and DIM+KU55933, 1.0 ± 0.4%; Hs578Bst: vehicle, 5.5 ± 1.6%; KU55933, 5.1 ± 2.1%; DIM, 46.1 ± 7.9%; and DIM+KU55933, 3.8 ± 1.3%. Comparison of DIM vs. vehicle yielded P < 0.001. In other cell lines, DIM-treated cells showed 30–60% strand rejoining, whereas control cells gave 0–2%.
Fig. 6.
DIM stimulates DNA repair and inhibits apoptosis. (A and B) The 184A1 (A) or Hs578Bst (B) cells were pretreated ± DIM (0.3 μM x 24 h), exposed to radiation (3 Gy) on ice, and subjected to neutral comet assays at different times after irradiation. Values of % tail DNA are means ± ranges of two determinations. (C) Plasmid strand-rejoining assays were performed by using nuclear lysates from 184A1 cells that were treated as indicated for 24 h. (D) The 184A1 cells were pretreated ± DIM for 24 h and irradiated (6 Gy). Twenty-four hours after irradiation, cells were analyzed by flow cytometry for apoptosis, by measuring membrane redistribution of phosphatidylserine. The percentage of early and late apoptotic cells for unirraduated control cells were as follows: vehicle treated, 1.3% and 1.3%; and DIM-treated, 0.5% and 1.0%, respectively. Images are representative of three independent experiments.
Pretreatment with DIM (24 h) blocked radiation-induced apoptosis in 184A1 cells, determined by flow cytometry of annexin V-labeled cells (Fig. 6D). Based on three independent experiments, the percent of apoptotic cells were as follows: radiation: early apoptosis, 24.5 ± 3.7%; late apoptosis, 37.0 ± 5.5%; and DIM+radiation: early apoptosis, 2.4 ± 0.6% (P < 0.001); late apoptosis, 4.5 ± 1.1% (P < 0.001). Protection of 184A1 cells against radiation-induced apoptosis was blocked by a selective cell permeant NF-κB activation inhibitor (CAS 545380-34-5), as was the protection observed in clonogenic survival assays, suggesting that in addition to ATM, BRCA1, and MRE11, DIM radioprotection also depends on the survival-promoting transcription factor NF-κB (Fig. S5 A and B). We also found that DIM potentiated radiation-induced stimulation of NF-κB reporter activity (Fig. S5C). These findings are consistent with the ability of ATM to stimulate NF-κB survival signaling in irradiated cells (38).
DIM Inhibits PP2A Activity.
Previous studies showed that protein phosphatase 2A (PP2A; a three subunit protein) acts as an upstream regulator of ATM. PP2A is normally bound to ATM in undamaged cells and maintains ATM in the unphosphorylated state (39). In response to ionizing radiation, PP2A dissociates from ATM, allowing its autophosphorylation. Using a commercial assay kit, we found that a 30-min exposure to DIM caused a dose-dependent inhibition of PP2A activity in 184A1 cells, with 70% inhibition at 0.3 μM DIM (Fig. S6). As a control, 5 nM okadaic acid, a selective PP2A inhibitor, gave approximately 80% inhibition of PP2A activity.
Discussion
DIM protects against γ radiation by a unique mechanism: stimulation of an ATM-driven DDR-like response, without causing DNA damage. This response involves signaling through an MRN/ATM/BRCA1 pathway. Because multiple doses of DIM after radiation were superior to a single dose in protecting rodents against TBI, it seems likely that in vivo radioprotection is due to more than acute stimulation of DNA repair. Most DSBs are repaired by 2–3 h after irradiation, but some (∼15%) are repaired very slowly, and these breaks may cause delayed cell death (40–42). Thus, DSB repair might contribute to mitigation, even when DIM is started 24 h after TBI.
Oxidative stress in irradiated tissues contributes to tissue damage, which can be ameliorated by antioxidants (e.g., superoxide dismutase and tocopherols) (28, 29). DIM stimulates and BRCA1 mediates antioxidant defenses, in part, by stimulating antioxidant gene expression via the transcription factor NFE2L2 (25, 43). BRCA1 also protects against oxidative stress by up-regulating the base excision repair pathway, which mediates repair of oxidative DNA lesions (44). A role for ATM in the antioxidant response is well established (45–47). Studies suggest ATM acts as a redox sensor and is activated by oxidative stress (48, 49). Thus, DIM-stimulated ATM/BRCA1 signaling to the DNA repair and antioxidant machinery could contribute to tissue radioprotection.
Our findings suggest that DIM function as a radioprotector/mitigator is due, in part, to its ability to activate ATM. Whether the physical target for DIM is ATM, one of its upstream regulators, or a component of chromatin, is uncertain. However, the finding that DIM inhibits PP2A, a negative regulator of ATM activity (39), suggests that DIM may activate ATM, in part, by inhibiting PP2A or a PP2A-like phosphatase.
The finding that NF-κB participates in DIM radioprotection is consistent with a known role for NF-κB in modulating survival in irradiated cells (38). DIM-induced NF-κB signaling may proceed through ATM-dependent (38) and/or ATM-independent activation processes. The finding that NF-κB is required for DIM radioprotection is consistent with the finding that DIM likely stimulates DSB repair, because DSBs are the major driver of radiation-induced cell killing and DSB repair takes time. Thus, a survival mechanism serves to prevent DSB-driven apoptosis.
As a radioprotector, DIM has several desirable features. It is a small molecule (246 Da) and can be administered safely by oral route to humans (9–12, 23). Although we used i.p. dosing for convenience, DIM can be given to mice (250 mg/kg) by oral gavage, with no acute toxicity and excellent bioavailability (7). One potential use is to mitigate radiation sickness in individuals accidentally exposed to radiation. The ability of DIM to protect against TBI when administered starting 24 h after TBI is important, because access to treatment may be delayed. Delivery by a route other than oral (e.g., s.c.) is useful, because GI syndrome may preclude oral administration after whole body exposures. DIM exhibits suitable bioavailability and strong radioprotection when administered s.c. to mice (50–52). We demonstrated 40% survival in rats given DIM s.c. after 13 Gy of TBI.
Although TBI affects multiple organ systems, death within the first 30-d is primarily due to (i) GI syndrome, which usually causes death within 10 d after exposure to 8–20 Gy of γ-rays, due to fluid and electrolyte imbalance and sepsis; and (ii) hematopoietic syndrome, which causes death within 30 d after exposure to 3–8 Gy, due to neutropenia and thrombocytopenia. DIM improved survival over a wide range of doses (5–13 Gy), suggesting that it can mitigate both GI and hematopoietic injury.
Methods
Cells Lines and Culture.
Brca1-deficient and wild-type MEFs were provided by Chuxia Deng (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD). MRC5 and AT5 cells were a gift from F. d'Adda di Fagagna [Wellcome Cancer Research Campaign (CRC) Institute, Cambridge, UK]; and Hs578Bst, 184A1 and MDA-MB-231 cells were obtained from the ATCC. CWAT, ATLD2, and ATLD3 cells were provided by M. Taylor (CRC Institute for Cancer Research, Birmingham, UK). Hs578Bst cells were cultured in Hybri-Care Medium (American Type Culture Collection), supplemented with 30 ng/mL mouse EGF and 10% (vol/vol) FCS. The 184A1 cells were cultured in mammary epithelium basal medium supplemented with the MEGM BulletKit (CC-3150; BioWhittaker). MRC5, AT5, and CWAT were cultured in Ham's F-10 medium with 15% (vol/vol) FCS and antibiotics. ATLD2 and ATLD3 cells were maintained in DMEM with high glucose and 10% (vol/vol) FCS. MDA-MB-231 cells were grown in DMEM plus 5% (vol/vol) FCS, l-glutamine (5 mM), nonessential amino acids (5 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL) (BioWhittaker). Further details are given in SI Methods.
Supplementary Material
Acknowledgments
These studies used the shared resources of Georgetown University (flow cytometry, tissue culture, and microscopy shared resources). This work was supported by US Public Health Service Grants CA104546 and CA150646, a grant from the Center for Drug Discovery at Georgetown University, and a Dean’s Pilot Research Award.
Footnotes
Conflict of interest statement: S.F., M.L.B., and E.M.R. and Georgetown University have submitted a patent application for the usage of DIM (3,3′-diindolylmethane) and DIM-related compounds as radioprotectors.
This article is a PNAS Direct Submission.
See Commentary on page 18355.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308206110/-/DCSupplemental.
References
- 1.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5(9):733–748. [PubMed] [Google Scholar]
- 2.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradlow HL, Michnovicz J, Telang NT, Osborne MP. Effects of dietary indole-3-carbinol on estradiol metabolism and spontaneous mammary tumors in mice. Carcinogenesis. 1991;12(9):1571–1574. doi: 10.1093/carcin/12.9.1571. [DOI] [PubMed] [Google Scholar]
- 4.Kojima T, Tanaka T, Mori H. Chemoprevention of spontaneous endometrial cancer in female Donryu rats by dietary indole-3-carbinol. Cancer Res. 1994;54(6):1446–1449. [PubMed] [Google Scholar]
- 5.Jin L, et al. Indole-3-carbinol prevents cervical cancer in human papilloma virus type 16 (HPV16) transgenic mice. Cancer Res. 1999;59(16):3991–3997. [PubMed] [Google Scholar]
- 6.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4(9):1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 7.Anderton MJ, et al. Physiological modeling of formulated and crystalline 3,3′-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab Dispos. 2004;32(6):632–638. doi: 10.1124/dmd.32.6.632. [DOI] [PubMed] [Google Scholar]
- 8.Anderton MJ, et al. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res. 2004;10(15):5233–5241. doi: 10.1158/1078-0432.CCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 9.Reed GA, et al. Single-dose and multiple-dose administration of indole-3-carbinol to women: Pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2477–2481. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- 10.Reed GA, et al. Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3′-diindolylmethane in healthy subjects. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2619–2624. doi: 10.1158/1055-9965.EPI-08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heath EI, et al. A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3′- Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am J Transl Res. 2010;2(4):402–411. [PMC free article] [PubMed] [Google Scholar]
- 12.Del Priore G, et al. Oral diindolylmethane (DIM): Pilot evaluation of a nonsurgical treatment for cervical dysplasia. Gynecol Oncol. 2010;116(3):464–467. doi: 10.1016/j.ygyno.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 13.Kong D, et al. Mammalian target of rapamycin repression by 3,3′-diindolylmethane inhibits invasion and angiogenesis in platelet-derived growth factor-D-overexpressing PC3 cells. Cancer Res. 2008;68(6):1927–1934. doi: 10.1158/0008-5472.CAN-07-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, et al. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282(29):21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 15.Garikapaty VP, Ashok BT, Tadi K, Mittelman A, Tiwari RK. 3,3′-Diindolylmethane downregulates pro-survival pathway in hormone independent prostate cancer. Biochem Biophys Res Commun. 2006;340(2):718–725. doi: 10.1016/j.bbrc.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 16.Firestone GL, Bjeldanes LF. Indole-3-carbinol and 3-3′-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions. J Nutr. 2003;133(7) Suppl:2448S–2455S. doi: 10.1093/jn/133.7.2448S. [DOI] [PubMed] [Google Scholar]
- 17.Firestone GL, Sundar SN. Minireview: Modulation of hormone receptor signaling by dietary anticancer indoles. Mol Endocrinol. 2009;23(12):1940–1947. doi: 10.1210/me.2009-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulvey L, et al. Interplay of genes regulated by estrogen and diindolylmethane in breast cancer cell lines. Mol Med. 2007;13(1-2):69–78. doi: 10.2119/2006-00038.Mulvey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter TH, et al. Diindolylmethane alters gene expression in human keratinocytes in vitro. J Nutr. 2002;132(11):3314–3324. doi: 10.1093/jn/132.11.3314. [DOI] [PubMed] [Google Scholar]
- 20.Meng Q, et al. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. J Nutr. 2000;130(12):2927–2931. doi: 10.1093/jn/130.12.2927. [DOI] [PubMed] [Google Scholar]
- 21.Sun S, et al. Endoplasmic reticulum stress as a correlate of cytotoxicity in human tumor cells exposed to diindolylmethane in vitro. Cell Stress Chaperones. 2004;9(1):76–87. doi: 10.1379/CSC-2R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan S, Meng Q, Auborn K, Carter T, Rosen EM. BRCA1 and BRCA2 as molecular targets for phytochemicals indole-3-carbinol and genistein in breast and prostate cancer cells. Br J Cancer. 2006;94(3):407–426. doi: 10.1038/sj.bjc.6602935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF. Pilot study: Effect of 3,3′-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50(2):161–167. doi: 10.1207/s15327914nc5002_5. [DOI] [PubMed] [Google Scholar]
- 24.Le HT, Schaldach CM, Firestone GL, Bjeldanes LF. Plant-derived 3,3′-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J Biol Chem. 2003;278(23):21136–21145. doi: 10.1074/jbc.M300588200. [DOI] [PubMed] [Google Scholar]
- 25.Fan S, Meng Q, Saha T, Sarkar FH, Rosen EM. Low concentrations of diindolylmethane, a metabolite of indole-3-carbinol, protect against oxidative stress in a BRCA1-dependent manner. Cancer Res. 2009;69(15):6083–6091. doi: 10.1158/0008-5472.CAN-08-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitagawa R, Kastan MB. The ATM-dependent DNA damage signaling pathway. Cold Spring Harb Symp Quant Biol. 2005;70:99–109. doi: 10.1101/sqb.2005.70.002. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Paull TT. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26(56):7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- 28.Thompson JS, Chu Y, Glass J, Tapp AA, Brown SA. The manganese superoxide dismutase mimetic, M40403, protects adult mice from lethal total body irradiation. Free Radic Res. 2010;44(5):529–540. doi: 10.3109/10715761003649578. [DOI] [PubMed] [Google Scholar]
- 29.Epperly MW, et al. Antioxidant-chemoprevention diet ameliorates late effects of total-body irradiation and supplements radioprotection by MnSOD-plasmid liposome administration. Radiat Res. 2011;175(6):759–765. doi: 10.1667/RR2398.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cucinotta FA, Pluth JM, Anderson JA, Harper JV, O’Neill P. Biochemical kinetics model of DSB repair and induction of γ-H2AX foci by non-homologous end joining. Radiat Res. 2008;169(2):214–222. doi: 10.1667/RR1035.1. [DOI] [PubMed] [Google Scholar]
- 31.Jakob B, et al. DNA double-strand breaks in heterochromatin elicit fast repair protein recruitment, histone H2AX phosphorylation and relocation to euchromatin. Nucleic Acids Res. 2011;39(15):6489–6499. doi: 10.1093/nar/gkr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Jung M, Dritschilo A, Jung M. Enhancement of radiation sensitivity of human squamous carcinoma cells by histone deacetylase inhibitors. Radiat Res. 2004;161(6):667–674. doi: 10.1667/rr3192. [DOI] [PubMed] [Google Scholar]
- 33.Hickson I, et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64(24):9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14(12):1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308(5721):551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 36.Taylor AM, Groom A, Byrd PJ. Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis. DNA Repair (Amst) 2004;3(8-9):1219–1225. doi: 10.1016/j.dnarep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Martin FL, et al. DNA damage in breast epithelial cells: Detection by the single-cell gel (comet) assay and induction by human mammary lipid extracts. Carcinogenesis. 1997;18(12):2299–2305. doi: 10.1093/carcin/18.12.2299. [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, Dimtchev A, Lavin MF, Dritschilo A, Jung M. A novel ionizing radiation-induced signaling pathway that activates the transcription factor NF-kappaB. Oncogene. 1998;17(14):1821–1826. doi: 10.1038/sj.onc.1202088. [DOI] [PubMed] [Google Scholar]
- 39.Goodarzi AA, et al. Autophosphorylation of ataxia-telangiectasia mutated is regulated by protein phosphatase 2A. EMBO J. 2004;23(22):4451–4461. doi: 10.1038/sj.emboj.7600455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeggo PA, Löbrich M. Artemis links ATM to double strand break rejoining. Cell Cycle. 2005;4(3):359–362. doi: 10.4161/cc.4.3.1527. [DOI] [PubMed] [Google Scholar]
- 41.Goodarzi AA, Jeggo P, Lobrich M. The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax. DNA Repair (Amst) 2010;9(12):1273–1282. doi: 10.1016/j.dnarep.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Iliakis G, et al. Mechanisms of DNA double strand break repair and chromosome aberration formation. Cytogenet Genome Res. 2004;104(1-4):14–20. doi: 10.1159/000077461. [DOI] [PubMed] [Google Scholar]
- 43.Bae I, et al. BRCA1 induces antioxidant gene expression and resistance to oxidative stress. Cancer Res. 2004;64(21):7893–7909. doi: 10.1158/0008-5472.CAN-04-1119. [DOI] [PubMed] [Google Scholar]
- 44.Saha T, Rih JK, Roy R, Ballal R, Rosen EM. Transcriptional regulation of the base excision repair pathway by BRCA1. J Biol Chem. 2010;285(25):19092–19105. doi: 10.1074/jbc.M110.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barzilai A, Rotman G, Shiloh Y. ATM deficiency and oxidative stress: A new dimension of defective response to DNA damage. DNA Repair (Amst) 2002;1(1):3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 46.Watters DJ. Oxidative stress in ataxia telangiectasia. Redox Rep. 2003;8(1):23–29. doi: 10.1179/135100003125001206. [DOI] [PubMed] [Google Scholar]
- 47.Reliene R, Schiestl RH. Antioxidants suppress lymphoma and increase longevity in Atm-deficient mice. J Nutr. 2007;137(1) Suppl:229S–232S. doi: 10.1093/jn/137.1.229S. [DOI] [PubMed] [Google Scholar]
- 48.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330(6003):517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 49.Krüger A, Ralser M. ATM is a redox sensor linking genome stability and carbon metabolism. Sci Signal. 2011;4(167):pe17. doi: 10.1126/scisignal.2001959. [DOI] [PubMed] [Google Scholar]
- 50.Chang X, et al. 3,3′-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis. 2005;26(4):771–778. doi: 10.1093/carcin/bgi018. [DOI] [PubMed] [Google Scholar]
- 51.Nachshon-Kedmi M, Fares FA, Yannai S. Therapeutic activity of 3,3′-diindolylmethane on prostate cancer in an in vivo model. Prostate. 2004;61(2):153–160. doi: 10.1002/pros.20092. [DOI] [PubMed] [Google Scholar]
- 52.Fares F, Azzam N, Appel B, Fares B, Stein A. The potential efficacy of 3,3′-diindolylmethane in prevention of prostate cancer development. Eur J Cancer Prev. 2010;19(3):199–203. doi: 10.1097/CEJ.0b013e328333fbce. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.