Significance
Sperm capacitation enables spermatozoa to undergo the acrosome reaction and to exhibit vigorous motility called hyperactivation. At the molecular level, capacitation is associated with activation of a cAMP-dependent pathway and with the increase of intracellular pH and Ca2+ concentrations. Ca2+ ionophore A23187 elevates intracellular Ca2+ and induces the acrosome reaction but renders the spermatozoa motionless. However, when the ionophore was washed away, spermatozoa recovered motility, showed hyperactivation, and were able to fertilize cumulus-intact eggs. In these conditions, sperm acquired fertilizing capacity even when the cAMP pathway was inactivated. Fertilized oocytes with A23187-treated sperm developed into normal offspring. These data indicate that a short elevation of intracellular Ca2+ overcomes other necessary signaling pathways during capacitation and renders sperm fertile.
Keywords: sperm capacitation, calcium
Abstract
Ca2+ ionophore A23187 is known to induce the acrosome reaction of mammalian spermatozoa, but it also quickly immobilizes them. Although mouse spermatozoa were immobilized by this ionophore, they initiated vigorous motility (hyperactivation) soon after this reagent was washed away by centrifugation. About half of live spermatozoa were acrosome-reacted at the end of 10 min of ionophore treatment; fertilization of cumulus-intact oocytes began as soon as spermatozoa recovered their motility and before the increase in protein tyrosine phosphorylation, which started 30–45 min after washing out the ionophore. When spermatozoa were treated with A23187, more than 95% of oocytes were fertilized in the constant presence of the protein kinase A inhibitor, H89. Ionophore-treated spermatozoa also fertilized 80% of oocytes, even in the absence of HCO3−, a component essential for cAMP synthesis under normal in vitro conditions. Under these conditions, fertilized oocytes developed into normal offspring. These data indicate that mouse spermatozoa treated with ionophore are able to fertilize without activation of the cAMP/PKA signaling pathway. Furthermore, they suggest that the cAMP/PKA pathway is upstream of an intracellular Ca2+ increase required for the acrosome reaction and hyperactivation of spermatozoa under normal in vitro conditions.
Mammalian spermatozoa, unlike spermatozoa of many other animals, are not able to fertilize on leaving the male body. They must undergo physiological changes, collectively called “capacitation,” to become fertilization-competent. Under normal conditions, sperm capacitation takes place within the female tract, but it can also occur in chemically defined media, as first demonstrated by Toyoda et al. (1) in the mouse. Although compositions of media necessary for successful in vitro capacitation vary between species, most are basically modified Tyrode’s and Krebs–Ringer’s solutions containing HCO3− and Ca2+, supplemented with energy metabolites and a cholesterol acceptor such as serum albumin. Capacitation enables spermatozoa to undergo the acrosome reaction and to exhibit vigorous motility called hyperactivation (2, 3). Both the acrosome reaction and hyperactivation are believed to be essential for successful sperm penetration into oocytes (4). Molecular changes associated with capacitation include an increase in intracellular pH (pHi) (5), an increase in intracellular Ca2+ concentration [Ca2+]i (6), activation of a cAMP/PKA pathway (7, 8), hyperpolarization of the sperm plasma membrane potential (9–11), loss of membrane cholesterol (12, 13) and modifications of other membrane lipids (14), and an increase in protein tyrosine phosphorylation (8, 15). How these events interact with each other to render spermatozoa capable of initiating the acrosome reaction and hyperactivation is not well understood. Recent studies using gene knock-out mice revealed that both cAMP- (14–17) and Ca2+-regulated signaling pathways (16–18) are intricately involved in these processes.
Involvement of Ca2+ in the sperm acrosome reaction and in hyperactivation has been known for a long time (4). Ca2+ ionophore, which transports extracellular Ca2+ into cells or releases Ca2+ from intracellular stores (19), induces increased respiration (20), motility (21), and the acrosome reaction (22) in mammalian spermatozoa. Several studies have shown that Ca2+ ionophore A23187 increases intracellular Ca2+ concentration excessively, rendering spermatozoa immotile (23–26). However, immobilized spermatozoa are not dead, as demonstrated by Suarez et al. (25), who found that spermatozoa could regain motility when high concentrations of BSA were added to the medium. The high affinity of BSA for hydrophobic A23187 could explain this recovery. However, the question as to whether these spermatozoa are capable of pursuing their physiological function (fertilization) remained unanswered. We report here that mouse spermatozoa treated with ionophore A23178 did indeed become immotile, but soon after the ionophore was removed, they began to move vigorously (hyperactivated) and quickly fertilized oocytes. More surprisingly, ionophore-treated spermatozoa fertilize oocytes under the constant presence of H89, a PKA inhibitor, and also in the absence of HCO3− in the medium, an ion that is absolutely necessary for fertilization under normal in vitro conditions (27).
Results
Effect of Ca2+ Ionophore on Sperm Motility, Acrosome Reaction, and Protein Phosphorylation.
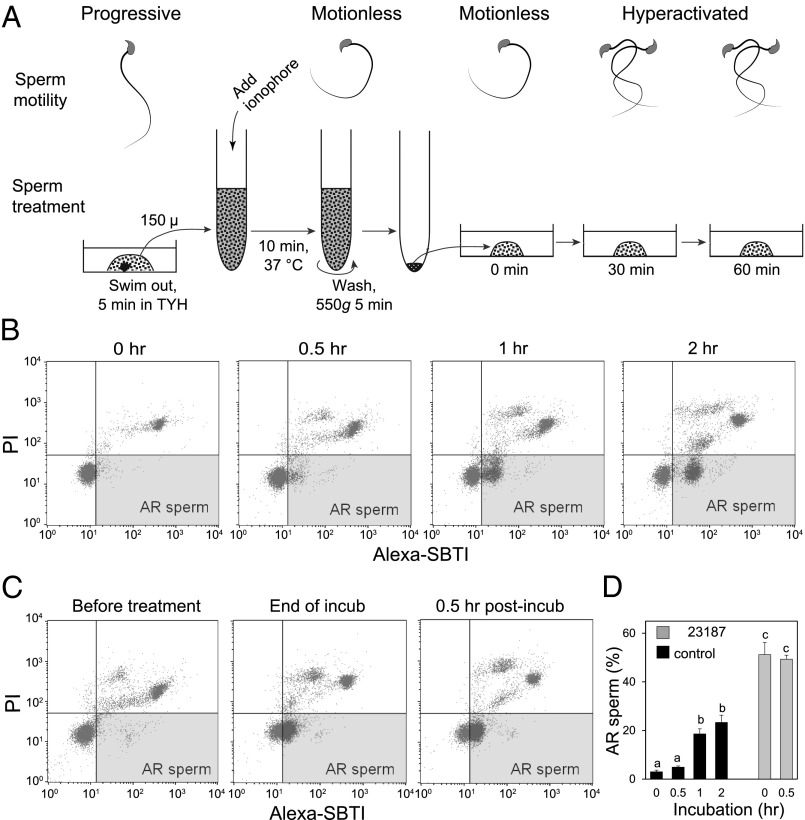
Fig. 1A illustrates how spermatozoa behaved before and after A23187 treatment. On suspension of spermatozoa in capacitating Toyoda–Yokoyama–Hosi (TYH) medium, spermatozoa began to move progressively (Movies S1–S4). As reported by many others, spermatozoa became completely motionless on the addition of ionophore and remained so during 10 min of treatment (Movies S5 and S6). Spermatozoa were motionless even after washing by centrifugation, but some began to display hyperactivated motility by as early as 5 min (Movie S7). Most sperm were hyperactivated by 30 min and remained so for the next 2 h (Movies S8 and S9). In a Ca2+-free medium, the ionophore did not stop sperm movement (Movies S10 and S11), indicating that extracellular Ca2+ is involved in this event. Because PKA activation is known to regulate sperm motility, the effect of A23187 on phosphorylation pathways was investigated by Western blot analysis, using antiphosphorylated PKA substrate antibodies (α-pPKAs) and antiphosphotyrosine antibody (α-pY). Under ordinary sperm-capacitating conditions, a quick activation of PKA occurred in less than 1 min, followed by a slow increase in tyrosine phosphorylation, which started at about 30 min, as reported earlier (28) (Fig. S1A). The addition of A23187 to sperm-capacitating medium 5 min after the beginning of sperm incubation quickly decreased phosphorylation of PKA substrates and blocked tyrosine phosphorylation (Fig. S1B). Because A23187 in Ca2+-free medium does not immobilize spermatozoa (Movies S10 and S11), the excessive amount of Ca2+ entering spermatozoa must be what immobilizes them. Intracellular Ca2+ measurement in Fluo-4–loaded spermatozoa on a laminin-coated coverslip confirmed that intracellular Ca2+ was increased rapidly by A23187 and then decreased in both head and midpiece regions after washing (Fig. S2 and Movies S12–S15). In the single-cell measurements, removal of A23187 was conducted by perfusion with ionophore-free media instead of a fast wash by centrifugation. Therefore, the sperm recovery in single-cell measurements was slower. Interestingly, tail movement was observed only when the intracellular Ca2+ started to decrease at 30 min (Movies S14 and S15).
Fig. 1.
Behavior of mouse spermatozoa before and after Ca2+ -ionophore treatment. (A) Diagram showing sperm behavior and treatment. Spermatozoa were incubated in TYH for 5 min before exposure to 20 µM Ca2+ ionophore A2187. After 10 min treatment with ionophore, spermatozoa were washed and resuspended in fresh TYH. They became motionless on ionophore treatment. After washing by centrifugation, most spermatozoa showed hyperactivation by 30 min and remained so for few hours (Movies S5–S9). (B) Spermatozoa, incubated in TYH for 0–2 h, were stained with both Alexa-SBTI and PI to evaluate acrosomal status by cytometry, Two-dimensional plots differentiated between spermatozoa with low (live) and high (dead) PI staining (y axis) and those with low (intact) or high (acrosome-reacted) SBTI staining (x axis). The lower-right quadrant represented live, acrosome-intact spermatozoa; almost all live spermatozoa at 0 h of incubation were acrosome-intact. The upper-right quadrant represented dead, acrosome-disrupted spermatozoa. The lower right quadrant (colored) represents live, acrosome-reacted spermatozoa. Cytometric assays were repeated three times. Live, acrosome-reacted spermatozoa had green fluorescence in their acrosomal cap (Fig. S3). Nuclei of live spermatozoa remained dark. (C) Acrosomal status of spermatozoa before, at the end of ionophore treatment, and 0.5 h after washing the ionophore. (D) Proportions of live, acrosome-reacted spermatozoa in capacitating media at various times after starting incubation (black columns) and at 0 and 0.5 h after ionophore treatment (gray columns).
FACScan (Becton Dickinson) analyses of spermatozoa using Alexa-soybean trypsin inhibitor (SBTI) (stains the acrosomal cap region of spermatozoa that have undergone the acrosome reaction; Fig. S3) and propidium iodide (labels dead spermatozoa) revealed that a few control spermatozoa (those not treated with A23187) started their acrosome reaction as early as 30 min after the onset of incubation, whereas the reaction became more prevalent after 1 h of incubation (Fig. 1 B and D). In contrast, the acrosome reaction was distinct in about half of spermatozoa at the end of a 10-min treatment with A23187 (Fig. 1 C and D).
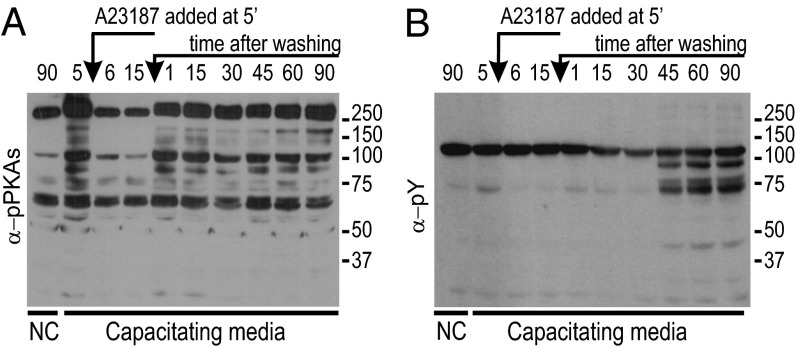
Western blots using antiphospho PKA substrates (Fig. 2A) indicated that PKA was activated on suspension of spermatozoa in capacitating medium (Fig. 2A, second lane). It was inhibited during ionophore treatment (Fig. 2A, third and fourth lanes) but recovered quickly after washing (Fig. 2A, fifth lane and onward). The recovery kinetics were similar to the initial activation kinetics observed without ionophore treatment (Fig. S1A). When the same blots were stripped (Methods) and probed with anti-pY antibodies, they showed that tyrosine phosphorylation started between 30 and 45 min after the end of ionophore treatment (Fig. 2B, seventh and eighth lanes). As reported previously (15), such a slow onset of tyrosine phosphorylation was also observed in normal conditions. As another control, spermatozoa were incubated in HCO3−-free medium, a condition that does not support capacitation. As expected, in the absence of HCO3−, neither phosphorylation of PKA substrates (Fig. 2A, first lane) nor any increase in tyrosine phosphorylation (Fig. 2B, first lane) occurred even after 90 min of incubation.
Fig. 2.
Immunodetection of PKA activity α-pPKAs and α-pY in spermatozoa at various times (in minutes) before and after Ca2+-ionophore treatment. Spermatozoa were either cultured in HCO3-containing capacitation medium or HCO3-free NC. (A) In NC, PKA remained inactive (first lane). In capacitating medium, PKA became active after sperm suspension in capacitation medium (second lane). It was inhibited by ionophore treatment (third and fourth lanes), but its activity was restored on washing (fifth lane and onward). (B) In NC, protein phosphorylation (α-pY) did not occur even after 90 min incubation (first lane). In capacitating medium, protein phosphorylation began sometime between 30 and 45 min after ionophore removal by washing (eighth lane and onward).
Fertilization by Ionophore-Treated Spermatozoa.
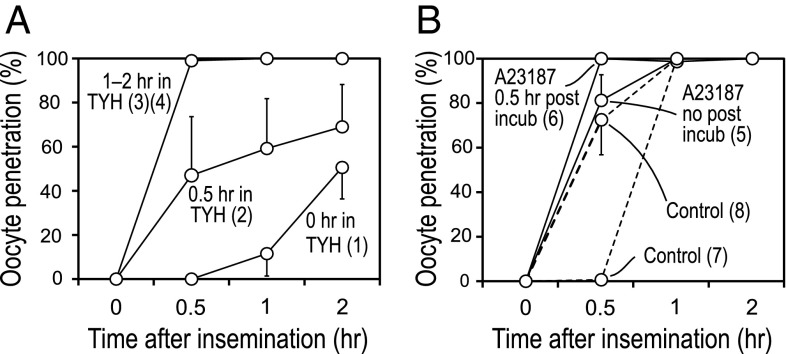
Fig. 3 indicates schematically the points at which spermatozoa were tested for their fertilizing ability with or without ionophore treatment. Fig. 4 shows that fresh spermatozoa with no preincubation in TYH medium (0 h in TYH) began to fertilize between 0.5 and 1 h after insemination (Fig. 4A, treatment 1). The fastest fertilization took place when spermatozoa were preincubated in TYH for 1–2 h (Fig. 4A, treatments 3 and 4). A23187-treated spermatozoa, and in particular those mixed with eggs after 0.5 h posttreatment incubation, penetrated eggs as fast as control spermatozoa that were preincubated in TYH for 1–2 h (Fig. 4B, treatment 6). The somewhat slower penetration of A23187-treated spermatozoa without posttreatment (Fig. 4B, treatment 5) could be attributed to the time (10–30 min) required to recover motility after washing.
Fig. 3.
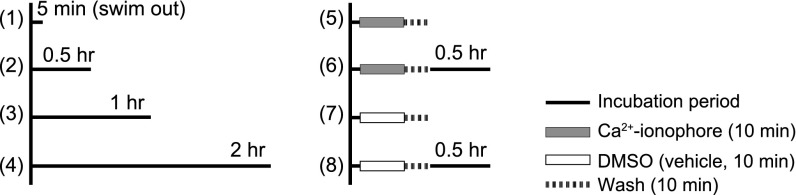
Diagram illustrating how spermatozoa were incubated in the standard TYH (1–4) or in Ca2+ ionophore solution (5 and 6) to determine how quickly they could fertilize oocytes. (1) Spermatozoa were dispersed in TYH for 5 min without further incubation. (2–4) Spermatozoa were incubated for 0.5–2 h before being use for insemination. (5 and 6) Spermatozoa were dispersed in TYH and then exposed to 20 µM Ca2+ ionophore and 1% DMSO for 10 min and washed for 5 min with or without 0.5 h posttreatment incubation.
Fig. 4.
Proportion of oocytes fertilized by spermatozoa with or without ionophore treatment. (A) Oocytes fertilized by control spermatozoa without ionophore treatment. Oocytes were fertilized more rapidly as the time of preincubation in the standard TYH was increased. The fastest sperm penetration occurred when spermatozoa were preincubated in TYH for 1–2 h (3 and 4). (B) Oocytes fertilized by ionophore-treated spermatozoa with (6) or without (5) 0.5 h posttreatment incubation. One percent DMSO was present in the medium during ionophore treatment. The fastest sperm penetration occurred when ionophore-treated spermatozoa were washed and incubated in TYH for 0.5 h before insemination (6). Dotted lines show fertilization by spermatozoa treated with 1% DMSO-containing TYH (no ionophore) for 10 min, with (8) or without (7) 0.5 h posttreatment incubation.
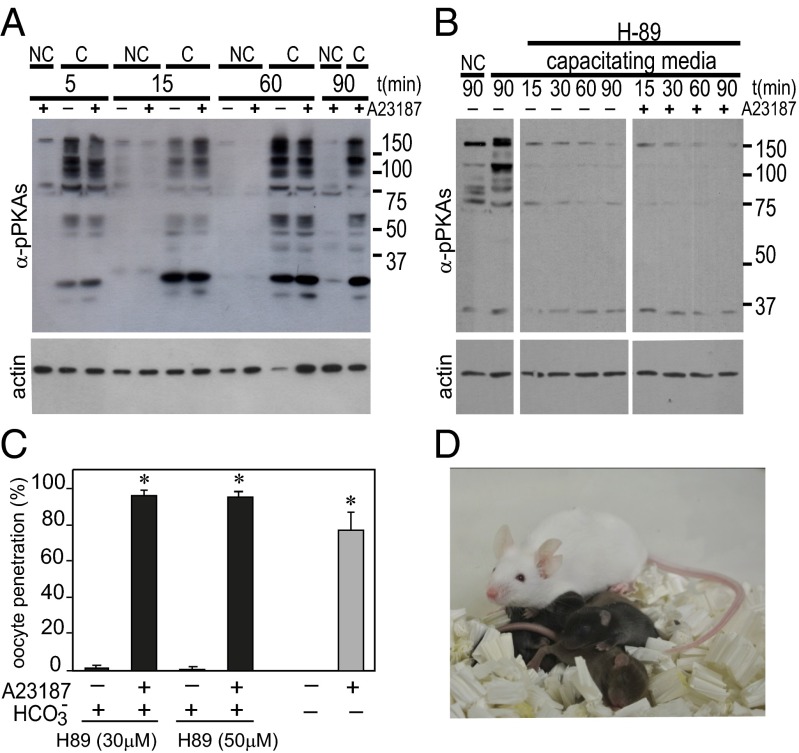
HCO3− in the medium is essential for sperm hyperactivation (29) and fertilization (27). It is known that this anion plays a key role in cAMP synthesis during sperm capacitation (30). In addition, it is known that H89, which blocks cAMP-dependent PKA, prevents spermatozoa from capacitation (30). Because we already showed that ionophore-treated spermatozoa fertilized all oocytes by 30 min after insemination (Fig. 4B), before protein tyrosine phosphorylation became evident (Fig. 2B), we examined whether ionophore treatment could bypass cAMP-dependent processes. We examined PKA activation in spermatozoa incubated in media lacking HCO3− (noncapacitating conditions) or in media containing H89. Sperm PKA activity was inhibited under noncapacitating conditions (Fig. 5A), as well as with the constant presence of H89 in the medium (Fig. 5B), regardless of any treatment with ionophore. Ionophore-treated spermatozoa fertilized the majority of oocytes in both capacitating (HCO3–-containing) and noncapacitating (HCO3−-free) media, which was in marked contrast with control spermatozoa not treated with ionophore (Fig. 5C). Similarly, oocytes were fertilized by ionophore-treated spermatozoa incubated in the constant presence of H89. In all tested conditions, the majority of fertilized oocytes developed into normal offspring after transfer of two-cell embryos to surrogate mothers (Fig. 5D and Table 1).
Fig. 5.
Ca2+-ionophore treatment overcomes the need for PKA activation in spermatozoa. (A) PKA activation in spermatozoa with (+) or without (−) Ca2+ ionophore treatment in the presence (C) or absence (NC) of HCO3−. Times in the figure represent minutes after washing. Note that PKA was activated in capacitating medium regardless of sperm treatment with ionophore (A23187 for 10 min and washed). No PKA activation occurred in NC irrespective of ionophore treatment. (B) PKA activity was weak at 90 min after sperm incubation in noncapacitating medium (first lane) and was strong in capacitating medium (second lane). When H89 was added to the capacitating medium (third to sixth lane) PKA remained inactive for up to 90 min. When sperm were exposed for 10 min to ionophore and then incubated in capacitating medium containing H89, PKA remained inactive for up to 90 min (seventh to tenth lanes). (C) Fertilization rates after insemination with spermatozoa with (+) or without (−) Ca2+ ionophore treatment (A23187 for 10 min and then washed). When spermatozoa were not treated with ionophore (−), no oocytes were fertilized under the constant presence of H89 (black bars, A23187) or in noncapacitating medium (gray bars, A23187). Ionophore-treated spermatozoa (A23187 for 10 min and washed) fertilized most oocytes even under the constant presence of H89 (black bars + A23187) and in HCO3−-free, noncapacitating medium (gray bars, + A23187). (D) Live offspring (black) developed from the oocytes fertilized with sperm treated with ionophore (A23187 for 10 min and then washed) in the constant presence of H89 in TYH.
Table 1.
Production of live offspring after transfer of two-cell embryos developed from in vitro fertilized oocytes
| Sperm treatment | No. of transferred embryos (No. of experiments) | Live offspring (%) |
| Control | 30 (2) | 27 (90.0) |
| Ionophore | 31 (2) | 22 (71.0) |
| Ionophore + H89 (30 μM) | 19 (2) | 14 (73.7) |
| Ionophore + H89 (50 μM) | 23 (2) | 17 (73.9) |
| Ionophore without HCO3− | 30 (2) | 27 (90.0) |
These oocytes were inseminated with spermatozoa with or without ionophore treatment. Both spermatozoa and oocytes were in the same media containing various combinations of H89 and HCO3– (for details, see Methods).
Discussion
Ionophores are lipid-soluble molecules that complex cations and transport them across a variety of membranes (19). A23187, used in this work, transports extracellular Ca2+ into spermatozoa (25). This ionophore induces the acrosome reaction of spermatozoa of a variety of animal species (2), but it can also immobilize them (23–26). Because A23187 in Ca2+-free medium did not immobilize spermatozoa (Movies S10 and S11), it must have been the excessive amount of Ca2+ entering spermatozoa that rendered them motionless. Bovine spermatozoa immobilized by A23187 become motile again after the addition of a high concentration of BSA to the medium (25). This finding suggests that BSA reduces the effective A23187 concentration, and as a consequence, the sperm [Ca2+]i is lowered to more physiological levels. Consistent with this explanation, here we showed that removal of A23187 from Fluo-4–loaded spermatozoa decreased intracellular Ca2+ (Movies S14 and S15). In addition, it is important to note that after 10 min of treatment with A23187, nearly half of the live mouse spermatozoa were acrosome-reacted (Fig. 1C) and began to enter oocytes as soon as they became motile.
The most interesting findings in this study were that A23187-treated spermatozoa were able to fertilize cumulus-intact oocytes within 30 min after insemination, when tyrosine phosphorylation had not yet begun (Figs. 2B and 4B), and that the spermatozoa were able to fertilize in HCO3−-free and H89-containing media, although control (normal) spermatozoa could never do so (Fig. 5C). These results indicate that ionophore bypasses signaling events associated with sperm capacitation that are necessary for normal fertilization in vivo and in vitro. Taken together, these data suggest that the cAMP-dependent pathway in spermatozoa is upstream of the intracellular Ca2+ increase required for spermatozoa to become fertilization-competent.
Fig. 6 represents a working model of the sequence of events that occur during capacitation, acrosome reaction, and hyperactivation of spermatozoa under normal conditions (solid lines for known events, dashed lines for probable events). Fig. 6 also suggests a way by which Ca2+ ionophore overcomes other signaling events and induces both the acrosome reaction and hyperactivation of spermatozoa (thin dotted arrows). When fresh spermatozoa are placed in capacitation-supporting medium, the atypical adenylyl cyclase Adcy10 (see 1 in Fig. 6) is quickly stimulated by the combined action of Ca2+ and HCO3−, which enter spermatozoa through an initial Ca2+ transporter, not yet identified (2), and a Na+/HCO3− cotransporter (3), respectively (31) (underlined numbers in parentheses cited in this paragraph refer to the underlined numbers in Fig. 6). Elevated cAMP (4) activates PKA (5) and the Na+/H+ antiporter (6), resulting in a [pH]i rise (7) (30, 32), which stimulates Catsper (9). It is not clear which sperm components are phosphorylated during capacitation, although some candidates have been proposed (33). We hypothesize that they include a secondary Ca2+ transporter (8) and the calcium channel protein Catsper (9) (32). The former, when stimulated by factors from the oviduct, cumulus, or zona pellucida, transports extracellular Ca2+ into the sperm head to induce the acrosome reaction. Ca2+ entering sperm flagellum through activated Catsper (9) induces hyperactivation (16). Alternatively, phosphorylation pathways could regulate Ca2+ channels by inducing hyperpolarization of the sperm plasma membrane potential (Em Hyperpol) (10). Ionophore A23187 (11) raises the [Ca2+]i of spermatozoa to induce both the acrosome reaction and hyperactivation, bypassing all ion transporters and cAMP/PKA pathways in spermatozoa (bold dotted arrows). Apparently all sperm head components necessary for the acrosome reaction (e.g., the outer acrosomal and overlying plasma membrane components) and sperm tail components necessary for hyperactivated motility (e.g., microtubule, dynein and calcium sensor, calaxin) are fully assembled in mature spermatozoa.
Fig. 6.
Working model of the sequence of events involved in preparation of spermatozoa for the acrosome reaction and hyperactivation under normal conditions and after Ca2+ ionophore treatment. See Discussion for an explanation of underlined numbers shown in the model.
According to McPartlin et al. (34), stallion spermatozoa are barely capable of fertilizing oocytes in ordinary media, but they become fertilization-competent in a medium containing ∼5 mM procaine. Although the exact mechanism is unknown, this reagent induces hyperactivated motility in spermatozoa, perhaps by increasing [Ca2+]i (35). Because procaine can have deleterious effects on embryonic development (34), a brief temporal exposure of spermatozoa to a very low concentration of calcium ionophore, as we accomplished in this study, would be preferable to prepare them for insemination. All researchers engaged in in vitro fertilization (IVF) are aware that there is no single technique that works for all species. The conditions necessary for successful sperm capacitation and IVF must be worked out for each species, which is cumbersome and time-consuming. If Ca2+ ionophore can enable spermatozoa of a variety of species to be fertilization-competent without damaging gametes or embryos, we might be able to use this or a similar reagent to prepare spermatozoa for IVF. This will be particularly useful for species in which IVF has been unsuccessful for unknown reasons or for those species whose gametes are seldom accessible for IVF (e.g., exotic or endangered species).
Intracytoplasmic sperm injection (ICSI) has been used successfully for the production of live offspring for human clinical purposes, as well as for basic studies of mammalian fertilization. Spermatozoa of some animals have very large acrosomes, and their injection seriously damages oocytes (36). This might arise from the introduction of large amounts of acrosomal enzymes into oocyte’s cytoplasm, which disrupts the cytoskeleton system (37). Mechanical or chemical removal of acrosomes before ICSI improves both survival and embryonic development of oocytes in the hamster (36), mouse (38), and rat (39). Efficient removal of acrosomes from live spermatozoa using ionophore, as we did here, might increase success rates of ICSI in various species.
Methods
Animals.
B6D2F1 hybrid mice, 7–12 wk of age, were used. Surrogate mothers were CD1 females, 10–12 wk of age. All experiments were carried out according to the Guidelines of Animal Experiments of Asahikawa Medical University (Institutional Animal Care and Use Committee Nos. 11036 and 12062). In experiments in which phosphorylation by PKA and tyrosine phosphorylation was investigated, CD1 male mice were used.
Media.
The medium used for sperm capacitation and fertilization was Toyoda–Yokoyama–Hosi (standard TYH) medium (1), consisting of (in millimolar) 119.37 NaCl, 4.78 KCl, 1.71 CaCl2, 1.19 KH2PO4, 1.19 MgSO4, 25.07 NaHCO3, 1.00 Na-pyruvate, 5.56 glucose, and 4 mg/mL BSA (AlbuMax, GibcoBRL), 50 μg/mL streptomycin sulfate, and 75 μg/mL penicillin G potassium at pH 7.4 when equilibrated with 5% (vol/vol) CO2. Hepes-buffered TYH (H-TYH) was prepared according to Tateno and Kamiguchi (40) and used in pure air. Ca2+ ionophore A23187 (Calbiochem-Merck) was used at 20 μM in 2 × Ca2+ TYH (3.42 mM Ca2+). Noncapacitating H-TYH was prepared by replacing 25 mM NaHCO3 with 25 mM Na-Hepes, containing 4 mg/mL BSA.
Preparation of Oocytes for Insemination.
Female mice, 7–12 wk old, were each injected with 8–10 IU equine chorionic gonadotropin and 8–10 IU human chorionic gonadotropin 48 h apart. Approximately 16 h after hCG injection, mature unfertilized oocytes in cumulus oophorus were collected from oviducts and placed in TYH (37 °C under 5% CO2 in air) and inseminated immediately.
Preincubation of Sperm to Test Their Fertility.
A dense sperm mass, collected from the cauda epididymis of a mature male mouse, was placed in 100 μL standard TYH under paraffin oil (Merck Japan) in a Petri dish at 37 °C, allowing motile sperm to disperse for 5 min (Fig. 3, sperm sample 1). Other sperm were incubated in the same medium for 0.5–2 h before insemination (Fig. 3, samples 2–4). The concentration of sperm during this preincubation was 107–108 cells per mL To treat sperm with ionophore, epididymal sperm were allowed to disperse in a drop (200 μL) of standard TYH for 5 min at 37 °C. An aliquot (150 μL) of the sperm suspension was transferred to a test tube and exposed to 20 μM Ca2+ ionophore in 2 × Ca2+ TYH. After 10 min, sperm were centrifuged for 5 min at 550 × g and resuspended in standard TYH with or without further 30 min posttreatment incubation (Fig. 3, samples 5 and 6). Alternatively, sperm were treated with Ca2+ ionophore in noncapacitating H-TYH and incubated for 30 min in H-TYH before insemination. In experiments using H89, sperm were continuously exposed to this inhibitor, including incubation in A23187 and washing steps.
Incubation of Sperm Before Western Blotting.
Sperm from caudae epididymides were allowed to swim out in noncapacitating H-TYH. After 5 min, epididymal tissues were removed and sperm were diluted with capacitating or noncapacitating H-TYH medium to a final concentration of 2 × 107 cells per mL For ionophore treatment, spermatozoa were exposed for 10 min to 20 μM Ca2+ ionophore in either standard or H-TYH media containing 3.4 mM Ca2+. Washing was done by two cycles of centrifugation (5 min, 550 × g). Sperm were resuspended in standard or H-TYH at 2 × 107 cells per mL before incubation in closed, 2-mL, round-bottom tubes at a concentration of 5 × 106 cells per mL, in a 37 °C water bath.
Examination of Sperm Acrosome Reaction.
Acrosome reaction was assessed, using flow cytometry, by staining sperm for 10 min with 1 μg/mL SBTI conjugated to Alexa Fluor 488 (Molecular Probes) and 8 μg/mL propidium iodide (PI) (41). After washing, live acrosome-reacted spermatozoa displayed green fluorescence in their acrosomal cap region. Nuclei of live spermatozoa remained dark. In contrast, the nuclei of dead spermatozoa showed strong red fluorescence. Sperm samples were analyzed using a BD FACS-Calibur (Becton Dickinson) equipped with an argon laser emitting light at 488 nm and a red diode laser emitting light at 635 nm. Fluorescence from Alexa Fluor 488 and from PI were detected at 515–545 nm and over 670 nm, respectively. Flow rate was adjusted to 720 cells per s, and 10,000 cells were counted. All data were analyzed using CELLQuest Pro software.
Ca2+ Image Recordings.
Sperm were incubated in TYH medium containing 2 µM Fluo-4:00 AM (Invitrogen) and 0.05% Pluronic acid for 45 min at 37 °C before wash by centrifugation. Sperm heads were allowed to stick to a mouse laminin-coated coverslip (11). Sperm were exposed to 10 μM A23187 for 10 min before they were washed several times, using A23187-free TYH medium. Calcium imaging of sperm (1 min each) was performed immediately after exposure to ionophore, as well as 10–45 min after washing. Recordings were performed at 37 °C, using a temperature controller model 202A (Harvard Apparatus). Sperm were viewed using an inverted microscope (Nikon Eclipse Ti-U) equipped with an oil immersion fluorescence Nikon plan Apo TIRF objective (60×/1.45) DIC H/N2. An EL6000 Leica mercury short-arc reflector lamp with an integrated shutter was connected via a liquid light guide to the microscope. For excitation and emission collection of Fluo-4, the filter set GFP 96343, Dichroic Mirror 495, Excitation 470/40, and Barrier 525/50 was used (Nikon). Fluorescent images were acquired with an Andor Ixon3 EMCCD camera model DU-897D-C00#B (Andor Technology) under protocols written in Andor iQ 1.10.2 software v4.0. Images were acquired at 0.4–0.5 Hz with an exposure/illumination time of 30 ms. Movies were processed and analyzed in Image J (v1.38, NIH). Total fluorescence levels in the head and midpiece regions, respectively, were normalized against total area of these compartments.
Insemination and Examination of Oocytes.
Cumulus-enclosed oocytes (70–140) were placed in 100-μL droplets of TYH under paraffin oil in a Petri dish. Insemination was performed by adding 5–10 μL of sperm to these droplets (final sperm concentration, 1–2 ×106 cells per mL). At 0.5, 1, and 2 h after insemination, oocytes were removed from the dish. Those taken at 0.5 h after insemination were still surrounded by cumulus cells, which were removed by 2–3 min treatment with 0.1% hyaluronidase (Sigma-Aldrich) in H-TYH. When the effect of H89 on fertilization was being examined, H89 was kept in the media continuously (from sperm treatments to examination of inseminated oocytes). To examine whether oocytes were fertilized, they were first freed from zonae pellucidae by 5 min treatment with 0.5% pronase (1,000,000 tyrosine units/g; Kaken Pharmaceuticals) in PBS, fixed with acetic-alcohol (1:3), and spread on glass slide by an air-drying method (42) before staining with Giemsa. An egg was considered fertilized when it contained a swollen sperm head or a compact sperm head stained dark with Giemsa. According to Krzanowska (43) and Miller and Masui (44), mouse sperm heads within acetic alcohol-fixed eggs are stained dark by Giemsa or toluidine blue even before their nuclei decondense (45).
SDS/PAGE and Western Blotting.
After treatments, sperm were centrifuged, washed in 1 mL PBS, and prepared for SDS/PAGE and further transferred to PVDF membranes, as described (8). For anti-pY and anti-pPKA immunodetections, membranes were blocked with 20% fish skin gelatin (Sigma-Aldrich) in TBS containing 0.1% Tween-20 (T-TBS). Antibodies were diluted in T-TBS as follows: 1/10,000 for anti-pY (clone 4G10) and 1/5,000 for anti-pPKAS (clone 100G7E). Secondary antibodies were diluted 1/10,000 in T-TBS and developed using an enhanced chemiluminescence detection kit (ECL plus, Amersham), according to the manufacturer’s instructions. When necessary, PVDF membranes were stripped as described (8).
Embryo Transfer.
Eight hours after insemination, fertilized eggs with two pronuclei and the second polar body were collected and cultured until the two-cell stage. These embryos were transferred into the oviducts of CD1 albino female mice that had been mated with vasectomized males of the same strain during the previous night. Females were allowed to deliver and raise pups.
Statistical Analysis.
Percentile data on acrosome reacted spermatozoa and oocyte penetration were transformed into arcsine values for statistical analysis. When the data were compared among different groups, one-way analysis of variance followed by Student t test or Welch’s method was used. For multiple comparisons, one-way analysis of variance and the Tukey-Kramer method were used. Results of embryo transfer were analyzed using Tukey’s method. Differences were considered significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Kim Tremblay (University of Massachusetts) and Dr. James Cummins (Murdoch University) who read the original manuscript and gave us invaluable editorial advice. This work was supported by grants from the Akiyama Science Foundation (to H.T.), the Agencia Nacional de Promoción Cientifica y Tecnológica of Argentina (Proyecto de Investigación Científica y Tecnológica 2011-0540 to D.K.), the University of Hawaii Foundation (to R.Y.), and the National Institutes of Health (HD38082 and HD44044 to P.E.V.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317113110/-/DCSupplemental.
References
- 1.Toyoda Y, Yokoyama M, Hosi T. Studies on the fertilization of mouse eggs in vitro. Jpn. J. Anim. Reprd. 1971;16:147–157. [Google Scholar]
- 2. Yanagimachi R (1994) Mammalian fertilization. The Physiology of Reproduction, eds Knobil E, Neill JD (Raven Press, Ltd., New York), Vol 1, pp 189–317.
- 3.Visconti PE, Krapf D, de la Vega-Beltrán JL, Acevedo JJ, Darszon A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl. 2011;13(3):395–405. doi: 10.1038/aja.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yanagimachi R. Fertility of mammalian spermatozoa: Its development and relativity. Zygote. 1994;2(4):371–372. doi: 10.1017/s0967199400002240. [DOI] [PubMed] [Google Scholar]
- 5.Zeng Y, Oberdorf JA, Florman HM. pH regulation in mouse sperm: Identification of Na(+)-, Cl(-)-, and HCO3(-)-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev Biol. 1996;173(2):510–520. doi: 10.1006/dbio.1996.0044. [DOI] [PubMed] [Google Scholar]
- 6.Ruknudin A, Silver IA. Ca2+ uptake during capacitation of mouse spermatozoa and the effect of an anion transport inhibitor on Ca2+ uptake. Mol Reprod Dev. 1990;26(1):63–68. doi: 10.1002/mrd.1080260110. [DOI] [PubMed] [Google Scholar]
- 7.Harrison RA. Rapid PKA-catalysed phosphorylation of boar sperm proteins induced by the capacitating agent bicarbonate. Mol Reprod Dev. 2004;67(3):337–352. doi: 10.1002/mrd.20028. [DOI] [PubMed] [Google Scholar]
- 8.Krapf D, et al. Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J Biol Chem. 2010;285(11):7977–7985. doi: 10.1074/jbc.M109.085845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng Y, Clark EN, Florman HM. Sperm membrane potential: Hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev Biol. 1995;171(2):554–563. doi: 10.1006/dbio.1995.1304. [DOI] [PubMed] [Google Scholar]
- 10.Escoffier J, Krapf D, Navarrete F, Darszon A, Visconti PE. Flow cytometry analysis reveals a decrease in intracellular sodium during sperm capacitation. J Cell Sci. 2012;125(Pt 2):473–485. doi: 10.1242/jcs.093344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De La Vega-Beltran JL, et al. Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. J Biol Chem. 2012;287(53):44384–44393. doi: 10.1074/jbc.M112.393488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis BK, Byrne R, Bedigian K. Studies on the mechanism of capacitation: Albumin-mediated changes in plasma membrane lipids during in vitro incubation of rat sperm cells. Proc Natl Acad Sci USA. 1980;77(3):1546–1550. doi: 10.1073/pnas.77.3.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross NL. Effect of cholesterol and other sterols on human sperm acrosomal responsiveness. Mol Reprod Dev. 1996;45(2):212–217. doi: 10.1002/(SICI)1098-2795(199610)45:2<212::AID-MRD14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Gadella BM, Harrison RA. The capacitating agent bicarbonate induces protein kinase A-dependent changes in phospholipid transbilayer behavior in the sperm plasma membrane. Development. 2000;127(11):2407–2420. doi: 10.1242/dev.127.11.2407. [DOI] [PubMed] [Google Scholar]
- 15.Visconti PE, et al. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121(4):1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- 16.Ren D, et al. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413(6856):603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quill TA, Ren D, Clapham DE, Garbers DL. A voltage-gated ion channel expressed specifically in spermatozoa. Proc Natl Acad Sci USA. 2001;98(22):12527–12531. doi: 10.1073/pnas.221454998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi H, et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci USA. 2007;104(4):1219–1223. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed PW, Lardy HA. A23187: A divalent cation ionophore. J Biol Chem. 1972;247(21):6970–6977. [PubMed] [Google Scholar]
- 20.Storey BT. Energy metabolism of spermatozoa. IV. Effect of calcium on respiration of mature epididymal sperm of the rabbit. Biol Reprod. 1975;13(1):1–9. doi: 10.1095/biolreprod13.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Babcock DF, First NL, Lardy HA. Action of ionophore A23187 at the cellular level. Separation of effects at the plasma and mitochondrial membranes. J Biol Chem. 1976;251(13):3881–3886. [PubMed] [Google Scholar]
- 22.Yanagimachi R. Acceleration of the acrosome reaction and activation of guinea pigs spermatozoa by detergents and other reagents. Biol Reprod. 1975;13(5):519–526. doi: 10.1095/biolreprod13.5.519. [DOI] [PubMed] [Google Scholar]
- 23.Visconti PE, et al. Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm. Biol Reprod. 1999;61(1):76–84. doi: 10.1095/biolreprod61.1.76. [DOI] [PubMed] [Google Scholar]
- 24.Hong CY, Chiang BN, Ku J, Wei YH, Fong JC. Calcium antagonists stimulate sperm motility in ejaculated human semen. Br J Clin Pharmacol. 1985;19(1):45–49. doi: 10.1111/j.1365-2125.1985.tb02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suarez SS, Vincenti L, Ceglia MW. Hyperactivated motility induced in mouse sperm by calcium ionophore A23187 is reversible. J Exp Zool. 1987;244(2):331–336. doi: 10.1002/jez.1402440218. [DOI] [PubMed] [Google Scholar]
- 26.Tateno H, Mikamo K. A chromosomal method to distinguish between X- and Y-bearing spermatozoa of the bull in zona-free hamster ova. J Reprod Fertil. 1987;81(1):119–125. doi: 10.1530/jrf.0.0810119. [DOI] [PubMed] [Google Scholar]
- 27.Lee MA, Storey BT. Bicarbonate is essential for fertilization of mouse eggs: Mouse sperm require it to undergo the acrosome reaction. Biol Reprod. 1986;34(2):349–356. doi: 10.1095/biolreprod34.2.349. [DOI] [PubMed] [Google Scholar]
- 28.Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci USA. 2009;106(3):667–668. doi: 10.1073/pnas.0811895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neill JM, Olds-Clarke P. A computer-assisted assay for mouse sperm hyperactivation demonstrates that bicarbonate but not bovine serum albumin is required. Gamete Res. 1987;18(2):121–140. doi: 10.1002/mrd.1120180204. [DOI] [PubMed] [Google Scholar]
- 30.Visconti PE, et al. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995;121(4):1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- 31.Demarco IA, et al. Involvement of a Na+/HCO-3 cotransporter in mouse sperm capacitation. J Biol Chem. 2003;278(9):7001–7009. doi: 10.1074/jbc.M206284200. [DOI] [PubMed] [Google Scholar]
- 32.Ren D, Xia J. Calcium signaling through CatSper channels in mammalian fertilization. Physiology (Bethesda) 2010;25(3):165–175. doi: 10.1152/physiol.00049.2009. [DOI] [PubMed] [Google Scholar]
- 33.Jha KN, et al. Biochemical and structural characterization of apolipoprotein A-I binding protein, a novel phosphoprotein with a potential role in sperm capacitation. Endocrinology. 2008;149(5):2108–2120. doi: 10.1210/en.2007-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McPartlin LA, Suarez SS, Czaya CA, Hinrichs K, Bedford-Guaus SJ. Hyperactivation of stallion sperm is required for successful in vitro fertilization of equine oocytes. Biol Reprod. 2009;81(1):199–206. doi: 10.1095/biolreprod.108.074880. [DOI] [PubMed] [Google Scholar]
- 35.Chang H, Suarez SS. Two distinct Ca(2+) signaling pathways modulate sperm flagellar beating patterns in mice. Biol Reprod. 2011;85(2):296–305. doi: 10.1095/biolreprod.110.089789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamauchi Y, Yanagimachi R, Horiuchi T. Full-term development of golden hamster oocytes following intracytoplasmic sperm head injection. Biol Reprod. 2002;67(2):534–539. doi: 10.1095/biolreprod67.2.534. [DOI] [PubMed] [Google Scholar]
- 37.Morozumi K, Yanagimachi R. Incorporation of the acrosome into the oocyte during intracytoplasmic sperm injection could be potentially hazardous to embryo development. Proc Natl Acad Sci USA. 2005;102(40):14209–14214. doi: 10.1073/pnas.0507005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morozumi K, Shikano T, Miyazaki S, Yanagimachi R. Simultaneous removal of sperm plasma membrane and acrosome before intracytoplasmic sperm injection improves oocyte activation/embryonic development. Proc Natl Acad Sci USA. 2006;103(47):17661–17666. doi: 10.1073/pnas.0608183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seita Y, Ito J, Kashiwazaki N. Removal of acrosomal membrane from sperm head improves development of rat zygotes derived from intracytoplasmic sperm injection. J Reprod Dev. 2009;55(5):475–479. doi: 10.1262/jrd.20216. [DOI] [PubMed] [Google Scholar]
- 40.Tateno H, Kamiguchi Y. Evaluation of chromosomal risk following intracytoplasmic sperm injection in the mouse. Biol Reprod. 2007;77(2):336–342. doi: 10.1095/biolreprod.106.057778. [DOI] [PubMed] [Google Scholar]
- 41.Tollner TL, Yudin AI, Cherr GN, Overstreet JW. Soybean trypsin inhibitor as a probe for the acrosome reaction in motile cynomolgus macaque sperm. Zygote. 2000;8(2):127–137. doi: 10.1017/s0967199400000903. [DOI] [PubMed] [Google Scholar]
- 42.Mikamo K, Kamiguchi Y. Primary incidences of spontaneous chromosomal anomalies and their origins and causal mechanisms in the Chinese hamster. Mutat Res. 1983;108(1-3):265–278. doi: 10.1016/0027-5107(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 43.Krzanowska H. Toluidine blue staining reveals changes in chromatin stabilization of mouse spermatozoa during epididymal maturation and penetration of ova. J Reprod Fertil. 1982;64(1):97–101. doi: 10.1530/jrf.0.0640097. [DOI] [PubMed] [Google Scholar]
- 44.Miller MA, Masui Y. Changes in the Stainability and Sulfhydryl Level in the Sperm Nucleus during Sperm-Oocyte Interaction in Mice. (Translated from English) Gamete Res. 1982;5(2):167–179. [Google Scholar]
- 45.Kosower NS, Katayose H, Yanagimachi R. Thiol-disulfide status and acridine orange fluorescence of mammalian sperm nuclei. J Androl. 1992;13(4):342–348. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.