Abstract
Epidemiological studies suggest that living close to the natural environment is associated with long-term health benefits including reduced death rates, reduced cardiovascular disease, and reduced psychiatric problems. This is often attributed to psychological mechanisms, boosted by exercise, social interactions, and sunlight. Compared with urban environments, exposure to green spaces does indeed trigger rapid psychological, physiological, and endocrinological effects. However, there is little evidence that these rapid transient effects cause long-term health benefits or even that they are a specific property of natural environments. Meanwhile, the illnesses that are increasing in high-income countries are associated with failing immunoregulation and poorly regulated inflammatory responses, manifested as chronically raised C-reactive protein and proinflammatory cytokines. This failure of immunoregulation is partly attributable to a lack of exposure to organisms (“Old Friends”) from mankind’s evolutionary past that needed to be tolerated and therefore evolved roles in driving immunoregulatory mechanisms. Some Old Friends (such as helminths and infections picked up at birth that established carrier states) are almost eliminated from the urban environment. This increases our dependence on Old Friends derived from our mothers, other people, animals, and the environment. It is suggested that the requirement for microbial input from the environment to drive immunoregulation is a major component of the beneficial effect of green space, and a neglected ecosystem service that is essential for our well-being. This insight will allow green spaces to be designed to optimize health benefits and will provide impetus from health systems for the preservation of ecosystem biodiversity.
Numerous studies demonstrate that living close to the natural rural or coastal environment, often denoted “green space or “blue space,” respectively, is beneficial for human health. It reduces overall mortality, cardiovascular disease, and depressive symptoms and increases subjective feelings of well-being (1–8). The beneficial effects are particularly prominent in individuals of low socioeconomic status (1–3, 8). It is often suggested that the mechanism of this effect is psychological. Looking at green spaces or walking in parkland or forests cause rapid psychological and physiological changes that can be demonstrated not only by psychological testing (4) but also by mobile elecroencephalograms (5) and by measurements of cerebral blood flow, various cardiac parameters, blood pressure, and salivary cortisol (6, 7). Even looking at the natural environment as images or through a window is said to have beneficial effects (9). Some authors explain this from an evolutionary perspective (10). The relaxation and satisfaction derived from the natural environment might represent the equivalent of “habitat selection” in other species (11). As shown recently by analyzing carbon isotopes in tooth enamel, from as early as 3–4 Mya, hominins were evolving in wooded grassland (12) and followed rivers and coastlines or settled near lakes. Thus, humans will have evolved to obtain psychological rewards from approaching these ideal hunter-gatherer habitats (10).
This psychological explanation is often supplemented by other factors: social interactions, exercise, and sunlight. For example, the natural environment might promote social interactions and a sense of community (13) when the natural environment is an important contributor to the social capital of the individual (14). Similarly green spaces sometimes encourage physical activity (15), although city-dwellers can walk to most resources and tend to do so, whereas individuals living in leafy suburbs are often forced to use their cars to get anywhere at all, so exercise can paradoxically decrease (16, 17). Sunlight is thought to counteract seasonal affective disorder (SAD) and has been used to treat tuberculosis and heal infected wounds (18, 19).
Although all these factors may contribute to the beneficial effects, there are two major uncertainties about the psychological component. First, there is the issue of specificity. Most psychological studies fail to include appropriate controls. It is not sufficient to compare exposure to a city street with exposure to a green space. Would any suitably relaxing environment—a quiet comfortable café, or a cinema showing a feel-good film in the urban environment—have the same psychological effects as green space when tested in comparison with a busy city street? [The other suggested benefits—social interaction, exercise, and sunlight—are clearly not specific for green space. Social capital usually derives from urban social interactions, and it has not been possible, using currently available data, to determine whether exercise taken in a green space is more beneficial than similar exercise taken in a city gym (20): there are undoubtedly health effects of exercise that do not depend on green space (21)].
The second uncertainty about the psychological explanation is the absence of evidence that the measurable rapid short-term psychological and physiological changes that follow exposure to natural environments (whether specific for such environments or not) translate into long-term health benefits. In other words, are these short-term psychological effects related in any way to the suggested health benefits of living close to green space for prolonged periods (reduced mortality, cardiovascular disease, chronic inflammatory disorders, and depression) (1, 2, 22, 23), or are they a separate transient phenomenon?
Summarizing the previous paragraphs, there is suggestive evidence that living close to the natural environment (defined here as nonbuilt, including gardens and agricultural land) has long-term health benefits (1, 2, 8). These benefits might be an additive consequence of several effects—an evolved psychological need, plus perhaps exercise, sunlight, and social interactions—but there are no conclusive data. This issue is crucially important because urban planners need to know whether urban green space is really the best way to achieve the beneficial effects, and assuming that it is, they then need to know the mechanism so that the health advantage derived from green spaces can be optimized. We suggest here that humans do indeed have an evolutionarily predetermined need for exposure to the natural environment, but that this has two distinct components. There is an immunological component that runs in parallel with the psychological one discussed above. This discussion is needed because the field is split into two distinct trains of thought that involve different aspects of our physiology (the brain and the immune system) and different scientific and medical disciplines. We hope here to break down these interdisciplinary barriers and show that these two pathways are likely to work together in ways that can usefully benefit urban planning for human well-being.
Hygiene Hypothesis and the “Old Friends” Mechanism
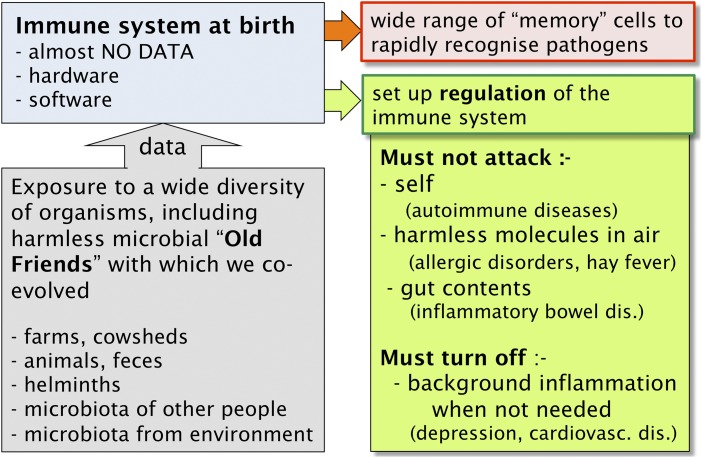
The high-income countries are undergoing massive increases in chronic inflammatory disorders (24–27). The cause is at least partly a failure of immunoregulation, so that the immune system is attacking inappropriate targets, such as self, harmless airborne antigens (allergens) and gut contents. At birth the immune system is like a computer (anatomical structures) that contains programs (genetics) but almost no data in terms of knowledge of molecular structures in the environment into which the child is born. It has some knowledge of self, acquired as lymphocytes mature in the thymus, and minimal knowledge of the outside world, transferred from the mother across the placenta. After birth, it needs microbial exposures to provide teaching inputs for several crucial reasons. First, exposure to a broad biodiversity of organisms builds up memory of diverse molecular structures that accelerates subsequent rapid recognition of novel dangerous organisms (28, 29). Second, microbial components taken up systemically from the gut maintain an essential background level of activation of the innate immune system (30). Third, and most important in the current context, the system needs to develop a network of regulatory pathways and regulatory T cells (Tregs) that stop inappropriate immune attacks on (i) self; (ii) harmless allergens; and (iii) gut contents (Fig. 1). If immunoregulation fails to stop immune attack on any of these categories of forbidden targets, the consequences are (i) autoimmune diseases such as multiple sclerosis; (ii) allergic disorders such as hay fever and atopic asthma; and (iii) inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease (Fig. 1).
Fig. 1.
The immune system does not develop normally in the absence of microbial inputs. In addition to a repertoire of potential effector cells, the system also requires regulatory circuits that inhibit damaging responses to inappropriate targets (such as self, trivial antigens in air, and gut contents) and that terminate inflammatory responses that are no longer needed. The disease groups that occur when immunoregulation fails are indicated in parentheses.
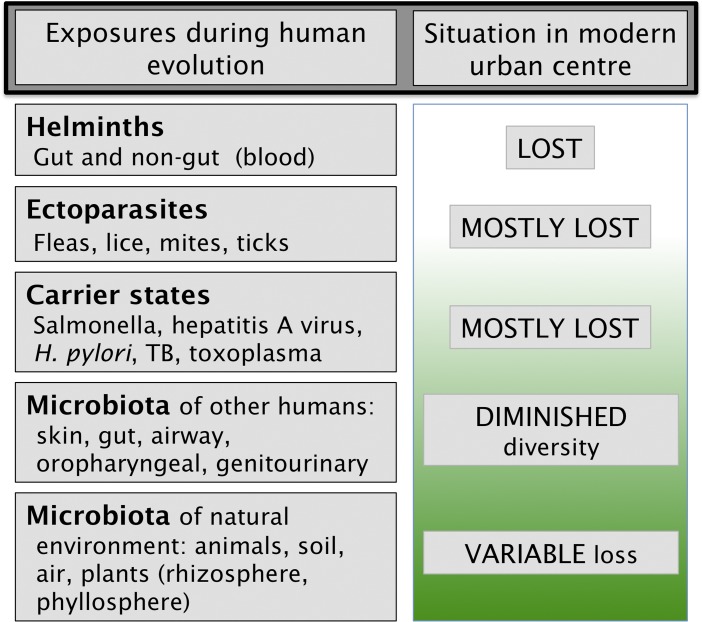
Finally, the immunoregulatory systems must also turn off inflammatory responses completely when they are not needed. A failure to do this is regularly seen in high-income countries where persistently raised levels of C-reactive protein (CRP) are common (discussed in ref. 31). Persistently raised inflammatory mediators lead to increased risk of cardiovascular disease (32) and depression (27, 33, 34). In contrast, a longitudinal study of adults in a rural low-income country where there is exposure to high microbial burdens in childhood showed that they were able to shut off the inflammatory response when there was no need for it (31). More work is needed to discover whether the same is true in other low-income settings, whether biomass or biodiversity was more important, and whether the effect was attributable to bacteria, fungi, protozoa, helminths, or other organisms. Fig. 2 illustrates some of the categories of organisms (Old Friends) implicated in driving the immunoregulatory mechanisms (reviewed and referenced in ref. 35). The crucial point is that all these organisms needed to be tolerated. Some were part of our physiology (human microbiota). Others were harmless but inevitably contaminating food and water (environmental microbiota). Similarly, there were carrier states due to organisms picked up soon after birth and helminths that persist for life. Helminthic parasites needed to be tolerated because, although not always harmless, once they were established in the host, any effort by the immune system to eliminate them was futile and merely caused tissue damage such as elephantiasis (36). Thus, helminths are powerfully immunoregulatory and act as Treg adjuvants. For example, when patients suffering from early relapsing multiple sclerosis (MS) become infected with helminths, the disease stops progressing, and circulating myelin-recognizing Tregs appear in the peripheral blood (37, 38), an exciting observation that has led to formal clinical trials (39). This view is now well supported by experimental data and molecular mechanisms. Old Friends can be shown to drive immunoregulation and to block or treat models of allergies, autoimmune disease, and inflammatory bowel disease (40–42). Some Old Friends (including members of the human gut microbiota such as Bacteroides fragilis) or molecules that they secrete are known to specifically expand Treg populations (42–45) or to cause dendritic cells (DCs) to switch to regulatory DCs that preferentially drive immunoregulation (46). However, it is important to remember that the gut is not the only site where immunoregulation can be induced by macro- and microorganisms. Helminths such as blood nematodes that never enter the gut are powerfully immunoregulatory (36), and recent data implicate the skin and airways also (29, 47).
Fig. 2.
A simple classification of organisms and parasites with which humans coevolved and that have been implicated by epidemiology or experimental models in the modulation of immunoregulation (although those listed as carrier states might be biomarkers of exchange of microbiota with other humans). The modern urban environment eliminates the first three categories, thus increasing our dependence on microbiota from other humans and from the natural environment.
In summary, it is now understood that the various classes of organism (Fig. 2) that had to be tolerated were entrusted by evolutionary processes with the role of setting up the immunoregulatory mechanisms and Treg populations. If this process fails, we develop susceptibility to chronic inflammatory diseases (24, 26, 37, 48), cardiovascular disease (49, 50), and some forms of inflammation-associated depression (27, 33, 51). This concept constitutes the Darwinian reformulation of the hygiene hypothesis in which the emphasis is on lifestyle changes that reduce our exposure to these immunoregulation-inducing Old Friends.
Progressive Loss of Microbial Inputs
Fig. 2 points out that in modern high-income societies, we have lost many of these categories of immunoregulatory Old Friends, so we are now much more dependent on the immunoregulation-inducing effects of the microbiota of other humans and on organisms from the natural environment. These sources of biodiversity are all that remain. However, increasing trends toward agricultural monoculture are likely to decrease rural microbial biodiversity because each crop is associated with strikingly different populations of bacteria, archaea, fungi, protozoa, nematodes, etc. (52). Similarly, the chronic inflammatory disorders that have risen strikingly in prevalence in developed high-income countries are usually found to be still more common in urban environments from which the immunoregulatory Old Friends are essentially absent (Fig. 2). This urban increase is true for allergies (53, 54), inflammatory bowel disease (55), and for autoimmune diseases such as MS (56–58). These urban-rural differences are equally obvious in psychiatric disorders (59). For example, a meta-analysis of high-quality studies performed in high-income countries since 1985 found that the prevalence of depression in urban areas was 39% higher than in rural areas. Similarly, the prevalence of anxiety disorders was 21% higher in urban than in rural areas (60), although a small minority of studies fails to find this urban-rural difference (61). Peen and colleagues also noted an increased urban prevalence of psychiatric disorders in general (38% more in urban communities) (60), and this is strikingly true for schizophrenia (62) and autism (63)—both of which involve an inflammatory component (64). It is usually suggested that these results are explained by greater stressfulness of urban life, or poor urban social networks, but data to establish these explanations are not provided (59). Meanwhile, an increasingly strong case can be made for the involvement of inflammation, secondary to failing immunoregulation in urban environments (27).
It should, however, be noted that not all studies find higher disease prevalences in urban rather than in rural environments. A study performed in the United States found no link between city-level greenness and heart disease and found greater overall mortality in greener cities, possibly attributable to greater car use (17). However, this city-level study did not provide data at the level of the proximity of the individual to green space. Moreover, it combined data from cities in cool wet environments with data from deserts, and apart from heart disease, did not document the chronic inflammatory disorders that are influenced by the immunological mechanisms discussed here.
Another situation that leads to a loss of exposure to microbial biodiversity is immigration from a developing country to a high-income urban center. This migration leads to rapid loss of the first three categories of organism shown in Fig. 2. In such immigrant populations, there are large increases in autoimmunity (27, 65–69), inflammatory bowel disease (55, 70, 71), depression (27, 72, 73), and allergic disorders (74–77). For allergic disorders, this has been rigorously documented for children adopted into Sweden from low/middle-income countries (75), for Mexican immigrants to the United States (76), and for immigrants to Israel from the former Soviet Union or Ethiopia (77).
Natural Environment and Immunoregulation
Some aspects of the Old Friends mechanism (such as immunoregulatory effects of helminths) are clearly not directly relevant to the green space phenomenon in high-income countries. Their absence merely increases our dependence on immunoregulation-inducing exposures from elsewhere, and the focus of this paper is the natural environment. Observations linking exposure to green environments and agriculture to protection from illness were made as early as the 19th century by Blackley who noticed that hay fever was rare among farmers (78). This observation has now been confirmed in many countries using rigorous epidemiological methods (54, 79). Moreover exposure to farms also protects from juvenile forms of inflammatory bowel disease (80). In agreement with the theme of this paper, recent studies indicate that the mechanism of this protection from allergic disorders involves exposure to microbial biodiversity (81). Mattress dust was screened for bacterial DNA (48) and in a separate study samples of settled dust from children’s rooms were gathered with electrostatic dust samplers and evaluated for bacterial and fungal taxa using culture techniques. In both studies, the diversity of microorganisms found was inversely related to the risk of asthma (48). Some of the microorganisms found in dust collected from protective farms have been shown to exert potent antiallergic effects in animal models (82–84) and constitute environmental Old Friends. Recently Hanski and colleagues recorded the skin microbiota as well as allergic sensitization to common allergens in an ecologically mixed area of Finland. Subjects living close to agricultural land rather than urban agglomerations had higher generic diversity of proteobacteria in their skin microbiota and less atopic sensitization (47). Similarly, a genetically homogeneous population living in Karelia is partitioned between Finland and Russia. In Finnish Karelia, the prevalence of type 1 diabetes is sixfold higher and childhood atopy is fourfold higher than in Russian Karelia. These differences are associated with strikingly different microbial populations in the home, with much greater diversity and many more animal-associated strains in Russian homes and more plant-associated species in the Finnish homes (85, 86). We cannot at this stage know whether the crucial factor is biodiversity, total microbial biomass, animal-derived organisms, or organisms from other environmental sources.
Microbial Diversity and the Air
What are the microbial exposures that result from proximity to the natural environment or farms or that fall onto settle plates in a child’s bedroom? First, the air itself contains large numbers of microorganisms, some of which may actively metabolize and replicate in the air (87). Particulate matter in the air such as pollen carries a load of bacteria (88). Many airborne particles are more than 5 μm and will therefore be deposited in the upper airways, so that after being carried up the trachea by the action of cilia, they will be swallowed. Therefore, airborne microorganisms end up on the skin, in the airways, and in the gut where they modulate the immune system.
When total numbers of organisms in air were counted (i.e., not only the cultivable ones) levels of 105/m3 or more were regularly encountered over a grassy field on clear sunny days, and estimates approaching 106/m3 have been reported above shrubs and some grasslands (reviewed in ref. 89). The air in facilities housing agricultural animals can contain still higher numbers, reaching 107–108 archaea and bacteria/m3 (90). Aerosols collected in Texas contained at least 1,800 different bacterial types, representing diversity comparable to that seen in some soils (91). Indeed bacteria commonly found in soil and water are abundant in outdoor air (92, 93). Recent samples from the upper troposphere contained variable proportions of bacteria thought to originate from soil, feces, fresh water, or the sea (94). Thus, blue space is another source of microbial biodiversity. Living by the coast does yield health benefits (8), and marine spray is a rich source of usually harmless microorganisms (95, 96). Interestingly, an organism that is not harmless provides proof of physiologically significant levels of intake of marine aerosols and their contained life forms. There is rapid (<1 h) onset of symptoms of brevetoxin poisoning while walking on beaches during algal blooms (97) and parallel increases in pulmonary problems at some distance inland from such beaches (98).
The microbial diversity that we encounter in the natural environment comes mostly from the soil and from plants and from any animals that are present (89, 92, 93). The microbiota of the soil has huge complexity, and is only now beginning to be explored in a global effort (www.earthmicrobiome.org/) (99). Our ignorance of what is out there remains profound, and these gaps in our understanding have been referred to as microbial “dark matter” (100). Tens of thousands of microbial species are associated with the rhizosphere (the below ground microbial habitat constituted by plant root systems) and the phyllosphere (above ground microbial habitats provided by plants). The crucial point is that plants are able to shape the microbiota of their rhizospheres (101). Thus, the nature of the vegetation in a green space will directly modulate the microbiota present in the soil, rhizosphere, and phyllosphere (102) and indirectly modulate the microbiota available from coexisting animal life. The nature, quantity, and diversity of microorganisms present is strikingly affected by agricultural practices (52), and it is likely that the modern trend toward vast areas of monoculture will reduce that diversity and further decrease exposure to immunoregulation-inducing organisms in wealthy countries.
Microbiota from Animals and Other People
The issue of microbiota derived from animals deserves further comment. The microbiota in dust from households with dogs is significantly richer and more diverse than that found in homes without pets (93, 103). This observation is interesting because exposure to pets, particularly dogs, in early life, protects against allergic sensitization and allergic disorders (104, 105). Moreover dogs were domesticated between 33,000 and 11,000 y ago, so humans have coevolved with dog microbiota for many millennia (106). Considered together with the protective effects of early exposure to cowsheds (79), other animal-derived strains (85), or animal feces (31), this might suggest that animals are a particularly important component of the natural environment. However, other humans are also relevant in this context. Some consider that social interactions are an important consequence of access to the natural environment, and it may be true that such interactions promote well-being by boosting social capital (13, 14), but they will also increase the diversity of organisms to which the individual is exposed. Teammates playing a contact sport tended to share a microbiota, but this converged with that of the opposing team after a match (107). Similarly cohabiting individuals tend to share microbiota (108). Interestingly people tend to share even more microbiota with their dogs (108), increasing exposure to the dog-associated microbial diversity mentioned above (93, 103). In sharp contrast, elderly people shut up in care homes with little variety in human contact (and little exposure to the natural environment) have diminished gut microbiota diversity that correlates with poor health outcomes and increased levels of biomarkers of inflammation such as IL-6 (109).
Microbiota of the Built Environment
The airborne microbiota of buildings is still poorly defined but can be different from that of the natural environment. The phylogenetic diversity of the airborne bacteria in mechanically ventilated rooms was lower than that seen in rooms ventilated via open windows or in the outdoor air itself (92, 93). Similarly the organisms derived from soil and water were abundant in samples of outdoor air but rare or absent from the indoor samples, which were dominated by organisms related to human pathogens and commensals (92). Air in cities tends to contain more particulate matter, such as diesel particulates and metallic fragments from subway wheels. Micrococcus species often dominate in urban air, perhaps associated with such particles (87, 110). The crucial point in the current context is that people living close to agricultural land have more biodiverse microbial populations on their skin than those living close to urban centers (47), and this correlates with immunoregulatory differences and reduced atopy. These organisms are also encountered via the airways and gut. By contrast, the use of biocides in the home may decrease microbial biodiversity (111).
Detrimental Microbiota in Unhealthy Buildings
This point leads on to a further problem with the environment in modern buildings. Humans evolved in a natural environment and in contact with animals. Until recently even our homes were constructed with timber, mud, animal hair, animal dung, thatch, and other natural products and were ventilated by outside air. By contrast, modern buildings are constructed with synthetic materials, plastics, and concrete, and the timber and cardboard are treated with adhesives and biocides, and the buildings are ventilated by air conditioning systems. When these modern structures degrade, become damp, or accumulate condensation in cavity walls, they do not become colonized with the bacterial strains with which we coevolved. They become habitats for unusual strains that we did not encounter during our evolutionary history, some of which synthesize toxic molecules that we are unable to inactivate (112, 113). Some examples of “sick building syndrome” have been tentatively attributed to prolonged exposure to these inappropriate airborne microbiota (112, 113).
Environment and the Human Microbiota
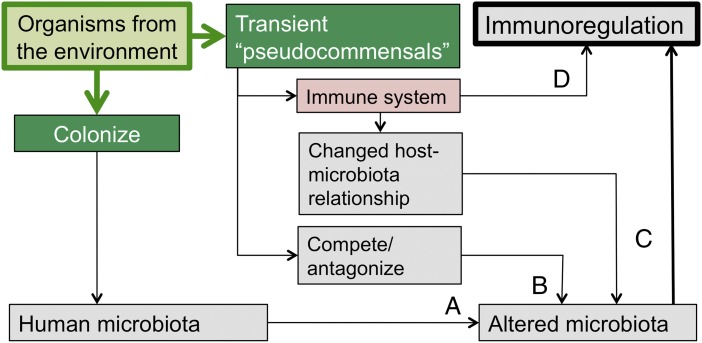
To what extent does exposure to these various environmental sources of microbial diversity directly modulate the human microbiota? Do organisms from green space become members of the human microbiota, or are these organisms “pseudocommensals” that impinge on the skin, airways, and gut and have independent immunoregulatory properties? Both mechanisms probably occur, although there are rather limited data on these issues. The environment does play an important role in the formation and maintenance of our microbiota. Fig. 3 illustrates several potential mechanisms.
Fig. 3.
Multiple ways in which the microbiota of the natural environment can modulate the immune system. This modulation may or may not involve colonization. There can be direct interaction with the immune system (pathway D) or indirect effects secondary to changes in the microbiota (pathways A, B, and C, fully explained in text).
From birth, our microbiota are constituted by colonization with organisms from our mothers, from other social contacts (107, 108), and from the environment, and then further modified by factors such as diet and antibiotics (114, 115). Thus, the lifestyle of the individual has major effects on that individual’s microbiota. The gut microbiota of children from traditional villages in Burkina Faso is totally different from that of Europeans (114). An interesting animal experiment compared piglets that were housed in a natural outdoor environment with genetically similar piglets that had been reared in a very clean indoor facility. Firmicutes, in particular Lactobacillus strains, were dominant in the gut microbiotas of the outdoor piglets, whereas the hygienic indoor piglets had reduced Lactobacillus and more potentially pathogenic phylotypes (116). The indoor piglets also had dramatically different patterns of gene expression in the ileum, discussed in the next section (116). Were these effects due to direct colonization by immunoregulation-inducing organisms from the outdoor environment (pathway A in Fig. 3), or did these organisms fail to colonize but exerted indirect effects on the immune system? The answer is unclear, but indirect effects certainly can occur in several ways. Some organisms compete with or antagonize established organisms (pathway B) and therefore alter the microbiota (117). Others alter the immune system directly (pathway D) or modulate the immune system in ways that lead secondarily to a change in the host–microbiota relationship, which in turn leads to changes in the microbiota (pathway C in Fig. 3). The last mechanism is well established in experimental models. Genetic manipulations of the innate immune system that have profound effects on immune function (such as gene knockout) often operate indirectly by altering the gut microbiota. The phenotypic effects can then be transferred to WT mice that have not been genetically modified, by transferring the altered microbiota (118, 119). It is the altered microbiota that is the proximate cause of the altered immunoregulation (118, 119). At least one environmental saprophyte that will not colonize (and is dead when used in experimental models and in human clinical trials), can be shown to evoke immunoregulatory effects that suppress allergic responses whether injected s.c. (120) or given orally (121) and also exerts antidepressant-like effects on the CNS (122). Probiotic strains such as some lactobacilli can at least temporarily colonize the gut and induce changes in the microbiota via a variety of mechanisms discussed elsewhere (117).
Microbiota Diversity and Regulation of Inflammation
The piglets discussed in the previous section, which had been reared in clean interiors without exposure to the natural environment, had different patterns of gene expression in the ileum, much of it related to the immune system. For example, they had increased type 1 IFN activity, increased MHC class 1, and up-regulation of many chemokines (116), implying a more inflammatory state in the guts of animals whose microbiota had not been modified and diversified by exposure to the natural environment. This correlation between reduced gut microbial biodiversity and poor control of inflammation is a common finding. Mice exhibit at least two enterotypes (bacterial ecosystems in the gut microbiota), one of which has low biodiversity and correlates with biomarkers of inflammation (123). Gut microbiota of limited diversity is also characteristic of human inflammation-associated conditions such as obesity and inflammatory bowel disease (124, 125). Similarly, diminished microbiota biodiversity in institutionalized elderly people correlates with diminished health and raised levels of peripheral inflammatory markers such as IL-6 (109). Adequate microbial inputs are required to maintain diversity of the gut microbiota, and such diversity plays a role in the regulation of inflammation.
Microbial Biodiversity as an Ecosystem Service
Interestingly, provision of microbial biodiversity is not conventionally listed as an “ecosystem service.” Ecosystem services are ecologically mediated functions essential to sustaining healthy human societies. Major reviews of these services do not contain the words inflammation or immunity (126, 127), despite the fact that the immunoregulatory roles of microorganisms have been known for decades. It is hoped that this perspective article will help to bridge the chasm between ecology and medicine/immunology, so that when ecologists consider how to maximize the services obtained from ecosystems, the contained microbial biodiversity is taken into consideration.
Psychology vs. Immunology
The major conclusion of this review is that the beneficial effects of exposure to natural environments are likely to have two separate but interacting components. First, there are well-established rapid psychological effects that might be explained by an evolved psychological reward from contemplating the ideal hunter-gatherer habitat. However, the specificity of the effect for green space has not been proven by comparison with other relaxing environments, and the relevance of such rapid transient changes for long-term health benefits is unknown.
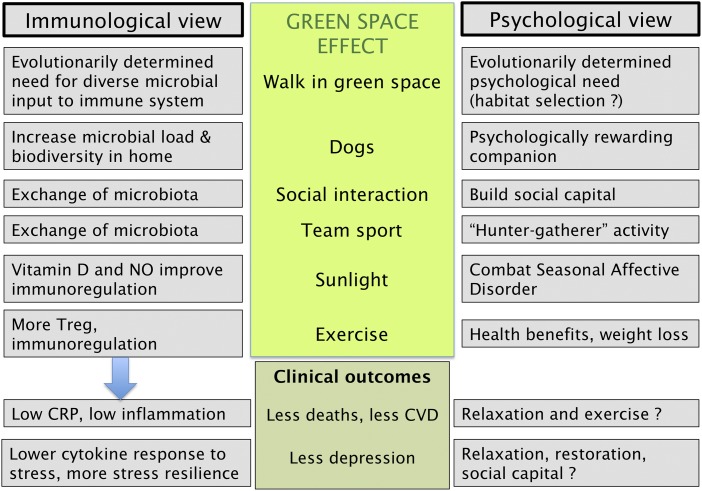
However, there is good evidence that the long-term benefit of exposure to the natural environment is one component of a broad range of effects that fall under the umbrella terms hygiene hypothesis or Old Friends mechanism or biodiversity hypothesis. These terms refer to the evolved need for the immune system to receive inputs provided by microbial biodiversity, and in particular, by organisms that need to be tolerated, and therefore have coevolved roles as inducers of the immunoregulatory pathways (26, 27). These immunoregulatory mechanisms help to stop chronic inflammation and its associated chronic inflammatory diseases, cardiovascular problems, and depression (27). Unlike the rapid psychological effects, this requires prolonged exposures, particularly important during childhood when much of the education of the immune system occurs. It might not be sufficient to encounter only the biased microbiota of the modern synthetic indoor environment that lacks the Old Friends and probably bears little resemblance to the microbiota we encountered throughout our evolutionary history. As illustrated in Fig. 2, modern life deprives us of many of the inputs that our immune systems evolved to anticipate, so we are now more dependent on the microbiota of other people and the microbiota of the natural environment and green spaces. Fig. 4 provides a list of probable components of the beneficial effects of the natural environment, with the parallel psychological and immunological explanations. It seems likely that both types of explanation are important. The underlying principle of the immunological explanation is that for many reasons, exposure to green spaces will lead to increased immunoregulation, resulting in lower background inflammation, manifested as lower resting CRP. Improved control of inflammation results in lower prevalence of inflammatory disorders, cardiovascular disease, and depression and increased stress resilience (27). It is interesting that sunlight and exercise both contribute to this effect. Sunlight enhances production of vitamin D (128) and nitric oxide (129), both of which play critical roles in immunoregulation. Similarly exercise increases the activity of Tregs (21, 130). Therefore, multiple physiological consequences of exposure to the natural environment will supplement the immunoregulatory effects of microbial biodiversity.
Fig. 4.
Immunological and psychological explanations for the health benefits derived from contact with the natural environment. (NO, nitric oxide). There are many studies of exposures during the perinatal period that point to the immunological mechanisms, whereas most studies in adult life have been orientated toward psychological explanations, and have not included investigation of the immunoregulatory aspects.
Urgent Questions and Research Issues
Much research will be needed to consolidate this immuno-microbiological view of the health benefits of exposure to the natural environment. We need new epidemiological studies that concentrate on inflammatory disorders attributable to defective immunoregulation. More studies that use high sensitivity CRP levels as a surrogate biomarker for background inflammation will be valuable. We need more information about the organisms people encounter in their own homes and how this is affected by the proximity to natural environments and by the nature of that environment. Humans evolved as a grassland species, so we can guess, for example, that deserts and some types of monoculture might be less beneficial than grassland and its associated animals. The exposures of individuals can be monitored by sampling skin and gut microbiota and perhaps by studying antibodies. We have worryingly little knowledge about the relationship between environmental strains and those that colonize humans, because current methods of studying microbiota usually give only a broad taxonomic grouping.
If it turns out that the immuno-microbiological view is correct, we will also need to know when the educational and immunoregulatory inputs to our immune systems need to occur. At least some of the immunoregulatory effects of Old Friends are exerted very early in life. For example, factors that delay (caesarian births) or distort (perinatal antibiotic use) the establishment of the gut microbiota of the infant increase the frequency of allergic disorders (131, 132). Similarly, the reduced prevalence of allergic disorders after exposure to the farm environment only occurs if the exposure is during pregnancy or the neonatal period (79, 133). Does this mean that all these immunoregulatory effects occur during the perinatal period? Might it be sufficient to design preschool daycare centers so that infants are exposed to relevant microbiota? This view is unlikely because later childhood might also be important. For example, environmental factors, probably microbial, that influence the risk of developing MS in later life seem to operate during childhood up to the age of 10–15 y (65, 68, 69, 134). Therefore, could the problem be solved by ensuring the presence of appropriate organisms in homes and schools until after adolescence? This possibility also seems unlikely because we know that helminth infections, even when they did not enter the gut, were powerfully immunoregulatory even in adults (36–38), and there is mounting evidence that dysbiosis or diminished biodiversity of the gut microbiota is associated with a variety of inflammatory conditions (109, 124, 125). There is a worrying lack of data on this point, but overall, it seems probable that most education of the immune system occurs in the perinatal period but that there is also an ongoing requirement for microbial inputs throughout life.
Conclusions
It is interesting that the beneficial effects of proximity to the natural environment are particularly prominent in individuals of low socioeconomic status (1–3, 8). Perhaps wealthier individuals are better able to supplement such exposures with holiday travel and rural second homes. This disturbing health gradient emphasizes the need for more research that will enable us to design urban green spaces that provide not only the psychological input to our brains but also an optimized microbial input to our immune systems. The research outlined above will help us to know what is needed and when. If a significant part of the role of the natural environment is to provide an appropriate airborne microbiota, then multiple, small, widely distributed urban green spaces of high microbial quality might suffice as supplements to a core of large recreational parks. There is already huge interest in the construction of roof gardens, vertical gardens and urban green spaces motivated by aesthetics and by organizations wishing to promote urban habitats for birds and insects and by urban planners wishing to delay the entry of rain downpours in sewer systems. However, we suggest that combating the epidemic of inflammation-associated illnesses in high-income urban environments provides another compelling motive for creating green spaces, and we hope that this paper will enhance collaboration between the medical profession, ecologists, and urban planners.
Acknowledgments
G.A.R. is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Maas J, Verheij RA, Groenewegen PP, de Vries S, Spreeuwenberg P. Green space, urbanity, and health: how strong is the relation? J Epidemiol Community Health. 2006;60(7):587–592. doi: 10.1136/jech.2005.043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell R, Popham F. Effect of exposure to natural environment on health inequalities: An observational population study. Lancet. 2008;372(9650):1655–1660. doi: 10.1016/S0140-6736(08)61689-X. [DOI] [PubMed] [Google Scholar]
- 3.Dadvand P, et al. Green space, health inequality and pregnancy. Environ Int. 2012;40:110–115. doi: 10.1016/j.envint.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Berman MG, Jonides J, Kaplan S. The cognitive benefits of interacting with nature. Psychol Sci. 2008;19(12):1207–1212. doi: 10.1111/j.1467-9280.2008.02225.x. [DOI] [PubMed] [Google Scholar]
- 5.Aspinall P, Mavros P, Coyne R, Roe J. The urban brain: Analysing outdoor physical activity with mobile EEG [published online ahead of print March 6, 2013] Br J Sports Med. 2013 doi: 10.1136/bjsports-2012-091877. bjsports-2012-091877. [DOI] [PubMed] [Google Scholar]
- 6.Tsunetsugu Y, Park BJ, Miyazaki Y. Trends in research related to “Shinrin-yoku” (taking in the forest atmosphere or forest bathing) in Japan. Environ Health Prev Med. 2010;15(1):27–37. doi: 10.1007/s12199-009-0091-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park BJ, Tsunetsugu Y, Kasetani T, Kagawa T, Miyazaki Y. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ Health Prev Med. 2010;15(1):18–26. doi: 10.1007/s12199-009-0086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheeler BW, White M, Stahl-Timmins W, Depledge MH. Does living by the coast improve health and wellbeing? Health Place. 2012;18(5):1198–1201. doi: 10.1016/j.healthplace.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Ulrich RS. View through a window may influence recovery from surgery. Science. 1984;224(4647):420–421. doi: 10.1126/science.6143402. [DOI] [PubMed] [Google Scholar]
- 10.Frumkin H. Beyond toxicity: Human health and the natural environment. Am J Prev Med. 2001;20(3):234–240. doi: 10.1016/s0749-3797(00)00317-2. [DOI] [PubMed] [Google Scholar]
- 11. Morris DW (2011) Adaptation and habitat selection in the eco-evolutionary process. Proc Biol Sci 278(1717):2401–2411. [DOI] [PMC free article] [PubMed]
- 12.Sponheimer M, et al. Isotopic evidence of early hominin diets. Proc Natl Acad Sci USA. 2013;110(26):10513–10518. [Google Scholar]
- 13.Kuo FE, Sullivan WC, Coley RL, Brunson L. Fertile ground for community: Inner city neighbourhood common spaces. Am J Community Psychol. 1998;26(6):823–851. [Google Scholar]
- 14.Nyqvist F, Nygård M, Steenbeek W. Social capital and self-rated health amongst older people in Western Finland and Northern Sweden: A multi-level analysis. Int J Behav Med. 2013 doi: 10.1007/s12529-013-9307-0. [DOI] [PubMed] [Google Scholar]
- 15.Maas J, Verheij RA, Spreeuwenberg P, Groenewegen PP. Physical activity as a possible mechanism behind the relationship between green space and health: A multilevel analysis. BMC Public Health. 2008;8:206. doi: 10.1186/1471-2458-8-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oakes JM, Forsyth A, Schmitz KH. The effects of neighborhood density and street connectivity on walking behavior: The Twin Cities walking study. Epidemiol Perspect Innov. 2007;4:16. doi: 10.1186/1742-5573-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson EA, et al. Green cities and health: a question of scale? J Epidemiol Community Health. 2012;66(2):160–165. doi: 10.1136/jech.2011.137240. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal NE, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41(1):72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 19.Hobday RA. Sunlight therapy and solar architecture. Med Hist. 1997;41(4):455–472. doi: 10.1017/s0025727300063043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson Coon JT, et al. Does participating in physical activity in outdoor natural environments have a greater effect on physical and mental wellbeing than physical activity indoors? A systematic review. Environ Sci Technol. 2011;45(5):1761–1772. doi: 10.1021/es102947t. [DOI] [PubMed] [Google Scholar]
- 21.Gleeson M, et al. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 22.de Vries S, Verheij RA, Groenewegen PP, Spreeuwenberg P. Natural environments–healthy environments? An exploratory analysis of the relationship between greenspace and health. Environ Plan A. 2003;35(10):1717–1731. [Google Scholar]
- 23.Maas J, et al. Morbidity is related to a green living environment. J Epidemiol Community Health. 2009;63(12):967–973. doi: 10.1136/jech.2008.079038. [DOI] [PubMed] [Google Scholar]
- 24.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 25.von Hertzen L, Hanski I, Haahtela T. Natural immunity. Biodiversity loss and inflammatory diseases are two global megatrends that might be related. EMBO Rep. 2011;12(11):1089–1093. doi: 10.1038/embor.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rook GA. Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42(1):5–15. doi: 10.1007/s12016-011-8285-8. [DOI] [PubMed] [Google Scholar]
- 27.Rook GAW, Lowry CA, Raison CL. Microbial old friends, immunoregulation and stress resilience. Evol Med Public Health. 2013;1(1):46–64. doi: 10.1093/emph/eot004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38(2):373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naik S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337(6098):1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke TB, et al. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16(2):228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDade TW, et al. Analysis of variability of high sensitivity C-reactive protein in lowland Ecuador reveals no evidence of chronic low-grade inflammation. Am J Hum Biol. 2012;24(5):675–681. doi: 10.1002/ajhb.22296. [DOI] [PubMed] [Google Scholar]
- 32.Rietzschel E, De Buyzere M. High-sensitive C-reactive protein: Universal prognostic and causative biomarker in heart disease? Biomarkers Med. 2012;6(1):19–34. doi: 10.2217/bmm.11.108. [DOI] [PubMed] [Google Scholar]
- 33.Gimeno D, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39(3):413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 35.Rook GAW. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clin Exp Immunol. 2010;160(1):70–79. doi: 10.1111/j.1365-2249.2010.04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176(5):3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 37.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61(2):97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 38.Correale J, Farez MF. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol. 2011;233(1-2):6–11. doi: 10.1016/j.jneuroim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Fleming JO, et al. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult Scler. 2011;17(6):743–754. doi: 10.1177/1352458511398054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osada Y, Kanazawa T. Parasitic helminths: New weapons against immunological disorders. J Biomed Biotechnol. 2010;2010:743758. doi: 10.1155/2010/743758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med. 2009;179(3):186–193. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- 43.Round JL, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332(6032):974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grainger JR, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207(11):2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atarashi K, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smits HH, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115(6):1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 47.Hanski I, et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci USA. 2012;109(21):8334–8339. doi: 10.1073/pnas.1205624109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ege MJ, et al. GABRIELA Transregio 22 Study Group Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364(8):701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 49.Kuiper J, et al. Immunomodulation of the inflammatory response in atherosclerosis. Curr Opin Lipidol. 2007;18(5):521–526. doi: 10.1097/MOL.0b013e3282efd0d4. [DOI] [PubMed] [Google Scholar]
- 50.McDade TW, Rutherford J, Adair L, Kuzawa CW. Early origins of inflammation: Microbial exposures in infancy predict lower levels of C-reactive protein in adulthood. Proc Biol Sci. 2010;277(1684):1129–1137. doi: 10.1098/rspb.2009.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McDade TW, Hoke M, Borja JB, Adair LS, Kuzawa CW. Do environments in infancy moderate the association between stress and inflammation in adulthood? Initial evidence from a birth cohort in the Philippines. Brain Behav Immun. 2013;31:23–30. doi: 10.1016/j.bbi.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turner TR, et al. Comparative metatranscriptomics reveals kingdom level changes in the rhizosphere microbiome of plants [published online ahead of print July 18, 2013] ISME J. 2013 doi: 10.1038/ismej.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicolaou N, Siddique N, Custovic A. Allergic disease in urban and rural populations: Increasing prevalence with increasing urbanization. Allergy. 2005;60(11):1357–1360. doi: 10.1111/j.1398-9995.2005.00961.x. [DOI] [PubMed] [Google Scholar]
- 54.MacNeill SJ, et al. GABRIELA study group Asthma and allergies: Is the farming environment (still) protective in Poland? The GABRIEL Advanced Studies. Allergy. 2013;68(6):771–779. doi: 10.1111/all.12141. [DOI] [PubMed] [Google Scholar]
- 55.Hou JK, El-Serag H, Thirumurthi S. Distribution and manifestations of inflammatory bowel disease in Asians, Hispanics, and African Americans: A systematic review. Am J Gastroenterol. 2009;104(8):2100–2109. doi: 10.1038/ajg.2009.190. [DOI] [PubMed] [Google Scholar]
- 56.Beebe GW, Kurtzke JF, Kurland LT, Auth TL, Nagler B. Studies on the natural history of multiple sclerosis. 3. Epidemiologic analysis of the army experience in World War II. Neurology. 1967;17(1):1–17. doi: 10.1212/wnl.17.1.1. [DOI] [PubMed] [Google Scholar]
- 57.Antonovsky A, et al. Epidemiologic Study of Multiple Sclerosis in Israel. I. An Overall Review of Methods and Findings. Arch Neurol. 1965;13:183–193. doi: 10.1001/archneur.1965.00470020073010. [DOI] [PubMed] [Google Scholar]
- 58.Lowis GW. The social epidemiology of multiple sclerosis. Sci Total Environ. 1990;90:163–190. doi: 10.1016/0048-9697(90)90192-w. [DOI] [PubMed] [Google Scholar]
- 59.Sundquist K, Frank G, Sundquist J. Urbanisation and incidence of psychosis and depression: Follow-up study of 4.4 million women and men in Sweden. Br J Psychiatry. 2004;184:293–298. doi: 10.1192/bjp.184.4.293. [DOI] [PubMed] [Google Scholar]
- 60.Peen J, Schoevers RA, Beekman AT, Dekker J. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr Scand. 2010;121(2):84–93. doi: 10.1111/j.1600-0447.2009.01438.x. [DOI] [PubMed] [Google Scholar]
- 61.Kovess-Masfety V, Lecoutour X, Delavelle S. Mood disorders and urban/rural settings: Comparisons between two French regions. Soc Psychiatry Psychiatr Epidemiol. 2005;40(8):613–618. doi: 10.1007/s00127-005-0934-x. [DOI] [PubMed] [Google Scholar]
- 62.McGrath J, et al. A systematic review of the incidence of schizophrenia: The distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004;2:13. doi: 10.1186/1741-7015-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lauritsen MB, Pedersen CB, Mortensen PB. Effects of familial risk factors and place of birth on the risk of autism: A nationwide register-based study. J Child Psychol Psychiatry. 2005;46(9):963–971. doi: 10.1111/j.1469-7610.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- 64.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: Both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011;69(5 Pt 2):26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahlgren C, Oden A, Lycke J. A nationwide survey of the prevalence of multiple sclerosis in immigrant populations of Sweden. Mult Scler. 2012;18(8):1099–1107. doi: 10.1177/1352458511433062. [DOI] [PubMed] [Google Scholar]
- 66.Cabre P. Environmental changes and epidemiology of multiple sclerosis in the French West Indies. J Neurol Sci. 2009;286(1-2):58–61. doi: 10.1016/j.jns.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 67.Söderström U, Aman J, Hjern A. Being born in Sweden increases the risk for type 1 diabetes - a study of migration of children to Sweden as a natural experiment. Acta Paediatr. 2012;101(1):73–77. doi: 10.1111/j.1651-2227.2011.02410.x. [DOI] [PubMed] [Google Scholar]
- 68.Milo R, Kahana E. Multiple sclerosis: Geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9(5):A387–A394. doi: 10.1016/j.autrev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 69.Gale CR, Martyn CN. Migrant studies in multiple sclerosis. Prog Neurobiol. 1995;47(4-5):425–448. [PubMed] [Google Scholar]
- 70.Li X, Sundquist J, Hemminki K, Sundquist K. Risk of inflammatory bowel disease in first- and second-generation immigrants in Sweden: A nationwide follow-up study. Inflamm Bowel Dis. 2011;17(8):1784–1791. doi: 10.1002/ibd.21535. [DOI] [PubMed] [Google Scholar]
- 71.Carr I, Mayberry JF. The effects of migration on ulcerative colitis: A three-year prospective study among Europeans and first- and second- generation South Asians in Leicester (1991-1994) Am J Gastroenterol. 1999;94(10):2918–2922. doi: 10.1111/j.1572-0241.1999.01438.x. [DOI] [PubMed] [Google Scholar]
- 72.Breslau J, Borges G, Hagar Y, Tancredi D, Gilman S. Immigration to the USA and risk for mood and anxiety disorders: Variation by origin and age at immigration. Psychol Med. 2009;39(7):1117–1127. doi: 10.1017/S0033291708004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vega WA, Sribney WM, Aguilar-Gaxiola S, Kolody B. 12-month prevalence of DSM-III-R psychiatric disorders among Mexican Americans: Nativity, social assimilation, and age determinants. J Nerv Ment Dis. 2004;192(8):532–541. doi: 10.1097/01.nmd.0000135477.57357.b2. [DOI] [PubMed] [Google Scholar]
- 74.Rottem M, Szyper-Kravitz M, Shoenfeld Y. Atopy and asthma in migrants. Int Arch Allergy Immunol. 2005;136(2):198–204. doi: 10.1159/000083894. [DOI] [PubMed] [Google Scholar]
- 75.Hjern A, Rasmussen F, Hedlin G. Age at adoption, ethnicity and atopic disorder: a study of internationally adopted young men in Sweden. Pediatr Allergy Immunol. 1999;10(2):101–106. doi: 10.1034/j.1399-3038.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 76.Eldeirawi K, et al. Associations of doctor-diagnosed asthma with immigration status, age at immigration, and length of residence in the United States in a sample of Mexican American School Children in Chicago. J Asthma. 2009;46(8):796–802. [PubMed] [Google Scholar]
- 77.Pereg D, et al. Prevalence of asthma in a large group of Israeli adolescents: Influence of country of birth and age at migration. Allergy. 2008;63(8):1040–1045. doi: 10.1111/j.1398-9995.2008.01661.x. [DOI] [PubMed] [Google Scholar]
- 78.Blackley CH. Experimental Researches on the Causes and Nature of Catarrhus aestivus (Hay-Fever and Hay-Asthma) London: Baillière Tindall and Cox; 1873. [Google Scholar]
- 79.Riedler J, et al. ALEX Study Team Exposure to farming in early life and development of asthma and allergy: A cross-sectional survey. Lancet. 2001;358(9288):1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 80.Radon K, et al. Chronische Autoimmunerkrankungen und Kontakt zu Tieren (Chronic Autoimmune Disease and Animal Contact) Study Group Contact with farm animals in early life and juvenile inflammatory bowel disease: A case-control study. Pediatrics. 2007;120(2):354–361. doi: 10.1542/peds.2006-3624. [DOI] [PubMed] [Google Scholar]
- 81.Heederik D, von Mutius E. Does diversity of environmental microbial exposure matter for the occurrence of allergy and asthma? J Allergy Clin Immunol. 2012;130(1):44–50. doi: 10.1016/j.jaci.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 82.Debarry J, et al. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J Allergy Clin Immunol. 2007;119(6):1514–1521. doi: 10.1016/j.jaci.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 83.Vogel K, et al. Animal shed Bacillus licheniformis spores possess allergy-protective as well as inflammatory properties. J Allergy Clin Immunol. 2008;122(2):307–312. doi: 10.1016/j.jaci.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 84.Hagner S, et al. Farm-derived Gram-positive bacterium Staphylococcus sciuri W620 prevents asthma phenotype in HDM- and OVA-exposed mice. Allergy. 2013;68(3):322–329. doi: 10.1111/all.12094. [DOI] [PubMed] [Google Scholar]
- 85.Pakarinen J, et al. Predominance of Gram-positive bacteria in house dust in the low-allergy risk Russian Karelia. Environ Microbiol. 2008;10(12):3317–3325. doi: 10.1111/j.1462-2920.2008.01723.x. [DOI] [PubMed] [Google Scholar]
- 86.Kondrashova A, et al. A six-fold gradient in the incidence of type 1 diabetes at the eastern border of Finland. Ann Med. 2005;37(1):67–72. doi: 10.1080/07853890410018952. [DOI] [PubMed] [Google Scholar]
- 87.Womack AM, Bohannan BJ, Green JL. Biodiversity and biogeography of the atmosphere. Philos Trans R Soc Lond B Biol Sci. 2010;365(1558):3645–3653. doi: 10.1098/rstb.2010.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heydenreich B, et al. Gram-positive bacteria on grass pollen exhibit adjuvant activity inducing inflammatory T cell responses. Clin Exp Allergy. 2012;42(1):76–84. doi: 10.1111/j.1365-2222.2011.03888.x. [DOI] [PubMed] [Google Scholar]
- 89.Burrows SM, Elbert W, Lawrence MG, Poeschl U. Bacteria in the global atmosphere—part 1: Review and synthesis of literature data for different ecosystems. Atmos Chem Phys. 2009;9:9263–9280. [Google Scholar]
- 90.Nehmé B, et al. Culture-independent characterization of archaeal biodiversity in swine confinement building bioaerosols. Appl Environ Microbiol. 2009;75(17):5445–5450. doi: 10.1128/AEM.00726-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brodie EL, et al. Urban aerosols harbor diverse and dynamic bacterial populations. Proc Natl Acad Sci USA. 2007;104(1):299–304. doi: 10.1073/pnas.0608255104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kembel SW, et al. Architectural design influences the diversity and structure of the built environment microbiome. ISME J. 2012;6(8):1469–1479. doi: 10.1038/ismej.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dunn RR, Fierer N, Henley JB, Leff JW, Menninger HL. Home life: Factors structuring the bacterial diversity found within and between homes. PLoS ONE. 2013;8(5):e64133. doi: 10.1371/journal.pone.0064133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DeLeon-Rodriguez N, et al. Microbiome of the upper troposphere: Species composition and prevalence, effects of tropical storms, and atmospheric implications. Proc Natl Acad Sci USA. 2013;110(7):2575–2580. doi: 10.1073/pnas.1212089110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leck C, Bigg K. Biogenic particles in the surface microlayer and overlaying atmosphere in the central Arctic Ocean during summer. Tellus. 2005;57B(4):305–316. [Google Scholar]
- 96.Prather KA, et al. Bringing the ocean into the laboratory to probe the chemical complexity of sea spray aerosol. Proc Natl Acad Sci USA. 2013;110(19):7550–7555. doi: 10.1073/pnas.1300262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fleming LE, et al. Aerosolized red-tide toxins (brevetoxins) and asthma. Chest. 2007;131(1):187–194. doi: 10.1378/chest.06-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kirkpatrick B, et al. Florida red tide and human health: Apilot beach conditions reporting system to minimize human exposure. Sci Total Environ. 2008;402(1):1–8. doi: 10.1016/j.scitotenv.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jansson JK, Prosser JI. Microbiology: The life beneath our feet. Nature. 2013;494(7435):40–41. doi: 10.1038/494040a. [DOI] [PubMed] [Google Scholar]
- 100.Rinke C, et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature. 2013;499(7459):431–437. doi: 10.1038/nature12352. [DOI] [PubMed] [Google Scholar]
- 101.Berendsen RL, Pieterse CM, Bakker PA. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17(8):478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 102.Kowalchuk GA, Buma DS, de Boer W, Klinkhamer PG, van Veen JA. Effects of above-ground plant species composition and diversity on the diversity of soil-borne microorganisms. Antonie van Leeuwenhoek. 2002;81(1-4):509–520. doi: 10.1023/a:1020565523615. [DOI] [PubMed] [Google Scholar]
- 103.Fujimura KE, et al. Man's best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010;126(2):410–413. doi: 10.1016/j.jaci.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288(8):963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 105.Aichbhaumik N, et al. Prenatal exposure to household pets influences fetal immunoglobulin E production. Clin Exp Allergy. 2008;38(11):1787–1794. doi: 10.1111/j.1365-2222.2008.03079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Axelsson E, et al. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature. 2013;495(7441):360–364. doi: 10.1038/nature11837. [DOI] [PubMed] [Google Scholar]
- 107.Meadow JF, Bateman AC, Herkert KM, O’Connor TK, Green JL. Significant changes in the skin microbiome mediated by the sport of roller derby. PeerJ. 2013;1:e53. doi: 10.7717/peerj.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song SJ, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013;2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Claesson MJ, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 110.Fang Z, Ouyang Z, Zheng H, Wang X, Hu L. Culturable airborne bacteria in outdoor environments in Beijing,China. Microb Ecol. 2007;54(3):487–496. doi: 10.1007/s00248-007-9216-3. [DOI] [PubMed] [Google Scholar]
- 111.McBain AJ, et al. Exposure of sink drain microcosms to triclosan: Population dynamics and antimicrobial susceptibility. Appl Environ Microbiol. 2003;69(9):5433–5442. doi: 10.1128/AEM.69.9.5433-5442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andersson MA, et al. The mitochondrial toxin produced by Streptomyces griseus strains isolated from an indoor environment is valinomycin. Appl Environ Microbiol. 1998;64(12):4767–4773. doi: 10.1128/aem.64.12.4767-4773.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sahlberg B, Wieslander G, Norbäck D. Sick building syndrome in relation to domestic exposure in Sweden—a cohort study from 1991 to 2001. Scand J Public Health. 2010;38(3):232–238. doi: 10.1177/1403494809350517. [DOI] [PubMed] [Google Scholar]
- 114.De Filippo C, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mulder IE, et al. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. 2009;7:79. doi: 10.1186/1741-7007-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6(1):39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wen L, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455(7216):1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Elinav E, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zuany-Amorim C, et al. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002;8(6):625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- 121.Hunt JR, Martinelli R, Adams VC, Rook GAW, Brunet LR. Intragastric administration of Mycobacterium vaccae inhibits severe pulmonary allergic inflammation in a mouse model. Clin Exp Allergy. 2005;35(5):685–690. doi: 10.1111/j.1365-2222.2005.02239.x. [DOI] [PubMed] [Google Scholar]
- 122.Lowry CA, et al. Identification of an immune-responsive mesolimbocortical serotonergic system: Potential role in regulation of emotional behavior. Neuroscience. 2007;146(2):756–772. doi: 10.1016/j.neuroscience.2007.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hildebrand F, et al. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biol. 2013;14(1):R4. doi: 10.1186/gb-2013-14-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rehman A, et al. Transcriptional activity of the dominant gut mucosal microbiota in chronic inflammatory bowel disease patients. J Med Microbiol. 2010;59(Pt 9):1114–1122. doi: 10.1099/jmm.0.021170-0. [DOI] [PubMed] [Google Scholar]
- 126.Reid WV. Millennium Ecosystem Assessment, 2005. Ecosystems and Human Well-being: Synthesis. Washington, DC: Island Press; 2005. [Google Scholar]
- 127.Mooney H, Cropper A, Reid W. Confronting the human dilemma. Nature. 2005;434(7033):561–562. doi: 10.1038/434561a. [DOI] [PubMed] [Google Scholar]
- 128.Milaneschi Y, et al. The association between low vitamin D and depressive disorders. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.36. [DOI] [PubMed] [Google Scholar]
- 129.Feelisch M, et al. Is sunlight good for our heart? Eur Heart J. 2010;31(9):1041–1045. doi: 10.1093/eurheartj/ehq069. [DOI] [PubMed] [Google Scholar]
- 130.Lowder T, Dugger K, Deshane J, Estell K, Schwiebert LM. Repeated bouts of aerobic exercise enhance regulatory T cell responses in a murine asthma model. Brain Behav Immun. 2010;24(1):153–159. doi: 10.1016/j.bbi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38(4):629–633. doi: 10.1111/j.1365-2222.2007.02780.x. [DOI] [PubMed] [Google Scholar]
- 132.Stensballe LG, Simonsen J, Jensen SM, Bonnelykke K, Bisgaard H. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J Pediatr. 2013;162(4):832–838. doi: 10.1016/j.jpeds.2012.09.049. [DOI] [PubMed] [Google Scholar]
- 133.Ege MJ, et al. Prenatal exposure to a farm environment modifies atopic sensitization at birth. J Allergy Clin Immunol. 2008;122(2):407–412. doi: 10.1016/j.jaci.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 134.Cabre P. Migration and multiple sclerosis: The French West Indies experience. J Neurol Sci. 2007;262(1-2):117–121. doi: 10.1016/j.jns.2007.06.044. [DOI] [PubMed] [Google Scholar]