Significance
In this study, we show that a primitive brain hormone, arginine vasopressin (AVP), which has hitherto been implicated in regulating water balance in mammals, has a function in skeletal homeostasis. Using genetically modified mice that are lacking one of the AVP receptors as well as pharmacologic inhibitors, we show that AVP negatively regulates osteoblasts (cells that form new bone) and stimulates osteoclasts (cells that remove old bone). Our findings explain the bone loss that is known to accompany low blood sodium levels in patients when AVP levels are high.
Keywords: oxytocin, pituitary hormone, osteopenia
Abstract
Although hyponatremia is known to be associated with osteoporosis and a high fracture risk, the mechanism through which bone loss ensues has remained unclear. As hyponatremic patients have elevated circulating arginine-vasopressin (AVP) levels, we examined whether AVP can affect the skeleton directly as yet another component of the pituitary-bone axis. Here, we report that the two Avp receptors, Avpr1α and Avpr2, coupled to Erk activation, are expressed in osteoblasts and osteoclasts. AVP injected into wild-type mice enhanced and reduced, respectively, the formation of bone-resorbing osteoclasts and bone-forming osteoblasts. Conversely, the exposure of osteoblast precursors to Avpr1α or Avpr2 antagonists, namely SR49059 or ADAM, increased osteoblastogenesis, as did the genetic deletion of Avpr1α. In contrast, osteoclast formation and bone resorption were both reduced in Avpr1α−/− cultures. This process increased bone formation and reduced resorption resulted in a profound enhancement of bone mass in Avpr1α−/− mice and in wild-type mice injected with SR49059. Collectively, the data not only establish a primary role for Avp signaling in bone mass regulation, but also call for further studies on the skeletal actions of Avpr inhibitors used commonly in hyponatremic patients.
Over the past decade, studies by others and us have documented direct effects of pituitary hormones on the skeleton. We have identified functional receptors for thyroid stimulating hormone (TSH), follicle stimulating hormone (FSH), adrenocorticotropic hormone (ACTH), and oxytocin (OT) on murine and human bone cells, namely bone-forming osteoblasts and bone-resorbing osteoclasts (1–4). The genetic deletion of either the receptor or the ligand itself, as in the case of FSH and OT, results in overt skeletal abnormalities. Specifically, deleting OT or its receptor, the Oxtr, causes profound osteopenia, which primarily arises from a dramatic reduction in bone formation by the osteoblast (4). Such studies have helped establish a pituitary-bone axis, in which pituitary hormones bypass their known targets, such as the thyroid, ovaries, adrenal, and breast, to regulate bone directly (5).
This growing body of data not only informs us of novel functions of pituitary hormones, but also explains the hitherto poorly understood mechanisms of certain forms of osteoporosis, which have traditionally been attributed solely to changes in distal hormones. For example, we find that low TSH signaling contributes to the bone loss in hyperthyroidism, which was thought solely to be a result of elevated thyroid hormones (6). We have also speculated that the rapid bone loss that occurs during late perimenopause, at a time when estradiol levels are relatively normal, could—at least in part—be caused by elevated serum FSH levels. Thus, an antibody to FSH reduces bone loss in ovariectomized mice by stimulating bone formation and inhibiting bone resorption (7). Similarly, through its skeletal anabolic actions, elevated OT levels during pregnancy and lactation could play a major role in enabling fetal skeletal mineralization and allowing the mother to recover from the osteoporosis caused by the intergenerational transfer of calcium (8).
Here, we report studies on arginine-vasopressin (AVP), another posterior pituitary hormone, which differs from OT only by two amino acids (9). The direct skeletal actions of AVP have never been explored, despite multiple and recurring observations that hyponatremia, which is invariably accompanied by elevated plasma AVP levels, is associated with bone loss and a high fracture risk (10–16). It has been thought that, as bone is a large reservoir for sodium ions, hyponatremia will trigger sodium release from the skeleton by increasing bone resorption (17, 18). However, the molecular basis of any such effect remains unknown. Interestingly, a recent study has described a male patient with syndrome of inappropriate secretion of antidiuretic hormone- (SIADH) induced hyponatremia, who had severe osteoporosis, despite having no identifiable risk factors (19). Plasma AVP was elevated by ∼30-fold, raising the possibility that high circulating AVP levels may cause the profound bone loss.
We show that AVP is a key regulator of bone resorption and formation, the two principal components of bone remodeling. Both Avp receptors, Avpr1α and Avpr2, are expressed on osteoclasts and osteoblasts, and their stimulation triggers extracellular signal regulated kinase (Erk) activation, which in turn suppresses bone formation and stimulates bone resorption. This decoupling would favor bone loss, as noted in hyponatremic states. However, we also find that the genetic deletion of Avpr1 or the pharmacologic inhibition of Avpr1 or Avpr2 increases bone mass not only by stimulating osteoblastogenesis and new bone synthesis, but also by simultaneously inhibiting osteoclast formation and bone resorption. We speculate, therefore, that the targeted therapy of hyponatremia with aquaretics (or AVPR inhibitors) could result in overall bone gain. Purposefully designed clinical studies in populations in whom hyponatremia is a significant clinical problem (20), and whom are otherwise also at a high risk for fracture (21), should shed further light on the proposed osteoprotective action of AVPR antagonists in people.
Results
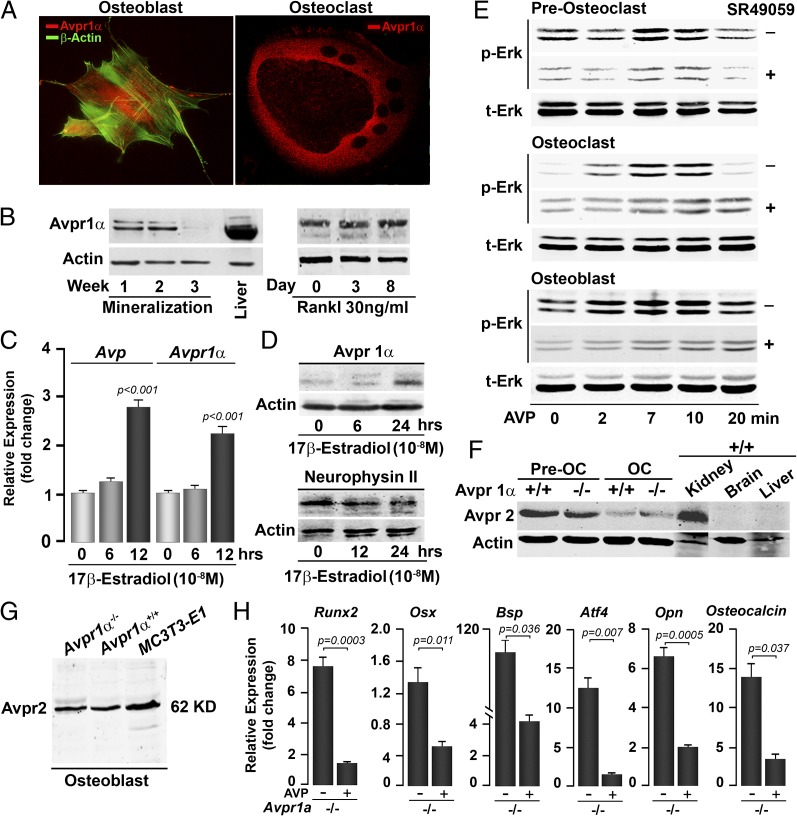
As part of our efforts in characterizing the posterior pituitary-bone axis (5), we have shown previously that functional Oxtrs and the ligand OT are expressed both in osteoclasts and osteoblasts (22, 23). Similarly, immunofluorescence microscopy and Western immunoblotting revealed Avpr1 expression in both cell types (Fig. 1 A and B). Avpr1α expression in the osteoblast decreased between weeks 2 and 3 of mineralization, but in the osteoclast, remained stable over 8 d of differentiation (Fig. 1B). Because the expression of OT and Oxtr were estrogen-sensitive (23), we studied the effect of 17β-estradiol on Avp and Avpr1α expression in osteoblasts. There was a significant increase in expression of Avp within 12 h of exposure to 17β-estradiol, but protein levels of its precursor, neurophysin II, remained unchanged (Fig. 1 C and D). Note that, as AVP is 9-aa long, we have measured levels in culture supernatants using ELISA (401 ± 133 pg/mL) to demonstrate production in bone marrow. In contrast, Avpr1α mRNA and protein expression were increased at 12 and 24 h, respectively (Fig. 1 C and D).
Fig. 1.
Bone cells express Avprs. Immunofluorescence micrographs (A) and Western immunoblotting (B) show the expression of Avpr1α in osteoblasts and osteoclasts, and as a function of osteoblast (mineralization) and osteoclast (with Rankl) differentiation. The expression of Avp (ligand) and Avpr1α (receptor) in osteoblasts is regulated by 17β-estradiol, as determined by quantitative PCR (C) and Western immunoblotting (D). (Magnification: A, 63×.) Because Avp is a small peptide, its precursor neurophysin II is measured. Statistics: Student t test, P values shown compared with 0 h. Stimulation of Erk phosphorylation (p-Erk) as a function of total Erk (t-Erk) by Avp (10−8 M) in osteoclast precursors (preosteoclasts), osteoclasts (OC) and osteoblasts establishes functionality of the Avpr1α in the presence or absence of the receptor inhibitor SR49059 (10−8 M) (E). Western immunoblotting showing the expression of Avpr2 in preosteoclasts, OCs (F), and osteoblasts (G) isolated from Avpr1α−/− mice, as well as in MC3T3.E1 osteoblast precursors (G). Functionality of Avpr2 was confirmed by the demonstration that cells from Avpr1a−/− mice remained responsive to AVP in reducing the expression of osteoblast differentiation genes, namely Runx2, Osx, Bsp, Atf4, Opn, and Osteocalcin (quantitative PCR, P values shown) (H).
To confirm the functionality of the detected Avpr1α, we measured Erk activation in response to AVP in the presence or absence of an Avpr1α inhibitor, SR49059. In osteoclast precursors, osteoclasts and osteoblasts, AVP produced a robust increase in Erk phosphorylation without changing total Erk (Fig. 1E). These responses were attenuated, but not abolished by the Avpr1α inhibitor SR49059 (Fig. 1E). That Erk activation persisted despite maximally effective inhibitor concentrations suggested that another Avpr isoform, Avrp2, was also expressed in bone cells.
To specifically examine for Avpr2 expression, we used bone marrow cells from Avpr1α−/− mice. Avpr1α−/− osteoclast precursors and mature osteoclasts expressed Avpr2 as did the kidney, but brain and liver, which express Avpr1α, did not (Fig. 1F). However, Avpr2 was expressed at lower levels in osteoclasts than in the precursors, but was equally detectable in Avpr1α+/+ and Avpr1α−/− cells (Fig. 1F). Similarly, calvarial osteoblasts from Avpr1α−/− mice expressed Avpr2 in addition to Avpr1α (Fig. 1 B and G). Functionality of osteoblastic Avpr2 was confirmed by studying the effect of AVP on the expression of differentiation genes, namely runt-related transcription factor 2 (Runx2), osterix (Osx), bone sialoprotein (Bsp), activating transcription factor 4 (Atf4), osteopontin (Opn), and Osteocalcin. Gene expression in Avp1α−/− osteoblasts was profoundly inhibited in response to AVP, suggesting that the Avpr isoform Avpr2 was also a functional regulator of osteoblastogenesis (Fig. 1H).
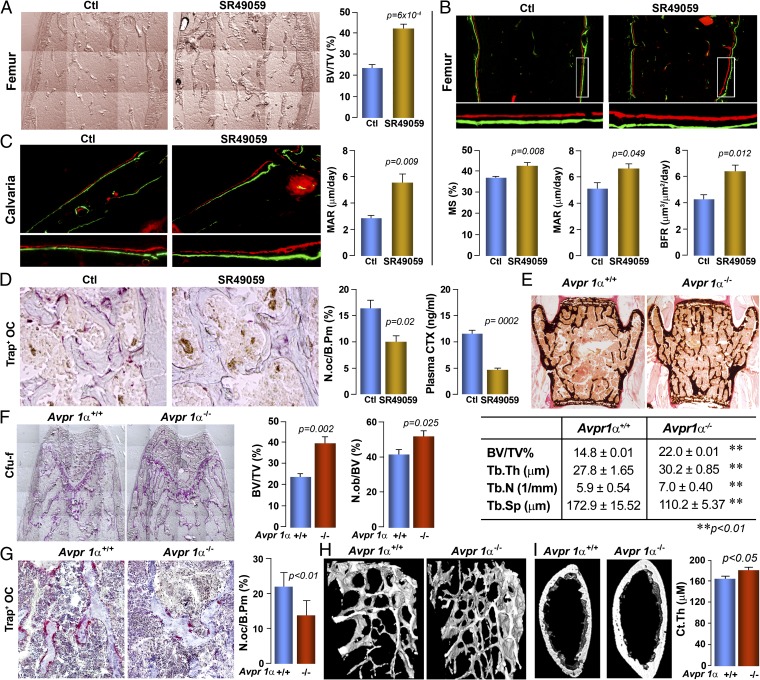
We then studied specifically the role of Avpr1α in regulating bone formation and bone mass. Injection of SR49059 into wild-type mice resulted in a dramatic increase in bone volume fraction, BV/TV (Fig. 2A). This finding was accompanied by a significant increase in bone formation parameters, notably mineralizing surface, mineral apposition rate (MAR), and bone formation rate, in double-labeled femur metaphyseal bone sections (Fig. 2B). Parallel studies with the double-labeled calvarial bone confirmed a substantial increase in MAR, which is normally measured in calvaria (Fig. 2C). Additionally, resorption parameters, including osteoclast surfaces (N.oc/B.Pm), as well as serum C-telopeptide levels were significantly reduced in inhibitor-treated mice compared with controls (Fig. 2D).
Fig. 2.
Avprs regulate bone remodeling and bone mass. Pharmacologic inhibition of Avpr1α by the injection of the receptor antagonist SR49059 (or vehicle, Ctl) increases bone mass (unstained sections, quantitated as BV/TV) (A); bone formation (photomicrograph of xylenol/calcein-labeled bones), measured as mineralizing surface, MAR, and bone formation rate in femur epiphyseal bone (B), and MAR in calvarial bone (C); and bone resorption in TRAP-labeled sections measured as osteoclast surfaces (N.oc/B.Pm) and serum C- telopeptide (CTX, by ELISA) (D). Statistics: Unpaired Student t test, P values shown, n = 4–5 mice per group. Consistent with this, Avpr1α−/− mice compared with Avpr1α+/+ littermates (E) showed a profound increase in trabecular bone in the vertebral column (von Kossa stained section; BV/TV, Tb.Th, Tb.N., and Tb.Sp.) and in the femur epiphysis (alkaline phosphatase-stained section, BV/TV); this is accompanied by an increase and decrease, respectively, in Cfu-f and N.ob./BPm (F), and osteoclast numbers (Trap-stained surface, N.oc/B.Pm) (G). Microtomography (H) confirms the increase in bone mass in both trabecular [femur metaphysis and cortical bone (Ct.Th)] (I). Unpaired Student t test, P values shown, n = 5 mice per group. (Magnification: A–C and F, 10×; D, 40×; E, 2×; G, 20×; H, 4×.)
These results from pharmacologic Avpr1α inhibition by SR49059 were closely mimicked by the genetic deletion of the Avpr1α. There was a highly significant increase in bone volume, BV/TV, observed on von Kossa staining of vertebral bone, as well as in femur metaphyseal bone (Figs. 2 E and F). Notably, there were accompanying increases in trabecular thickness (Tb.Th) and trabecular number (Tb.N), and decreases in trabecular spacing (Tb.Sp). Significant increases and decreases, respectively, in osteoblast (N.ob/BV) and osteoclast (N.oc/BPm) number were noted (Fig. 2 F and G). The increases in bone mass were further confirmed by 3D microtomography of femur metaphyseal trabecular bone (Fig. 2H), as well as in the femur diaphysis, where cortical thickness (Ct.Th) was significantly increased (Fig. 2I). Overall, the findings suggest a prominent role for Avpr1α in bone remodeling and bone mass regulation.
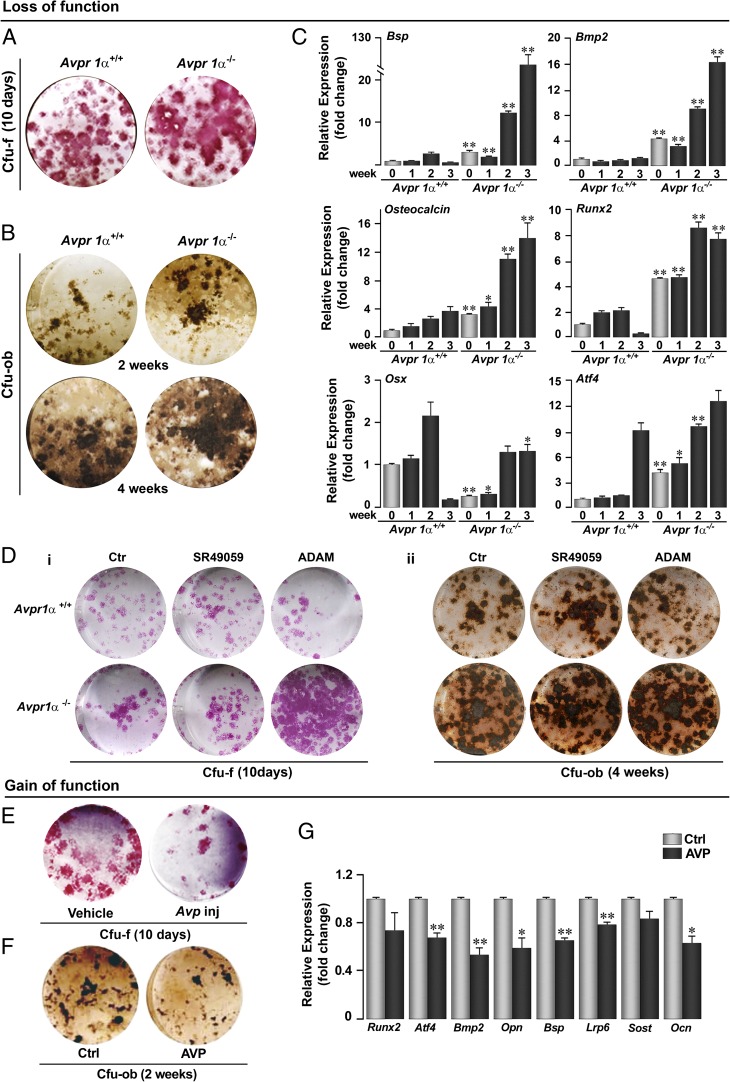
Consistent with the increase in bone formation noted in vivo, Avpr1α−/− bone marrow stromal cells showed a significant elevation in colony forming-fibroblastoid (Cfu-f) at day 10 (Fig. 3A), with colony forming-osteoblastoid (Cfu-ob) increasing progressively until 4 wk of culture (Fig. 3B). This pronounced increase in osteoblast differentiation was demonstrable further by measuring the expression of Bsp, bone morphogenetic protein-2 (Bmp2), Osteocalcin, Runx2, Osx, and Atf4. Although the expression of all of these genes increased as a function of osteoblast differentiation in wild-type mice, this phenomenon was exaggerated significantly in Avpr1α−/− cultures (Fig. 3C). Interestingly, with the exception of Runx2, these increases contrast the inhibitory effects of Oxtr deletion, reported previously (4). We suggest that Oxtr and Avpr have opposing actions on osteoblastogenesis, although the deletion of either receptor elevates Runx2 expression (4). The significance of this discordance remains unknown. Furthermore, gain-of-function studies showed that the injection of AVP into mice or exposure to AVP in vitro inhibited osteoblast formation (Fig. 3 E and F) and gene expression (Fig. 3G).
Fig. 3.
Avpr1α and Avpr2 regulate osteoblastogenesis. Genetic deletion of Avpr1α in Avpr1α−/− mice causes a dramatic increase in osteoblastogenesis, measured by alkaline phosphatase-positive Cfu-f (10 d) (A) and von Kossa-positive Cfu-ob (2 and 4 wk) (B). For Cfu-f and Cfu-ob assays, bone marrow stromal cells were grown in α-MEM and 15% (vol/vol) FCS with acorbate-2-phosphate (1 mM) (Materials and Methods). (C) The enhanced osteoblastogenesis was confirmed by a strong up-regulation of differentiation genes (quantitative PCR), namely Bsp, Bmp2, Osteocalcin, Runx2, Osx, and Atf4. (D) Effect of inhibitors of Avpr1α (SR49059) or Avpr2 (ADAM) on Cfu-f (i, 10 d) and Cfu-ob (ii, 4 wk) formation. (E) Ex vivo Cfu-f formation (10 d) in mice injected with AVP (4 µg per mouse, twice, 6-wk-old, killed 12 h after second injection). Cfu-ob formation (F) and expression of Runx2, Atf4, Bmp2, Opn, Bsp, Lrp6, Sost, and Ocn (G) (at 2 wk) in the presence of AVP in vitro. Statistics: Unpaired Student t test, corrected by Bonferroni’s; *P < 0.05; **P < 0.01, comparison between Avpr1α+/+ and Avpr1α−/− cells or between Ctl and AVP. (Magnification: all stained images, 1×.)
A functional role of Avpr2 in osteoblastogenesis was also explored using Avpr1α−/− cells and the inhibitors of the two receptors Avpr1α and Avpr2. Exposure of wild-type cells to the Avpr1α inhibitor SR49059 or deletion of Avpr1α resulted in enhanced Cfu-f and Cfu-ob formation (Fig. 3D), whereas no further increases were noted when Avpr1α−/− cells were treated with SR49059. However, a dramatic increase in Cfu-f was observed when Avpr1α−/− cells were exposed to the Avpr inhibitor ADAM (Fig. 3D), suggesting that both Avpr isoforms work in tandem to inhibit osteoblastogenesis.
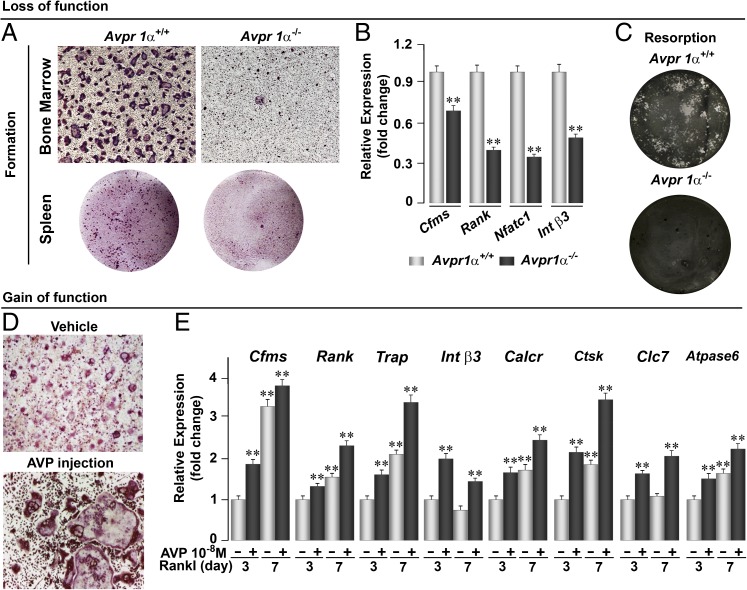
We next probed the effects of Avpr activation on osteoclasts in loss- and gain-of-function studies using Avpr1α−/− cells, and by administering either the Avpr1α antagonist SR49059 or AVP. Bone marrow cell or spleen cultures from Avrp1α−/− mice in the presence of Rankl showed a dramatic reduction in osteoclast formation compared with wild-type mice (Fig. 4A). This change was accompanied by reduced expression of certain osteoclast genes, notably Cfms, Rank, Nfatc1, and Integrin β3 (Int β3) in ex vivo bone marrow cell cultures (Fig. 4B). When osteoclasts were seeded on hydroxyapatite-coated coverslips, there was a striking attenuation in bone resorption, as evaluated by von Kossa staining (Fig. 4C). These findings were consistent with the significant decrease in osteoclast surface, N.oc/B.Pm, noted when wild-type mice were injected with SR49059 (Fig. 2C). Collectively, the data suggest that Avp is a proresorptive hormone in mice, as the loss of Avpr signaling inhibits osteoclast formation and bone resorption.
Fig. 4.
Avpr1α regulates osteoclast formation and function. Genetic deletion of Avpr1α in Avpr1α−/− mice causes a dramatic reduction in osteoclastogenesis in bone marrow and spleen cell cultures (A), which is accompanied by a reduction in the expression (quantitative PCR) of differentiation genes, namely Cfms, Rank, Nfatc1, and Int β3 (B), and by a reduction in bone resorption by mature osteoclasts plated on dentine slices (von Kossa-stained) (C). Gain-of-function experiments evaluating the stimulatory effect of AVP injections (4 µg per mouse, twice, 6-wk-old mice killed 12 h after second injection) on TRAP-positive osteoclast formation in bone marrow cell cultures treated with Rankl (30 ng/mL) (D), and on osteoclast genes, namely Cfms, Rank, Trap, Int β3, Calcr, Ctsk, Clc7, and Atpase6 (E). Statistics by Unpaired Student t test; **P < 0.01 vs. vehicle (−, no AVP) treated at 3 and 7 d of culture. (Magnification: A, Upper, and D, 10×; A, Lower, and C, 2×.)
We tested this putative proresorptive action directly by injecting AVP into wild-type mice. A profound increase in osteoclastogenesis was observed ex vivo (Fig. 4D). This increase was accompanied by a significant up-regulation in bone marrow cell cultures of osteoclast differentiation genes, namely Cfms, Rank, Trap, Int β3, Calcr, Ctsk, Clc7, and Atpase6 (Fig. 4E). Overall, these findings suggest that Avp enhances bone resorption by stimulating the formation and function of osteoclasts, although we cannot demonstrate a direct effect of AVP on bone in mice for technical reasons. These actions are in stark contrast to OT, which stimulates osteoclastogenesis, but inhibits the function of mature osteoclasts, with little change in overall bone resorption (4).
Discussion
The G protein-coupled receptor superfamily of receptors, to which the Avprs belong, is much more widely distributed than originally thought. The clearest example is ACTH, which is part of a widely distributed melanocortin receptor system that regulates a diverse array of functions, including pigment production, apatite, and sexual function (24, 25). These receptors are evolutionarily primitive in that lower organisms, such as coelenterates, express, for example, the TSH receptor (24). In fish, the TSH receptor is not only present in the thyroid gland, but also in heart muscle and brain. Oxtrs also have a widespread distribution in the central nervous system to control apatite, sexual function, and behavior (26). Similarly, in lower vertebrates, such as birds, amphibians, and fish, Avpr1α, expressed in the central nervous system, determines sexual and reproductive behavior (27). In mammals, Avpr1α regulates the vasculature, Avpr1β is involved in aggression, social memory, and stress responsiveness, and Avpr2, localized primarily to the kidney, controls body water (27). Here, we show in parallel with Oxtrs, that Avpr1α and Avpr2 are expressed in osteoblasts and osteoclasts. However, Avpr action on the skeleton, which is to inhibit bone formation, is opposed to Oxtr’s anabolic action.
More fascinating, however, is that the ligands for the aforementioned G protein-coupled receptors, the so called “pituitary hormones,” have an equally wide cellular distribution. ACTH is produced by macrophages, so that its anabolic action in skeletal homeostasis may in fact be exerted locally and independently of its pituitary origin (28). Macrophages also produce a TSHβ-variant, which is not suppressed by thyroid hormone and may help protect the skeleton from the effects of pituitary TSH deficiency in hyperthyroidism (6). In contrast, FSHβ has not been detected in bone cells, although OT is produced in abundance by osteoclasts and osteoblasts (22, 23). Furthermore, Oxtr and OT expression in bone cells, particularly in osteoblasts, is regulated by estrogen (22, 23). Although estrogen stimulates bone formation, in part, by increasing Oxtr/OT expression, it is likely also to enhance the expression of Avp and Avrp1α, which in turn will oppose the feed-forward loop that we have predicted for Oxtr/OT interactions (23). Thus, our demonstration of regulated Avp/Avpr expression in bone not only adds to the emerging complexity of the pituitary-bone axis, but also suggests that short-range circuits have evolved that use locally produced pituitary hormones to conduct skeletal surveillance.
Our studies also shed new light on the long-standing dogma that has centered round the mechanisms underlying the osteoporosis that accompanies hyponatremia (10–16). It is quite possible that the bone loss seen in the patient with SIADH had arisen from a 30-fold elevation in serum AVP levels, although the accompanying high aldosterone may have contributed (19). Hyperaldosteronism has been linked to bone loss in rodents (29, 30). The use of spirinolactone in patients with secondary hyperaldosteronism because of heart failure has also been shown to reduce fracture risk (31). Nonetheless, the complementary cell-based, pharmacological, and mouse genetic data we provide compels us to argue for a role for AVP in causing hyponatremia-induced bone loss.
Although we have yet to prove this hypothesis in people, our study does offer a strong rationale for the routine evaluation of skeletal health in patients with chronic hyponatremia, as well as for the potential of using an antiresorptive or anabolic agent for osteoprotection. However, as Avpr2 is expressed osteoblasts and osteoclasts, and is active functionally in regulating bone mass, it is possible that highly selective Avpr2 inhibitors, such as tolvaptan (32), when used as aquaretics in the therapy of chronic hyponatremia, could in fact themselves offer osteoprotection. Our data prompt future clinical studies to examine the skeletal actions of Avpr2 inhibitors in people receiving them.
Materials and Methods
Avpr1α−/− mice, originally on a C57BL/6J x 129Sv background, were crossed for >10 generations to eliminate the mixed background. For histomorphometry, the mice were injected with xylenol orange (90 mg/kg, i.p.) and calcein (15 mg/kg, i.p.) 10 and 2 d before being killed. Lumbar vertebrae, femora, and calvarial bone were dissected, processed, and analyzed for bone formation and resorption parameters, as described previously (7). Additionally, 10-µm longitudinal sections were stained by von Kossa for bone volume measurement, and 12-µm calvarial sections were analyzed for MAR. Blood (∼1 mL) was collected for measurement of C-telopeptisde levels (ELISA), and 3D microtomography was performed as described previously (7). The protocols were approved by the Institutional Animal Care and Use Committee, Faculty of Medicine, University of Bari and Mount Sinai School of Medicine.
Mouse cells were isolated from Avpr1α−/− and wild-type mouse bone marrow-adherent fraction of 5-d-old pups. Cells were cultured in phenol red free α-MEM (Gibco) with 15% (vol/vol) charcoal-stripped FCS in the presence of ascorbate-2-phosphate (1 mM; Sigma) for mRNA and protein analysis and von Kossa staining. To detect Avpr1α expression, immunofluorescence was performed using a rabbit polyclonal anti-Avpr1α antibody, and anti-rabbit Cy-3 conjugated secondary antibodies (Chemicon International) associated with 60 µg/mL fluorescein-labeled phalloidin (Sigma Aldrich). Western blotting was performed using rabbit polyclonal anti-Avpr1α and anti-Avpr2, goat polyclonal anti-neurophysin II, mouse monoclonal pERK, rabbit polyclonal tERK (Santa Cruz), and IRDye-labeled secondary antibodies (680/800CW) (LI-COR Biosciences). For immunobloting, Li-Cor Odyssey infrared imaging system was used. Quantitative PCR was performed as described previously (4).
Acknowledgments
This work was supported in part by National Institutes of Health Grants DK80459 (to M.Z. and L.S.), AR65932 (to M.Z.), and AG40132 (to M.Z.); the Maria I. New Children’s Hormone Foundation and the Chinese University of Hong Kong (M.I.N.); and the Italian Space Agency (ASI) [Osteoporosis and Muscular Atrophy (OSMA) Project] and European Space Agency (ESA) (Eristo Map Project) (to A.Z.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Abe E, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115(2):151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 2.Sun L, et al. FSH directly regulates bone mass. Cell. 2006;125(2):247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 3.Zaidi M, et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci USA. 2010;107(19):8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamma R, et al. Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci USA. 2009;106(17):7149–7154. doi: 10.1073/pnas.0901890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaidi M, et al. Primer on the Metabolic Bone Disease and Disorders of Mineral Metabolism. 2013. The pituitary-bone connection. 8th ed. eds Rosen CJ, Bouillon R, Compston JE, Rosen V (Wiley-Blackwell, Hoboken, NJ), pp 969–977. [Google Scholar]
- 6.Baliram R, et al. Hyperthyroid-associated osteoporosis is exacerbated by the loss of TSH signaling. J Clin Invest. 2012;122(10):3737–3741. doi: 10.1172/JCI63948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu LL, et al. Blocking antibody to the β-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc Natl Acad Sci USA. 2012;109(36):14574–14579. doi: 10.1073/pnas.1212806109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, et al. Oxytocin deficiency impairs maternal skeletal remodeling. Biochem Biophys Res Commun. 2009;388(1):161–166. doi: 10.1016/j.bbrc.2009.07.148. [DOI] [PubMed] [Google Scholar]
- 9.Hruby VJ, Chow MS, Smith DD. Conformational and structural considerations in oxytocin-receptor binding and biological activity. Annu Rev Pharmacol Toxicol. 1990;30:501–534. doi: 10.1146/annurev.pa.30.040190.002441. [DOI] [PubMed] [Google Scholar]
- 10.Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):e1–e8. doi: 10.1016/j.amjmed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Kinsella S, Moran S, Sullivan MO, Molloy MG, Eustace JA. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol. 2010;5(2):275–280. doi: 10.2215/CJN.06120809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbalis JG, et al. Hyponatremia-induced osteoporosis. J Bone Miner Res. 2010;25(3):554–563. doi: 10.1359/jbmr.090827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gankam Kengne F, Andres C, Sattar L, Melot C, Decaux G. Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM. 2008;101(7):583–588. doi: 10.1093/qjmed/hcn061. [DOI] [PubMed] [Google Scholar]
- 14.Sandhu HS, Gilles E, DeVita MV, Panagopoulos G, Michelis MF. Hyponatremia associated with large-bone fracture in elderly patients. Int Urol Nephrol. 2009;41(3):733–737. doi: 10.1007/s11255-009-9585-2. [DOI] [PubMed] [Google Scholar]
- 15.Barsony J, Sugimura Y, Verbalis JG. Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J Biol Chem. 2011;286(12):10864–10875. doi: 10.1074/jbc.M110.155002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoorn EJ, et al. Mild hyponatremia as a risk factor for fractures: The Rotterdam Study. J Bone Miner Res. 2011;26(8):1822–1828. doi: 10.1002/jbmr.380. [DOI] [PubMed] [Google Scholar]
- 17.Bergstrom WH, Wallace WM. Bone as a sodium and potassium reservoir. J Clin Invest. 1954;33(6):867–873. doi: 10.1172/JCI102959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergstrom WH. The participation of bone in total body sodium metabolism in the rat. J Clin Invest. 1955;34(7, Part 1):997–1004. doi: 10.1172/JCI103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sejling AS, Pedersen-Bjergaard U, Eiken P. Syndrome of inappropriate ADH secretion and severe osteoporosis. J Clin Endocrinol Metab. 2012;97(12):4306–4310. doi: 10.1210/jc.2012-2031. [DOI] [PubMed] [Google Scholar]
- 20.Miller M. Hyponatremia and arginine vasopressin dysregulation: Mechanisms, clinical consequences, and management. J Am Geriatr Soc. 2006;54(2):345–353. doi: 10.1111/j.1532-5415.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 21.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13(7):791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 22.Colaianni G, et al. Regulated production of the pituitary hormone oxytocin from murine and human osteoblasts. Biochem Biophys Res Commun. 2011;411(3):512–515. doi: 10.1016/j.bbrc.2011.06.158. [DOI] [PubMed] [Google Scholar]
- 23.Colaianni G, et al. Bone marrow oxytocin mediates the anabolic action of estrogen on the skeleton. J Biol Chem. 2012;287(34):29159–29167. doi: 10.1074/jbc.M112.365049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blair HC, et al. Skeletal receptors for steroid-family regulating glycoprotein hormones: A multilevel, integrated physiological control system. Ann N Y Acad Sci. 2011;1240:26–31. doi: 10.1111/j.1749-6632.2011.06287.x. [DOI] [PubMed] [Google Scholar]
- 25.Arnason BG, Berkovich R, Catania A, Lisak RP, Zaidi M. Mechanisms of action of adrenocorticotropic hormone and other melanocortins relevant to the clinical management of patients with multiple sclerosis. Mult Scler. 2013;19(2):130–136. doi: 10.1177/1352458512458844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bale TL, Dorsa DM. Regulation of oxytocin receptor messenger ribonucleic acid in the ventromedial hypothalamus by testosterone and its metabolites. Endocrinology. 1995;136(11):5135–5138. doi: 10.1210/endo.136.11.7588251. [DOI] [PubMed] [Google Scholar]
- 27.Hasunuma I, et al. Roles of arginine vasotocin receptors in the brain and pituitary of submammalian vertebrates. Int Rev Cell Mol Biol. 2013;304:191–225. doi: 10.1016/B978-0-12-407696-9.00004-X. [DOI] [PubMed] [Google Scholar]
- 28.Isales CM, Zaidi M, Blair HC. ACTH is a novel regulator of bone mass. Ann N Y Acad Sci. 2010;1192:110–116. doi: 10.1111/j.1749-6632.2009.05231.x. [DOI] [PubMed] [Google Scholar]
- 29.Balla T, Nagy K, Tarján E, Renczes G, Spät A. Effect of reduced extracellular sodium concentration on the function of adrenal zona glomerulosa: Studies in conscious rats. J Endocrinol. 1981;89(3):411–416. doi: 10.1677/joe.0.0890411. [DOI] [PubMed] [Google Scholar]
- 30.Chhokar VS, et al. Loss of bone minerals and strength in rats with aldosteronism. Am J Physiol Heart Circ Physiol. 2004;287(5):H2023–H2026. doi: 10.1152/ajpheart.00477.2004. [DOI] [PubMed] [Google Scholar]
- 31.Carbone LD, et al. Fracture risk in men with congestive heart failure risk reduction with spironolactone. J Am Coll Cardiol. 2008;52(2):135–138. doi: 10.1016/j.jacc.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 32.Hori M. Tolvaptan for the treatment of hyponatremia and hypervolemia in patients with congestive heart failure. Future Cardiol. 2013;9(2):163–176. doi: 10.2217/fca.13.3. [DOI] [PubMed] [Google Scholar]