Significance
Childhood poverty has been linked to emotion dysregulation, which is further associated with negative physical and psychological health in adulthood. The current study provides evidence of prospective associations between childhood poverty and adult neural activity during effortful attempts to regulate negative emotion. Adults with lower family income at age 9 exhibited reduced ventrolateral and dorsolateral prefrontal cortex activity and failure to suppress amygdala activation at age 24. Chronic stressor exposure across childhood mediated the relations between family income at age 9 and prefrontal cortex activity. The concurrent adult income, on the other hand, was not associated with neural activity. The information on the developmental timing of poverty effects and neural mechanisms may inform early interventions aimed at reducing health disparities.
Keywords: fMRI, childhood adversity, socioeconomic status, reappraisal
Abstract
Childhood poverty has pervasive negative physical and psychological health sequelae in adulthood. Exposure to chronic stressors may be one underlying mechanism for childhood poverty−health relations by influencing emotion regulatory systems. Animal work and human cross-sectional studies both suggest that chronic stressor exposure is associated with amygdala and prefrontal cortex regions important for emotion regulation. In this longitudinal functional magnetic resonance imaging study of 49 participants, we examined associations between childhood poverty at age 9 and adult neural circuitry activation during emotion regulation at age 24. To test developmental timing, concurrent, adult income was included as a covariate. Adults with lower family income at age 9 exhibited reduced ventrolateral and dorsolateral prefrontal cortex activity and failure to suppress amygdala activation during effortful regulation of negative emotion at age 24. In contrast to childhood income, concurrent adult income was not associated with neural activity during emotion regulation. Furthermore, chronic stressor exposure across childhood (at age 9, 13, and 17) mediated the relations between family income at age 9 and ventrolateral and dorsolateral prefrontal cortex activity at age 24. The findings demonstrate the significance of childhood chronic stress exposures in predicting neural outcomes during emotion regulation in adults who grew up in poverty.
Childhood poverty is related to increased risk of psychopathology (1–3) and physical illness in adulthood (4, 5). Furthermore, childhood poverty predicts adult morbidity irrespective of adult poverty (5–7). One possible mechanism to explain the far-reaching effects of childhood poverty on health is chronic stress (8). Chronic exposure to stressors associated with living in low-income families has long-term negative effects on physiological stress regulatory systems (9–12), eventually resulting in pathology (13, 14). Growing evidence suggests exposure to chronic stress and socioeconomic adversity produces lasting neurobiological changes (15, 16). However, little is known about whether childhood poverty is prospectively associated with central nervous system mechanisms involved in emotion regulation. Such knowledge may provide insights into identifying neural patterns for emotion regulatory dysfunction among adults who grew up in childhood poverty.
The amygdala and prefrontal cortex (PFC) play a critical role for stress and emotion regulation. The amygdala detects and responds to threats from the environment, activating physiological stress responses (17). The PFC is widely considered as a top-down region that regulates the amygdala (18, 19). More specifically, the ventrolateral PFC (VLPFC), dorsolateral PFC (DLPFC), and medial PFC (mPFC) implement cognitive strategies such as cognitive reappraisal involved in emotion regulation (18–20). During reappraisal of negative stimuli, increased activity in the VLPFC, DLPFC, and mPFC regions is associated with diminished amygdala reactivity to negative stimuli as well as decreased perceived negative affect (21). Amygdala and PFC dysregulation has also been observed in populations with mood dysregulation, including depression (22), anxiety disorders (23, 24) including posttraumatic stress disorder (25), impulsive aggression (26), and substance abuse (27). Aberrant amygdala reactivity and inefficient or blunted PFC regulatory function are considered a neurobiological mechanism involved in impaired emotion regulation in these psychiatric disorders.
Amygdala and PFC functions have also been shown to be affected by socioeconomic disparities (28, 29). In children, low socioeconomic status (SES) has been related to greater amygdala volume (30) and reduced PFC activity during cognitive tasks (31). In adults, retrospective reports of childhood SES were associated with elevated amygdala activity while processing negative facial expressions independently of adult SES (32) and reduced VLPFC activity while experiencing social exclusion (33). However, whether the amygdala and PFC functions associated with childhood poverty are directly related to effortful emotion regulation has never been examined.
At present, little is known about underlying mechanisms that account for the relation between childhood SES and neural functioning. Chronic stress is one hypothetical mediator of the negative link between childhood poverty and adult health outcomes (8, 10). For example, children living in poverty are more likely to be exposed to multiple chronic stressors including violence, family turmoil, separation from family members, and substandard living environments (34, 35). In our previous studies, poverty exposure at age 9 prospectively predicted physiological stress dysregulation (34) and emotion dysregulation (36, 37) in adolescence when concurrent levels of poverty exposure were controlled. In these studies, poverty exposure at age 9 was concurrently and prospectively associated with chronic stress exposure at age 9, 13, and 17 (37–39), and elevated chronic stress, in turn, mediated the association between childhood poverty and later outcomes. Furthermore, animal studies and recent human brain imaging studies demonstrate that repeated exposure to chronic stress impacts amygdala and PFC development, potentially leading to impaired emotion regulation (40–43).
Therefore, in this longitudinal study, we investigated whether childhood family income was associated prospectively with adult neural activity in the amygdala and PFC during emotion regulation. We also examined a stress pathway linking childhood poverty and the subsequent neural functions for emotion regulation. The current study used family income assessed at age 9 as a direct measure of childhood poverty exposure. To investigate the developmental timing of poverty and neural functioning, we examined the link between childhood poverty and adult neural functioning after controlling for adult income levels. We used a well-established emotion regulation functional magnetic resonance imaging (fMRI) paradigm (18, 44), in which participants are instructed to experience the natural emotional state (Maintain) or to decrease the intensity of their negative affect by using cognitive reappraisal (Reappraisal) while viewing negative images. We hypothesized that, in the contrast of Reappraisal vs. Maintain conditions, low family income at age 9 would be associated with increased amygdala and decreased PFC activation. The amygdala and PFC activation may also be associated with self-reports of emotion regulation (Materials and Methods). Furthermore, we assessed chronic stress by averaging exposure to multiple physical (i.e., substandard housing, crowding, and noise) and social (i.e., family turmoil, violence, and child–family separation) risk factors across ages 9–17. We hypothesized that the influence of childhood income on amygdala and PFC activity would be mediated by chronic stress exposure throughout childhood.
Results
Descriptive and Behavioral Data.
Ratings of negative affective state between two conditions, Reappraisal and Maintain, were significantly different, t(48) = 4.13, P < 0.001. The average ratings decreased from the Maintain (mean = 2.93 ± 0.81) to Reappraisal (mean = 2.48 ± 0.95). However, the success of regulating negative emotions (calculated by subtracting Reappraisal ratings from Maintain ratings) was not significantly associated with family income at age 9, chronic stress exposure across ages 9–17, or current adult income levels at age 24.
Family Income at Age 9 and Neural Emotion Regulation at Age 24.
Reappraisal of emotion (compared with maintaining one’s emotional response) produced greater activation in bilateral inferior/middle/superior frontal gyrus, precentral gyrus, striatum, insula, parietal lobe, and temporal gyrus (P < 0.05, corrected for multiple comparisons; Materials and Methods). However, no amygdala activation was detected in the contrast of Reappraisal vs. Maintain using a region of interest (ROI) approach, although bilateral amygdala activation was detected on Reappraisal (vs. Baseline) and Maintain (vs. Baseline) conditions (P < 0.05, corrected).
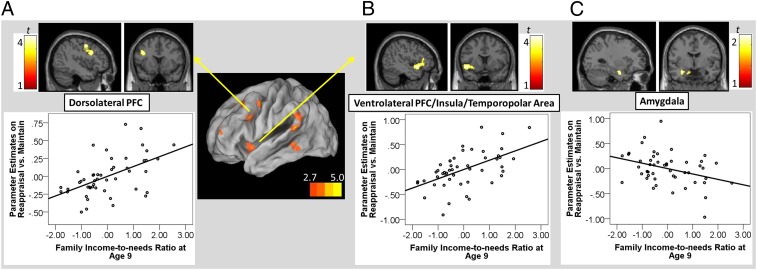
Enhanced neural activation during Reappraisal was predicted by family income at age 9. In particular, in the contrast of the Reappraisal vs. Maintain, lower family income at age 9 predicted reduced activation in the left DLPFC (Fig. 1A), VLPFC/insula/temporopolar area (Fig. 1B), precentral gyrus, and inferior parietal lobe/superior temporal gyrus (Ps < 0.05, corrected; Table 1; all analyses controlled for current income). No cluster was identified with a significant positive association with the current, adult income level.
Fig. 1.
The upper panels are regions showing a significant association with family income-to-needs ratio at age 9. (A) Dorsolateral prefrontal cortex (PFC) (x, y, z = –40, 12, 28; 343 voxels; P < 0.05, corrected). (B) Ventrolateral PFC, insula, temporopolar area (x, y, z = –46, 10, –8; 672 voxels; P < 0.05, corrected). (C) Amygdala (x, y, z = –30, –4, –22; 140 voxels; P < 0.05, uncorrected). The lower panels depict partial regression plots describing the associations between family income-to-needs ratio at age 9 and parameter estimates of a region in the contrast of Reappraisal vs. Maintain, controlling for adult income level.
Table 1.
Brain areas with the positive associations between family income-to-needs ratio at age 9 and neural activity in the Reappraise vs. Maintain contrast at age 24
| MNI coordinates |
|||||||
| Area of activation | Brodmann area | Side | # voxels | x | y | z | t(1, 46) |
| Dorsolateral PFC | 9, 46 | L | 343 | −40 | 12 | 28 | 3.99 |
| −46 | 0 | 48 | 3.92 | ||||
| −42 | 2 | 38 | 3.13 | ||||
| Ventrolateral PFC, | 47, 13, 38 | L | 672 | −46 | 10 | −8 | 4.04 |
| Insula, | −28 | 14 | −14 | 3.95 | |||
| Temporopolar area | −48 | 22 | −2 | 3.30 | |||
| Precentral gyrus | 6, 8 | R, L | 602 | 0 | 12 | 68 | 4.26 |
| 8 | 26 | 64 | 4.03 | ||||
| −2 | 20 | 58 | 3.20 | ||||
| Superior temporal | 22, 40 | L | 293 | −64 | −48 | 18 | 4.23 |
| gyrus, Inferior | −58 | −48 | 32 | 3.60 | |||
| parietal gyrus | −56 | −56 | 30 | 3.14 | |||
P < 0.05, corrected. L, left; MNI, Montreal Neurological Institute; PFC, prefrontal frontal cortex; R, right.
Amygdala ROI analysis revealed that activation in the Reappraisal vs. Maintain conditions was negatively associated with childhood income in the left amygdala [t(46) = 2.48, x, y, z = –30, –4, –22; 140 voxels] (P < 0.05, uncorrected) (Fig. 1C), controlling for adult income level. Besides the amygdala activity from ROI analysis, no other cluster showed a negative association with family income at age 9 or current adult income level.
Furthermore, we explored functional connectivity between the left amygdala and VLPFC/DLPFC regions using the psychophysiological interaction (PPI) analysis at P < 0.001, uncorrected, cluster size > 10 voxels (SI Text). The analysis revealed that amygdala activity was positively coupled with the left VLPFC [x, y, z = –58, 18, 8; 58 voxels; t(46) =3.97; Fig. S1] during Reappraisal among individuals with lower family income at age 9, whereas amygdala activity was negatively coupled with the left VLPFC during Reappraisal among individuals with higher family income at age 9 (Fig. S2). Family income at age 9 was not associated with the amygdala−DLPFC connectivity.
Finally, we calculated the correlation between regulation success scores and the amygdala, VLPFC, and DLPFC activity during Reappraisal. The correlation analysis revealed that Reappraisal success was positively correlated with both DLPFC [r(49) = 0.31, P < 0.05] and VLPFC [r(49) = 0.27, P < 0.05], but not with amygdala activity. Thus, DLPFC and VLPFC activity during Reappraisal was associated with greater success in downregulating negative emotions. However, the use of everyday reappraisal coping (Materials and Methods) was not associated with the neural activity.
Childhood Chronic Stressor Exposure as a Mediator.
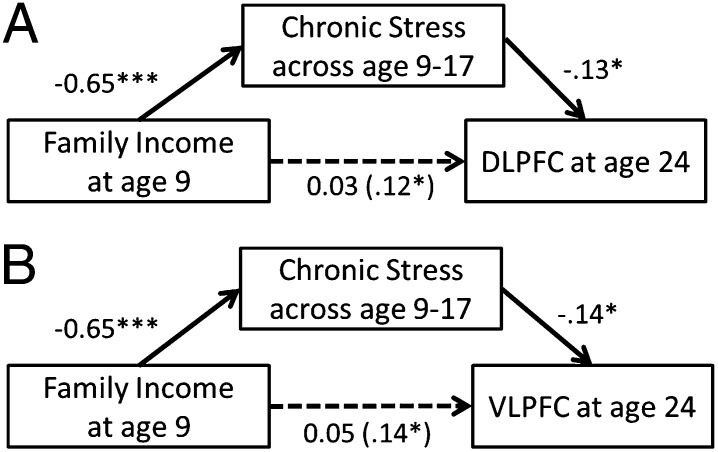
We next tested whether exposure to chronic stressors during childhood (ages 9–17) mediated the relations between family income at age 9 and adult DLPFC and VLPFC activity during Reappraisal. Elevated childhood chronic stress exposure mediated the associations between family income at age 9 and increased adult left DLPFC activity during Reappraisal, controlling for concurrent, adult income (Fig. 2A). The addition of childhood chronic stress shrank the beta weight for childhood income 75%, which was no longer significant and suggested full mediation [indirect effect = 0.09, 95% confidence intervals (CIs) = 0.02–0.18].
Fig. 2.
A path diagram showing a mediation model with the unstandardized coefficients for each association. Chronic stress exposure across ages 9–17 mediated the relationship between family income-to-needs ratio and neural activity during Reappraisal in (A) dorsolateral prefrontal cortex (DLPFC) and (B) ventrolateral prefrontal cortex (VLPFC). ***P < 0.001, *P < 0.05.
Because the size of the suprathreshold cluster including the left VLPFC was large and contained other parietal and temporal regions, an ROI approach was used to separate the estimated activity of the left VLPFC from the estimated activity of other regions. We placed an 8 mm radius sphere at the left VLPFC peak (x, y, z = –50, 22, 6) from a meta-analysis of fMRI reappraisal studies (19). We found elevated chronic stress exposure across ages 9–17 mediated the relations between family income at age 9 and increased left VLPFC activity during Reappraisal, controlling for concurrent adult income (Fig. 2B). The addition of childhood chronic stress shrank the beta weight for childhood income 64%, which was no longer significant and suggested full mediation (indirect effect = 0.09, 95% CIs = 0.02–0.16). An analysis performed on amygdala activity did not find a mediation effect.
Discussion
We examined whether childhood poverty was prospectively linked to adult neural activity in the PFC and amygdala, regions centrally involved in emotional regulation. We found a significant relation between childhood income and neural functions. During emotion regulation with cognitive reappraisal, lower family income at age 9 was associated with reduced activity in the adult DLPFC and VLPFC but increased amygdala activity. In contrast to childhood income level, current income level as an adult was not linked to neural activity during emotion regulation. When the individual’s stress history was incorporated into our model, exposure to chronic stressors throughout childhood (i.e., ages 9–17) mediated the links between family income at age 9 and reduced adult DLPFC and VLPFC activity. Reduced PFC and increased amygdala activity among adults who grew up in poverty provides evidence for neural embedding of childhood poverty. Furthermore, the mediating role of chronic stressor exposure in childhood may help account for the link between childhood poverty and adult neural functions, which may contribute to physiological and psychological stress regulation difficulties.
We found that lower family income at age 9 was associated with reduced DLPFC and VLPFC activity in 24-y-olds during emotion regulation using cognitive reappraisal. Both DLPFC and VLPFC are involved in cognitive control and executive functioning and facilitate goal-directed behaviors (45). Furthermore, increased DLPFC and VLPFC activity was associated with greater success in down-regulating negative emotions, further supporting the role of these cortical regions in emotion regulation. In contrast to reduced DLPFC and VLPFC activity, family income at age 9 was associated with increased adult amygdala activity during emotion regulation. More specifically, the data in Fig. 1C suggest that in adults who had higher family income at age 9, the negative values of neural activity suggest less amygdala activity during Reappraisal relative to the Maintain condition. However, in those with lower family income at age 9, the positive values of neural activity suggest greater amygdala activity during Reappraisal than Maintain, indicating potential failure of amygdala regulation using Reappraisal. The functional connectivity findings further suggested altered relations between the amygdala and VLPFC activity in the context of childhood poverty exposure. A negative amygdala−VLPFC coupling among individuals with lower family income at age 9 suggests that higher childhood family income is associated with greater VLPFC activity to suppress amygdala activity during emotion regulation. On the other hand, a positive amygdala−VLPFC coupling during Reappraisal suggests that lower childhood income is associated with ineffective amygdala activity suppression of the VLPFC activity during emotion regulation. Such failure of amygdala regulation, in part by the dampened VLPFC and DLPFC activity, has been suggested as neural deficits in many psychiatric illnesses associated with childhood exposure to chronic stress.
With regard to the amygdala findings, we found no main effect of Reappraisal (vs. Maintain) on diminishing amygdala activity; therefore, the potential interpretation of the reappraisal-related amygdala modulation is limited to the context of variability in childhood family income at age 9. Reduction in amygdala activation during Reappraisal has been inconsistently reported across previous studies (22, 44, 46). The reason for the inconsistent findings may be associated with different types of regulatory strategies used across different studies. All regulatory strategies require emotional appraisal and attention to emotional stimuli—processes strongly associated with amygdala activity (47). However, some strategies such as positive reinterpretation or distancing from the content of negative stimuli may recruit more amygdala activity than distraction (directing attention away from negative stimuli) (48). The current study focused on positive reinterpretation and distancing, and this may have produced no main effect on amygdala activity during Reappraisal vs. Maintain.
In addition to altered VLPFC and DLPFC functioning, childhood poverty predicted activity in several other frontal, parietal, and temporal regions of the adult brain, including the precentral gyrus, inferior parietal lobe, superior temporal gyrus, and temporopolar gyrus, during cognitive reappraisal. In all of these regions, low family income at age 9 predicted reduced activity during cognitive reappraisal. Each of these regions has been shown to be involved in emotion regulation via cognitive reappraisal (18, 19, 44). The precental gyrus contributes to the top-down control of cognitive and emotional processes through selective attention (49). Although we did not find a significant association between childhood income and mPFC, a region involved in reappraisal, the precentral gyrus is structurally interconnected and frequently activated with the mPFC as well as lateral PFC during Reappraisal (50). The inferior parietal lobe is a part of the attention system along with the DLPFC (51). Thus, the activity in the precentral gyrus and inferior parietal lobe may contribute to reappraisal by selectively monitoring information. Previously, adult retrospective reports of childhood SES have been associated with lower activity in the inferior partial lobe during monetary reward processing (52). Temporopolar area and superior temporal gyrus are related to the representation of perceptual and semantic information that likely assists in the reappraisal process (18). Thus, the significant associations between childhood family income and neural activity in these regions uncovered herein may reflect the pervasive effects of childhood SES disparities on neural functions across multiple regions involved in emotional regulation.
We also tested the hypothesis that exposure to chronic stressors across ages 9–17 would help explain the relations between childhood poverty and reduced DLPFC and VLPFC activity during emotion regulation. Chronic stressor exposure may be particularly significant for PFC plasticity because the PFC matures primarily during adolescence (ages 9–17) (53). However, in the amygdala, chronic stress exposure across ages 9–17 did not mediate the link between family income at age 9 and neural activity. This could be due to the weaker association of childhood income with amygdala responses, compared with PFC responses, during Reappraisal.
Our finding that family income at age 9 predicts adult neural function that is mediated by childhood chronic stressor exposure is consistent with the hypothesis that early experiences of poverty become embedded within the organism, setting individuals on lifelong trajectories that portend morbidity (5, 54). Furthermore, these trends hold independently of concurrent poverty during adulthood. The latter added no additional explanatory power to the prediction of adult neural functioning. Although in our study the poverty exposure data are available at age 9, when children were first recruited, children in poverty at age 9 are likely to have been disadvantaged at an earlier age as well. This earlier exposure to poverty may have impacted long-term neural functions. Growing animal and human evidence suggests exposure to chronic stress in early childhood produces lasting neurobiological changes in the amygdala and PFC when neural regions are immature and rapidly developing (41). For instance, institutionalization in infancy was associated with increased amygdala activity in response to negative expressions in children at age 10 (43). Exposure to cumulative risk including maternal depression and financial stress in infancy was also associated with decreased amygdala and PFC connectivity during rest in adolescent females (55). Therefore, it is critical that future research more directly investigates the developmental timing of poverty, chronic stressor exposure, and neural functioning at shorter time intervals across a wider range of maturation.
In addition to more in-depth assessments over time, the present results should be considered in light of several limitations. First, participants were Caucasian, had no psychiatric or neurological disease, and grew up in rural areas. Thus, our findings may not generalize to a more racially heterogeneous or urban population. Second, the VLPFC and DLPFC play an important role in cognitive control, including attention, executive function, and working memory. Therefore, reduced activity in the regions may be related to difficulties in cognitive processes, not specifically to emotional processing per se. Indeed, childhood SES has also been related to impaired cognitive functions such as lack of sustained attention and deficits in executive functioning (56, 57). Thus, it is important to further investigate common and unique patterns of neural activity during cognitive and emotional task demands and their associations with childhood SES. Third, we found that the associations among reduced negative affect, environmental, and neural factors were limited to DLPFC and VLPFC activity. Childhood income, chronic stress, and amygdala activity were not associated with regulation success based on subjective ratings. It may be possible that subjective ratings from the instructed emotion regulation were not sensitive to individual differences in childhood adversity and amygdala activity. Therefore, the interpretation of emotion dysregulation should be restricted to DLPFC and VLPFC activity. More studies using other methods such as self-report or observed measures of emotion regulation are necessary to confirm whether there are associations among childhood adversity, amygdala activity, and changes in negative affect. Fourth, the current study did not detect evidence that neural activity was associated with the use of reappraisal strategies in response to everyday stress. Thus, effects of childhood SES on neural activity may not generalize to everyday coping efforts using cognitive reappraisal. However, because the Reappraisal scale included only four items across any stressors in life, the scale may have had a limited ability to detect individual differences. Studies assessing coping strategies specifically in response to financial hardships suggest that children and adults living in poverty rely more on involuntary coping strategies in their daily lives, such as avoidance, than on active coping strategies, such as cognitive reappraisal (58). Furthermore, teaching coping skills including cognitive reappraisal helped adult, low-income women decrease their reliance on involuntary coping strategies and reduce their depressive symptoms (58). Thus, future studies should investigate whether childhood SES effects on the neural functioning during cognitive reappraisal are linked to everyday reappraisal efforts for particular stressors including financial hardships.
The current study revealed that childhood poverty is related to reduced activity in the VLPFC and DLPFC and increased activity in the amygdala during emotion regulation among young adults. Furthermore, in both the DLPFC and VLPFC, childhood chronic stressor exposure mediated the relations between childhood poverty and decreased activity during emotion regulation. The current study demonstrates the significance of childhood family income and stress exposure in predicting neural outcomes in young adults during emotion regulation. Greater knowledge about the developmental timing of risk exposures and brain development would be extremely valuable for informing interventions. Thus, future longitudinal studies should examine the timing of childhood poverty, stressor exposures, and brain development, ideally from conception throughout childhood.
Materials and Methods
Participants.
Participants were initially recruited for a longitudinal study on rural poverty and child development (mean age, 9.2 y) in rural counties in the Northeastern United States from public schools, the Cooperative Extension System, Head Start, and other antipoverty programs. One child per family participated, and low-income families were oversampled. The participants were followed up during wave 2 (mean age, 13.4 y) and wave 3 (mean age, 17.3 y). See Evans et al. (34, 35) for further details on subject recruitment and protocols.
Among individuals participating in these three waves of data collection, 54 participants were recruited for this study. This study was approved by the University of Michigan and Cornell University Institutional Review Boards, and all participants provided informed consent. All participants had no MRI contraindications (e.g., metallic/ferrous materials in their body), no prior or current treatment for any psychiatric disorder [clinician-conducted psychiatric evaluation based on the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)], and no current neurological condition. Approximately half of the participants were from low-income backgrounds at age 9, and half were from families with incomes two to four times above the poverty line. Of 54 participants, 49 participants completed the Emotion Regulation Task (ERT) task, and had a full set of usable fMRI data. Two participants did not complete the task, one participant had a severe artifact in fMRI data, and two participants had excessive movement in fMRI data beyond our criteria (2 mm/2° in any directions). The average age was 23.61 (SD = 1.30, range, 20–27), and 55.1% (27 out of 49) were males. The average income-to-need ratio at age 9 was 1.8 (SD = 1.1) and at age 24 was 3.2 (SD = 3.0).
Procedure.
In the longitudinal study, a pair of trained researchers visited children’s homes at each wave of data collection, independently interviewing the participant and his/her mother. Only one child per household was eligible for the study. Demographic information, measures of mental health, and chronic stress exposure were assessed during the home visits. In the fMRI study, participants visited the University of Michigan’s neuroimaging center, where trained researchers administered training and fMRI sessions.
Measures.
Income-to-needs.
The ratio of family income-to-needs was computed by dividing total family income by the poverty threshold at each wave of data collection. This ratio is an annually adjusted, per capita index of income that the US Census Bureau calculates using a standardized formula. The income-to-needs ratio at age 24 was calculated based on the participants’ own income.
Chronic stress.
Children’s exposure to chronic stress was assessed at wave 1 (age 9), wave 2 (age 13), and wave 3 (age 17). Chronic stressors included three psychosocial risk factors (child–family separation, violence, and family turmoil) and three physical risk factors (noise, crowding, and housing quality). Psychosocial risk factors were assessed by maternal reports at wave 1 and combined maternal and child reports at waves 2 and 3. Mothers completed the Life Events and Circumstances Checklist (59), with subscales on child–family separation, violence, and family turmoil. Mothers answered dichotomous items (yes/no) to indicate whether specific events or circumstances had happened to their child during the interval since the prior interview. Children also completed a life event scale based on a modified version of the Adolescent Perceived Events Scale (60), answering dichotomous items (yes/no) to specific events. An event was counted a single time if it was reported by the child, the mother, or both. As for physical risk factors, housing quality was rated by trained observers on a standardized scale (61). Noise was assessed by two, 2-h readings of average decibel levels (Leq) in the primary social space of the home (typically the living room). Crowding was defined as the ratio of occupants to number of rooms in the home.
For each participant, each of the six risk factors were coded dichotomously—1 if scores were in the upper quartile based on the data distribution of the entire sample at each age point, and 0 otherwise. Chronic stressor exposure at each wave of data collection was calculated by summing the dichotomous scores of all risk factors (range, 0–6). Additive indices of cumulative stress exposure are robust and consistently predict physical and mental health outcomes better than indices of singular stressor exposure or alternative multiple stressor exposure metrics (62). Chronic stress exposure scores were then averaged across the three waves.
Everyday reappraisal coping.
The use of reappraisal strategies in responses to everyday stress was assessed by the COPE Inventory (63). The measure has a 4-point scale ranging from “I don’t do this at all” to “I do this a lot.” The scale Positive Reinterpretation and Growth included four items (e.g., “I try to see it in a different light, to make it seem more positive”), and the summary score of the four items was included as an indicator of everyday use of reappraisal strategies.
fMRI paradigm.
Neural activity of participants was recorded while they were engaged in the ERT (44, 64). During the Look condition of the ERT, participants were asked to simply look at pictures with emotionally neutral valence. During the Maintain task, participants were instructed to attend to and experience naturally (without trying to change or alter) the emotional state elicited by the pictures. During the Reappraisal task, participants were instructed to voluntarily decrease the intensity of their negative affect by using the cognitive strategy of reappraisal. The participants were asked to use one of two strategies for each picture: (i) transforming the depicted scenario into less negative or positive terms (e.g., people crying outside the church are leaving a wedding and the tears are joyful) and (ii) rationalizing or objectifying the content of the pictures (e.g., an abused woman smoking a cigarette is an actress in a movie between scenes). During the practice session, participants were asked to go through the reappraisal process out loud. They were assisted in reevaluating the images if their strategies were judged as inappropriate by the experimenters. The fMRI sessions were conducted only after all participants demonstrated full understanding of the task.
The fMRI task involved a block-related design in which participants viewed 20 s blocks of aversive or neutral pictures; each picture was presented for 5 s consecutively without an interstimulus interval. Before each block of pictures, the instruction to “look,” “maintain,” or “reappraise” appeared at the center of a black screen for a duration of 5 s. Immediately following each Look, Maintain, or Reappraisal block, a rating scale appeared on a screen for 5 s asking participants to rate the intensity of their negative affect on a 5-point scale (1, least negative/neutral; 5, extremely negative) via button response. The look, maintain, and reappraise blocks were interspersed with 20 s baseline blocks consisting of a fixation cross. During this period, participants were asked to stop maintaining or reappraising their emotional experience and to relax. The total task duration was 10 min spread across two runs.
fMRI data acquisition and preprocessing.
Scanning took place in a 3.0 Tesla Philips magnet scanner in the fMRI laboratory at Veterans Affairs Ann Arbor using a standard eight-channel SENSE head coil. Functional data were acquired (300 T2*-weighted echo-planar-imaging (EPI) volumes; TR = 2,000 ms; TE = 30 ms; flip angle = 90; field of view = 220 mm; matrix size, 64 × 64; 42 axial slices; voxels = 3.44 × 3.44 × 2.80 mm). A high-resolution anatomical T1-weighted image with a 3D gradient recalled echo was also acquired. Functional imaging data were preprocessed and analyzed using Statistical Parametric Mapping 8 (Wellcome Trust Center for Neuroimaging, University College, London; www.fil.ion.ucl.ac.uk/spm). Five images at the beginning of each fMRI run were discarded. Slice timing correction was performed using a middle slice as a reference (slice 21), and then images within each run were realigned to the first image of the first run to correct for movement. The realigned functional images were spatially normalized to a functional template, resampled to 2 × 2 × 2 mm voxels, and then spatially smoothed using a Gaussian filter (full width at half maximum, 8 mm).
fMRI data analysis.
At the individual subject level, response amplitudes were estimated for each condition using the general linear model. A high-pass filter of 0.0078 Hz was used. Conditions that were modeled included look, maintain, and reappraise blocks as well as instruction and rating periods. The current study was primarily interested in emotion regulation; thus, for individual subjects, we contrasted images of the blood-oxygen-level–dependent (BOLD) signal changes associated with the Reappraisal vs. Maintain contrast, which estimated the neural functions involved in explicit regulation of negative emotion. For the group-level analysis, contrast images for individual subjects were entered into a random-effects analysis. To identify regions that were more active on Reappraisal, we first performed contrast compared activation in the Reappraisal vs. Maintain condition. To identify regions that were associated with childhood family income, a multiple regression was performed with the income-to-needs ratio at age 9 as an independent variable and the current income–to-needs ratio as a covariate of no interest. An initial voxel-wise threshold of P < 0.005 and a minimum cluster size of 265 voxels for the Reappraisal vs. Maintain contrast gave a corrected P < 0.05. This threshold was determined by Monte-Carlo simulations using the 3dClustSim program of the AFNI toolkit (3dClustSim –mask –both –prefix –fwhmxyz 10.31 10.82 10.02; http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html). The amygdala was a region of a priori interest and has a small structure; thus, an ROI approach was used. A threshold of P < 0.05, uncorrected, was applied, and estimates of signal change for each contrast averaged across the entire suprathreshold region were extracted for each participant using MarsBaR (Marseille boîte à région d'intérêt) (65) and were then entered into Statistical Package for the Social Sciences (SPSS, Inc.) for additional analyses.
In the additional analyses, we used neural activity during the Reappraisal task alone (vs. Fixation), rather than contrasting Reappraisal against Maintain. We sought to isolate the effects of Reappraisal as Maintain because the contrast confounds potential interactions between the two tasks, both of which involve appraisal and implicit and explicit control, whereas the fixation condition approximates noncognitive/nonemotional control “baseline.” The same approach was used in previous studies (44, 48, 64, 66). First, we estimated regulation success of negative emotions by subtracting Reappraisal ratings from Maintain ratings (21). Then, the regulation success as well as everyday reappraisal coping were correlated with amygdala and PFC activity. Second, in the PFC and amygdala, the indirect effect of chronic stress exposure was tested using 95% bias-corrected CIs with bootstrapping procedures (10,000 bootstrap resamples) (67). The 95% bias-corrected CIs without the inclusion of 0 indicates a statistically significant indirect relationship at P < 0.05 (67).
Supplementary Material
Acknowledgments
We thank Erika Blackburn, Sarah Garfinkel, and Robert Varney for assistance with data collection. The current study was supported by National Institutes of Health Grant RC2MD004767, the William T. Grant Foundation, the John D. and Catherine T. MacArthur Foundation Network on Socioeconomic Status and Health, and the Robert Wood Johnson Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308240110/-/DCSupplemental.
References
- 1.Grant KE, et al. Stressors and child and adolescent psychopathology: Moving from markers to mechanisms of risk. Psychol Bull. 2003;129(3):447–466. doi: 10.1037/0033-2909.129.3.447. [DOI] [PubMed] [Google Scholar]
- 2.Adler NE, Rehkopf DH. U.S. disparities in health: Descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 3.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53(1):371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 4.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: How and why do these relationships change with age? Psychol Bull. 2002;128(2):295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Ann N Y Acad Sci. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- 6.Poulton R, et al. Association between children’s experience of socioeconomic disadvantage and adult health: A life-course study. Lancet. 2002;360(9346):1640–1645. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blair C, Raver CC. Child development in the context of adversity: Experiential canalization of brain and behavior. Am Psychol. 2012;67(4):309–318. doi: 10.1037/a0027493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans GW. The environment of childhood poverty. Am Psychol. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 9.Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biol Psychiatry. 2000;48(10):976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- 10.Evans GW, Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Ann N Y Acad Sci. 2010;1186:174–189. doi: 10.1111/j.1749-6632.2009.05336.x. [DOI] [PubMed] [Google Scholar]
- 11.Evans GW, Chen E, Miller GE, Seeman TE. The Oxford Handbook of Poverty and Child Development. In: Maholmes V, King R, editors. How Poverty Gets Under the Skin: A Lifecourse Perspective. New York: Oxford Univ Press; 2012. pp. 13–36. [Google Scholar]
- 12.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 13. Adler NE, Marmot M, McEwen BS, Stewart J eds (1999) Socioeconomic Status and Health in Industrial Nations (New York Academy of Sciences, New York)
- 14.Kessler RC, Price RH, Wortman CB. Social factors in psychopathology: stress, social support, and coping processes. Annu Rev Psychol. 1985;36:531–572. doi: 10.1146/annurev.ps.36.020185.002531. [DOI] [PubMed] [Google Scholar]
- 15.Boyce WT, Sokolowski MB, Robinson GE. Toward a new biology of social adversity. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17143–17148. doi: 10.1073/pnas.1121264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEwen BS. Brain on stress: How the social environment gets under the skin. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 18.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalisch R. The functional neuroanatomy of reappraisal: Time matters. Neurosci Biobehav Rev. 2009;33(8):1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Kalisch R, Wiech K, Critchley HD, Dolan RJ. Levels of appraisal: A medial prefrontal role in high-level appraisal of emotional material. Neuroimage. 2006;30(4):1458–1466. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ball TM, Ramsawh HJ, Campbell-Sills L, Paulus MP, Stein MB. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychol Med. 2013;43(7):1475–1486. doi: 10.1017/S0033291712002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends Cogn Sci. 2007;11(10):413–418. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 25.New AS, et al. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry. 2009;66(7):656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry. 2007;62(2):168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 27.O’Daly OG, et al. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology. 2012;37(10):2267–2276. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11(9):651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 2012;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheridan MA, Sarsour K, Jutte D, D’Esposito M, Boyce WT. The impact of social disparity on prefrontal function in childhood. PLoS ONE. 2012;7(4):e35744. doi: 10.1371/journal.pone.0035744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gianaros PJ, et al. Potential neural embedding of parental social standing. Soc Cogn Affect Neurosci. 2008;3(2):91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanagisawa K, et al. Family socioeconomic status modulates the coping-related neural response of offspring. Soc Cogn Affect Neurosci. 2013;8(6):617–622. doi: 10.1093/scan/nss039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans GW, Kim P. Early childhood poverty and adult chronic physiological stress: The mediating role of childhood cumulative risk exposure. Psychol Sci. 2012;23(9):979–983. doi: 10.1177/0956797612441218. [DOI] [PubMed] [Google Scholar]
- 35.Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev Psychol. 2003;39(5):924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- 36.Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Dev. 2002;73(4):1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- 37.Doan SN, Fuller-Rowell TE, Evans GW. Cumulative risk and adolescent’s internalizing and externalizing problems: The mediating roles of maternal responsiveness and self-regulation. Dev Psychol. 2012;48(6):1529–1539. doi: 10.1037/a0027815. [DOI] [PubMed] [Google Scholar]
- 38.Wells NM, Evans GW, Beavis A, Ong AD. Early childhood poverty, cumulative risk exposure, and body mass index trajectories through young adulthood. Am J Public Health. 2010;100(12):2507–2512. doi: 10.2105/AJPH.2009.184291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans GW, Kim P. Childhood poverty and health: Cumulative risk exposure and stress dysregulation. Psychol Sci. 2007;18(11):953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 40.Davidson RJ, McEwen BS. Social influences on neuroplasticity: Stress and interventions to promote well-being. Nat Neurosci. 2012;15(5):689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 42.Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry. 2006;60(3):296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 43.Tottenham N, et al. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phan KL, et al. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 45.Wager TD, et al. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27(2):323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 46.Urry HL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phan KL, Sripada CS. Emotion Regulation. In: Armony J, Vuilleumier P, editors. The Cambridge Handbook of Human Affective Neuroscience. Cambidge, UK: Cambridge Univ Press; 2013. pp. 375–400. [Google Scholar]
- 48.McRae K, et al. The neural bases of distraction and reappraisal. J Cogn Neurosci. 2010;22(2):248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheth SA, et al. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488(7410):218–221. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 52.Gianaros PJ, et al. Parental education predicts corticostriatal functionality in adulthood. Cereb Cortex. 2011;21(4):896–910. doi: 10.1093/cercor/bhq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahl RE. Adolescent brain development: A period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- 54.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 55.Burghy CA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15(12):1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proc Natl Acad Sci USA. 2009;106(16):6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 58.Wadsworth ME, Santiago CD, Einhorn L, Etter EM, Rienks S, Markman H. Preliminary efficacy of an intervention to reduce psychosocial stress and improve coping in low-income families. Am J Community Psychol. 2011;48:257–271. doi: 10.1007/s10464-010-9384-z. [DOI] [PubMed] [Google Scholar]
- 59.Wyman PA, Cowen EL, Work WC, Parker GR. Developmental and family milieu correlates of resilience in urban children who have experienced major life stress. Am J Community Psychol. 1991;19(3):405–426. doi: 10.1007/BF00938033. [DOI] [PubMed] [Google Scholar]
- 60.Compas BE. Responses to Stress Questionnaire. Burlington, VT: Univ of Vermont; 1997. [Google Scholar]
- 61.Evans GW, Wells NM, Chan HY, Saltzman H. Housing quality and mental health. J Consult Clin Psychol. 2000;68(3):526–530. doi: 10.1037//0022-006x.68.3.526. [DOI] [PubMed] [Google Scholar]
- 62. Evans GW, Li D, Sepanski Whipple S (2013) Cumulative risk and child development. Psychol Bull, in press. [DOI] [PubMed]
- 63.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: A theoretically based approach. J Pers Soc Psychol. 1989;56(2):267–283. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- 64.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brett M, Anton J, Valabregue R, Poline J (2002) Region of interest analysis using an SPM toolbox. NeuroImage 16(2):CD-ROM, abstr 497.
- 66.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 67.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.