Significance
Familial dysautonomia (FD) is a devastating developmental peripheral autonomic and sensory neuropathy caused by a mutation in the gene inhibitor of kappa B kinase complex-associated protein (IKBKAP). It is marked by tachycardia, blood pressure lability, autonomic vomiting “crises,” and decreased pain and temperature sensation. FD is progressive, and affected individuals commonly die during early adulthood. To identify the cellular and molecular mechanisms that cause FD, we generated a mouse model for the disease in which Ikbkap expression is ablated in the neural crest lineage. This study is a mechanistic analysis of the cellular events that go awry in the developing peripheral nervous system in FD and identifies essential functions of IKAP protein in the peripheral nervous system.
Abstract
Familial dysautonomia (FD) is a devastating developmental and progressive peripheral neuropathy caused by a mutation in the gene inhibitor of kappa B kinase complex-associated protein (IKBKAP). To identify the cellular and molecular mechanisms that cause FD, we generated mice in which Ikbkap expression is ablated in the peripheral nervous system and identify the steps in peripheral nervous system development that are Ikbkap-dependent. We show that Ikbkap is not required for trunk neural crest migration or pathfinding, nor for the formation of dorsal root or sympathetic ganglia, or the adrenal medulla. Instead, Ikbkap is essential for the second wave of neurogenesis during which the majority of tropomyosin-related kinase A (TrkA+) nociceptors and thermoreceptors arise. In its absence, approximately half the normal complement of TrkA+ neurons are lost, which we show is partly due to p53-mediated premature differentiation and death of mitotically-active progenitors that express the paired-box gene Pax3 and give rise to the majority of TrkA+ neurons. By the end of sensory development, the number of TrkC neurons is significantly increased, which may result from an increase in Runx3+ cells. Furthermore, our data demonstrate that TrkA+ (but not TrkC+) sensory and sympathetic neurons undergo exacerbated Caspase 3-mediated programmed cell death in the absence of Ikbkap and that this death is not due to a reduction in nerve growth factor synthesis. In summary, these data suggest that FD does not result from a failure in trunk neural crest migration, but rather from a critical function for Ikbkap in TrkA progenitors and TrkA+ neurons.
Hereditary sensory and autonomic neuropathies (HSANs) are a group of five phenotypically diverse but overlapping disorders of the peripheral nervous system (PNS) that result from mutations in 12 distinct genes (1). HSAN type 3, or familial dysautonomia (FD) (also called Riley–Day syndrome), results from an intronic mutation (IVS20 + 6T > C; 99.5% of patients) in a gene called inhibitor of kappa B kinase complex-associated protein or IKBKAP, causing mis-splicing and subsequent tissue-specific reductions in IKAP protein (2, 3). FD is marked by tachycardia, blood pressure lability, autonomic vomiting “crises,” decreased pain and temperature sensation, and commonly death during early adulthood (4). The function of IKAP in the nervous system is unclear, nor is it understood why deletions in this broadly expressed gene primarily devastate the PNS. The earliest pathology study, performed on a 2-y-old child with FD, showed that ∼90% of cells in the dorsal root and sympathetic ganglia (SG) were missing (5). To identify IKAP’s function in the developing PNS, we first need to establish the steps in which it is essential.
The vertebrate PNS derives primarily from the neural crest, a multipotent, heterogeneous cell population that delaminates from the neural tube and migrates throughout the embryo (6). Those neural crest cells that stop laterally to the neural tube give rise to the chain of sensory dorsal root ganglia (DRG), whereas those that migrate further ventrally give rise to the vertebral chain of SG. Within the DRG, neural crest cells generate heterogeneous neuronal subpopulations including nociceptors, thermoreceptors, mechanoreceptors and proprioceptors. With the completion of neural crest migration, multiple steps ensue that are essential for normal PNS development, including proliferation of discrete sets of neuronal progenitor cells that derive from different waves of migrating neural crest cells, neuronal differentiation, axonogenesis, target innervation, and circuit formation. FD could theoretically result from failure in any or several of these key developmental processes.
Insight into the mechanisms that cause FD have been complicated by data that implicate functions for IKAP in both the nucleus and the cytoplasm. In yeast, the IKAP homolog, Elp1, serves as a scaffold protein within the multisubunit Elongator complex that binds RNA polymerase II and facilitates transcription via histone acetylation (7–9). Although studies indicate that Elongator also functions in the cytoplasm to acetylate α-tubulin (10, 11), recent findings suggest that Elongator may regulate tubulin acetylation indirectly through tRNA modification (12–15). Independent of its role in the Elongator complex, cytosolic IKAP has also been shown to regulate actin cytoskeletal organization and cell migration, which has prompted the suggestion that FD results from a failure in neural crest cell migration (16–19). Mice that are completely null for Ikbkap die early in embryogenesis [by embryonic day (E) 10.5] with failure in neurulation and vasculogenesis, precluding their usefulness for analyzing the aspects of PNS development that are most impacted in FD (20, 21). Although a mouse hypomorphic Ikbkap model was recently generated that recapitulates many of the phenotypic hallmarks of FD (22), the data presented here comprise a mechanistic analysis of the molecular and cellular events that go awry during sensory neurogenesis in the absence of Ikbkap.
Results
Generation of a Neural Crest-Specific Mouse Model for FD.
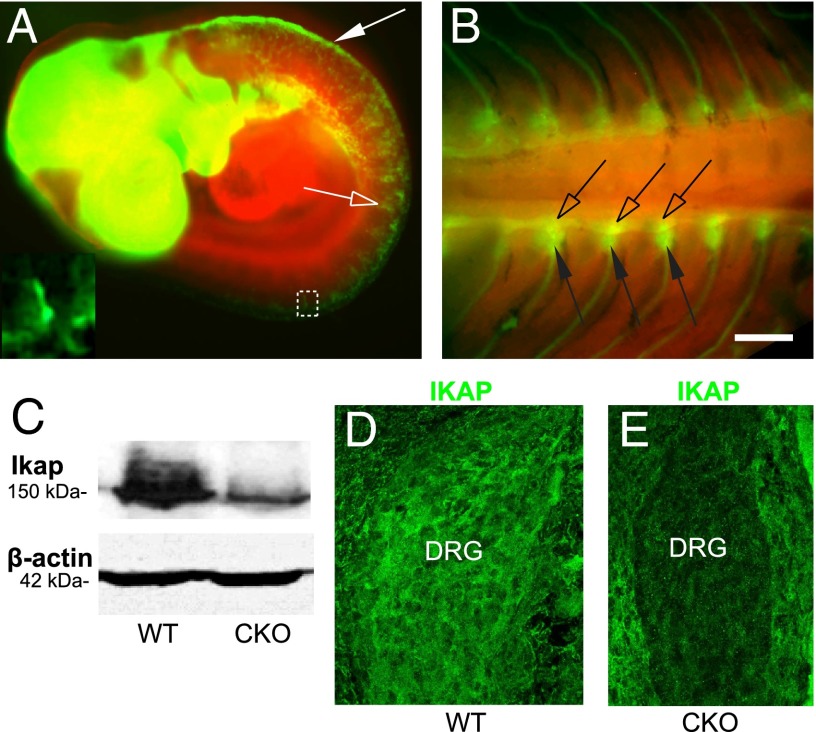
To determine the steps in PNS development that fail in FD, we used a Wnt1-Cre transgene to generate mice in which Ikbkap is selectively deleted in the neural crest lineage and in discrete regions of the CNS (Fig. 1 A and B) (23). Mice homozygous for a floxed allele of Ikbkap were crossed to mice heterozygous for both the floxed Ikbkap allele and for a Wnt1-Cre transgene (Fig. S1). Ikbkap conditional knockout (CKO) embryos, which expressed Wnt1-Cre and were homozygous for the IkbkapLoxP allele (Wnt1-Cre;IkbkapLoxP/LoxP), were generated at the expected 3-to-1 Mendelian ratio (255 expressing Ikbkap to 83 CKO; P > 0.95), as determined by PCR-based genotyping (Fig. S1 and SI Materials and Methods). Decreased expression of IKAP protein in CKO embryos was confirmed via Western blot and immunostaining for IKAP in the DRG (Fig. 1 C–E). Although CKO pups were born alive, they died within 24 h of birth. This early death was not attributable to an inability to suckle as they were often found with milk in their stomachs. Gross analysis of pups at E18.5, 1 d before birth, revealed an overtly normal development, although the Ikbkap CKO pups were smaller than their littermates and had a noticeably altered facial morphology (Fig. S2 A and B). Children with FD can exhibit retrognathism of the mandible and a resulting reduced inferior facial angle (24). Because the Wnt1+ cranial crest orchestrates and contributes to much of the cranial facial morphology (23), we measured the inferior facial angle and the position of the mandible relative to the most anterior point on the face (25) (Fig. S2 C and D). Both of these indices demonstrate that CKO pups exhibit significant retrognathism of the mandible compared with their control littermates.
Fig. 1.
Ikbkap CKO mice. (A and B) ROSAmT-mG; Wnt1-Cre embryos. Red cells convert to green following Cre recombination. (A) E9.5. Cre is active in the dorsal neural tube (arrow) and neural crest cells (open arrow and Inset). (B) E14.5. Cre is active in the SG (open arrows) and DRG (arrows). (C) CKO neural tubes exhibit reduced expression of IKAP, but normal levels of β-actin via Western blot. Because Wnt1 is only expressed in the dorsal neural tube, some residual IKAP is still present in the CKO neural tube. (D and E) E11.5. IKAP protein is not expressed in CKO DRG but is still expressed in surrounding somite. (Scale bar: A, Inset, D, and E, 20 μm; B, 400 μm.)
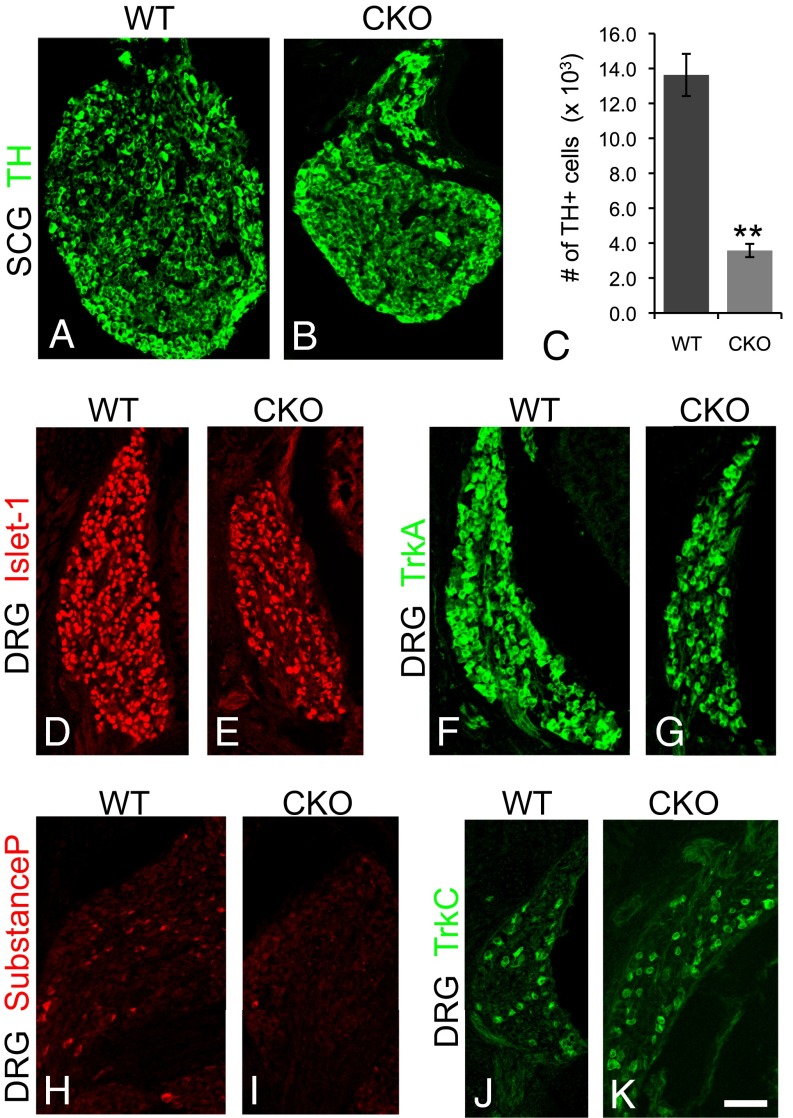
To determine whether ablating Ikbkap in the Wnt1 lineage was sufficient to recapitulate the neuronal hallmarks of FD, we quantified neuronal numbers in the sympathetic and parasympathetic ganglia and DRG using a battery of markers in E17.5 embryos (Fig. 2 and Table S1). We found that the number of tyrosine hydroxylase (TH)+ neurons in the superior cervical ganglion (SCG) was reduced by nearly 70% in Ikbkap CKO embryos compared with controls (Fig. 2 A–C). The total number of DRG neurons in CKO mice was also reduced by one-third compared with controls (Fig. 2 D and E and Table S1). To determine whether programmed cell death contributed to this reduction in neuronal number, we quantified the number of cleaved-Caspase 3+ cells in both the SCG [wild type (WT), 6.8 ± 2.20; CKO, 13.3 ± 3.70; P < 0.001] and DRG (Table S1) at E17.5 and found a significantly increased number of apoptotic cells in both ganglia types in CKO embryos relative to controls.
Fig. 2.
Reduced numbers of sympathetic and DRG neurons in CKO embryos. (A–K) E17.5. Neuron numbers are significantly reduced in the SCG (A–C) and DRG (D and E) (Table S1) in CKO embryos compared with controls. (F–K) TrkA+ and substance P+ DRG neurons are depleted in CKO embryos (G and I, respectively), compared with controls (F and H), whereas the number of TrkC+ DRG neurons is slightly increased in mutant embryos (J and K). (Scale bar: 40 μm.) **P < 0.01.
We next determined whether neuronal deficits in Ikbkap CKO mice were specific to particular subpopulations of DRG neurons, because a hallmark of FD is decreased pain and temperature sensation. Pain- and temperature-receptive neurons express the neurotrophin receptor TrkA, whereas proprioceptors and many mechanoreceptors express TrkC (26, 27). Our data demonstrate that the TrkA subpopulation was reduced by one half at E17.5 (Fig. 2 F and G and Table S1). Nociceptors synthesize and release the neuropeptide substance P and autopsy studies on FD patients show a severe reduction in substance P staining in the spinal cord dorsal horn (28). To further determine the fate of the nociceptive subpopulation of DRG neurons in the absence of Ikbkap, substance P expression was examined and found to be virtually absent in the Ikbkap CKO DRG (Table S1 and Fig. 2 H and I). TH is transiently expressed in the embryonic DRG, possibly by future VGLUT2+ thermal pain receptors, and in adult cutaneous low-threshold mechanoreceptors (29–32). This DRG subpopulation was also nearly nonexistent in the mutant DRG (Fig. S3). In contrast, not only was there no reduction in TrkC numbers, there was actually a small, but significant, increase in this subpopulation (Fig. 2 J and K and Table S1). FD is characterized by a decrease in deep tendon reflexes and a reduction in muscle spindles in FD patients has been reported (33). Because the nervous system progressively degenerates in FD, our data showing that proprioceptors arise and differentiate normally indicate that their eventual demise could potentially be thwarted with the appropriate therapeutics.
The substantia gelatinosa is reduced in FD patient pathology studies (28). Consistent with this phenotype, the central projections of TrkA+ DRG neurons were considerably reduced in the dorsal horn of the spinal cord in mutant embryos (Fig. S4). TrkA+ fibers did innervate the skin in the CKO at E17.5, albeit they were less prevalent than in their control littermates (Fig. S4 C and D). Parasympathetic cell bodies were reduced by 31% in the CKO submandibular gland (SMG) compared with controls (Fig. S5). Interestingly, we discovered that whereas in the control SMG, parasympathetic neuronal cell bodies were heavily innervated by TH+ sympathetic terminals (Fig. S5 A–C and H–J), TH+ axons were rarely present in mutant SMG (Fig. S5 E and F). This absence in TH innervation of the salivary glands may explain the impaired swallowing experienced by children with FD. Together, these data demonstrate that deletion of Ikbkap in the neural crest lineage is sufficient to recapitulate the major hallmarks of FD: severe reduction in DRG sensory neurons that detect pain and temperature and in sympathetic and parasympathetic neurons that are critical for homeostasis.
Ikbkap Is Expressed Throughout Neurogenesis in both Progenitors and Neurons.
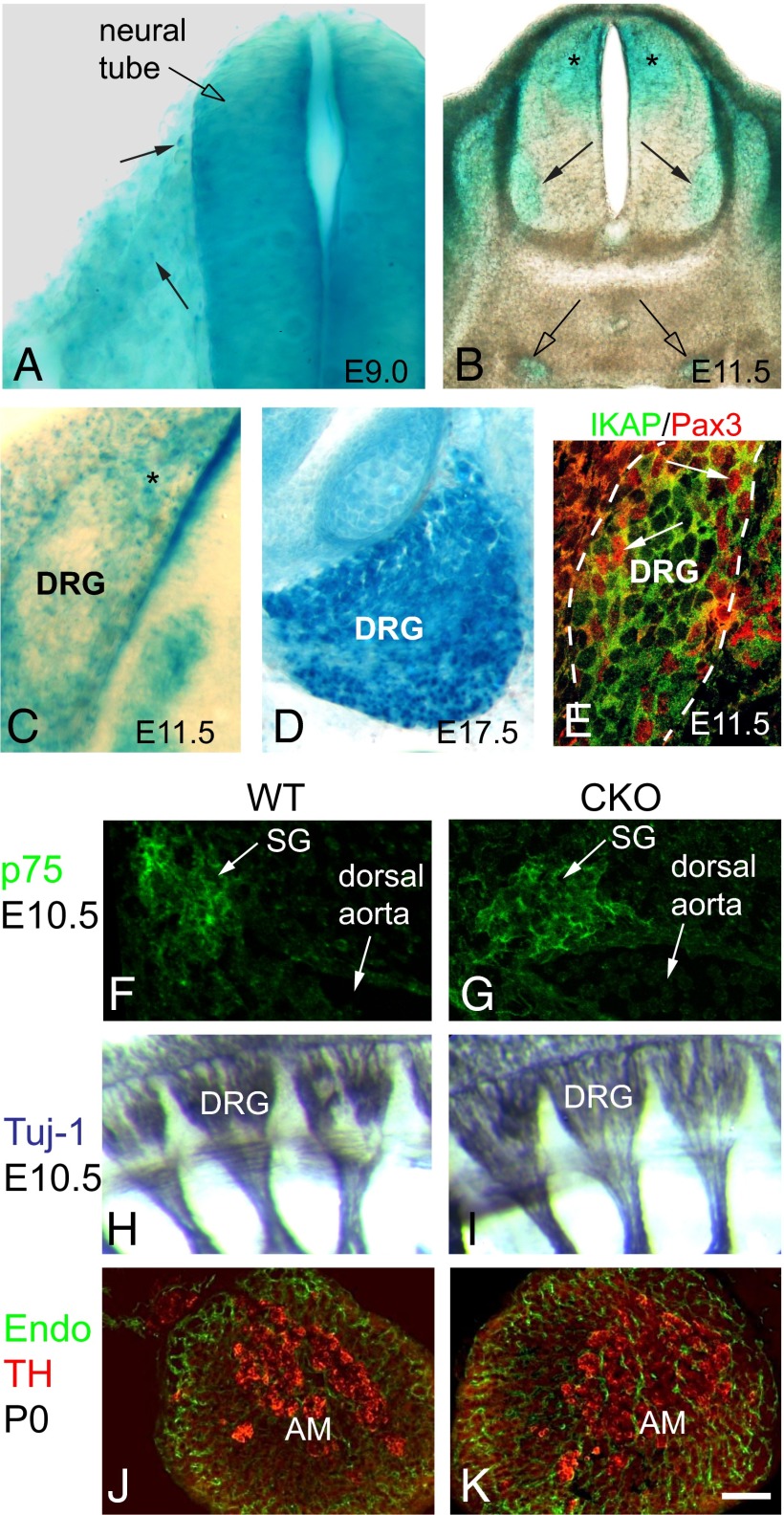
Although apoptosis was elevated in the DRG and SCG of CKO mice just before birth, we sought to determine whether disruption in a putative earlier Ikbkap function might also contribute to the neuronal deficit observed at birth. To gain insight into the developmental events that might require Ikbkap, we used an Ikbkap:LacZ reporter mouse and, here, present a developmental analysis of Ikbkap expression in mammalian tissue (Fig. 3). Although strongly expressed in the neural tube at the earliest ages examined, E8.5 to E9.0 (Fig. 3A), β-galactosidase (β-gal) was not prominently expressed in migrating trunk neural crest cells. By E11.5, the Ikbkap:LacZ cassette was clearly active in sympathetic neurons and DRG, in addition to being expressed in motor neurons, dorsal interneurons, and the dorsal ventricular zone of the spinal cord (Fig. 3B). Neurogenesis in the DRG occurs between E9.5 to E13.5 (34, 35) and comprises two overlapping waves, with the majority of TrkC+ neurons born during the first wave (E9.5 to E10.5) and the majority of TrkA+ neurons born during the second wave (E10.5 to E13.5) (36). During this time frame, the DRG is composed of mitotically active progenitor cells that comprise the DRG dorsal pole and DRG perimeter, with nascent neurons localized in the inner core (37, 38). Ikbkap:LacZ reporter embryos demonstrate that Ikbkap is expressed in both the progenitor and neuronal zones (Fig. 3C) and maintained in neurons through birth (Fig. 3D). To confirm IKAP protein expression in progenitor cells, we also immunostained E11.5 embryonic sections with antibodies to IKAP and to Pax3, a marker for progenitor cells that give rise to TrkA+ neurons (38). IKAP protein was strongly expressed in both Pax3+ progenitors and in postmitotic neurons (Fig. 3E). Thus, deletion of Ikbkap could potentially disrupt the development of either progenitors or postmitotic neurons or both.
Fig. 3.
Ikbkap expression in the developing PNS. (A–D) Ikbkap:LacZ reporter embryos. (A) E9.0. β-Gal is robustly expressed in the neural tube but minimally expressed in migrating NCCs. (B and C) At E11.5, Ikbkap is expressed in the dorsal half of the spinal cord including the ventricular zone (asterisks in B), in motor neurons (arrows in B), in the SG (open arrows in B), and in the DRG. (C) At E11.5, β-gal is expressed in the DRG dorsal pole (asterisk) and in the core neural zone. (D) At E17.5, Ikbkap is expressed in mature neurons. (E) IKAP protein is expressed in both the dorsal pole and perimeter in Pax3+ TrkA progenitors (arrows) and in neurons in the neural core. (F–K) NCC migration and patterning in the CKO is comparable to WT with normal formation of SG (F and G), DRG (H and I), and chromaffin cells in the adrenal medulla (J and K). (Scale bar: A, C–G, J, and K, 30 μm; B, 100 μm; H and I, 75 μm.)
Although we did not observe prominent Ikbkap:LacZ reporter activity in trunk neural crest, several reports have suggested that impaired neural crest cell (NCC) migration could underlie the neuronal phenotype of FD patients (17–19). To determine whether Ikbkap is indeed required at this stage, we examined CKO embryos at E9.5 and E10.5 and found that trunk NCCs migrated along their stereotypical ventral pathways and formed sympathetic ganglia, DRG, and adrenal medulla in their normal locations (Fig. 3 F–K). To verify the location and timing of Cre expression, as well as its activity, we analyzed E9 Wnt-Cre; ROSAmT-mG embryos and saw robust Cre activity in migrating NCCs (Fig. 1A, Inset), indicating that Ikbkap was likely deleted in NCCs in CKO embryos despite their normal behavior. Together, our data demonstrate that IKAP is not required in mice for trunk NCC migration, pathfinding, nor for the cessation of migration to form PNS derivatives in their stereotyped locations, consistent with our previous findings in chick PNS development (39).
Ikbkap Is Required for the Generation of TrkA+ Neurons.
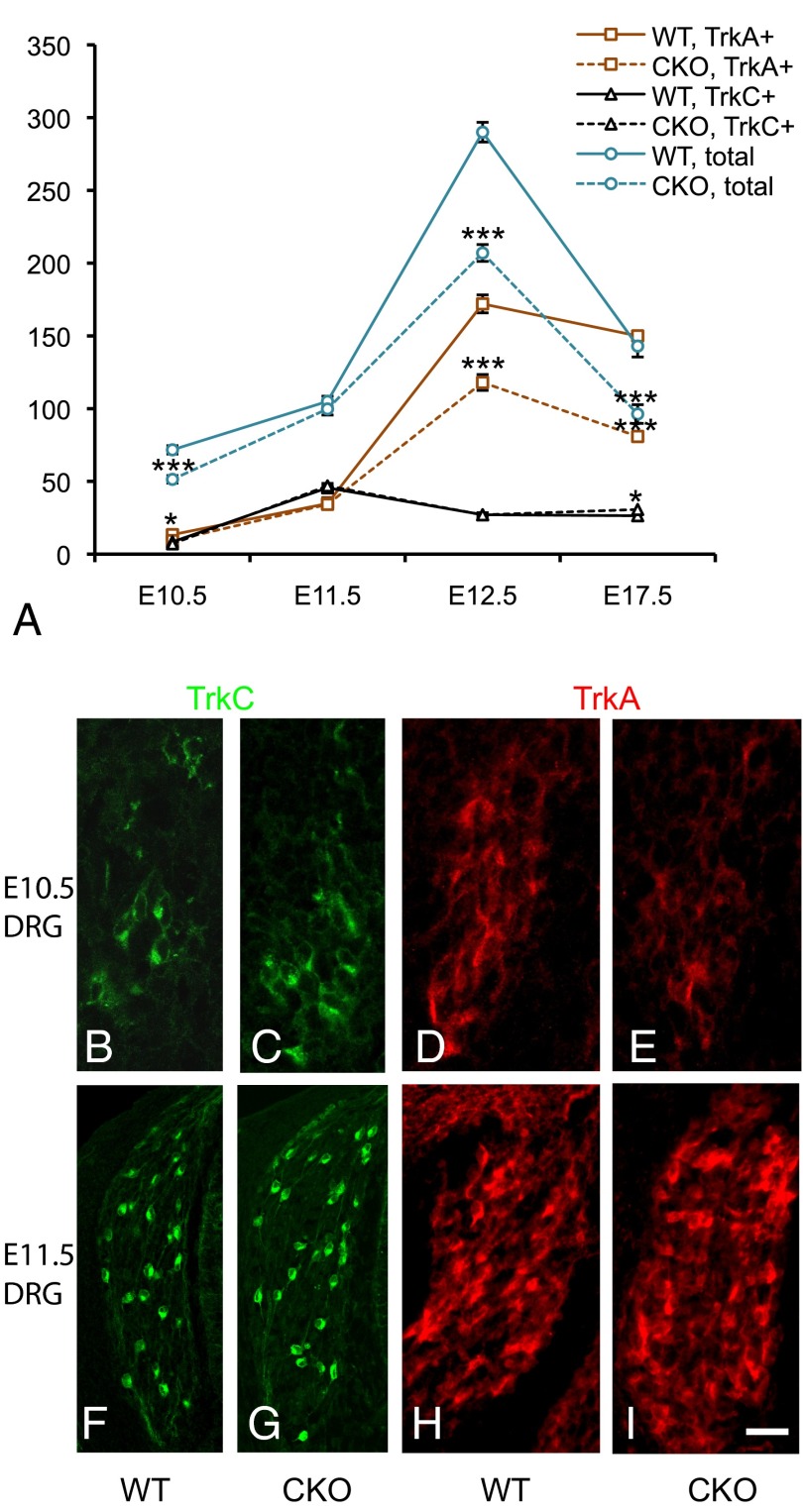
Given our expression analysis demonstrating that Ikbkap is expressed throughout sensory neurogenesis and our finding that DRG neuron numbers were reduced in its absence, we quantified neuronal number through both waves of neurogenesis. At E10.5, there was a significant decrease in the total number of DRG neurons in conjunction with a significant decrease in TrkA+ neurons but not in TrkC+ neurons (Fig. 4 A–E and Table S2). Interestingly, this deficit completely recovered within 24 h such that by E11.5, there was no significant difference between control and CKO DRG in either the total number of neurons or in the TrkA+ number (Fig. 4 A and F–I and Table S2). This recovery is reminiscent of the compensation that occurs in NT-3 knockout mice (35, 40) and is most parsimoniously explained by premature differentiation of TrkA progenitors into TrkA+ neurons. During normal DRG development, peak neuronal numbers are achieved by E12.5 to E13 (35), corresponding to the end of the second wave of neurogenesis (Fig. 4A). However, at E12.5, the DRG of Ikbkap CKO embryos contained 30% fewer total neurons and 30% fewer TrkA+ neurons compared with control DRG (Fig. 4A and Table S2). Together, these findings indicate that generation of TrkC+ neurons during the first wave of neurogenesis does not require Ikbkap because mutant mice have a normal number of TrkC+ neurons from E10.5 to E12.5. The generation of TrkA neurons, however, is critically dependent on Ikbkap because in its absence, the full complement of TrkA+ neurons is never obtained. Exacerbating the final deficit in TrkA numbers is the fact that the extent of apoptosis in CKO embryos far exceeds that in controls.
Fig. 4.
Ikbkap is required for the generation of TrkA+ neurons, but not TrkC neurons, in the DRG. (A–I) Quantification of total neurons, TrkC+ neurons, and TrkA+ neurons from E10.5 to E17.5. (B, C, F, and G) Immunostaining for TrkC at E10.5 (B and C) and E11.5 (F and G) shows no difference between mutant and control. (D, E, H, and I) Although TrkA numbers are reduced in the mutant at E10.5 (D and E), they recover by E11.5 (H and I). (Scale bar: 40 μm.) *P < 0.05; ***P < 0.001.
The transcription factor Runx3 is required for the maintenance of TrkC expression in the DRG (41). Ectopic overexpression of Runx3 causes an increase in the number of TrkC neurons (42), whereas its deletion results in a TrkC+ neuronal deficit (42, 43). Given that there was a slight but significant increase in the number of TrkC+ neurons in Ikbkap CKO embryos at E17.5, we asked whether that increase was preceded by an increase in the number of cells expressing Runx3. In fact, we found a significant increase in the number of Runx3+ cells in the CKO DRG at E11.5 compared with littermate controls (Fig. S6 and Table S2).
Second-Wave Pax3+, TrkA Progenitors Require Ikbkap.
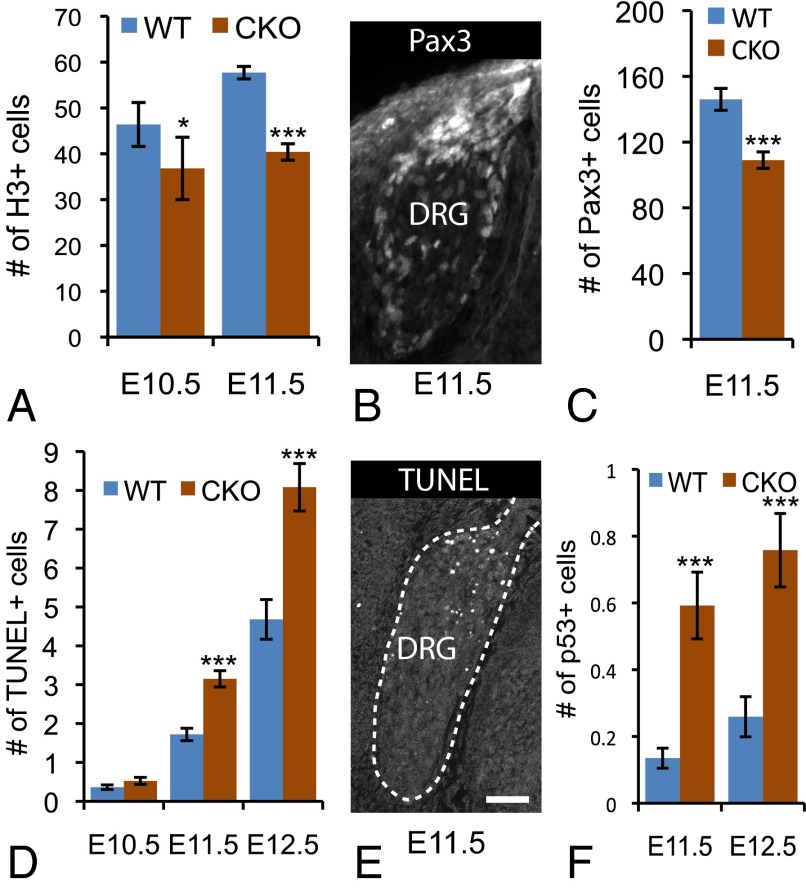
We next sought to determine why Ikbkap DRG fail to generate the normal complement of TrkA+ sensory neurons. Given our finding that Ikbkap is expressed in DRG progenitors (E11.5; Fig. 3 C and E), we tracked the proliferation and death of progenitors throughout neurogenesis (Fig. 5 A–F). At E10.5, we found a small but significant reduction in the number of mitotically active phosphorylated histone H3+ (pH3) progenitors in CKO DRG (P = 0.014) and a 30% decrease at E11.5 relative to controls (P < 0.0001) (Fig. 5A). Furthermore, analysis of the data demonstrates that the 25% increase in the number of cycling progenitors that normally occurs in the DRG between E10.5 and E11.5 does not occur in the absence of Ikbkap. Given that TrkA+ neurons are born last, these data suggest that one function of Ikbkap is to maintain the survival and/or proliferation of second-wave DRG progenitors; in its absence, TrkA progenitors prematurely differentiate between E10.5 and E11.5 to cause an abnormal increase in TrkA numbers at E11.5, followed by a steep decline because of the failure of TrkA progenitors to remain in the cell cycle.
Fig. 5.
Ikbkap is required for the second wave of neurogenesis in the DRG. (A) The number of H3+ progenitors is reduced in CKO embryos at E10.5 and E11.5. (B and C) E11.5. Pax3+ progenitors colonize the dorsal pole and perimeter of the murine DRG (B) and are depleted in Ikbkap CKO embryos (C). (D and E) The number of apoptotic cells is consistently higher in mutant embryos compared with controls (D), with TUNEL+ cells concentrated in the DRG progenitor zones (E). (F) Compared with controls, the number of p53+ cells is also dramatically higher in Ikbkap CKO embryos. (Scale bar: 40 μm.) *P < 0.05; ***P < 0.001.
We previously demonstrated that the majority of TrkA+ sensory afferents in chick derive from a specific subset of second-wave progenitors that express Pax3 and colonize the DRG dorsal pole and perimeter (38). Analysis of Pax3 expression in mouse demonstrates that these same geographically distinct progenitor zones exist in mammals (Fig. 5B). Because it is the TrkA+ pool that is specifically compromised in both FD and in our mouse model, we determined the fate of Pax3+ progenitor cells in Ikbkap CKO embryos. Quantification at E11.5 shows that the number of these progenitors was reduced by 25% in the CKO DRG relative to controls (P < 0.0001; Fig. 5C). To determine whether the reduced number of cycling progenitors and Pax3+ cells indicated a role for Ikbkap in progenitor cell survival, we quantified the number of apoptotic cells throughout neurogenesis in the DRG. Although there was no significant difference between CKO and control embryos at E10.5, by E11.5, there was significantly more programmed cell death in the DRG of Ikbkap CKO embryos compared with littermate controls (Fig. 5D). Our data showing equivalent neuronal numbers in mutant and control embryos at this same time point (E11.5), when the DRG is composed exclusively of mitotically active progenitor cells and of postmitotic neurons (34, 35), suggest that the dying cells were progenitors. In further support of a role for Ikbkap in the survival of neuronal progenitors, the distribution of apoptotic cells in the CKO DRG was concentrated in the dorsal pole and perimeter (Fig. 5E), zones densely colonized by Pax3+ cells (Fig. 5B). Together, these data indicate that Ikbkap is required for survival of the mitotically active progenitor population that generates TrkA+ nociceptors and thermoreceptors during the second wave of neurogenesis in the DRG. In addition, quantification of apoptotic cells at E12.5 and E17.5 demonstrates elevated levels of cell death in the CKO DRG throughout the rest of embryonic development (Table S2).
Nerve growth factor (NGF) is a critical target-derived survival factor for TrkA+ sensory and sympathetic neurons (26, 27, 44). To test whether sympathetic neurons die because of a reduction in NGF synthesis, we compared NGF mRNA levels in two sympathetic targets, the heart and the submandibular glands. These experiments show that rather than being reduced, NGF levels were actually elevated in the Ikbkap CKO mouse (Fig. S7).
We next sought to determine why Ikbkap deletion leads to the apoptosis of mitotically active, Pax3+ progenitors. Because Pax3 is known to destabilize the p53-mediated apoptosis pathway (45, 46), we determined whether p53 levels were altered in CKO embryos. In fact, we found that the number of p53+ cells was increased fourfold in the DRG of CKO versus control embryos from E11.5 to E12.5 (Fig. 5F and Fig. S8). We also found a significant increase in p53+ cells in the SG during this same timeframe (Fig. S8).
Discussion
In summary, these studies indicate that Ikbkap exerts pleiotropic effects in the developing PNS, including a critical function in neurogenesis and neuronal survival. The data reported here demonstrate that although Ikbkap is broadly expressed within the embryo, its ablation in the PNS is sufficient to generate the classic hallmarks of FD: devastation of the sympathetic and sensory nervous systems. These data also establish that this loss is not attributable to abrogation of trunk neural crest migration but, rather, to failure of DRG progenitor cells to generate the full complement of pain and temperature receptors, in addition to premature death of sensory and sympathetic progenitors and neurons. We show here that Ikbkap is expressed in both DRG progenitors, including Pax3+ TrkA progenitors, and in postmitotic neurons. Our data indicate that in the absence of Ikbkap, second-wave progenitors exit the cell cycle prematurely and either differentiate precociously into neurons (including, aberrantly, Runx3+ neurons) or die, leaving fewer progenitors available to generate the complete set of TrkA+ nociceptors and thermoreceptors that would normally be obtained by E13. The fact that Runx3+ cell numbers (a marker of first-wave neurons) increased at the same age when Pax3+ progenitors were reduced (a progenitor of second-wave neurons) implicates the presence of a coordinated feedback system between the two waves of DRG neurogenesis, which was observed in the Ngn1 and Ngn2 knockouts (40).
How IKAP functions to maintain the survival of postmitotic neurons remains to be elucidated. Depletion of IKBKAP activates several proapoptotic p53-mediated genes in colon cancer cells (43), and we did find a significant elevation in p53 expression in the immature DRG and SG of CKO embryos. Given that the key neurons that undergo apoptosis in FD are NGF-dependent, it was of interest to discover that, rather than being reduced, NGF levels were actually elevated in target tissues of CKO mice. Interestingly, HSAN types 4 and 5 result from mutations in the TrkA gene, NTRK1, and NGFβ, respectively. In the absence of Ikbkap, neurons could be dying before, during, or after target innervation; if the latter, this could suggest a requirement for IKAP in target-derived retrograde transport of NGF. Disruption in axonal transport has been observed in mutation of Elp1 in Caenorhabditis elegans (11) and in HSAN type 1 and type 2 (1). We did find fewer TrkA+ axons in target tissue, but additional studies will be required to determine whether this is attributable to the reduction in TrkA+ neuronal cell bodies and/or a requirement for IKAP in target innervation.
Our data also indicate that IKAP is required for proliferation and survival of Pax3+ progenitors. Given that acetylation of Pax3 regulates its ability to activate downstream targets, including Hes1 and Ngn2 (47), IKAP may play a role in Pax3 acetylation, either directly via Elongator-mediated acetylation or indirectly through Elongator-mediated tRNA modification. In support of a direct association between IKAP and Pax3, IKAP contains WD40 domains that have been shown in Gro proteins to interact with Pax and Runx family members (48, 49). Pax3, in turn, also directly associates with p53 and mediates its binding to the ubiquitin ligase Mdm2, triggering its degradation (46). Via this pathway, the genetic ablation of p53 rescues the apoptosis and neural tube defects that characterize Pax3 mutant Splotch embryos (46, 50). Another possible link between p53 and IKAP/Elongator is that p53 activity is also critically dependent on acetylation (51). Thus, multiple pathways point toward a role for IKAP in affecting the posttranslational modifications of one or more of these key proteins.
Although it has been posited that IKAP/Elp1 may be required for the Elongator subunit Elp3 to acetylate tubulin (10), we did not find any alteration in tubulin acetylation in our CKO mice. Pax3 mutations also cause Waardenburg syndrome type 1, which results from a failure in development of the cardiac outflow track and may explain the death of our CKO embryos perinatally. In summary, the Ikbkap CKO mouse model presented here provides an ideal system for identifying the molecular pathways that could be therapeutically targeted to thwart the developmental pathologies and progressive degeneration that marks FD and related HSANs.
Materials and Methods
Facial Morphology Measurements.
The inferior facial angle was defined on a sagittal view (Fig. S2 A and B) by the crossing of two lines: (i) a reference line from the inferior border of the eye to the anterior tip of the nose (solid line in Fig. S2 A and B); and (ii) a line joining the anterior tip of the nose and the anterior border of the chin. The position of the mandible was determined by measuring the distance between two parallel lines along the anterior tip of the nose and the anterior tip of the chin (double ended arrows in Fig. S2 A and B). These lines were drawn perpendicular to the reference line described above (solid line in Fig. S2 A and B).
Mice.
Ikbkap CKO mice were obtained from the International Mouse Consortium. For additional information on mice and other materials and methods, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Sylvia Arber, Louis Reichardt, and Eric Turner for the generous gift of antibodies; Drs. Steven Eiger and Lino Tessarollo for helpful discussion; and Dr. Ed Schmidt for the Rosa-EGFP/tomato mice and guidance. This work was supported by National Institutes of Health (NIH) Grants R01 35714 (to F.L.) and P01 NS041997 (to G.A.C.), NIH National Research Service Award F31 AG031630 (to M.O.), and the Dysautonomia Foundation (F.L.). In memory of Michael Kronick and Barbara Kronick.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Q.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308596110/-/DCSupplemental.
References
- 1.Rotthier A, Baets J, Timmerman V, Janssens K. Mechanisms of disease in hereditary sensory and autonomic neuropathies. Nat Rev Neurol. 2012;8(2):73–85. doi: 10.1038/nrneurol.2011.227. [DOI] [PubMed] [Google Scholar]
- 2.Anderson SL, et al. Familial dysautonomia is caused by mutations of the IKAP gene. Am J Hum Genet. 2001;68(3):753–758. doi: 10.1086/318808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slaugenhaupt SA, et al. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet. 2001;68(3):598–605. doi: 10.1086/318810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelrod FB. Familial dysautonomia. Muscle Nerve. 2004;29(3):352–363. doi: 10.1002/mus.10499. [DOI] [PubMed] [Google Scholar]
- 5.Pearson J, Pytel BA, Grover-Johnson N, Axelrod F, Dancis J. Quantitative studies of dorsal root ganglia and neuropathologic observations on spinal cords in familial dysautonomia. J Neurol Sci. 1978;35(1):77–92. doi: 10.1016/0022-510x(78)90103-x. [DOI] [PubMed] [Google Scholar]
- 6.Le Douarin NM, Kalcheim C. 1999. The Neural Crest (Cambridge Univ Press, New York), 2nd Ed.
- 7.Otero G, et al. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol Cell. 1999;3(1):109–118. doi: 10.1016/s1097-2765(00)80179-3. [DOI] [PubMed] [Google Scholar]
- 8.Winkler GS, Kristjuhan A, Erdjument-Bromage H, Tempst P, Svejstrup JQ. Elongator is a histone H3 and H4 acetyltransferase important for normal histone acetylation levels in vivo. Proc Natl Acad Sci USA. 2002;99(6):3517–3522. doi: 10.1073/pnas.022042899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wittschieben BO, et al. A novel histone acetyltransferase is an integral subunit of elongating RNA polymerase II holoenzyme. Mol Cell. 1999;4(1):123–128. doi: 10.1016/s1097-2765(00)80194-x. [DOI] [PubMed] [Google Scholar]
- 10.Creppe C, et al. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136(3):551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 11.Solinger JA, et al. The Caenorhabditis elegans Elongator complex regulates neuronal alpha-tubulin acetylation. PLoS Genet. 2010;6(1):e1000820. doi: 10.1371/journal.pgen.1000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akella JS, et al. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 2010;467(7312):218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer F, Hermand D. A coordinated codon-dependent regulation of translation by Elongator. Cell Cycle. 2012;11(24):4524–4529. doi: 10.4161/cc.22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci USA. 2010;107(50):21517–21522. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer F, et al. Translational control of cell division by Elongator. Cell Rep. 2012;1(5):424–433. doi: 10.1016/j.celrep.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheishvili D, et al. IKAP/Elp1 involvement in cytoskeleton regulation and implication for familial dysautonomia. Hum Mol Genet. 2011;20(8):1585–1594. doi: 10.1093/hmg/ddr036. [DOI] [PubMed] [Google Scholar]
- 17.Close P, et al. Transcription impairment and cell migration defects in elongator-depleted cells: Implication for familial dysautonomia. Mol Cell. 2006;22(4):521–531. doi: 10.1016/j.molcel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Johansen LD, et al. IKAP localizes to membrane ruffles with filamin A and regulates actin cytoskeleton organization and cell migration. J Cell Sci. 2008;121(Pt 6):854–864. doi: 10.1242/jcs.013722. [DOI] [PubMed] [Google Scholar]
- 19.Naumanen T, Johansen LD, Coffey ET, Kallunki T. Loss-of-function of IKAP/ELP1: Could neuronal migration defect underlie familial dysautonomia? Cell Adhes Migr. 2008;2(4):236–239. doi: 10.4161/cam.2.4.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YT, et al. Loss of mouse Ikbkap, a subunit of elongator, leads to transcriptional deficits and embryonic lethality that can be rescued by human IKBKAP. Mol Cell Biol. 2009;29(3):736–744. doi: 10.1128/MCB.01313-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietrich P, Yue J, e S, Dragatsis I. Deletion of exon 20 of the Familial Dysautonomia gene Ikbkap in mice causes developmental delay, cardiovascular defects, and early embryonic lethality. PLoS ONE. 2011;6(10):e27015. doi: 10.1371/journal.pone.0027015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietrich P, Alli S, Shanmugasundaram R, Dragatsis I. IKAP expression levels modulate disease severity in a mouse model of familial dysautonomia. Hum Mol Genet. 2012;21(23):5078–5090. doi: 10.1093/hmg/dds354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brault V, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128(8):1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 24.Mass E, Brin I, Belostoky L, Maayan C, Gadoth N. A cephalometric evaluation of craniofacial morphology in familial dysautonomia. Cleft Palate Craniofac J. 1998;35(2):120–126. doi: 10.1597/1545-1569_1998_035_0120_aceocm_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 25.Schubert J, Jahn H, Berginski M. Experimental aspects of the pathogenesis of Robin sequence. Cleft Palate Craniofac J. 2005;42(4):372–376. doi: 10.1597/03-166.1. [DOI] [PubMed] [Google Scholar]
- 26.Lindsay RM. Role of neurotrophins and trk receptors in the development and maintenance of sensory neurons: An overview. Philos Trans R Soc Lond B Biol Sci. 1996;351(1338):365–373. doi: 10.1098/rstb.1996.0030. [DOI] [PubMed] [Google Scholar]
- 27.Huang EJ, Reichardt LF. Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearson J, Brandeis L, Cuello AC. Depletion of substance P-containing axons in substantia gelatinosa of patients with diminished pain sensitivity. Nature. 1982;295(5844):61–63. doi: 10.1038/295061a0. [DOI] [PubMed] [Google Scholar]
- 29.Brumovsky P, Villar MJ, Hökfelt T. Tyrosine hydroxylase is expressed in a subpopulation of small dorsal root ganglion neurons in the adult mouse. Exp Neurol. 2006;200(1):153–165. doi: 10.1016/j.expneurol.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Lagerström MC, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68(3):529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindeberg J, et al. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40(2):67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- 32.Li L, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147(7):1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macefield VG, Norcliffe-Kaufmann L, Gutiérrez J, Axelrod FB, Kaufmann H. Can loss of muscle spindle afferents explain the ataxic gait in Riley-Day syndrome? Brain. 2011;134(Pt 11):3198–3208. doi: 10.1093/brain/awr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawson SN, Biscoe TJ. Development of mouse dorsal root ganglia: An autoradiographic and quantitative study. J Neurocytol. 1979;8(3):265–274. doi: 10.1007/BF01236122. [DOI] [PubMed] [Google Scholar]
- 35.Fariñas I, Yoshida CK, Backus C, Reichardt LF. Lack of neurotrophin-3 results in death of spinal sensory neurons and premature differentiation of their precursors. Neuron. 1996;17(6):1065–1078. doi: 10.1016/s0896-6273(00)80240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fariñas I, Wilkinson GA, Backus C, Reichardt LF, Patapoutian A. Characterization of neurotrophin and Trk receptor functions in developing sensory ganglia: Direct NT-3 activation of TrkB neurons in vivo. Neuron. 1998;21(2):325–334. doi: 10.1016/s0896-6273(00)80542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.George L, Chaverra M, Todd V, Lansford R, Lefcort F. Nociceptive sensory neurons derive from contralaterally migrating, fate-restricted neural crest cells. Nat Neurosci. 2007;10(10):1287–1293. doi: 10.1038/nn1962. [DOI] [PubMed] [Google Scholar]
- 38.George L, Kasemeier-Kulesa J, Nelson BR, Koyano-Nakagawa N, Lefcort F. Patterned assembly and neurogenesis in the chick dorsal root ganglion. J Comp Neurol. 2010;518(4):405–422. doi: 10.1002/cne.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunnicutt BJ, Chaverra M, George L, Lefcort F. IKAP/Elp1 is required in vivo for neurogenesis and neuronal survival, but not for neural crest migration. PLoS ONE. 2012;7(2):e32050. doi: 10.1371/journal.pone.0032050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13(13):1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura S, et al. Dynamic regulation of the expression of neurotrophin receptors by Runx3. Development. 2008;135(9):1703–1711. doi: 10.1242/dev.015248. [DOI] [PubMed] [Google Scholar]
- 42.Kramer I, et al. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron. 2006;49(3):379–393. doi: 10.1016/j.neuron.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Levanon D, et al. The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. EMBO J. 2002;21(13):3454–3463. doi: 10.1093/emboj/cdf370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci. 2004;24(3):743–751. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan SC, et al. Cardiac outflow tract septation failure in Pax3-deficient embryos is due to p53-dependent regulation of migrating cardiac neural crest. Mech Dev. 2008;125(9-10):757–767. doi: 10.1016/j.mod.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang XD, Morgan SC, Loeken MR. Pax3 stimulates p53 ubiquitination and degradation independent of transcription. PLoS ONE. 2011;6(12):e29379. doi: 10.1371/journal.pone.0029379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakazaki H, et al. Key basic helix-loop-helix transcription factor genes Hes1 and Ngn2 are regulated by Pax3 during mouse embryonic development. Dev Biol. 2008;316(2):510–523. doi: 10.1016/j.ydbio.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Jennings BH, Ish-Horowicz D. The Groucho/TLE/Grg family of transcriptional co-repressors. Genome Biol. 2008;9(1):205. doi: 10.1186/gb-2008-9-1-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jennings BH, et al. Molecular recognition of transcriptional repressor motifs by the WD domain of the Groucho/TLE corepressor. Mol Cell. 2006;22(5):645–655. doi: 10.1016/j.molcel.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 50.Pani L, Horal M, Loeken MR. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: Implications for Pax-3- dependent development and tumorigenesis. Genes Dev. 2002;16(6):676–680. doi: 10.1101/gad.969302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133(4):612–626. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.