Abstract
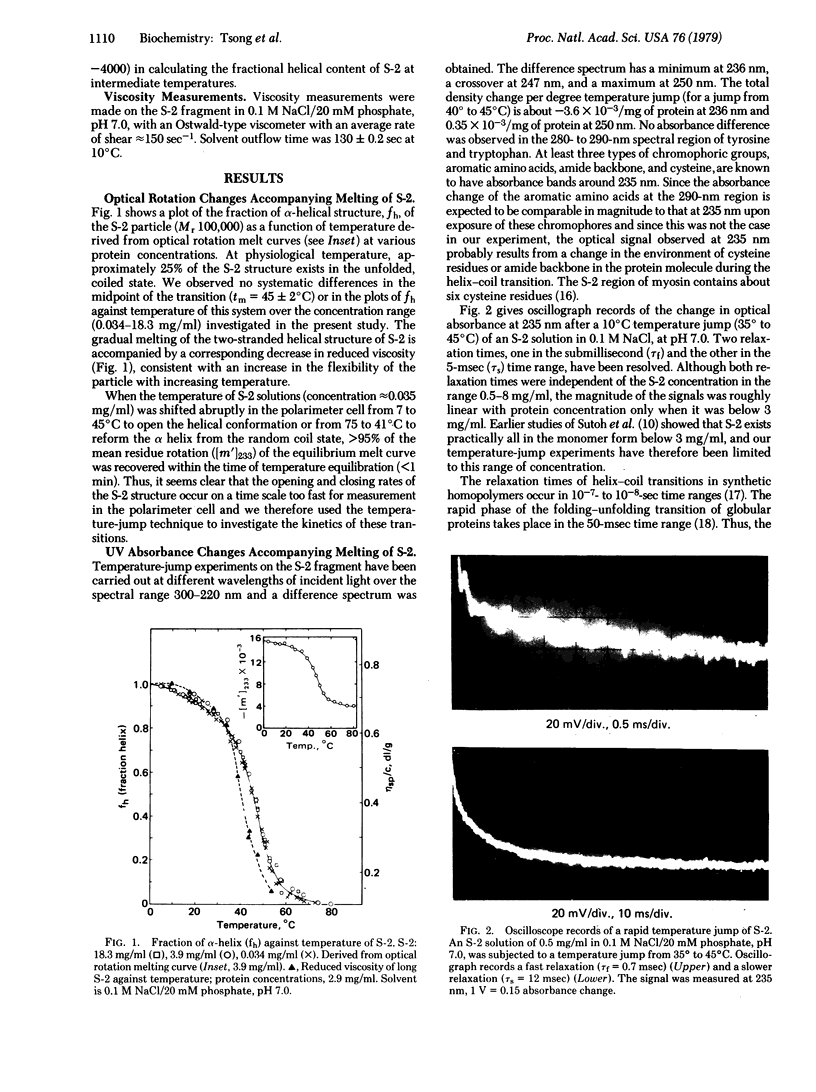
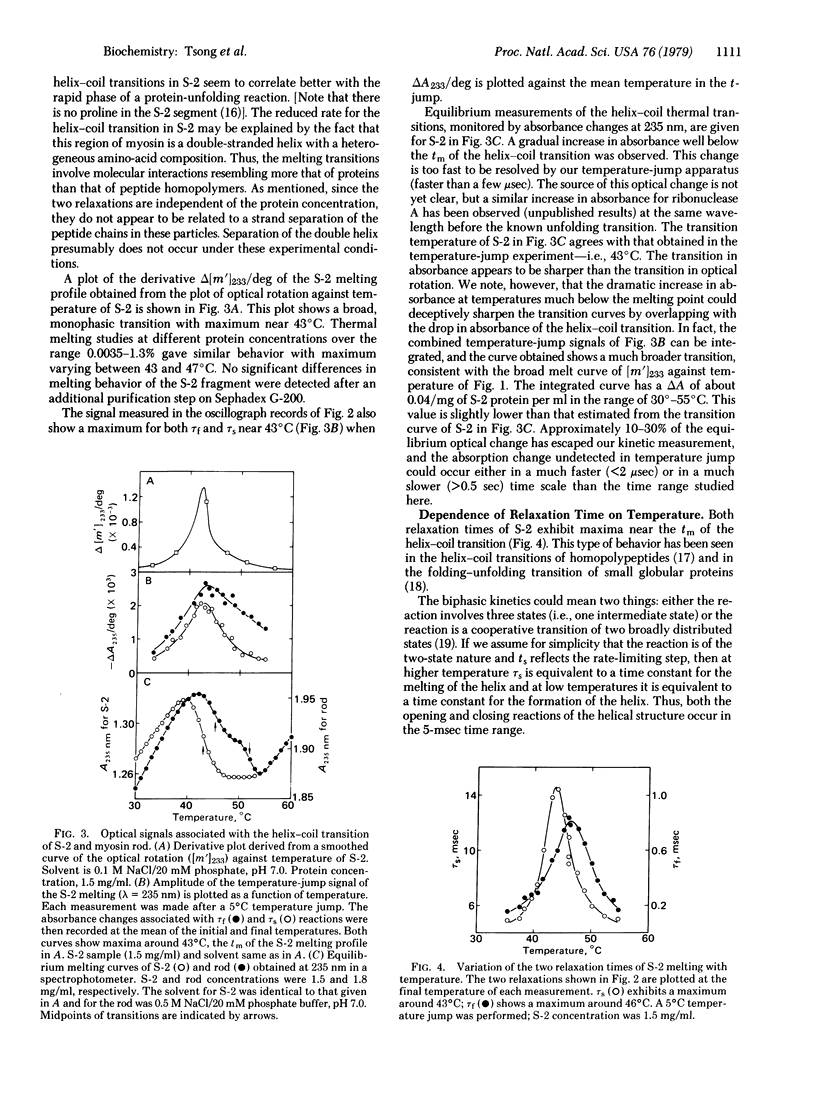
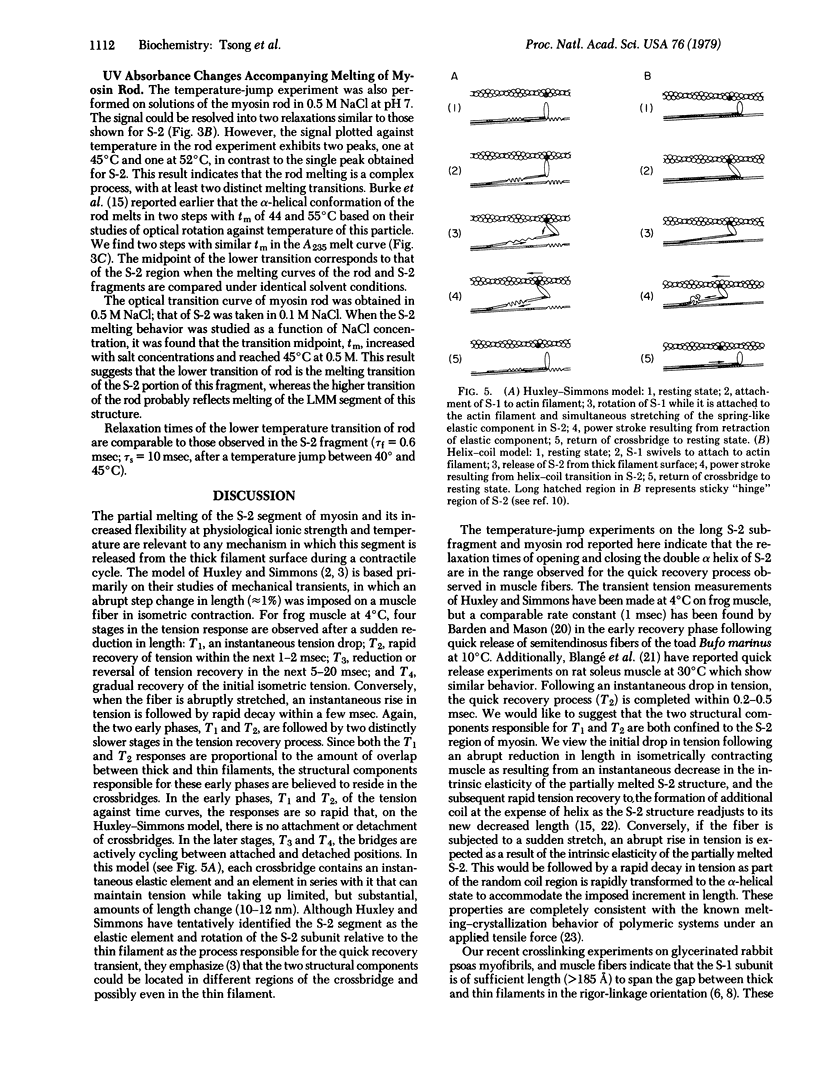
Temperature-jump studies on the long S-2 fragment (100,000 daltons) isolated from myosin show that this structure can undergo alpha-helix--random coil transitions in a time range approximating the cycle time of a crossbridge. Two relaxation times are observed after temperature jumps of 5 degrees C over the range 35--55 degrees C, one in the submillisecond (tau f) and the other in the millisecond (tau s) time ranges. Both processes exhibit maxima near the midpoint of the helix--coil transition (tm = 45 +/- 2 degrees C) as determined by optical rotation melt experiments. Similar results were observed for the low temperature transition (tm = 45 degrees C) of the myosin rod. Viscosity studies reveal that the S-2 particles has significant flexibility at physiological temperature. Results are considered in terms of the Huxley--Simmons and helix--coil transition models for force generation in muscle.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. L. Intermediates in protein folding reactions and the mechanism of protein folding. Annu Rev Biochem. 1975;44:453–475. doi: 10.1146/annurev.bi.44.070175.002321. [DOI] [PubMed] [Google Scholar]
- Barden J. A., Mason P. Muscle crossbridge stroke and activity revealed by optical diffraction. Science. 1978 Mar 17;199(4334):1212–1213. doi: 10.1126/science.415364. [DOI] [PubMed] [Google Scholar]
- Blangé T., Karemaker J. M., Kramer A. E. Elasticity as an expression of cross-bridge activity in rat muscle. Pflugers Arch. 1972;336(4):277–288. doi: 10.1007/BF00586953. [DOI] [PubMed] [Google Scholar]
- Burke M., Himmelfarb S., Harrington W. F. Studies on the "hinge" region of myosin. Biochemistry. 1973 Feb;12(4):701–710. doi: 10.1021/bi00728a020. [DOI] [PubMed] [Google Scholar]
- Flory P. J. Role of Crystallization in Polymers and Proteins. Science. 1956 Jul 13;124(3211):53–60. doi: 10.1126/science.124.3211.53. [DOI] [PubMed] [Google Scholar]
- Harrington W. F. A mechanochemical mechanism for muscle contraction. Proc Natl Acad Sci U S A. 1971 Mar;68(3):685–689. doi: 10.1073/pnas.68.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington W. F., Burke M. Geometry of the myosin dimer in high-salt media. I. Association behavior of rod segments from myosin. Biochemistry. 1972 Apr 11;11(8):1448–1455. doi: 10.1021/bi00758a019. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Kanehisa M. I., Tsong T. Y. Mechanism of the multiphasic kinetics in the folding and unfolding of globular proteins. J Mol Biol. 1978 Sep 5;124(1):177–194. doi: 10.1016/0022-2836(78)90155-9. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Rome E. X-ray diffraction studies of the filament lattice of striated muscle in various bathing media. J Mol Biol. 1968 Oct 28;37(2):331–344. doi: 10.1016/0022-2836(68)90272-6. [DOI] [PubMed] [Google Scholar]
- Schwarz G., Seelig J. Kinetic properties and the electric field effect of the helix-coil transition of poly(gamma-benzyl L-glutamate) determined from dielectric relaxation measurements. Biopolymers. 1968;6(9):1263–1277. doi: 10.1002/bip.1968.360060904. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Chiao Y. C., Harrington W. F. Effect of pH on the cross-bridge arrangement in synthetic myosin filaments. Biochemistry. 1978 Apr 4;17(7):1234–1239. doi: 10.1021/bi00600a016. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Harrington W. F. Cross-linking of myosin thick filaments under activating and rigor conditions. A study of the radial disposition of cross-bridges. Biochemistry. 1977 May 31;16(11):2441–2449. doi: 10.1021/bi00630a020. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Sutoh K., Karr T., Harrington W. F. Isolation and physico-chemical properties of a high molecular weight subfragment-2 of myosin. J Mol Biol. 1978 Nov 25;126(1):1–22. doi: 10.1016/0022-2836(78)90276-0. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Tsong T. T., Kingsley E., Siliciano R. Relaxation phenomena in human erythrocyte suspensions. Biophys J. 1976 Sep;16(9):1091–1104. doi: 10.1016/S0006-3495(76)85757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]




