Significance
This report shows that the reducing power in the environment influences oxidation of glutamate in a neuronal tissue. Glutamate is a neurotransmitter, and it is especially important as a metabolite because it is required for synthesis of glutathione, other amino acids, and proteins. Glutamate also is a key intermediate in glutamine-dependent anaplerosis, now considered to be a principal source of citric acid cycle intermediates in cancer cells. Our analyses also show that the reducing power in the environmental can influence glutamate oxidation in cancer cells.
Abstract
Glutamate in neurons is an important excitatory neurotransmitter, but it also is a key metabolite. We investigated how glutamate in a neural tissue is protected from catabolism. Flux analysis using 13C-labeled fuels revealed that retinas use activities of the malate aspartate shuttle to protect >98% of their glutamate from oxidation in mitochondria. Isolation of glutamate from the oxidative pathway relies on cytosolic NADH/NAD+, which is influenced by extracellular glucose, lactate, and pyruvate.
Glutamate is especially important as a metabolite because it is required for the synthesis of glutathione, other amino acids, and proteins. Glutamate also is a key intermediate in glutamine-dependent anaplerosis, now known to be a principal source of citric acid cycle intermediates in cancer cells (1).
When it is released as a neurotransmitter at brain synapses, glutamate that escapes from the synapse is taken up by astrocytes. There it is converted to glutamine and is delivered back to neurons in a process called the “glutamate/glutamine cycle” (2). Uptake of glutamate and conversion to glutamine within astrocytes stimulates glycolysis and synthesis of lactate. Astrocytes export the lactate to neurons as fuel in a process called the “astrocyte neuron lactate shuttle” (ANLS) (3).
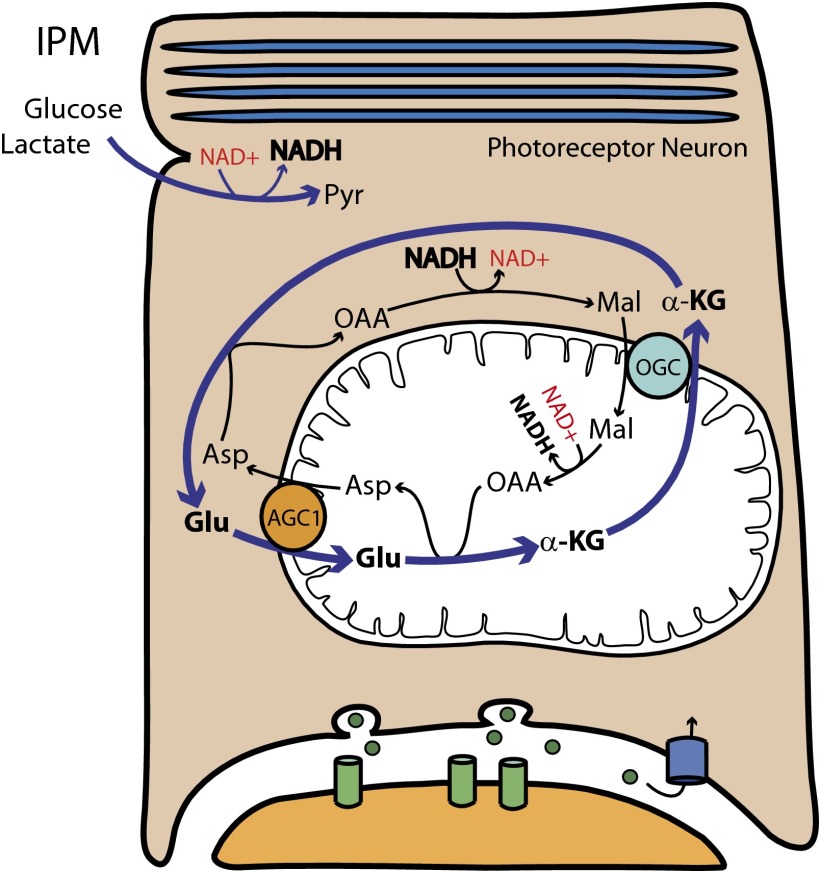
Synaptic terminals of rod and cone photoreceptors have characteristics that appear incompatible with the ANLS. The photoreceptor terminal is enriched with transporters for reuptake of glutamate (4), and it encapsulates the synapse. It is unlikely that much glutamate can escape the synapse before being sequestered back into the photoreceptor. We initiated a study to evaluate the role of ANLS in retina. However, the unusual metabolic features of retina revealed a surprising feature of neuronal metabolism, that >98% of glutamate is protected from catabolism. We investigated this protection and show here that the protection is provided by activities associated with the metabolic pathway known as the “malate aspartate shuttle” (MAS) (shown schematically in Fig. 1).
Fig. 1.
How the malate aspartate shuttle isolates glutamate. The glutamate/α-ketoglutarate cycle in retina isolates the carbon atoms of glutamate from the oxidative pathway inside mitochondria. In this report we demonstrate the influence that cytosolic reducing power and Aralar/AGC1 activity have on this pathway. ASP, aspartate; OAA, oxaloacetate; MAL, malate; GLU, glutamate; AKG, α-ketoglutarate; OGC, oxoglutarate carrier, IPM, interphotoreceptor matrix.
MAS activity regenerates cytosolic NAD+ that is needed to support glycolysis. To do so, it uses two important transporters to trap the reducing power from cytosolic NADH and shuttle it into the mitochondrial matrix. One transporter is the neuronal aspartate/glutamate carrier (AGC1 or Aralar) (Fig. 1, orange circle); the other transporter is the oxoglutarate carrier (OGC) (Fig. 1, light blue circle). AGC1 transports glutamate from the cytoplasm into the mitochondrial matrix in exchange for aspartate from the matrix (Fig. 1). OGC transports α-ketoglutarate from the matrix into the cytoplasm in exchange for malate from the cytoplasm (Fig. 1) (5). An important consequence of MAS activity is that it diverts metabolic flux in mitochondria away from succinyl CoA, succinate, and fumarate (Fig. 1). Most importantly, glutamate that completes a MAS cycle functions as a catalyst for the importation of reducing power into the mitochondria. The carbon atoms of glutamate are isolated from the oxidative pathway in the mitochondrial matrix. To determine the extent of that isolation in a neuronal tissue, we used 13C-labeled fuels to identify metabolic networks in mouse retinas and quantify their metabolic flux.
Results
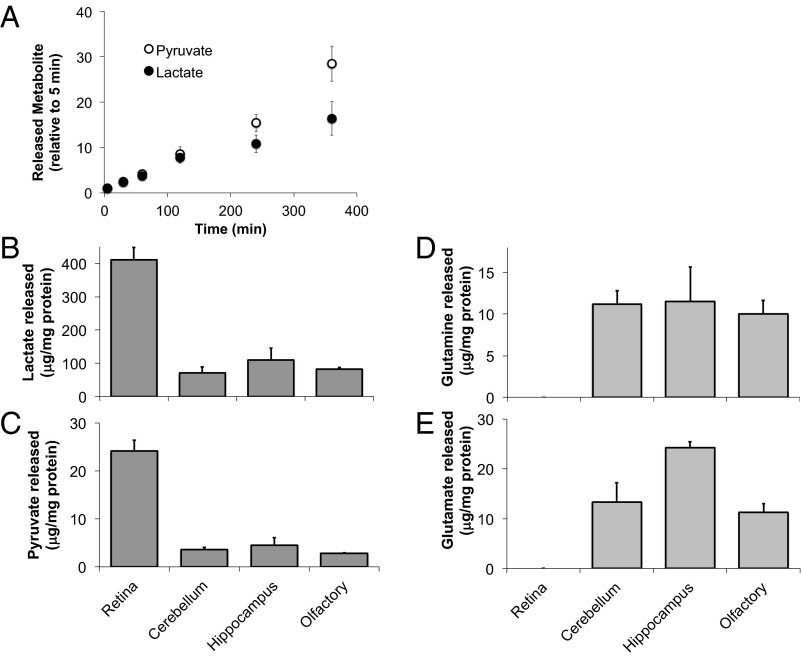
We began by using gas chromatography/mass spectrometry (GC/MS) to analyze metabolites released into the medium from mouse retinas cultured in 5 mM glucose. Fig. 2A shows that lactate and pyruvate accumulate in the culture medium at a ratio of ∼20:1. Release of monocarboxylates from retina is fast, four to five times faster than from brain slices (Fig. 2 B and C). In contrast, retinas release much less glutamate and glutamine than brain slices (Fig. 2 D and E).
Fig. 2.
Mouse retina exports lactate and pyruvate but not glutamate or glutamine. (A) Time-dependent release of pyruvate and lactate into culture medium from retinas incubated with glucose. The ion intensity of pyruvate or lactate at 5 min was used to normalize subsequent time points. (B–E) Retina secreted more lactate and pyruvate but less glutamate and glutamine than brain slices. After incubation with glucose for 2 h, lactate, pyruvate, glutamate, and glutamine in the media of retinas and other neurons were measured by GC/MS. Concentrations of released metabolites were normalized by protein concentration (n = 3.5).
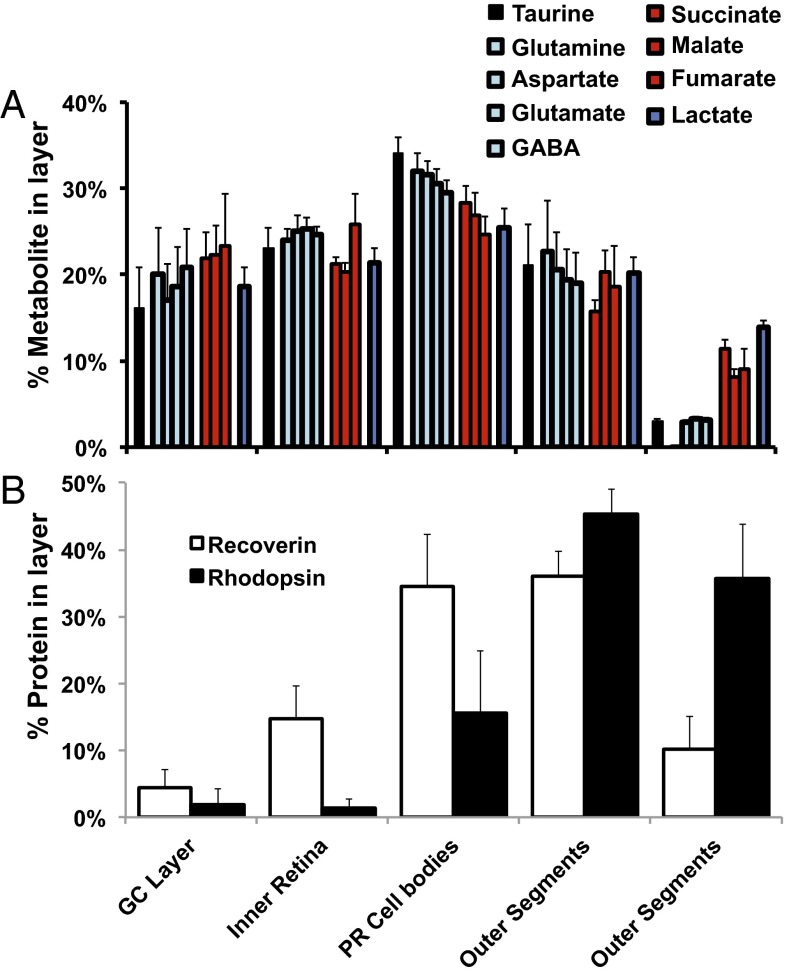
Next we analyzed metabolites within retinas. Retinas were incubated with 5 mM glucose and were washed; then metabolites were extracted. The outer retina is composed mostly of photoreceptors, but metabolites in retinal homogenates also come from other retinal neurons and glia. We estimated the portion of metabolites from the outer retina by serial sectioning followed by GC/MS and found that more than half of the glutamate in the retina comes from the outer retina (Fig. 3).
Fig. 3.
Distribution of metabolites in the retina. Most of the metabolites in the retina are in photoreceptor inner segments and cell bodies. Rat retina sections were analyzed for (A) metabolites by GC/MS and (B) protein by quantitative immunoblots. Results are expressed as percent of total ion intensity for each metabolite in each section (n = 3). Rhodopsin and recoverin were used as landmarks. GC, ganglion cell; PR, photoreceptor.
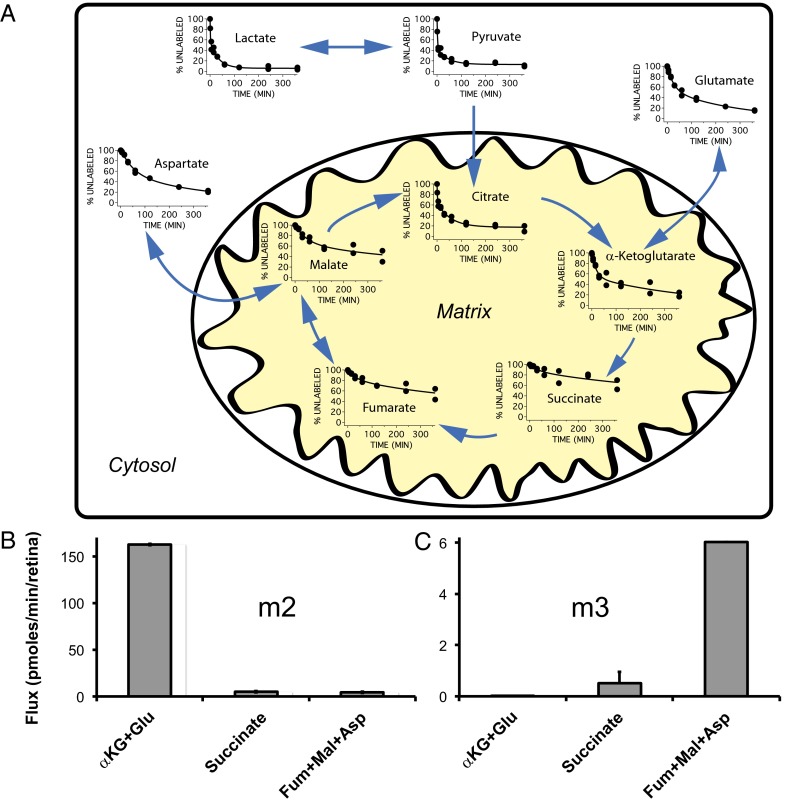
We then used GC/MS to measure the rates at which 13C from uniformly labeled 13C (U-13C) glucose incorporates into retinal metabolites. Fig. 4A shows the displacement of endogenous 12C isotopomers by isotopomers in which two or more 12C atoms are replaced by 13C (6). Glucose is taken up and oxidized by glycolysis so quickly that half the endogenous pyruvate and lactate in the retina are replaced within 5 min.
Fig. 4.
Fluxes through glycolytic and citric acid cycle intermediates labeled with U-13C glucose. (A) Retinas were incubated with 5 mM U-13C glucose, and the amounts of each 12C metabolite not yet replaced by its 13C-labeled counterpart were determined at the indicated times. Continuous curves are double-exponential fits. Blue arrows highlight the predominant direction of each reaction. Data are from two sets of identical experiments. (B and C) Flux measured 1.0 min after 12C-glucose was replaced with U-13C glucose. (B) The m2 isotopomers are derived from the glycolysis, pyruvate dehydrogenase, citrate synthase pathway (Fig. S1, Right). (C) The m3 isotopomers are derived from the glycolysis, pyruvate carboxylase pathway (Fig. S1, Left).
Table 1 summarizes the metabolic fluxes calculated from the rate constants and the total level of each metabolite in the retina. Flux of 13C through citrate and α-ketoglutarate/glutamate is 3–4% of the flux through lactate/pyruvate, as is consistent with previous reports (7, 8). Remarkably, flux of 13C through succinate is nearly 100-fold slower. This result indicates that >98% of α-ketoglutarate in mitochondria is removed from the matrix before it can be oxidized by α-ketoglutarate dehydrogenase. The most likely path for this removal is OGC activity (9–11).
Table 1.
Metabolic fluxes calculated from total metabolites per retina and the rate constants determined from the first 30 min of the data shown in Fig. 4
| Total metabolites per retina, nmol | Rate, min | Flux per retina, nmol/min | |
| Lactate + pyruvate | 22.9 ± 1.1 | 0.26 ± 0.11 | 5.96 ± 2.5 |
| Citrate | 1.1 ± 0.0 | 0.21 ± 0.07 | 0.23 ± 0.08 |
| α-Ketoglutarate + glutamate | 11.1 ± 1.4 | 0.017 ± 0.005 | 0.19 ± 0.06 |
| Succinate | 1.0 ± 0.1 | 0.003 ± 0.003 | 0.003 ± 0.003 |
| Fumarate + malate | 1.3 ± 0.0 | 0.007 ± 0.012 | 0.009 ± 0.015 |
Total metabolites per retina, rate constants, and calculated fluxes are shown. Monocarboxylates were pooled because their rate constants indicate they are in rapid equilibrium. Similarly, glutamate and α-ketoglutarate were pooled, and fumarate and malate were pooled.
The data in Fig. 4A show that aspartate and malate accumulate label faster than succinate and fumarate. A limitation of the type of kinetic analysis shown in Fig. 4A is that it does not distinguish citric acid cycle intermediates labeled by citrate synthase from those labeled by pyruvate carboxylase. To distinguish those pathways, we analyzed the data in more detail. We quantified incorporation of either two 13C atoms (“m2”) via the pyruvate dehydrogenase/citrate synthase route or three 13C atoms (“m3”) via pyruvate carboxylase. The pathways and the patterns of labeling from U-13C–labeled glucose are shown schematically in Fig. S1. The measurements were made after a very short incubation time (1 min) to ensure that much less than a single citric acid cycle could occur. Analysis of the m2 isotopomers (Fig. 4B) showed that the rate of succinate formation is <2% of the rate of glutamate formation, in agreement with the conclusions from the data in Table 1. Analysis of the m3 isotopomers (Fig. 4C) showed that the pyruvate carboxylase pathway is ∼25 times slower than the pyruvate dehydrogenase pathway.
Retinas also consume fuels other than glucose. When glutamine is the only fuel in the culture medium, it is effective in raising levels of α-ketoglutarate, succinate, fumarate, and malate in retinas (blue bars in Fig. 5A). However, when glucose is included with the glutamine, those retinal metabolites decrease, and glutamate increases (green bars). This effect of glucose occurs even when U-13C–labeled glutamine is used as fuel. Fig. S2 shows that incorporation of 13C from labeled glutamine into mitochondrial metabolites is suppressed by the presence of unlabeled glucose. Glucose decreases the incorporation of 13C atoms from glutamine into the citric acid cycle in both the “clockwise” direction (succinate, fumarate, malate) and in the “counterclockwise” direction [citrate via reversal of the isocitrate dehydrogenase reaction (12–14)]. The unexpected effect of glucose led us to hypothesize that cytosolic reducing power, which is generated from glucose by glycolysis, has a dominant influence on retinal metabolism.
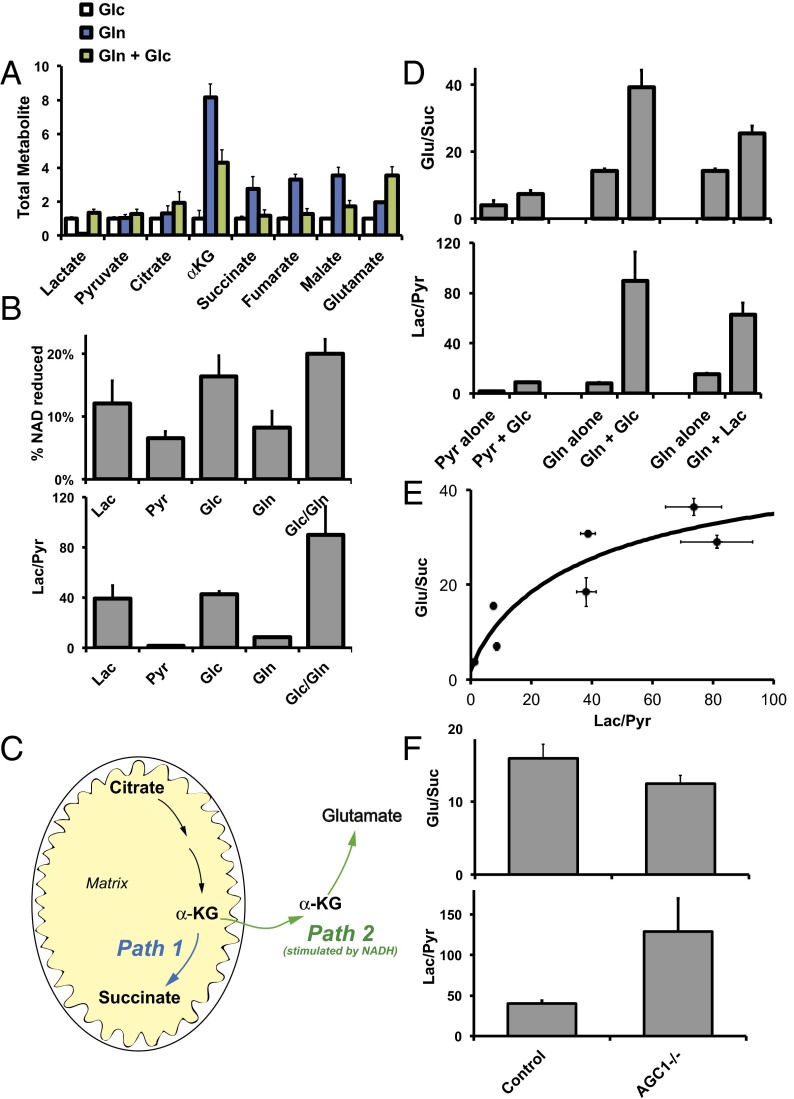
Fig. 5.
Cytosolic reducing power influences the distribution of citric acid cycle intermediates and amino acids. (A) Retinas were incubated for 2 h with glucose alone, with glutamine alone, or with a mix of glucose and glutamine (5 mM each). Metabolites were extracted and quantified by GC/MS. Glutamine alone caused several-fold increases of α-ketoglutarate, succinate, fumarate, and malate. Addition of glucose counteracted this effect of glutamine. Remarkably, glucose and glutamine together also caused a nearly fourfold increase in the total amount of glutamate in the retina (n = 3). (B) Different types of fuel or fuel combinations affect NADH levels and lactate/pyruvate ratios similarly. Each fuel was used at 5 mM (n = 4–6). (C) Schematic of two competing pathways from α ketoglutarate (α-KG). Path 1 produces succinate and occurs independently of NADH. Path 2 produces glutamate and is stimulated by NADH. (D) Glutamate/succinate correlates with the lactate/pyruvate ratio. The reducing power of glucose and lactate increases both lactate/pyruvate and glutamate/succinate ratios (n = 3). (E) Summary of the relationship between the lactate/pyruvate ratio and the glutamate/succinate ratio. Data are taken from the set of experiments shown in D. (F) Disruption of AGC1 activity in Aralar/AGC1−/− retinas disrupts the relationship between the glutamate/succinate and lactate/pyruvate ratios. In the absence of AGC1 activity NADH accumulates in the cytoplasm. The elevated NADH level drives up the lactate/pyruvate ratio. In striking contrast to the results summarized in E, the substantially elevated lactate/pyruvate ratio in AGC1−/− retinas does not increase the glutamate/succinate ratio. In fact, the glutamate/succinate ratio decreases slightly. The effects of AGC1 inactivation on levels of other metabolites are shown in Fig. S4B. Aralar/AGC1−/− retinas have normal expression of photoreceptor and mitochondrial proteins (Fig. S7).
We tested this hypothesis directly by incubating retinas with mixtures of glucose, glutamine, pyruvate, or lactate. Glucose can reduce cytosolic NAD+ to NADH by fueling glycolysis. Lactate can reduce cytosolic NAD+ to NADH via lactate dehydrogenase activity. Glutamine has no effect on cytosolic reducing power, whereas pyruvate oxidizes NADH to NAD+ via lactate dehydrogenase. We confirmed these predictions by measuring total NADH and NAD+ in retinas incubated with various fuel combinations (Fig. 5B, Upper). We expect that cytosolic NADH and NAD+ equilibrate rapidly with intracellular lactate and pyruvate. Therefore the lactate/pyruvate ratio (Fig. 5B, Lower) more accurately reflects the average relative cytosolic NADH/NAD ratio under the various incubation conditions.
This influence of fuels on cytosolic NADH/NAD+ allowed us to investigate relationships between cytosolic reducing power, amino acids, and citric acid cycle intermediates. We focused on the glutamate/succinate ratio because it reflects the relative activities of two competing metabolic paths (Fig. 5C). Path 1 is oxidation of α-ketoglutarate to succinyl CoA followed by conversion to succinate. Path 2 is removal of α-ketoglutarate from mitochondria by OGC followed by conversion to glutamate. Path 2 is part of the MAS, and it relies on reducing power from NADH.
We predicted that high cytosolic reducing power would favor path 2 over path 1, thereby increasing glutamate/succinate. To test this prediction, we incubated retinas in pyruvate with or without glucose and evaluated the ratios of glutamate/succinate and lactate/pyruvate. We also used glutamine with or without glucose and glutamine with or without lactate. When glucose or lactate was present, lactate/pyruvate increased, indicating higher cytosolic NADH/NAD+. Fig. 5 D and E shows a direct relationship between glutamate/succinate and lactate/pyruvate. Fig. S3 shows the effects on the absolute levels of metabolites. We conclude from these data that high reducing power in the cytosol protects glutamate. This protection is especially important for neurons, because glutamate is a neurotransmitter and is an essential precursor for the synthesis of glutathione and proteins.
To confirm this metabolic relationship between glutamate and cytosolic reducing power, we examined the consequence of disrupting it. OGC-mediated exchange of mitochondrial α-ketoglutarate for cytosolic malate requires synthesis of cytosolic malate from cytosolic oxaloacetate. Cytosolic oxaloacetate can be generated by transport of matrix aspartate to the cytosol via Aralar/AGC1 followed by transamination (Fig. S4A). We disrupted this pathway by using retinas from Aralar/AGC1−/− mice.
Aralar/AGC1 deficiency blocks a major path for NADH oxidation in the cytoplasm, so we expected cytosolic NADH to accumulate and drive the reduction of pyruvate to lactate. Consistent with this notion, we found that Aralar/AGC1 deficiency increases the lactate/pyruvate ratio (Fig. 5F). Consistent with our model, Aralar/AGC1 deficiency also lowers the glutamate/succinate ratio even at very high lactate/pyruvate ratios (Fig. 5F). The effect on other metabolites is summarized in Fig. S4B.
We asked if the metabolic relationship between glutamate and cytosolic reducing power occurs in cells other than the neurons and glia in retinas. We incubated an immortal cancer cell line (HeLa) with a similar set of fuel combinations and analyzed lactate/pyruvate and glutamate/succinate ratios. Fig. S5 suggests that this metabolic relationship may be generalizable to other cells and tissues.
Discussion
We show in this report that an α-ketoglutarate/glutamate circuit in retinas protects glutamate from oxidation. Glutamate exchanges into neuronal mitochondria via Aralar/AGC1, and α-ketoglutarate exchanges out via OGC (Fig. 1). OGC competes with α-ketoglutarate dehydrogenase activity to remove α-ketoglutarate from the matrix before it can be oxidized. Protection of glutamate by OGC activity ensures that glutamate is available for neurotransmission, synthesis of glutathione, and synthesis of other amino acids and proteins.
Our serial section analysis showed that more than half the metabolites that we measure from whole-retina homogenates comes from the photoreceptor layer, consistent with other studies that used a different type of method to map distributions of amino acids in retina (15, 16). However, the experimental approach we used in our study does not distinguish the metabolites from photoreceptors from the metabolites from other neurons and from glia. The metabolic pathways we analyzed are not identical in all cells in the retina because AGC1 is present only in neurons and not in Müller cells (11). Nevertheless, we find from whole retina that only 1–2% of the carbon atoms that flow through the α-ketoglutarate/glutamate pool undergo oxidation to succinate. This result is consistent with the ubiquitous presence of OGC. OGC in neurons removes α-ketoglutarate from the matrix before it can be oxidized so that it can be used as a neurotransmitter. OGC activity in Müller cells protects the α-ketoglutarate/glutamate pool to ensure it can be used for glutamine synthesis, one of the primary functions of glia. OGC-mediated protection does not require AGC activity. It requires only that there be sufficient cytosolic malate to drive the exchange. Because Müller cells lack AGC1, the source of carbon atoms for cytosolic malate may be aspartate from neurons (17). Further studies are underway to confirm this possibility in retina.
The measurements we report here were made on isolated retinas in culture so that we could control their fuel supply. It is important to ask whether the metabolic relationships we identified also occur in vivo. To answer this question, we compared effects of AGC1 inactivation from our in vitro experiments with the corresponding effects under in vivo conditions. We compared metabolite levels from our study in which retinas were incubated in culture with metabolite levels reported in a previous study that used freshly isolated brain tissue (17). The effects of AGC1 inactivation were nearly identical under in vitro and in vivo conditions (Fig. S6). Another study showed that glutamate levels are reduced by 29–48% in brains from AGC1−/− mice (18). Glutamine and GABA levels also are reduced substantially. These changes are consistent with our findings, but glutathione levels increase slightly in the study by Llorente-Folch et al. (18). The ratio of reduced to oxidized glutathione decreases by half in AGC1−/− brains, suggesting that an additional response to AGC1 inactivation might stimulate glutathione synthesis.
Metabolic dysregulation can be a significant factor in diseases that cause retinal degeneration and loss of vision (19). We show here that the extent to which glutamate is protected depends on the cytosolic reducing power, which in turn depends on extracellular lactate, pyruvate, and glucose. Consistent with this result, a recent study used a fluorescent NADH sensor to show that extracellular lactate and pyruvate directly and rapidly influence cytosolic NADH/NAD+ (20). When retinas consume glucose, 80–96% of the glucose is converted to lactate (8, 21). Lactate is released from retinas into the interphotoreceptor matrix (IPM) that bathes the retina and the neighboring retinal pigment epithelium (22). Our findings suggest that the lactate/pyruvate ratio in the IPM can influence retinal metabolism, function, and viability.
Taken together, these results show that reducing power in the environment influences glutamate levels in a neuronal tissue. Our preliminary analysis of HeLa cells shows that this influence of extracellular fuels may extend to other types of cells and tissues. For example, metabolic adaptations that lead cancer cells to prefer glutamine as a fuel (23) also may be influenced by the metabolic relationships we have described in this report.
Methods
Reagents.
U-13C glucose was obtained from Cambridge Isotope Laboratories, Inc. Other 13C tracers and reagents were from Sigma unless otherwise specified.
Animals.
All animal experiments were conducted in accordance with the guidelines of the University of Washington Animal Care and Use Committee after Institutional Animal Care and Use Committee approval and with procedures approved in the Directive 86/609/EEC of the European Union and the Ethics Committee of the Universidad Autónoma de Madrid. C57BL mice (6–8 wk old) were purchased from The Jackson Laboratory. Aralar/AGC1+/− mice (24) were crossed to produce Aralar/AGC1−/− and Aralar/AGC1+/+ control littermates in the laboratory of J.S.
Retina and Brain Tissue Culture.
Mice were killed by cervical dislocation. Eyes were removed, and retinas were separated from retinal pigment epithelium in cold HBSS. All experiments in this report were performed under standard room illumination. For brain tissues, after decapitation, the whole brain was removed quickly from the skull and was placed on wet filter paper with HBSS. The cerebellum (consisting of the crus 1 ansiform, crus 2 ansiform, and paramedian lobules), hippocampus, and olfactory bulb were isolated. The retina and other brain tissue were cultured in Krebs–Ringer bicarbonate medium (25) with 5 mM glucose or 5 mM other 13C tracers in a 37 °C, 5.0% CO2 incubator.
GC/MS Analysis of Metabolites.
A single mouse retina was rinsed in cold 0.9% sodium chloride and snap frozen in liquid nitrogen. The retina was homogenized in 120 μL of a 700:200:50 cold mixture of methanol/chloroform/water with 0.1 mM methylsuccinate as an internal standard and was placed on ice for 15 min. The extracts were centrifuged at 16,000 × g for 15 min, and the supernatant was transferred into glass inserts. The extracts were dried under vacuum, derivatized with 25 μL of freshly prepared methylhydroxylamine HCl in pyridine (20 mg/mL), and incubated at 37 °C for 90 min. The extracts were further derivatized with 25 μL of N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide at 70 °C for 30 min.
The medium metabolites were analyzed by drying 20 μL of medium and following the procedure in the retina samples except for reduction of the derivatization volume to 20 μL total. The rat section samples were homogenized in the mixture of methanol/chloroform/water and were derivatized in 10 μL of methylhydroxylamine HCl in pyridine and 10 μL of N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide.
An Agilent 7890/5975C GC/MS system (Agilent Technologies) with an Agilent HP-5MS column (30 m × 0.25 mm × 0.25 μm film) column was used for GC separation and analysis of metabolites. Ultra–high-purity helium was the carrier gas at a constant flow rate of 1 mL/min. One microliter of sample was injected in split-less mode by the autosampler. The temperature gradient started at 100 °C with a hold time of 4 min and then increased at a rate of 5 °C/min to 300 °C, where it was held for 5 min. The temperatures were set as follows: inlet 250 °C, transfer line 280 °C, ion source 230 °C, and quadrupole 150 °C. Mass spectra were collected from m/z 50–600 at a rate of 1.4 spectra/s after a 6.5-min solvent delay. The chromatograms were analyzed using Agilent Chemstation software. Abundances of the following ions were extracted: m/z 174–177 for pyruvate, m/z 261–264 for lactate, m/z 432–437 for glutamate, m/z 431–436 for glutamine, m/z 418–422 for aspartate, m/z 287–291 for fumarate, m/z 289–293 for succinate, m/z 346–351 for α-ketoglutarate, m/z 419–423 for malate, m/z 591–597 for citrate, and m/z 260–263 for alanine. The measured distribution of mass isotopomers was corrected the natural abundance of isotopes using the software IsoCor (26, 27) and was defined with standards and verified by mass after each experiment. Labeled metabolite data were expressed using relative ion abundances or as a concentration (in micrograms per milligram) determined using an external calibration curve.
Protein Concentration Assay.
After metabolites were extracted, tissue pellets were dissolved in 200 μL of 0.1 M NaOH overnight at 37 °C. Protein concentration was determined by the BCA assay kit (Thermo Fisher Scientific).
NADH Cycling Assay.
Mouse retinas were extracted and rinsed once in HBSS before incubation in fuels (lactate, pyruvate, glucose, glutamine, and glucose + glutamine) for 1 h. Samples were homogenized in DMSO, lyophilized, and resuspended in the NADP/NADPH extraction buffer from the NADP/NADPH Assay Kit (Abcam). To measure NADH alone, samples were heated at 60 °C for 20 min to degrade NAD+. NADH alone and total NADH/NAD+ were quantified using a cycling assay (28, 29). Measurements were taken every minute for 20 min at 570 nm (25).
Statistical Analysis.
Data are expressed as mean ± SD. Significance of differences between means was determined by unpaired two-tailed t tests or ANOVA with an appropriate post hoc test. A P value <0.05 was considered to be significant.
Supplementary Material
Acknowledgments
We thank Ken Lindsay, Ian Sweet, and Jack Saari for helpful comments and suggestions. This work was supported by Grants EY06641, EY017863, and EY023346 from the National Institutes of Health (to J.B.H.), and by Grants BFU2011-30456-C02-01/BMC from Ministerio de Economía y Competitividad and S2010/BMD-2402 from Coumunidad Autónoma de Madrid (to J.S.). This work also was funded by the Centro de Investigación Biomédica en Red de Enfermedades Raras, an initiative from the Instituto de Salud Carlos III, and an institutional grant from the Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311193110/-/DCSupplemental.
References
- 1.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 3. Pellerin L. Magistretti PJ (2012) Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32(7):1152–1166. [DOI] [PMC free article] [PubMed]
- 4.Hasegawa J, Obara T, Tanaka K, Tachibana M. High-density presynaptic transporters are required for glutamate removal from the first visual synapse. Neuron. 2006;50(1):63–74. doi: 10.1016/j.neuron.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Satrústegui J, et al. Role of aralar, the mitochondrial transporter of aspartate-glutamate, in brain N-acetylaspartate formation and Ca(2+) signaling in neuronal mitochondria. J Neurosci Res. 2007;85(15):3359–3366. doi: 10.1002/jnr.21299. [DOI] [PubMed] [Google Scholar]
- 6.Yuan J, Bennett BD, Rabinowitz JD. Kinetic flux profiling for quantitation of cellular metabolic fluxes. Nat Protoc. 2008;3(8):1328–1340. doi: 10.1038/nprot.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkler BS. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981;77(6):667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkler BS, Starnes CA, Twardy BS, Brault D, Taylor RC. Nuclear magnetic resonance and biochemical measurements of glucose utilization in the cone-dominant ground squirrel retina. Invest Ophthalmol Vis Sci. 2008;49(10):4613–4619. doi: 10.1167/iovs.08-2004. [DOI] [PubMed] [Google Scholar]
- 9.Contreras L, Satrústegui J. Calcium signaling in brain mitochondria: Interplay of malate aspartate NADH shuttle and calcium uniporter/mitochondrial dehydrogenase pathways. J Biol Chem. 2009;284(11):7091–7099. doi: 10.1074/jbc.M808066200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monné M, Miniero DV, Iacobazzi V, Bisaccia F, Fiermonte G. The mitochondrial oxoglutarate carrier: From identification to mechanism. J Bioenerg Biomembr. 2013;45(1-2):1–13. doi: 10.1007/s10863-012-9475-7. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, et al. Energy sources for glutamate neurotransmission in the retina: Absence of the aspartate/glutamate carrier produces reliance on glycolysis in glia. J Neurochem. 2007;101(1):120–131. doi: 10.1111/j.1471-4159.2006.04349.x. [DOI] [PubMed] [Google Scholar]
- 12. Fendt SM, et al. (2013) Reductive glutamine metabolism is a function of the alpha-ketoglutarate to citrate ratio in cells. Nat Commun 4:2236. [DOI] [PMC free article] [PubMed]
- 13.Metallo CM, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481(7381):380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullen AR, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481(7381):385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones BW, et al. Retinal remodeling in the Tg P347L rabbit, a large-eye model of retinal degeneration. J Comp Neurol. 2011;519(14):2713–2733. doi: 10.1002/cne.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marc RE, et al. Neural reprogramming in retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48(7):3364–3371. doi: 10.1167/iovs.07-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pardo B, et al. (2011) Brain glutamine synthesis requires neuronal-born aspartate as amino donor for glial glutamate formation. J Cereb Blood Flow Metab 31(1):90–101. [DOI] [PMC free article] [PubMed]
- 18.Llorente-Folch I, et al. AGC1-malate aspartate shuttle activity is critical for dopamine handling in the nigrostriatal pathway. J Neurochem. 2013;124(3):347–362. doi: 10.1111/jnc.12096. [DOI] [PubMed] [Google Scholar]
- 19.Punzo C, Xiong W, Cepko CL. Loss of daylight vision in retinal degeneration: Are oxidative stress and metabolic dysregulation to blame? J Biol Chem. 2012;287(3):1642–1648. doi: 10.1074/jbc.R111.304428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung YP, Albeck JG, Tantama M, Yellen G. Imaging cytosolic NADH-NAD(+) redox state with a genetically encoded fluorescent biosensor. Cell Metab. 2011;14(4):545–554. doi: 10.1016/j.cmet.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ames A, 3rd, Li YY, Heher EC, Kimble CR. Energy metabolism of rabbit retina as related to function: High cost of Na+ transport. J Neurosci. 1992;12(3):840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler AJ, Southwick RE. Distribution of glucose and lactate in the interphotoreceptor matrix. Ophthalmic Res. 1992;24(4):243–252. doi: 10.1159/000267174. [DOI] [PubMed] [Google Scholar]
- 23.Wise DR, Thompson CB. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jalil MA, et al. Reduced N-acetylaspartate levels in mice lacking aralar, a brain- and muscle-type mitochondrial aspartate-glutamate carrier. J Biol Chem. 2005;280(35):31333–31339. doi: 10.1074/jbc.M505286200. [DOI] [PubMed] [Google Scholar]
- 25.Chertov AO, et al. Roles of glucose in photoreceptor survival. J Biol Chem. 2011;286(40):34700–34711. doi: 10.1074/jbc.M111.279752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millard P, Letisse F, Sokol S, Portais JC. IsoCor: Correcting MS data in isotope labeling experiments. Bioinformatics. 2012;28(9):1294–1296. doi: 10.1093/bioinformatics/bts127. [DOI] [PubMed] [Google Scholar]
- 27.van Winden WA, Wittmann C, Heinzle E, Heijnen JJ. Correcting mass isotopomer distributions for naturally occurring isotopes. Biotechnol Bioeng. 2002;80(4):477–479. doi: 10.1002/bit.10393. [DOI] [PubMed] [Google Scholar]
- 28.Bernofsky C, Swan M. An improved cycling assay for nicotinamide adenine dinucleotide. Anal Biochem. 1973;53(2):452–458. doi: 10.1016/0003-2697(73)90094-8. [DOI] [PubMed] [Google Scholar]
- 29.Giblin FJ, Reddy VN. Pyridine nucleotides in ocular tissues as determined by the cycling assay. Exp Eye Res. 1980;31(5):601–609. doi: 10.1016/s0014-4835(80)80019-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.