Hypoxia and inflammation share an interdependent relationship (1). Many recent publications implicate hypoxia-elicited inflammation, or inflammation during hypoxic conditions in the outcomes of a wide array of human diseases (2). On the one hand, inflammatory disease states are frequently characterized by tissue hypoxia, or stabilization of hypoxia-dependent transcription factors, such as hypoxia-inducible factor (HIF) (3). For example, intestinal inflammation, such as occurs during inflammatory bowel disease, is characterized by the occurrence of severe hypoxia of the mucosal surface, and concomitant stabilization of HIF (4, 5). Stabilization of HIF1A during intestinal inflammation is caused by alterations in metabolic supply and demand ratios, particularly for oxygen, leading to “inflammatory hypoxia” (6). Similarly, lung inflammation, such as occurs during acute lung injury, is associated with metabolic alterations leading to the stabilization of HIF1A (7). On the other hand, disease conditions that are primarily caused by a lack of oxygen are characterized by secondary inflammatory changes. For example, ischemia and reperfusion injury is characterized by inflammatory responses that lead to subsequent organ dysfunction (8). The functional role of hypoxia-signaling and stabilization of HIFs during acute inflammatory or hypoxic disease states has been the focus of many recent studies. Surprisingly, many of these studies show that pharmacologic stabilization of HIF functions in an adaptive manner by increasing ischemia tolerance (9) and controlling excessive inflammation (10). Many of the studies have linked the anti-inflammatory effects of hypoxia-signaling to a transcriptional program that is under the control of HIF, where pharmacologic activators of HIFs provide tissue protection (11, 12). However, a recent study by Scholz et al. provides a different perspective on the mechanism of how hypoxia-signaling can function to dampen tissue inflammation (13). Scholz et al. provide compelling evidence that hydroxylases, which are inhibited by hypoxic conditions, modulate inflammation via key posttranslational modifications in the IL-1β pathway, with important implications for the use of hydroxylase inhibitors for the treatment of inflammatory disorders.
Hydroxylases play a central role for the link between hypoxia and inflammation. Specifically, a set of four different hydroxylases—prolyl-hydroxylases (PHD)-1, PHD2, and PHD3, and the asparagine-hydroxylase factor-inhibiting HIF (FIH)—have been implicated in the posttranslational regulation of hypoxic and inflammatory signaling pathways (2). These four enzymes are known for their functional role in controlling the stability of the α-subunit of HIF. Both FIH and PHDs require oxygen as a cofactor for hydroxylation and if oxygen levels fall, FIH and PHDs are functionally inactive, thereby leading to the stabilization of HIF and subsequent activation of hypoxia-elicited genes. Therefore, hydroxylases, such as FIH and PHDs, play an important functional role as cellular oxygen sensors, by sensing the presence or absence of oxygen and controlling the subsequent stabilization of HIFs (Fig. 1) (2). Importantly, HIF-elicited gene programs have been shown in a wide variety of inflammatory disease models to dampen hypoxia-induced inflammation, for example, through the enhanced production and signaling effects of anti-inflammatory signaling molecules, such as adenosine (6) or netrin-1 (14).
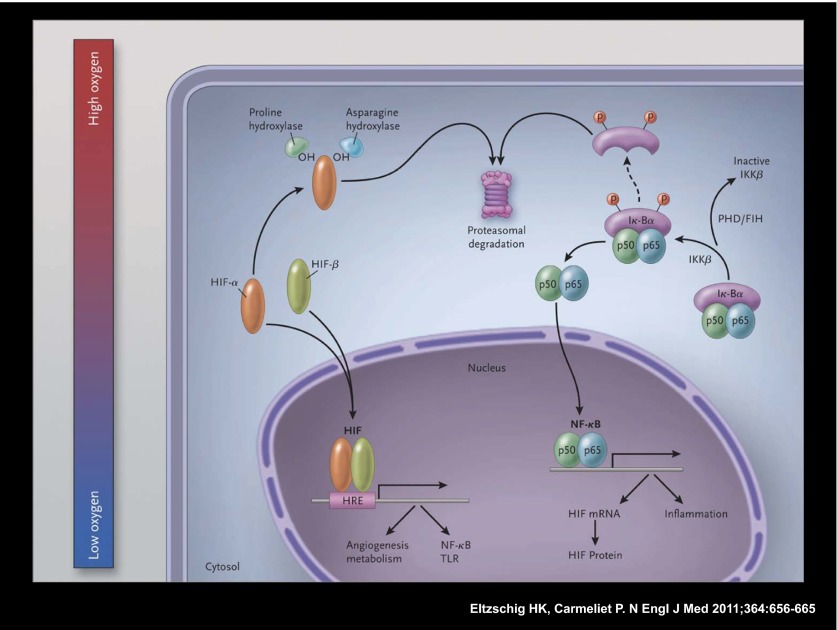
Fig. 1.
Schematic overview of the molecular interaction between the HIF and the NF-κB pathways. In hypoxic conditions (Left), hypoxia-inducible factor (HIF) α and HIF-β subunits translocate to the nucleus, where they bind as heterodimers to a hypoxia response promoter element (HRE), inducing transcription of numerous genes, including those of nuclear factor κB (NF-κB) and toll-like receptors (TLRs). In normoxia, HIF-α is hydroxylated by prolyl-hydroxylases (PHDs) and factor-inhibiting HIF (FIH), and is thereby targeted for proteasomal degradation (in the case of PHDs) or rendered transcriptionally less active (in the case of FIH; not shown here). In resting cells (Right), NF-κB, a heterodimer consisting of p50 and p65 subunits, is inactive in the cytosol because it is associated with nuclear factor of kappa light polypeptide gene enhancer in B cells alpha (IκBα), a regulatory component of NF-κB. At the time of cellular activation, the beta subunit of the IκB kinase complex (IKKβ) phosphorylates the inhibitor IκBα, which thereby becomes degraded and liberates NF-κB for translocation in the nucleus, where it can activate the transcription of inflammatory genes as well as of HIF (genes involved in tissue protection and homeostasis are not shown). PHDs and FIH regulate NF-κB activation by controlling the activity of IKKβ. From Eltzschig and Carmeliet (2). Reprinted with permission from the Massachusetts Medical Society.
However, there are many additional links between hypoxia-signaling and inflammatory gene expression. For example, a recent study contributed to the definition of the relationship between NF-κB and HIF-1 by showing that NF-κB is a critical transcriptional activator of HIF-1 (15). Similarly, previous studies from Taylor’s laboratory have significantly contributed to our understanding of the relationship between hypoxia signaling and the NF-κB pathway. Based on the observation that the transcription factor NFκB is activated in hypoxia, Taylor’s research team demonstrated that hypoxia activates NF-κB through a pathway involving activation of IκB kinase-β (IKKβ), leading to phosphorylation-dependent degradation of IκBα and liberation of NF-κB (16). These findings indicate that hypoxia leads to NF-κB activation through decreased PHD-dependent hydroxylation (resulting in evasion of degradation) of IKKβ (16).
These results are somewhat in contrast to many reports that consistently demonstrate an anti-inflammatory role of hydroxylase inhibitors, such as dimethyloxallyl glycine (DMOG) (7, 10, 17–19). Indeed, in their most recent work, Scholz et al. confirm that hydroxylase inhibitor treatment using DMOG is associated with attenuated IL-1β–induced NF-κB activity in vitro or in vivo (13). In other words, these findings indicate that the activity of hydroxylases is required for IL-β–induced NF-κB activation. To identify the responsible hydroxylase, the authors next performed siRNA-mediated repression of individual hydroxylases, including PHD1 to -3 and FIH. These findings indicated that a combination of PHD1 and FIH knockdown is associated with attenuated IL-1β–induced NF-κB activity (13). Having demonstrated that hydroxylase inhibitors regulate IL-1β–induced NF-κB signaling, the next step was to identify possible substrates for hydroxylation in the IL-1β pathway. For this purpose, Scholz et al. used an unbiased mass spectrometry-based approach to identify proteins that coimmunoprecipitate with individual hydroxylase isoforms. These studies demonstrated that several proteins of the IL-1β signaling pathway form complexes with
Scholz et al. contribute significantly to our understanding of how hydroxylase inhibitors function to dampen excessive inflammation at the interface between hypoxia and inflammation.
either PHD1 or FIH, and subsequent studies provide indirect evidence that several of these proteins are hydroxylated. Taken together, the present studies of Taylor’s research team demonstrate that PHD1 and FIH play a central role in modulating IL-1β–induced NF-κB activity, and several proteins in the IL-1β signaling pathway were found to be associated with hydroxylases. Furthermore, peptides from these (and other) IL-1β signaling proteins are found in the hydroxylated state. As such, these studies shed a different light on the mechanism of how hydroxylase inhibitors can function to dampen inflammation. Importantly, these anti-inflammatory effects of hydroxylase inhibitors are independent of HIF1A and indicate a more global role of PHDs and FIH outside of the HIF pathway. These findings are very timely and important from a translational perspective. Indeed, a recent phase 1 clinical trial successfully used a hydroxylase inhibitor in the treatment of renal anemia (20), indicating that these compounds can be used safely in patients. Moreover, many preclinical studies demonstrate therapeutic effects of hydroxylase inhibitors in pathologic conditions characterized by hypoxia-associated inflammation (e.g., ischemia and reperfusion injury) (9, 17, 19) or during inflammatory diseases characterized by tissue hypoxia or HIF activation (5, 7). As such, the present studies of Scholz et al. (13) contribute significantly to our understanding of how hydroxylase inhibitors function to dampen excessive inflammation at the interface between hypoxia and inflammation.
Acknowledgments
This work is supported by National Institute of Health Grants R01-DK097075, R01-HL0921, R01-DK083385, R01-HL098294, and P0IHL114457-01; a grant by the Crohn’s and Colitis Foundation of America (to H.K.E.); and an American Heart Association grant (to A.G.).
Footnotes
The authors declare no conflict of interest.
See companion article on page 18490.
References
- 1.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol. 2008;586(Pt 17):4055–4059. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor CT, McElwain JC. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology (Bethesda) 2010;25(5):272–279. doi: 10.1152/physiol.00029.2010. [DOI] [PubMed] [Google Scholar]
- 4.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol. 2012;74:153–175. doi: 10.1146/annurev-physiol-020911-153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karhausen J, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114(8):1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367(24):2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckle T, et al. HIF1A reduces acute lung injury by optimizing carbohydrate metabolism in the alveolar epithelium. PLoS Biol. 2013;11(9):e1001665. doi: 10.1371/journal.pbio.1001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eltzschig HK, Eckle T. Ischemia and reperfusion—From mechanism to translation. Nat Med. 2011;17(11):1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckle T, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18(5):774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clambey ET, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA. 2012;109(41):E2784–E2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummins EP, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134(1):156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Robinson A, et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134(1):145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholz CC, et al. Regulation of IL-1β–induced NF-κB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc Natl Acad Sci USA. 2013;110:18490–18495. doi: 10.1073/pnas.1309718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberger P, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10(2):195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 15.Rius J, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453(7196):807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummins EP, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci USA. 2006;103(48):18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart ML, et al. Hypoxia-inducible factor-1α-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol. 2011;186(7):4367–4374. doi: 10.4049/jimmunol.0903617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 2009;136(2):607–618. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 19.Eckle T, Köhler D, Lehmann R, El Kasmi KC, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: A new paradigm for ischemic preconditioning. Circulation. 2008;118(2):166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 20.Bernhardt WM, et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol. 2010;21(12):2151–2156. doi: 10.1681/ASN.2010010116. [DOI] [PMC free article] [PubMed] [Google Scholar]