Significance
Periodontal disease (gum disease) is an extremely prevalent inflammatory disease initiated by persistent bacterial insult, leading to the destruction of bone and gingival tissues. Current clinical treatments focus solely on the removal of bacteria. In this study, we put forth a strategy to address the underlying inflammatory imbalance in periodontal disease by harnessing the body’s own sophisticated immunoregulatory mechanisms through the recruitment of regulatory T cells (Tregs). This is accomplished by controllably releasing small quantities (nanogram/kilogram range) of chemokine recognized by Tregs using biodegradable, resorbable polymers with an excellent track record of regulatory approval. Administration of Treg-recruiting treatments to the gingiva of mice and canines reduces clinical scores of disease as well as hard and soft tissue destruction.
Abstract
The hallmark of periodontal disease is the progressive destruction of gingival soft tissue and alveolar bone, which is initiated by inflammation in response to an invasive and persistent bacterial insult. In recent years, it has become apparent that this tissue destruction is associated with a decrease in local regulatory processes, including a decrease of forkhead box P3-expressing regulatory lymphocytes. Accordingly, we developed a controlled release system capable of generating a steady release of a known chemoattractant for regulatory lymphocytes, C-C motif chemokine ligand 22 (CCL22), composed of a degradable polymer with a proven track record of clinical translation, poly(lactic-co-glycolic) acid. We have previously shown that this sustained presentation of CCL22 from a point source effectively recruits regulatory T cells (Tregs) to the site of injection. Following administration of the Treg-recruiting formulation to the gingivae in murine experimental periodontitis, we observed increases in hallmark Treg-associated anti-inflammatory molecules, a decrease of proinflammatory cytokines, and a marked reduction in alveolar bone resorption. Furthermore, application of the Treg-recruiting formulation (fabricated with human CCL22) in ligature-induced periodontitis in beagle dogs leads to reduced clinical measures of inflammation and less alveolar bone loss under severe inflammatory conditions in the presence of a diverse periodontopathogen milieu.
Periodontitis is characterized by destructive inflammation of the periodontal tissue including the gingiva, periodontal ligament, and alveolar bone, and it is considered the most pressing oral health concern today, affecting more than 78 million individuals in the United States alone (1). Importantly, this disease affects not only tooth loss, but also may impact the incidence of diabetes; cardiovascular, kidney, rheumatologic, and respiratory diseases; and even premature childbirth (2). To date, clinical approaches have been focused on abrogation of invasive bacterial species that trigger local and systemic inflammatory and immune responses (3). Specifically, the current standard of care involves debridement and is sometimes accompanied by local or systemic administration of broad-spectrum antibiotic agents (4). However, given that reestablishment of periodontal lesions is common, patients must repetitively undergo these procedures. In addition, treatment is ineffective in as much as 30% of the population (5, 6).
A large body of literature now suggests that bacterial species (albeit protagonists) are secondary to the host immune response in regard to the etiology of periodontal disease progression (7–9). Specifically, various lymphocyte subsets can accumulate in the periodontium, leading to the local expression of soft tissue-destroying matrix metalloproteinases (10) (MMPs) and receptor activator of NF-κB (RANK) ligand (RANKL) (11) (the primary activation factor for osteoclasts), initiating alveolar bone resorption. Several recent reports have also shown that another lymphocyte subset called regulatory T cells (Tregs) can accumulate in the gingival tissues during periodontal disease in humans and in experimental models (12–15), and helps protect the host from harmful inflammation. However, it appears that, when Tregs are present in insufficient numbers, progression of the disease is accelerated (15).
Accordingly, we sought to develop a strategy for increasing local numbers of regulatory lymphocytes through the recruitment of endogenous Tregs (16) [mimicking a mechanism tumors use to evade immune responses (17)]. Specifically, a natural gradient of a known chemoattractant for regulatory lymphocytes, C-C motif chemokine ligand 22 (CCL22) (16, 18), could be artificially reproduced by using controlled release from a local site. Recently, we developed polymer microspheres capable of steadily releasing CCL22 by using a model-aided design process that specifies the requisite formulation properties (such as porosity) and polymer composition (16). Importantly, this process permits the tuning of release behavior using degradable polymers such as poly(lactic-co-glycolic) acid (PLGA) that are already known to be safe and biocompatible and also exhibit a proven track record of clinical translation (19, 20). This CCL22-releasing formulation has been shown to be effective at recruiting Tregs in vitro and in vivo (16). These recruited Tregs have the potential to influence the local immunological milieu, shifting it toward homeostasis (16). Based on these observations, we hypothesized that this biodegradable, controlled-release formulation of CCL22 administered locally in the periodontium, may recruit Tregs and effectively abrogate periodontal disease symptoms without necessarily reducing local bacterial numbers. Furthermore, the presence of Tregs may actually help to balance the proinflammatory response and generate an environment that is conducive to periodontal tissue regeneration as well as bone regeneration, possibly through expression of IL-10 and osteocalcin (15).
By using Actinobacillus actinomycetemcomitans (Aa)-induced murine and ligature-induced canine models, we demonstrate that the Treg recruiting formulation significantly halts the progression of periodontitis as determined by significant decreases in alveolar bone resorption (mice and dogs), as well as clinical scores of disease progression (dogs). Furthermore, in mice, our Treg recruiting formulation leads to a significant decrease in the production of proinflammatory cytokines in the periodontal tissues (along with an increase in anti-inflammatory cytokines) as well as a decrease in markers of soft and hard tissue destruction (along with an increase in markers of soft and hard tissue regeneration). Overall, the Treg-recruiting formulations described herein may serve as a tool for the study of the role of Treg in periodontal disease, and even suggest a unique treatment modality that intends to harness the body’s own sophisticated immune regulatory mechanisms through the recruitment of cells.
Results
CCL22-Releasing Microparticles Reduce Bone Resorption and Inflammatory Cell Infiltration in Vivo.
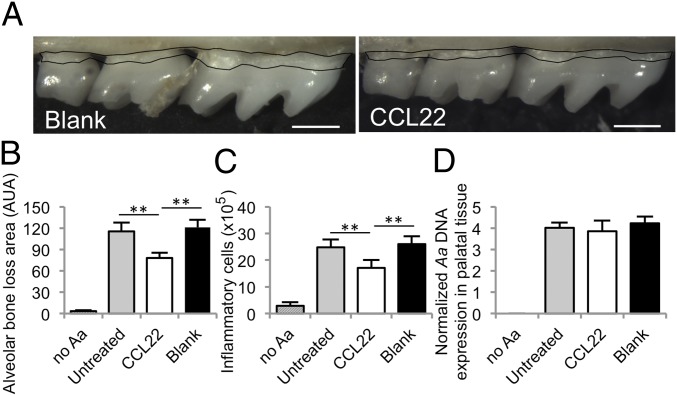
First, we tested the ability of the Treg-recruiting formulation (16) to reduce alveolar bone resorption and decrease inflammatory cell infiltration into the periodontium in an experimental mouse model of periodontal disease. We observed that mice receiving CCL22-releasing microparticles injected into the gingiva [day −1 (before infection) and days 10 and 20 postinfection, based on previous observations that a single treatment was effective in mice for at least 10 d (16)] exhibited less alveolar bone resorption (Fig. 1A) as indicated by area measurements between the cementoenamel junction (CEJ) and the alveolar bone crest (ABC; Fig. 1B). Additionally, we observed a significant decrease in total inflammatory cells within the periodontium of mice receiving the CCL22 formulation (Fig. 1C). Notably, CCL22 microparticle administration did not appear to affect the levels of periodontopathogen Aa (or total bacterial counts; Fig. S1) detected in the resected tissues 30 d after initial colonization (Fig. 1D).
Fig. 1.
The Treg-recruiting formulation significantly diminished inflammatory cell infiltrate and alveolar bone resorption in mice, while not influencing bacterial load. (A) Representative stereoscope images of defleshed maxilla from C57BL/6 mice infected with Aa 30 d after inoculation. Results of treatment with blank (unloaded) PLGA microparticles (Left) and CCL22 microparticles (Right) injected into maxillary gingiva on days −1, 10, and 20 relative to the first Aa inoculation (Scale bar: 0.5 mm.) (B) Area measurement between the CEJ and ABC on the buccal root surface. Untreated mice were infected but did not receive microparticles, and no Aa served as uninfected controls. (C) Inflammatory cell counts in the periodontal tissue following treatments. (D) PCR expression of Aa-specific bacterial 16s ribosomal DNA in the periodontal tissue normalized by palatal tissue weight following treatment (n = 5–8 mice; **P < 0.05 by one-way ANOVA followed by Bonferroni multiple-comparisons test). Untreated, CCL22, and blank groups were statistically different from no Aa infection.
CCL22 Releasing Microparticles Recruit Tregs to the Periodontium.
We hypothesized that the observed reduction in alveolar bone loss was a result of an increased presence of Tregs in the periodontium. To test this hypothesis, expression of canonical Treg markers and associated molecules (mRNA expression) were measured within the palatal gingival tissues 30 d after disease induction. We observed that mice receiving the CCL22-releasing formulation expressed significantly higher mRNA levels of the hallmark Treg markers forkhead box P3 (FOXP3), IL-10, TGF-β, and cytotoxic T lymphocyte antigen 4 (CTLA-4; Fig. 2A and Fig. S2A) compared with untreated or blank (i.e., vehicle-only) controls. Furthermore, CCL22 microparticle treatments led to observable FOXP3+ cell presence in the periodontium as revealed by immunohistochemistry analysis whereas controls produced no such observable staining (Fig. S3 C and D). We did not observe any residual PLGA microparticles (or remnants of particles) in the histological sections 20 d after the last microparticle injection (Fig. S3 C and D), which is to be expected given the relatively low molecular weight distribution of polymer used (9–12 kDa). Quantification of IL-10, CTLA-4, and TGF-β protein levels, extracted from gingival tissue, confirmed the mRNA trends, showing that mice receiving the CCL22-releasing formulation produced higher levels of the anti-inflammatory proteins compared with the untreated and blank controls (Fig. 2B and Fig. S2B). Notably, the experimental administration of microparticles had no significant effect on Th type 2 (Th2)-associated marker IL-4 expression or endogenous CCL22 production within the periodontium (Fig. S2A), suggesting that the local release of CCL22 primarily affected the presence of Tregs as opposed to Th2 cells. Although many activated T cells will express some level of C-C chemokine receptor type 4 (CCR4; the receptor for CCL22), it has been previously shown that CCR4 expression in Tregs is significantly higher than in other T-cell subsets (21), possibly accounting for the preferential recruitment of Tregs seen here and in our earlier studies (16).
Fig. 2.
The Treg-recruiting formulation led to an increased expression of Treg-associated markers and anti-inflammatory cytokines. Periodontal tissues were resected from C57BL/6 mice infected with Aa 30 d after inoculation. Microparticles were injected into maxillary gingiva on days −1, 10, and 20 relative to the first Aa inoculation. CCL22 microparticle-treated mice, blank (unloaded) microparticle, and untreated mice served as infected experimental and control groups, and uninfected no-Aa mice served as positive controls. (A) mRNA expression of Foxp3, IL-10, and TGF-β in periodontal tissue as measured by quantitative PCR. The mRNA expression levels were compared by the value of 2(−ΔCt) − 1, compared with β-actin reference. (B) IL-10 and TGF-β protein levels in periodontal tissues as determined by ELISA from digested periodontal tissues of mice (n = 5 mice; **P < 0.05 by one-way ANOVA followed by Bonferroni multiple-comparisons test). Untreated, CCL22, and blank groups were statistically different from no Aa.
Systemic Inhibition of Treg Reverses the Therapeutic Effects of CCL22 Microparticles.
To confirm that Tregs are indeed responsible for the decreased disease symptoms and anti-inflammatory cytokine expression, endogenous Tregs were systemically inhibited by using anti–glucocorticoid-induced tumor necrosis factor receptor (GITR) antibodies during the onset of periodontitis. Anti-GITR monoclonal antibodies bind to the GITR receptor (which is highly expressed on the surface of Tregs), resulting in reduced Treg functionality in mice (15, 22). Indeed, administration of anti-GITR antibodies along with the application of CCL22-releasing microparticles resulted in dramatically increased levels of alveolar bone destruction and a corresponding statistical increase of inflammatory cells into the periodontal tissues 30 d after disease initiation (Fig. 3 A and B). Furthermore, Treg-impaired mice exhibited a marked decrease in the protein levels of anti-inflammatory cytokines IL-10 and TGF-β found in the periodontal tissues after CCL22 microparticle administration compared with mice with unaltered Treg populations (Fig. 3C). Strikingly, Treg inhibition by using anti-GITR appears to result in complete reversal (and, more so, exacerbated disease symptoms) of the beneficial effects seen with CCL22 microparticles.
Fig. 3.
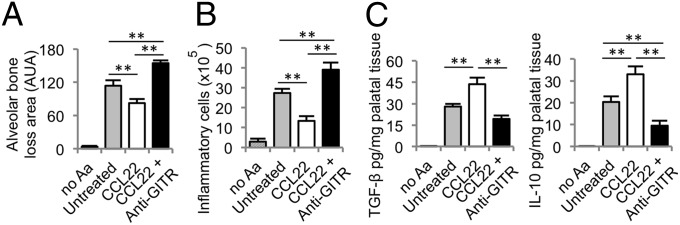
Anti-GITR induced ablation of Tregs increases inflammatory cell infiltrate and bone loss and decreases anti-inflammatory cytokine production. Systemic blockage of Tregs was performed by i.p. injection of anti-GITR antibodies in C57BL/6 mice infected with Aa, and disease indicators were measured after 30 d. CCL22 microparticle-injected mice, CCL22 microparticle plus anti-GITR, and untreated mice served as infected experimental and control groups, and uninfected no-Aa mice served as positive controls. Microparticles were delivered on days −1, 10, and 20 relative to the first Aa inoculation. (A) Alveolar bone loss as determined by the measuring the area between CEJ to the ABC of resected, defleshed maxilla. (B) The number of inflammatory cells in the periodontal tissues was determined after digesting the periodontal tissues. (C) IL-10 and TGF-β protein levels in digested palatal tissues determined by ELISA (n = 5 mice; **P < 0.05 by one-way ANOVA followed by Bonferroni multiple-comparisons test). Untreated, CCL22, and CCL22 plus anti-GITR were statistically different from no Aa.
Administration of the Treg-Recruiting Formulation Alters the Expression of Osteogenic Markers, Cytokines, Chemokines, and Growth Factors.
To explore the underlying molecular mechanisms responsible for disease amelioration after CCL22 microparticle administration, we screened for changes in the expression of cytokines, chemokines, growth factors, and osteogenic markers in the periodontal tissues. Results suggest that administration of CCL22 microparticles is associated with up-regulation of anti-inflammatory cytokine IL-10 and TGF-β (as previously shown in Fig. 2) and a down-regulation of the proinflammatory cytokines IL-1, IL-2, IL-12, IL-17, IFN-γ, and TNF-α compared with untreated infected mice (Fig. 4). In line with evidence of the recruitment of Tregs seen in Fig. 2 and Fig. S3, we observed that the CCR4, the receptor for CCL22 [expressed highly by Tregs (21)], was significantly up-regulated after CCL22 microparticle treatments (Fig. S4).
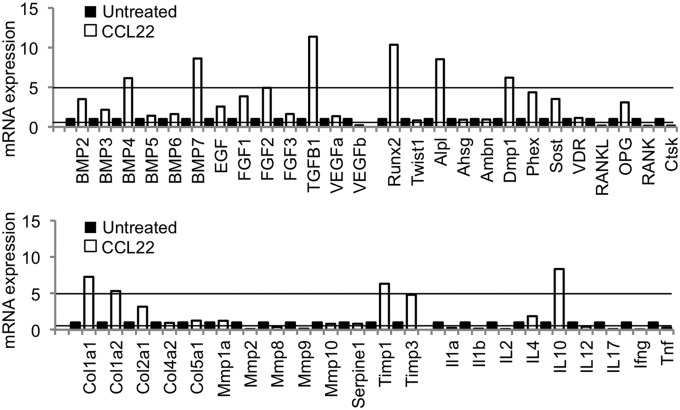
Fig. 4.
The Treg-recruiting formulation enhanced the expression of osteogenic, regenerative, and anti-inflammatory markers in the periodontium. The expression mRNA in the periodontal tissue of mice 30 d after Aa inoculation was analyzed by using a custom-designed, exploratory PCR array. Samples were collected from periodontal tissue of C57BL/6 mice that were infected with Aa without treatment (untreated control, solid bars) and also infected with Aa and injected with CCL22-releasing microparticles (unfilled bars). Microparticles were delivered on days −1, 10, and 20 relative to the first Aa inoculation. The threshold for up-regulation (more than fivefold increase) and down-regulation (less than 0.5 fold decrease) in the CCL22-treated group compared with the control untreated group is indicated with lines.
Interestingly, the CCL22 microparticle formulation not only led to an up-regulation of Treg-recruiting chemokine receptor and associated anti-inflammatory molecules, but also a significant and marked up-regulation of bone growth factors, i.e., bone morphogenic proteins (BMPs) 4 and 7, and TGF-β (Fig. 4). Furthermore, we observed a significant up-regulation of markers of bone formation, including runt-related transcription factor 2 (RUNX2; an important transcription factor for bone forming osteoblasts), alkaline phosphatase, and dentin matrix protein 1 (DMP1), in the CCL22-treated mice (Fig. 4). Correspondingly, we recorded a significant down-regulation of RANK and RANKL, the receptor and ligand important for osteoclastogenesis leading to bone resorption, after CCL22 microparticle administration. In correlation with the down-regulation of RANKL, the expression of RANKL extracellular inhibitor osteoprotegerin (OPG) was moderately up-regulated, although not significantly. Moreover, mice receiving the CCL22 formulation exhibited a significant up-regulation of ECM protein collagen type 1 (COL1A1 and COL1A2) and tissue inhibitors of metalloproteinase (TIMPs) TIMP1 and TIMP3 (Fig. 4). Again, correspondingly, mice receiving the CCL22 formulation showed a down-regulation of ECM-degrading enzymes MMP2, MMP8, and MMP9 compared with untreated control mice 30 d after infection (Fig. 4).
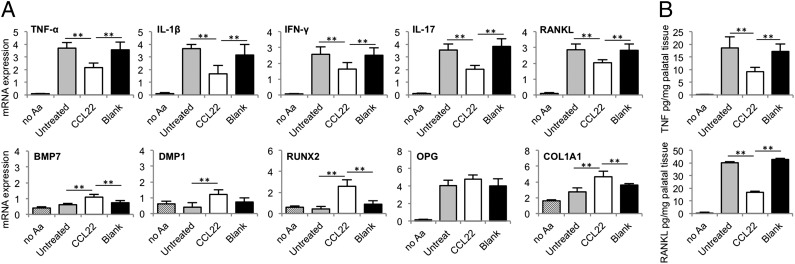
To verify the most important results observed with the exploratory PCR array, we used a traditional quantitative PCR assay that enables the confirmation of statistical significance. Consistent with data obtained from the PCR array, we observed marked decrease in proinflammatory cytokines IL-1, TNF (confirmed at the protein level; Fig. 5B), IFN-γ, and IL-17 in the CCL22 microparticle-treated animals compared with blank or untreated controls (Fig. 5A). Furthermore, CCL22 microparticle administration led to statistical increases in proregenerative factors in ECM protein collagen type 1, osteoblast transcription factor RUNX2, BMP7, and DMP1. Accordingly, a decrease in osteoclast maturing RANKL at the transcription and protein level (Fig. 5) was indeed observed in the CCL22 microparticle-administered mice.
Fig. 5.
The Treg recruiting formulation decreased inflammatory cytokine expression and increased the expression of proregenerative factors. Periodontal tissues were resected from C57BL/6 mice infected with Aa 30 d after inoculation. CCL22 microparticle-injected mice, blank (unloaded) microparticle, and untreated mice served as infected experimental and control groups, and uninfected no-Aa mice served as positive controls. Microparticles were delivered on days −1, 10, and 20 relative to the first Aa inoculation. (A) mRNA expression of TNF-α, IL-1β, IFN-γ, IL-17, RANKL, BMP7, DMP1, RUNX2, COL1A1, and OPG in periodontal tissue was analyzed by quantitative PCR. mRNA expression levels were compared by the value of 2(−ΔCt) − 1 with reference to β-actin. (B) TNF and RANKL protein levels were measured in digested palatal tissues by ELISA (n = 5 mice; **P < 0.05 by one-way ANOVA followed by Bonferroni multiple-comparisons test). Untreated, CCL22, and blank groups were statistically different from no Aa except for the mRNA expression of BMP7, DMP1, and RUNX2.
Administration of the Treg-Recruiting Formulation Diminishes Clinical Severity of Inflammation and Reduces Alveolar Bone Resorption in a Canine Periodontal Disease Model.
To explore the potential for clinical translatability of Treg-recruiting treatments, we used a ligature-induced canine model that tested the ability of the CCL22-releasing formulation to perform under severe inflammatory conditions and a diverse periodontopathogen milieu (23, 24). Specifically, disease was induced in nine beagle dogs (n = 3 dogs per group, left and right mandibles) by placement of ligature at the gingival cervix. The study was terminated 8 wk subsequent to ligature placement, and plaque had manifested on the ligatures and cervical gingiva appeared inflamed in all animals (Fig. 6A). Whereas the mouse experiments relied on CCL22 microparticles encapsulating recombinant mouse CCL22 (16), for canine experiments, we used a formulation that released recombinant human CCL22 (Fig. S5) deposited in the subgingival pocket at time points 0 and 4 wk, which mirrors the duration of human CCL22 release from the treatment (Fig. S5). To assess periodontal health and the degree of inflammation, we measured the gingival pocket probing depth and recorded the bleeding on probing sites at 0, 4, and 8 wk after the ligature placement (Fig. S6). CCL22 microparticle treatments administered during disease induction led to noticeable reduction in probing depths compared with blank and untreated controls at 4 and 8 wk after disease initiation (Fig. S6A). Additionally, bleeding on probing score was lower at 4 and 8 wk in dogs receiving CCL22 microparticles compared with controls (Fig. S6B).
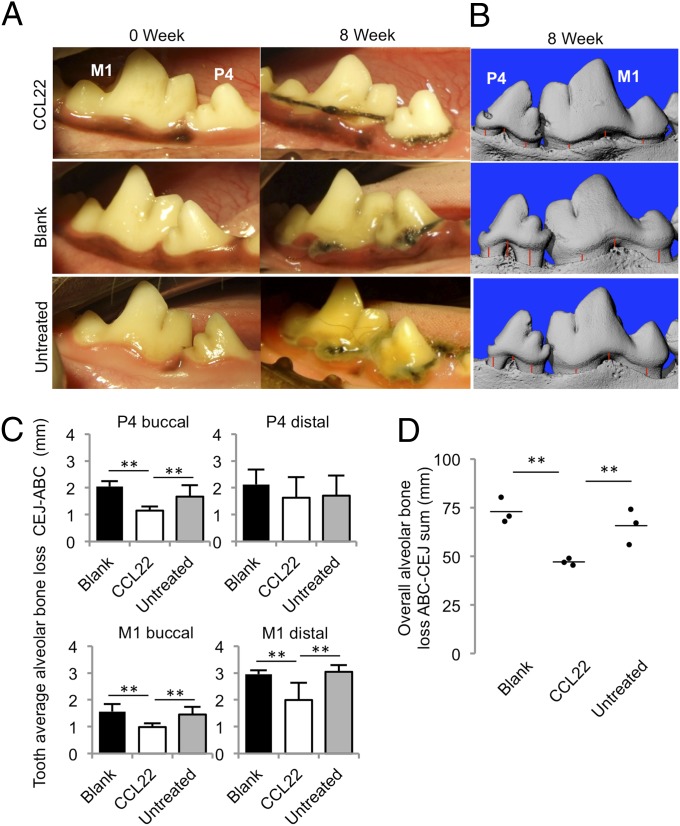
Fig. 6.
Administration of the Treg-recruiting CCL22 formulation diminished the clinical severity of inflammation in gingival tissue in a preclinical canine model of periodontitis. Dogs received scaling and root planing followed by ligature placement (untreated) or by ligature placement and CCL22 microparticle treatment or blank (unloaded) microparticle controls. Microparticles were deposited into the subgingival pocket at 0 and 4 wk after ligature placement. (A) Representative digital pictures as taken on the date of ligature placement (0 wk) and after the 8 wk of treatment (terminal endpoint) of the premolars and carnassial teeth of beagle dogs. (B) Representative 3D images of left mandibular buccal surface of treated and control fourth premolar and carnassial teeth taken postmortem with microCT scans. Red lines illustrate representative CEJ–ABC distances, which were measured by using 2D slices of the tooth as described in Fig. S8. (C) Quantification of the average linear bone loss on the P4 and M1 distal and molar sites, calculated on a per-tooth basis. (D) Quantification of overall alveolar bone loss per dog determined as a summation of linear distances between the ABC and CEJ on the buccal and distal sites of the fourth premolar (P4) and carnassial (first molar, M1; n = 3 dogs per group; **P < 0.05 by ANOVA followed by Tukey honestly significant difference multiple-comparisons posttest).
CCL22 microparticle administration also appears to significantly reduce alveolar bone resorption compared with controls (Fig. 6 B–D). By using X-ray microtomography (i.e., microCT) at the termination of the study 8 wk after ligature placement, we quantified alveolar bone resorption. Substantial bone loss was apparent on all dogs, particularly at the buccal side of fourth premolar and first molar (Fig. 6A and Figs. S7 and S8). Quantifying total alveolar bone loss (on a per-animal basis) as a summation of the linear distance between CEJ and ABC, CCL22-treated dogs revealed significantly reduced bone resorption after 8 wk (Fig. 6D). More specifically, CCL22-treated dogs displayed on average 0.5 to 1.0 mm less CEJ–ABC bone loss than controls at buccal and distal tooth sites (Fig. 6C), akin to the linear reduction in clinical attachment loss reported after clinical periodontal flap surgery (25).
Discussion
As an alternate strategy to current antimicrobial treatments for periodontal disease, we sought to explore the local enrichment of regulatory T lymphocytes (i.e., Tregs) in the periodontal space. Tregs have been known to balance inflammation through a number of mechanisms, including anti-inflammatory cytokine secretion, including, but not limited to, IL-10, TGF-β, and CTLA-4 (26–28); the metabolic disruption of inflammatory cells; perforin-mediated direct killing of inflammatory cells; and inhibiting dendritic cell function (reviewed in ref. 28). Coincidentally, the anti-inflammatory cytokine IL-10 appears to be associated with less aggressive form of periodontitis in humans (29) and in mice (7). Furthermore, the anti-inflammatory cytokine TGF-β has been studied as a therapeutic in context of periodontal healing and bone regeneration (30). Previous attempts to harness Tregs in situ involved the use of adenoviral vectors to induce the expression of CCL22 in murine autoimmune diabetes (18). Here we describe a strategy to recruit Tregs via engineered release of CCL22 protein by using biodegradable and biocompatible polymers with an excellent track record of approval by the Food and Drug Administration. More specifically, we used rationally designed controlled-release systems to sustain presentation of CCL22 chemokine from an injectable point source, which has previously been shown to successfully localize Tregs in vivo (16).
Indeed, we observe that administration of the CCL22-releasing formulation effectively recruits functional Tregs to the periodontium (Fig. 2 and Fig. S3), leading to a net decrease of the local inflammatory response and a reduction in alveolar bone loss in a mouse periodontitis model (Fig. 1). Interestingly, the Treg recruiting formulation is able to achieve this tissue-protective effect without a corresponding increase in bacterial load (Aa or total bacterial levels) in the gingival tissues (Fig. 1 and Fig. S1) or increase in systemic inflammation to the infection (C-reactive protein levels in serum; Fig. S1), whereas traditional blocking of immune responses can impair the host’s protective defenses (31).
It was also apparent that impairing Treg function (via anti-GITR administration) reverses the beneficial effects of the Treg-recruiting formulation (Fig. 3). These results further underscore the importance of Treg in periodontal health. It is also clear from our data (Figs. 2 and 4) that Treg recruitment to the periodontium is accompanied by an increase in IL-10 and TGF-β expression. It is well known that these regulatory cytokines are secreted by Tregs, and are a mode by which Tregs exert a protective effect (28). For instance, these Treg-associated mediators are known to diminish the presence of inflammatory cytokines such as IFN-γ and IL-17 (32, 33). Notably, Th type 1 (Th1) and Th17 cells, which are associated with high levels of IFN-γ and IL-17 expression, have been previously implicated to exacerbate periodontal disease symptoms (31–35). Consistent with the idea that recruited Tregs may regulate this process, our data (Figs. 4 and 5) reveal decreased levels of IFN-γ and IL-17 in treated mice. In addition, Treg secretion of IL-10 and TGF-β are also known to modulate the inflammatory markers TNF (36) and IL1-β (37), which appear to also be down-regulated in CCL22 microparticle-treated mice (Figs. 4 and 5). Most importantly, it is known that decreased levels of inflammatory cytokines such as IFN-γ, IL-17, TNF, and IL-1β are associated with reduced levels of RANKL (also observed in Figs. 4 and 5), with decreasing amounts of this maturation factor previously being correlated with decreased amounts of bone loss (8). Finally, we also observed an increase in CTLA-4 expression in the periodontal space of CCL22 microparticle-treated mice (Fig. S2), suggesting that Tregs recruited to the periodontium may suppress inflammation via a cell–cell contact mechanism, as observed previously (38).
In conjunction with their ability to regulate inflammation, Tregs are also being identified as effective promoters of tissue regeneration (36, 39). For instance, Tregs negatively modulate proinflammatory cytokines responsible for inhibiting various processes during tissue regeneration (36). Accordingly, CCL22 microparticle treatments led to decreased levels of inflammatory tissue destroying factors, and importantly led to the up-regulation of tissue regenerative growth factors (Figs. 4 and 5). We also observed that the C-X-C motif chemokine 12 (CXCL12; also known as stromal cell-derived factor 1) is up-regulated upon CCL22 microparticle treatment (Fig. S4), which may mediate the migration of potentially regenerative stem cells into tissues (40). This result may be particularly important given that the inhibition of CXCL12 or corresponding C-X-C chemokine receptor type 4 was recently reported to delay bone fracture repair (41). Similarly, the parallel up-regulation of the C-X3-C chemokine motif ligand 1 observed after CCL22 microparticle treatment (Fig. S4) also could account for mesenchymal stem cell recruitment (42). However, further studies would need to be performed to evaluate whether stem cells play a role in the effects observed in this study. Regardless, the results described here suggest that recruitment of Tregs may not only halt destructive inflammation but also promote an environment amenable to tissue regeneration.
To confirm the effects of the Treg recruiting formulation in a model that is more representative of human disease, we used a widely accepted canine model, in which disease is established by placement of ligatures followed by a soft diet, encouraging retention of a more diverse microbial insult and, correspondingly, more complex disease progression (43). CCL22 microparticles were administered as a dry powder directly to the periodontal pocket, much in the same way as minocycline-loaded particles are delivered as adjunctive treatment to scaling and root planing in the clinic today (6). Consistent with the results in our mouse model, we observed slower disease progression with reduced clinical measures of probing depth and bleeding on probing in canine subjects treated with CCL22-releasing particles (Fig. S6). Clinical probing depths and bleeding on probing scores are typically sufficient to determine the therapeutic efficacy of potential treatments in large animal models (44). In addition, we also quantified alveolar bone resorption by using microCT image analysis (Fig. 6 and Fig. S8). By using these methods, we observed that alveolar bone loss was significantly reduced in groups treated with CCL22 microparticles (Fig. 6) by as much as 1 mm, even at extremely low doses of active protein (less than 1 µg of CCL22 per kilogram body weight). This amount of bone loss is significant given that current clinical gold standard periodontal flap surgeries appear to halt ∼0.5 mm of clinical attachment level after 1 y (25). As possible limitations of this study, one animal in the CCL22 treatment group exhibited an abnormality (crookedness) of the fourth premolar that likely contributed to the observed statistical variance with the P4 distal sites (Fig. 6C). In addition, another animal in the CCL22 treatment group arrived with a missing premolar as a congenital defect. In this animal, an analysis was performed to ensure that the plaque burden and bone resorption was not reduced at adjacent sites, and, in fact, bone resorption was actually the greatest at this site compared with other animals. Overall, future studies using microCT analysis should prioritize the selection of animals to exclude these sources of variance before initiation of the study.
The treatment of inflammatory and autoimmune diseases through the use of Tregs is an emerging trend with significant potential (45). Indeed, several methods are currently being investigated in preclinical models (18) and clinical trials for autoimmune disease such as type 1 diabetes and transplant rejection (46). However, these current methods involve complicated ex vivo Treg expansion protocols, and have been difficult to replicate in humans (46). Here we describe a method to harness endogenous Tregs and recruit them to specific sites of aberrant inflammation in a stable, dry powder form that can be stored and easily administered in the clinic. We also foresee that these Treg recruiting microparticles may be useful for the in situ expansion of Tregs (47) for a number of other inflammatory and autoimmune diseases in which local reestablishment of immune hyporesponsiveness or inflammatory homeostasis would be beneficial to halting destructive inflammation and even establishing a proregenerative milieu.
Materials and Methods
More detailed descriptions of the experimental procedures are provided in SI Materials and Methods.
Fabrication, Characterization, and Administration of CCL22 Microparticles.
PLGA microparticles containing CCL22 were prepared as described (16). Microparticles were suspended in viscous saline solution and injected into the maxillary periodontal tissue maxilla mice. Dogs received dry microparticles deposited into subgingival pocket of teeth that had ligatures.
Periodontal Disease Induction.
Periodontitis was induced by Aa or Porphyromonas gingivalis bacterial inoculation in mice as described previously (15, 34). Periodontitis was induced in beagle dogs by placing silk ligatures (sutures) at the cervix of the gingiva of mandibular teeth as described previously (44).
Murine Anti-GITR Treatment.
Anti-GITR (DTA-1) hybridomas were purified as described (15) and injected i.p. 15 d after bacterial inoculation in mice.
Assessment of Periodontal Disease.
Murine maxillary alveolar bone loss was quantified as the area between the CEJ and the ABC after defleshing resected tissues as described previously (15). Total RNA was extracted from murine periodontal tissues as described previously (15), and fold change was calculated as 2(−ΔΔCT) − 1. Periodontal probing depths and bleeding upon probing in beagle dogs was assessed at 0, 4, and 8 wk after ligature placement. After 8 wk, canine mandibles were resected, and the alveolar bone loss was determined by using microCT as the linear distance between the CEJ and ABC.
Supplementary Material
Acknowledgments
We thank Bernard Moncla and Kara Pryke (Magee Research Institute, University of Pittsburgh Medical Center) and Mario J. Ávila-Campos (Department of Microbiology, Institute of Biomedical Sciences, São Paulo University) for providing bacteria cultures and Konstantinos Verdelis for guidance on microCT use and image analysis. This work was supported by National Institutes of Health (NIH) Grants 1R01DE021058-01 A1 and 1R56DE021058-01 through the National Institute of Dental and Craniofacial Research (to S.R.L., C.S., and G.P.G.), Wallace H. Coulter Foundation (S.R.L. and C.S.), Camille and Henry Dreyfus Foundation (to S.R.L), Arnold and Mabel Beckman Foundation (S.R.L.), funding from the Commonwealth of Pennsylvania (S.R.L), and NIH Award F31 DE021297-01 (to A.J.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302829110/-/DCSupplemental.
References
- 1.Eke PI, Genco RJ. CDC Periodontal Disease Surveillance Project: Background, objectives, and progress report. J Periodontol. 2007;78(7) Suppl:1366–1371. doi: 10.1902/jop.2007.070134. [DOI] [PubMed] [Google Scholar]
- 2.Pizzo G, Guiglia R, Lo Russo L, Campisi G. Dentistry and internal medicine: From the focal infection theory to the periodontal medicine concept. Eur J Intern Med. 2010;21(6):496–502. doi: 10.1016/j.ejim.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 4.Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy. A systematic review. Ann Periodontol. 2003;8(1):115–181. doi: 10.1902/annals.2003.8.1.115. [DOI] [PubMed] [Google Scholar]
- 5.Varela VM, et al. Systemic antimicrobials adjunctive to a repeated mechanical and antiseptic therapy for aggressive periodontitis: A 6-month randomized controlled trial. J Periodontol. 2011;82(8):1121–1130. doi: 10.1902/jop.2011.100656. [DOI] [PubMed] [Google Scholar]
- 6.Williams RC, et al. Treatment of periodontitis by local administration of minocycline microspheres: A controlled trial. J Periodontol. 2001;72(11):1535–1544. doi: 10.1902/jop.2001.72.11.1535. [DOI] [PubMed] [Google Scholar]
- 7.Garlet GP, et al. Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol. 2006;21(1):12–20. doi: 10.1111/j.1399-302X.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 8.Garlet GP. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89(12):1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 9.Graves DT, Oates T, Garlet GP. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J Oral Microbiol. 2011;3:1–15. doi: 10.3402/jom.v3i0.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birkedal-Hansen H, et al. Matrix metalloproteinases: A review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 11.Taubman MA, Valverde P, Han X, Kawai T. Immune response: The key to bone resorption in periodontal disease. J Periodontol. 2005;76(11) suppl:2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 12.Cardoso CR, et al. Characterization of CD4+CD25+ natural regulatory T cells in the inflammatory infiltrate of human chronic periodontitis. J Leukoc Biol. 2008;84(1):311–318. doi: 10.1189/jlb.0108014. [DOI] [PubMed] [Google Scholar]
- 13.Dutzan N, Gamonal J, Silva A, Sanz M, Vernal R. Over-expression of forkhead box P3 and its association with receptor activator of nuclear factor-kappa B ligand, interleukin (IL) -17, IL-10 and transforming growth factor-beta during the progression of chronic periodontitis. J Clin Periodontol. 2009;36(5):396–403. doi: 10.1111/j.1600-051X.2009.01390.x. [DOI] [PubMed] [Google Scholar]
- 14.Okui T, et al. Characterization of CD4+ FOXP3+ T-cell clones established from chronic inflammatory lesions. Oral Microbiol Immunol. 2008;23(1):49–54. doi: 10.1111/j.1399-302X.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- 15.Garlet GP, et al. Regulatory T cells attenuate experimental periodontitis progression in mice. J Clin Periodontol. 2010;37(7):591–600. doi: 10.1111/j.1600-051X.2010.01586.x. [DOI] [PubMed] [Google Scholar]
- 16.Jhunjhunwala S, et al. Bioinspired controlled release of CCL22 recruits regulatory T cells in vivo. Adv Mater. 2012;24(35):4735–4738. doi: 10.1002/adma.201202513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 18.Montane J, et al. Prevention of murine autoimmune diabetes by CCL22-mediated Treg recruitment to the pancreatic islets. J Clin Invest. 2011;121(8):3024–3028. doi: 10.1172/JCI43048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothstein SN, Federspiel WJ, Little SR. A unified mathematical model for the prediction of controlled release from surface and bulk eroding polymer matrices. Biomaterials. 2009;30(8):1657–1664. doi: 10.1016/j.biomaterials.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothstein SN, Little SR. A “tool box” for rational design of degradable controlled release formulations. J Mater Chem. 2011;21(1):29–39. [Google Scholar]
- 21.Mailloux AW, Young MRI. NK-dependent increases in CCL22 secretion selectively recruits regulatory T cells to the tumor microenvironment. J Immunol. 2009;182(5):2753–2765. doi: 10.4049/jimmunol.0801124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohm AP, Williams JS, Miller SD. Cutting edge: Ligation of the glucocorticoid-induced TNF receptor enhances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J Immunol. 2004;172(8):4686–4690. doi: 10.4049/jimmunol.172.8.4686. [DOI] [PubMed] [Google Scholar]
- 23.Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35(2):89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellegrini G, Seol YJ, Gruber R, Giannobile WV. Pre-clinical models for oral and periodontal reconstructive therapies. J Dent Res. 2009;88(12):1065–1076. doi: 10.1177/0022034509349748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaldahl WB, Kalkwarf KL, Patil KD, Molvar MP, Dyer JK. Long-term evaluation of periodontal therapy: I. Response to 4 therapeutic modalities. J Periodontol. 1996;67(2):93–102. doi: 10.1902/jop.1996.67.2.93. [DOI] [PubMed] [Google Scholar]
- 26.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6(4):353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 27.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat Immunol. 2008;9(3):239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garlet GP, Martins W, Jr, Ferreira BR, Milanezi CM, Silva JS. Patterns of chemokines and chemokine receptors expression in different forms of human periodontal disease. J Periodontal Res. 2003;38(2):210–217. doi: 10.1034/j.1600-0765.2003.02012.x. [DOI] [PubMed] [Google Scholar]
- 30.Wikesjö UM, et al. Periodontal repair in dogs: Effect of recombinant human transforming growth factor-beta1 on guided tissue regeneration. J Clin Periodontol. 1998;25(6):475–481. doi: 10.1111/j.1600-051x.1998.tb02476.x. [DOI] [PubMed] [Google Scholar]
- 31.Garlet GP, et al. The essential role of IFN-gamma in the control of lethal Aggregatibacter actinomycetemcomitans infection in mice. Microbes Infect. 2008;10(5):489–496. doi: 10.1016/j.micinf.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Huber S, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3⁻ and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34(4):554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sojka DK, Fowell DJ. Regulatory T cells inhibit acute IFN-γ synthesis without blocking T-helper cell type 1 (Th1) differentiation via a compartmentalized requirement for IL-10. Proc Natl Acad Sci USA. 2011;108(45):18336–18341. doi: 10.1073/pnas.1110566108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu JJ, et al. An essential role for IL-17 in preventing pathogen-initiated bone destruction: Recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109(9):3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eskan MA, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13(5):465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, et al. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011;17(12):1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74(3):391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 38.Kawai T, et al. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J Immunol. 2000;164(4):2102–2109. doi: 10.4049/jimmunol.164.4.2102. [DOI] [PubMed] [Google Scholar]
- 39.Gandolfo MT, et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76(7):717–729. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 40.Ratajczak MZ, Kim C. The use of chemokine receptor agonists in stem cell mobilization. Expert Opin Biol Ther. 2012;12(3):287–297. doi: 10.1517/14712598.2012.657174. [DOI] [PubMed] [Google Scholar]
- 41.Toupadakis CA, et al. Long-term administration of AMD3100, an antagonist of SDF-1/CXCR4 signaling, alters fracture repair. J Orthop Res. 2012;30(11):1853–1859. doi: 10.1002/jor.22145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87(9) suppl:S42–S45. doi: 10.1097/TP.0b013e3181a28533. [DOI] [PubMed] [Google Scholar]
- 43.Weinberg MA, Bral M. Laboratory animal models in periodontology. J Clin Periodontol. 1999;26(6):335–340. doi: 10.1034/j.1600-051x.1999.260601.x. [DOI] [PubMed] [Google Scholar]
- 44.Martuscelli G, Fiorellini JP, Crohin CC, Howell TH. The effect of interleukin-11 on the progression of ligature-induced periodontal disease in the beagle dog. J Periodontol. 2000;71(4):573–578. doi: 10.1902/jop.2000.71.4.573. [DOI] [PubMed] [Google Scholar]
- 45.June CH, Blazar BR. Clinical application of expanded CD4+25+ cells. Semin Immunol. 2006;18(2):78–88. doi: 10.1016/j.smim.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: Take a billion or so and call me in the morning. Immunity. 2009;30(5):656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jhunjhunwala S, et al. Controlled release formulations of IL-2, TGF-β1 and rapamycin for the induction of regulatory T cells. J Control Release. 2012;159(1):78–84. doi: 10.1016/j.jconrel.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.