Significance
The development of acquired chemoresistance is often a clinical problem limiting the successful treatment of cancers. RNAi is showing promising results in human clinical trials. The combination of chemotherapy with RNAi approaches to suppress the expression of proteins involved in the emergence of drug resistance represents a promising synergistic strategy to circumvent or reverse acquired chemoresistance. Such combination therapy approaches require specific delivery vehicles to encapsulate and deliver chemotherapy and siRNA therapeutics simultaneously in a controlled manner. Herein, we describe a nanoparticle technology to codeliver a DNA-damaging chemotherapeutic and siRNAs that impair the cell’s ability to repair the DNA damage. This combination therapeutic approach can sensitize cancer cells to chemotherapeutics and shows superior tumor inhibition compared with monochemotherapy.
Keywords: siRNA delivery, chemosensitivity, combination therapy
Abstract
Cisplatin and other DNA-damaging chemotherapeutics are widely used to treat a broad spectrum of malignancies. However, their application is limited by both intrinsic and acquired chemoresistance. Most mutations that result from DNA damage are the consequence of error-prone translesion DNA synthesis, which could be responsible for the acquired resistance against DNA-damaging agents. Recent studies have shown that the suppression of crucial gene products (e.g., REV1, REV3L) involved in the error-prone translesion DNA synthesis pathway can sensitize intrinsically resistant tumors to chemotherapy and reduce the frequency of acquired drug resistance of relapsed tumors. In this context, combining conventional DNA-damaging chemotherapy with siRNA-based therapeutics represents a promising strategy for treating patients with malignancies. To this end, we developed a versatile nanoparticle (NP) platform to deliver a cisplatin prodrug and REV1/REV3L-specific siRNAs simultaneously to the same tumor cells. NPs are formulated through self-assembly of a biodegradable poly(lactide-coglycolide)-b-poly(ethylene glycol) diblock copolymer and a self-synthesized cationic lipid. We demonstrated the potency of the siRNA-containing NPs to knock down target genes efficiently both in vitro and in vivo. The therapeutic efficacy of NPs containing both cisplatin prodrug and REV1/REV3L-specific siRNAs was further investigated in vitro and in vivo. Quantitative real-time PCR results showed that the NPs exhibited a significant and sustained suppression of both genes in tumors for up to 3 d after a single dose. Administering these NPs revealed a synergistic effect on tumor inhibition in a human Lymph Node Carcinoma of the Prostate xenograft mouse model that was strikingly more effective than platinum monotherapy.
Advances in genomics and cell biology have highlighted the heterogeneity and complexity of cancer. It is generally accepted that cancer is usually the result of a combination of interconnected disease pathways that may not be treated effectively with 1D therapeutic mechanisms (1). The inhibition of a pathway by a single-drug therapy often results in the emergence of drug resistance and tumor relapse, largely because of pathway redundancy, cross-talk, compensatory and neutralizing actions, and antitarget activities that commonly occur with single-drug cancer therapy (2). In some cases, relapse can result in the emergence of phenotypically distinct and possibly more virulent tumors. For example, treatment of prostatic adenocarcinoma with androgen ablation therapies, such as abiraterone or enzalutamide, results in the development of abiraterone or enzalutamide refractory castration-resistant prostate cancer that is phenotypically nonadenocarcinoma and represents a rare and often lethal form of prostate cancer with a neuroendocrine phenotype (3).
Platinum agents are among the most widely used cytotoxic agents for cancer therapy. Cisplatin and other DNA adduct-forming chemotherapeutics cause DNA damage as their primary mechanism of cellular cytotoxicity. However, several cellular pathways are activated in response to their interaction with DNA, which include DNA repair pathways that remove the damage and translesion DNA synthesis (TLS) by specialized DNA polymerases that helps the cells tolerate the DNA damage (4, 5). The Rev1/Rev3L/Rev7-dependent error-prone TLS pathway has been shown to play an important role in cisplatin-induced mutations that improve the capacity of tumor cells to either repair or tolerate DNA damage, resulting in acquired chemoresistance (6). Rev1 is a translesion DNA polymerase, while Rev3 is the catalytic subunit of the translesion DNA polymerase Polζ (Rev3L/Rev7). Recent studies using mouse lymphoma and lung cancer models have shown that the suppression of error-prone TLS activity in mammalian cells by knocking down Rev1 or Rev3L can inhibit drug-induced mutagenesis so that relapsed tumors remain sensitive to subsequent treatment (6, 7). It has been suggested that combining conventional chemotherapy with newly emerging siRNA therapeutics could be a promising strategy for improving the efficacy of chemotherapy through additive or synergistic effects (8).
Since the discovery of RNAi, synthetic siRNA has emerged as a class of attractive therapeutics for treatment of various diseases, including cancer (9, 10). Given the ability to target and silence nearly any gene of interest, specific siRNA can be constructed to target genes encoding proteins involved in DNA repair and the acquisition of multidrug resistance (6, 11). Naked siRNA cannot readily cross cellular membranes due to its polyanionic and macromolecular characteristics, and it is susceptible to degradation by endogenous enzymes (12). Therefore, considerable efforts have been made to develop safe and effective vehicles to facilitate the delivery of siRNA into cells (13–15). Similarly, the methods by which chemotherapeutics are delivered also have a significant effect on the efficacy (16, 17). Recent research has begun to explore the feasibility of combining chemotherapeutics with siRNA using a variety of nanocarrier platforms (18, 19). One of the earliest efforts using this therapeutic paradigm involved cancer treatment by targeted minicells containing specific siRNA followed by drug-loaded minicells, which efficiently reversed drug resistance in drug-resistant tumors and produced enhanced therapeutic efficacy in inhibiting tumor growth (20). However, to exert optimal synergistic effects, both the drug and siRNA may need to be temporally colocalized in the tumor cells. As a result, nanocarrier platforms that are capable of simultaneously delivering siRNA and anticancer drugs to the same tumor cells are emerging as a promising nanomedicine approach for improved cancer therapy (21, 22).
Nanoparticles (NPs) self-assembled from biodegradable PLGA-PEG block copolymers represent a promising class of potential delivery vehicles due to several unique properties: PLGA-PEG copolymers (i) are biocompatible and biodegradable and used in many U.S. Food and Drug Administration-approved products, (ii) are capable of encapsulating small- and macromolecular payloads with a wide range of physiochemical properties, and (iii) can be designed for controlled release through a combination of polymer degradation and drug diffusion (23). Recently, a docetaxel-containing formulation termed BIND-014 (BIND Biosciences), which has been selected from an NP library composed of poly(d,l-lactide), PLGA, and PEG, is currently in phase I clinical trials (24). Another NP system based on PLGA-PEG has been developed by Kolishetti et al. (25) for codelivery of cisplatin and docetaxel, two drugs with different characteristics and metabolic targets, to prostate cancer cells. However, there remains a pressing need to engineer nanocarriers that are capable of delivering combination therapeutics involving siRNA because systemic delivery of siRNA still remains challenging. Herein, we describe an integrated nanodelivery system capable of simultaneously delivering cisplatin prodrug and siRNAs against REV1 and REV3L to enhance chemosensitivity of tumors. PLGA-PEG was formulated with a cationic lipid-like molecule designated as G0-C14 into NPs that comprise three components: an aqueous inner core, a cationic and hydrophobic layer composed of PLGA and G0-C14, and a hydrophilic PEG corona (Fig. 1A). The G0-C14 compound is synthesized with cationic head groups that can efficiently bind siRNA via electrostatic interactions and flexible hydrophobic tails for self-assembly with PLGA-PEG to form Pt(IV)-prodrug encapsulating NPs (Fig. 1A). In this study, we applied a Pt(IV)-prodrug approach previously used in our laboratory to deliver cisplatin (26). In this approach, a unique Pt(IV) precursor compound, c,c,t [Pt(NH3)2Cl2(O2C(CH2)8CH3)2] (compound 1; Fig. 1B), was developed to allow the release of cisplatin at a lethal dose upon intracellular reduction. The linear decanoyl chains in compound 1 also enable efficient encapsulation within the hydrophobic layer of NPs and controlled release without compromising either feature (26). We investigated the ability of these polymer/lipid hybrid NPs to down-regulate the expression of target genes as well as to induce diminished resistance and enhanced therapeutic profile both in vitro and in vivo. Using a human Lymph Node Carcinoma of the Prostate (LNCaP) xenograft mouse model of prostate cancer, we further demonstrated that these hybrid NPs containing Pt(IV)-prodrug and REV1/REV3L-specific siRNAs (siREV1, siREV3L) cooperatively suppress tumor growth through synergistic effects.
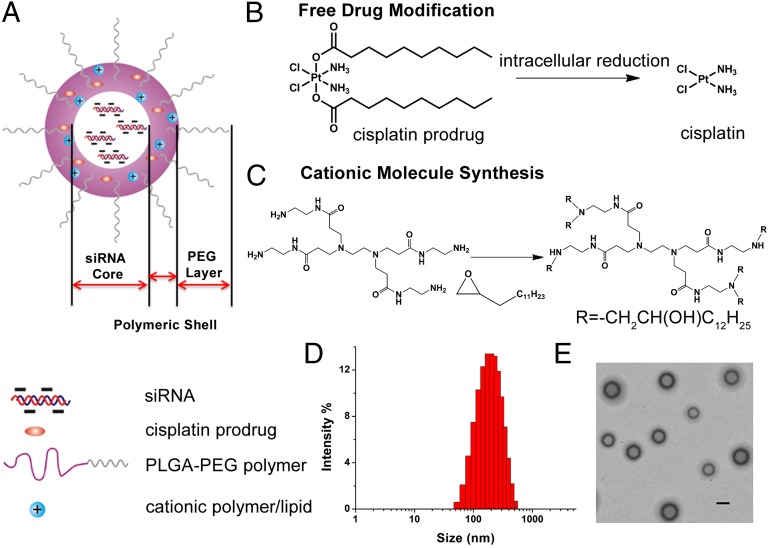
Fig. 1.
(A) Chemical structure of PLGA-PEG/G0-C14 NPs. The particle consists of three components: (i) an outer PEG surface, (ii) a PLGA/G0-C14 layer that plays two roles: (a) acting as a polymer matrix loaded with nonpolar drugs and (b) protecting and promoting siRNA molecule retention inside the NP core and controlling drug release, and (iii) an aqueous inner core containing siRNA. (B) Chemical structure of the hydrophobic platinum(IV) compound 1 and the chemistry by which the active drug cisplatin is released after reduction in the cell. (C) Synthesis of G0-C14 through ring opening of 1,2-epoxytetradecane by ethylenediamine core-PAMAM generation 0 dendrimer. (D) Size distribution of the NPs containing both compound 1 and siRNA determined by dynamic light scattering. (E) Representative transmission EM image of the NPs. (Scale bar, 200 nm.)
Results and Discussion
Design and Synthesis of NPs.
The design and preparation of polymer/G0-C14 hybrid NPs are shown in Fig. 1. To facilitate siRNA encapsulation, a cationic lipid-like molecule termed G0-C14 was synthesized through ring opening of 1,2-epoxytetradecane by generation 0 of poly(amidoamine) (PAMAM) dendrimers (Fig. 1C) (27). NPs were prepared through self-assembly of PLGA-PEG and the resulting G0-C14 using a double-emulsion solvent evaporation method. We chose to use the generation 0 of PAMAM dendrimer for the cationic lipid preparation due to its minimal cytotoxicity compared with higher generations, while still providing a sufficient positive charge to entrap the siRNA therapeutic. Previous research has demonstrated that lipid-like materials, termed lipidoids, containing a 14 carbon tail are idea for siRNA delivery; thus, we chose to use 1,2-epoxytetradecane for synthesis of G0-C14 (27). Compound 1 was designed and synthesized as a Pt(IV) prodrug because its hydrophobicity allowed for encapsulation within PLGA-PEG NPs, while being sufficiently soluble in organic solvents like DMSO and dichloromethane, which are required for Pt(IV)-encapsulated NP preparation. Additionally, Pt(IV) complexes can be reduced in the intracellular milieu to yield the cytotoxic Pt(II) species through a reductive elimination of axial ligands (28). The redox potential for the reduction of a Pt(IV) prodrug, which is an analog of compound 1, has been investigated previously at various pH conditions (26). Electrochemical studies have demonstrated a positive shift of its reduction potential at pH 6, indicating that the acidic intracellular environment in cancer cells will facilitate reduction of the Pt(IV) compound and the release of cisplatin. Thus, Pt(IV) prodrugs provide an attractive alternative to the existing portfolio of Pt(II) drugs.
After the incorporation of G0-C14, the resultant hybrid NPs exhibited simultaneous entrapment of siRNA (up to 99%) and compound 1. In contrast, PLGA-PEG NPs were only able to encapsulate ∼6–10% of the initial siRNA, suggesting that G0-C14 drastically enhanced the entrapment of siRNA within NPs. The entrapment efficiencies of Cy3-labeled siRNA at various weight ratios of G0-C14 to siRNA are calculated based on fluorescence measurement and are shown in Table S1. The size of the NPs ranges from 180 to 220 nm with the polydispersity index at or lower than 0.23. In addition, the zeta potential of the resultant NPs increased with the weight ratio of G0-C14 to siRNA. Within the range of our tested parameters, the weight ratio of G0-C14 to siRNA had little impact on the loading efficiency of compound 1, which remained around 10%. For the following studies, we used NPs with a fixed G0-C14/siRNA weight ratio of 20. The NPs showed a compact and spherical morphology with a mean diameter of around 200 nm (Fig. 1 D and E).
The release kinetics of the Pt(IV) prodrug 1 and siRNA from the NPs were measured. In this system, the Pt(IV) compound 1 is homogeneously dispersed by encapsulation throughout the hydrophobic PLGA layer and is released through a diffusion-controlled process and polymer degradation (29). We conducted release studies by dialyzing NPs containing both compound 1 and siRNA against 2 L of frequently renewed PBS at pH 7.4 and 37 °C to mimic physiological conditions. The amount of platinum released from the NPs was measured by atomic absorption spectroscopy (AAS). As shown in Fig. S1, 15.7% of the total platinum compound was rapidly released over the first 4 h, followed by a sustained release after 8 h. This controlled release of Pt(IV) from the NPs extended over 1 wk, reaching a maximum value of 95% thereafter. The release profile of Cy3-labeled siRNA was measured using fluorescent spectrophotometry, which showed that 50% of the total siRNA was released at 30 h and reached a maximum value of 91% over 10 d. The above results demonstrate that our designed NPs enable the dense loading and sustained release of a combination payload of siRNA and chemotherapeutic drugs.
NP-Mediated Gene Silencing in Vitro.
We evaluated the in vitro gene-silencing efficacy of siRNA-encapsulated hybrid NPs, termed NP(siRNA), in luciferase-expressing HeLa-derived cells (Dual-Luc HeLa). These cells are genetically modified to express both reporter proteins stably: firefly Photinus pyralis and Renilla reniformis luciferase (27). NPs containing various doses of anti-firefly luciferase siRNA [an siRNA-targeting luciferase (siLuc)] were incubated with cells in the presence of growth media, and the expression of both reporter proteins was measured 1 d posttransfection. In this assay, reduction in firefly luciferase expression in the absence of Renilla reduction was considered to be the consequence of siRNA-mediated silencing. Renilla expression was monitored as an internal control for nanocarrier-associated toxicity. The gene knockdown efficacy of NPs(siLuc) was determined by the comparison of detected protein expression levels in treated groups against the untreated control and expressed as relative firefly luciferase expression. The performance of the NPs is plotted as a function of siRNA dose as shown in Fig. 2A. An NP that lacked siRNA (blank NP) produced no silencing effects, whereas luciferase expression was significantly silenced with an increase of siLuc dose in NPs. When a dose of siRNA at or above 25 ng was used, NP(siLuc) achieved greater than 95% luciferase knockdown, a more efficient silencing efficacy than the commercially available liposome-based lipoplex [Lipofectamine 2000 (Lipo2000; Invitrogen)–siRNA complex]. It should also be noted that no evidence of cellular toxicity was observed by the XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] assay under all the conditions used for in vitro transfection experiments (Fig. 2B).
Fig. 2.
(A) Relative firefly luciferase expression of Dual-Luc HeLa cells transfected with NP(siLuc) at an escalating dose of siLuc. Relative firefly luciferase expression was determined by comparison of detected protein levels in treated groups vs. untreated control. Lipofectamine 2000 (Lipo2000)–siRNA complex containing 50 ng of siRNA was used as a positive control. (B) XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] assay of Dual-Luc HeLa cells incubated with an escalating dose of NP(siLuc).
Suppression of TLS Genes Sensitizes LNCaP Prostate Cancer Cells to Pt(IV)-Encapsulated NPs.
Several classes of cancer drugs, including platinum-based compounds and cyclophosphamide, attack cancer cells by damaging their DNA. This DNA damage can prevent cells from replicating their DNA before dividing, which usually induces apoptosis. However, cancer cells can use enzymes known as translesion DNA polymerases to carry out TLS, allowing them to escape apoptosis. This type of DNA replication is highly prone to errors, thereby introducing mutations into the DNA. These newly acquired mutations can allow cancer cells that survive chemotherapy to be much more drug-resistant and aggressive. Thus, error-prone TLS can induce massive genomic mutations after DNA-damaging chemotherapy, while also helping the cells avoid the cytotoxic effect of the treatment (5–7). In human cells, several gene products are major contributors to drug-induced mutagenesis. For example, Rev1 is a scaffolding protein that recruits other translesion DNA polymerases to DNA lesions. It is also a deoxy-CMP transferase that contributes to the bypass of certain lesions (30, 31). Rev3L is the catalytic subunit of DNA polymerase ζ, which plays a key role in extending replication termini across DNA damage. It has been found that human cells expressing reduced levels of Rev1 and Rev3L proteins are more sensitive to cytotoxicity by cisplatin (31). We thus hypothesized that the suppression of REV1 and REV3L by specific siRNA-containing NPs would inhibit TLS activity; impair drug-induced mutagenesis; and, consequently, sensitize cancer cells to chemotherapy.
We designed and conducted experiments based on previous work to investigate whether Rev1 and Rev3L depletion could sensitize tumor cells to platinum-based chemotherapy (6). Using human prostate cancer LNCaP cells and breast cancer MDA-MB-231 cells as two cell models, we first determined the knockdown efficiency of these target genes by NP(siREV1, siREV3L). After 24 h of incubation with NPs, the cells were washed with fresh medium, and the cellular levels of REV1 and REV3L mRNA were assessed on 3 consecutive days using quantitative real-time PCR (qRT-PCR). As shown in Fig. 3A, the qRT-PCR assay revealed a sustained knockdown efficiency of up to 87% for both genes REV1 and REV3L in LNCaP cells over the course of 3 d. A similar gene-silencing efficacy was observed in MDA-MB-231 cells (Fig. S2). Notably, these developed NPs are capable of simultaneously targeting multiple genes. We then performed an escalating-dose experiment in LNCaP cells to examine the effects of NP-mediated REV1/REV3L suppression on tumor cell chemosensitivity. We tested the following four different formulations: (i) compound 1 in solution form, (ii) compound 1-encapsulated NP [NP(compound 1)], (iii) NP(siREV1, siREV3L) with compound 1 in solution, and (iv) compound 1 within the NP(siREV1, siREV3L) [NP(siREV1, siREV3L, compound 1)]. Before treatment with the two siRNA-containing NP formulations, the cells were transfected with NP(siREV1, siREV3L) for 24 h to achieve substantial levels of Rev1 and Rev3L suppression. The transfected cells were then treated with the two different siRNA-containing formulations with an escalating dose of compound 1. The first formulation consists of NP(siREV1, siREV3L) with compound 1 in solution form, whereas the second contains NP(siREV1, siREV3L, compound 1). A comparison of the dose–response curves revealed significantly lower EC50 values for all three NP formulations compared with the free drug in solution form (Fig. 3B). As expected, the cells treated with NP(siREV1, siREV3L) exhibited a better dose response to free drug than the untreated cells, thus providing evidence for the enhanced chemosensitization of prostate cancer cells through REV1 and REV3L suppression. Similarly, inclusion of the REV1/REV3L siRNAs in NPs resulted in improved tumor cell response to compound 1-loaded NPs compared with NP(scrambled siRNA, compound 1) (Fig. S3). It is important to recognize that the lowest EC50 value was attributed to the NP formulation loaded with both siRNA and compound 1, suggesting a greater potency and efficacy compared with the other therapeutic formulations. Interestingly, we discovered that whether compound 1 is delivered free or within NPs plays a crucial role in cell survival. To investigate how encapsulation of 1 affects cell survival rates, LNCaP cells were incubated with an equivalent dose of the compound in solution or NP form, and the platinum content of the cell was determined by AAS. As shown in Fig. S4, cells treated with NP (compound 1) had taken up around 50% more platinum compared with cells treated with free compound 1. The enhanced cellular uptake of platinum is probably a consequence of the improved solubility of compound 1 in aqueous systems when encapsulated within NPs. Another possible explanation is that NPs shield compound 1 from unspecific interactions with albumin present in the cell growth medium, which are considered to be the main route for platinum binding in human blood plasma (32). Taken together, these results indicate that the simultaneous delivery of siRNAs and compound 1 is concurrently able to knock down expression levels of genes REV1 and REV3L and sensitize cultured cancer cells to platinum treatment. These findings are consistent with our previous studies, which have revealed that cells lacking either Rev1 or Rev3L displayed a reduced drug-induced mutation in response to DNA-damaging agents and an increased sensitivity to DNA-damaging chemotherapy (6, 7).
Fig. 3.
Enhanced sensitization of LNCaP cells to platinum treatment by codelivery of siREV1, siREV3L, and compound 1. (A) qRT-PCR confirmation of REV1 and REV3L gene suppression using NP(siREV1, siREV3L) in LNCaP human prostate adenocarcinoma cells. (B) Platinum dose–response curves in cells expressing normal [NP(compound 1)] or impaired [NP(siREV1, siREV3L, compound 1)] levels of REV1 and REV3L. The experiment was conducted in quadruplicate (n = 4). *Before treatment with the two siRNA-containing NP formulations, the cells were transfected with NP(siREV1, siREV3L) for 48 h. The transfected cells were then treated with the two different formulations with an escalating dose of compound 1.
NP-Mediated Gene Silencing in Vivo.
In the next set of experiments, we tested the ability of the polymer/lipid hybrid NPs to deliver siRNA in vivo. To this end, luciferase-expressing MDA-MB-231 cells were injected s.c. into the mammary fat pad of nude mice to develop xenograft tumors that stably express luciferase. Two weeks after development of the tumor xenograft, the gene knockdown efficacy experiments were initiated. Ten tumor-bearing nude mice were randomly divided into two groups (n = 5), with each group administered either NP(negative siRNA) (control group) or NP(siLuc) (experiment group) through a single intratumoral injection. We first obtained the initial bioluminescence images of the mice (day 0), after which the mice were injected with a single dose of NP(siLuc). The treated mice were then imaged thereafter for 3 consecutive days (Fig. 4A). The bioluminescence intensity from the control group mice increased rapidly from day 0 to day 3, with day 3 being a near 64% increase in intensity compared with day 0. In contrast, the tumors treated with NP(siLuc) showed a drastic decrease in bioluminescence intensity 1 d postinjection and increased in the following days. These results suggest that the NPs are capable of delivering siRNA to inhibit luciferase expression in vivo.
Fig. 4.
(A) In vivo bioluminescence imaging of mice bearing a luciferase-expression tumor treated either with NP(negative siRNA) (control group) or NP(siLuc) (experiment group) through a single intratumoral injection. Images were taken at days 0, 1, 2, and 3. Luminescence intensity is shown by the legend to the right. (B) Luciferase expression of each group (n = 5) was represented by the luminescence intensity (mean ± SE) relative to day 0. *P < 0.05 vs. NP(negative siRNA) at days 1, 2, and 3.
To quantify the gene-silencing efficacy of NP(siLuc), we normalized the bioluminescence intensity signals obtained for each tumor at different days by setting the initial bioluminescent signal (day 0) equal to 1. The relative luminescence intensity (n = 5, mean ± SE) was then plotted as a function of time (Fig. 4B). Notably, we observed an 80% decrease in luciferase expression in the treated group 1 d postinjection. Furthermore, the bioluminescence intensity of days 2 and 3 relative to day 0 remains a fractional part (71.4% and 85.7%, respectively), indicating a sustained gene-silencing effect. These results are consistent with our in vitro data. NP(siLuc) exhibited a remarkable in vivo efficacy in suppressing luciferase expression in the MDA-MB-231 cells, making our polymer/lipid hybrid NPs a suitable nanocarrier for siRNA delivery.
In Vivo Efficacy of NPs Containing siRNA and Pt(IV) Prodrug.
Using a well-established LNCaP xenograft mouse model of prostate cancer (26), we examined whether our PLGA-PEG/G0-C14 hybrid NPs are capable of inhibiting REV1 and REV3L expression in tumors. LNCaP cells were first retrovirally infected to express GFP stably before they were injected into SCID-beige mice to develop xenograft tumors. In this study, GFP was used as a marker for implanted LNCaP cells. After the tumors reached a sufficient size of ∼100–200 mm3, we randomly divided animals into two groups (n = 4) to minimize weight and tumor size differences between the groups. Each group was administered either NP(negative siRNA) (control group) or NP(siREV1, siREV3L) (experimental group) at a dose of 0.4 mg of siRNA per kilogram of animal weight via intratumoral injection. Tumors were harvested from the mice at the designated time points, and pure populations of LNCaP cells were isolated by GFP sorting before qRT-PCR. Fig. 5A shows that the cellular levels of REV1 and REV3L expression were significantly decreased by 78% relative to the control in the harvested GFP-labeled LNCaP cells 48 h posttreatment. Additionally, the gene-silencing effect mediated by a single dose of NP(siREV1, siREV3L) was still noticeable 72 h after administration (36% and 42% decrease in REV1 and REV3L, respectively). In this context, these results further demonstrate that our NPs are able to deplete REV1 and REV3L expression efficiently in tumors.
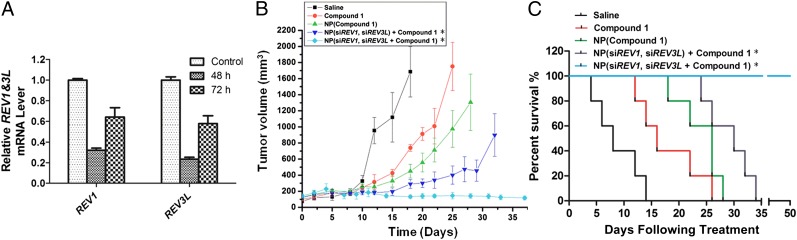
Fig. 5.
Enhanced in vivo therapeutic efficacy mediated by NP(siREV1, siREV3L, compound 1). (A) qRT-PCR confirmation of REV1 and REV3L gene suppression in LNCaP cells that were harvested from a xenograft tumor and isolated by GFP sorting 2 or 3 d after injection of NP(siREV1, siREV3L). (B) Inhibition of LNCaP xenograft tumor growth by formulation v [NP(siREV1, siREV3L, compound 1] in comparison to the following formulations: (i) saline, (ii) compound 1 in solution form, (iii) NP(compound 1), and (iv) NP(siREV1, siREV3L) with compound 1 in solution. The doses of compound 1 and siRNA per injection were 4 mg/kg and 0.4 mg/kg, respectively. (C) Survival curves of tumor-bearing mice treated with the aforementioned five formulations. Day 0 represents the first day of NP(siREV1, siREV3L) administration [n = 5 for groups i–iv, n = 8 for group v: P < 0.0136 for compound 1 vs. NP(siREV1, siREV3L) + compound 1; P < 0.008 NP(compound 1) vs. NP(siREV1, siREV3L, compound 1)]. P values for all survival studies were determined using log-rank curve comparison tests. *Before treatment with formulations iv and v, the tumor-bearing mice were injected on day 0 and day 2 with NP(siREV1, siREV3L). Starting from the fourth day, the mice received intratumoral injections of the aforementioned five formulations twice weekly. Day 0 represents the first day of NP(siREV1, siREV3L) administration.
We also investigated the efficacy of injecting NPs that contained both the Pt(IV) prodrug and the siRNAs directed against REV1 and REV3L [NP(siREV1, siREV3L, compound 1)] using the LNCaP xenograft mouse model as described above. Three weeks after inoculation with LNCaP cells, mice were randomly divided into five groups and treated with the following regimens with equivalent doses of compound 1 via intratumoral administration twice weekly for 5 wk: (i) saline, (ii) compound 1 in solution form, (iii) NP(compound 1), (iv) NP(siREV1, siREV3L) with compound 1 in solution, and (v) NP(siREV1, siREV3L, compound 1). It should be noted that before treatment with the two siRNA-containing NP formulations (regimens iv and v), mice were injected on day 0 and day 2 with NP(siREV1, siREV3L) to ensure that Rev1 and Rev3L depletion occurred at significant levels. At day 4, we started injections with the aforementioned five formulations. The aim of this study was to determine whether simultaneous delivery of REV1/REV3L-specific siRNAs and platinum chemotherapeutics would result in enhanced antitumor activity through synergistic effects. From Fig. 5B, it is clear that the administration of NP(siREV1, siREV3L, compound 1) resulted in virtually complete inhibition of tumor growth. Moreover, the tumors treated with NP(siREV1, siREV3L, compound 1) seemed to decline slightly in volume postinjection. Notably, tumors treated with a combination of NP(siREV1, siREV3L) and compound 1 in solution displayed delayed growth compared with their drug-only counterparts, again emphasizing that suppression of REV1/REV3L expression can improve the antitumor response of chemotherapy irrespective of whether 1 is delivered free or within NPs (Fig. 5B and Fig. S5). Additionally, inclusion of the REV1/REV3L siRNAs in NPs carrying compound 1 increased their effectiveness [compare v with iii and v with the NP(scrambled siRNA, compound 1) group], further emphasizing that suppressing REV1/REV3L expression can enhance tumor growth suppression (Fig. 5B and Fig. S6A). These results remain consistent with our in vitro escalating-dose experiment (Fig. 3B and Fig. S3). Strikingly, all mice treated with NP(siREV1, siREV3L, compound 1) survived the entire 50-d study duration without tumor growth (Fig. 5C). In contrast, no other group had animals survive the 34-d study (Fig. 5C and Fig. S6B). The combination of NP(siREV1, siREV3L) with compound 1 in solution (regimen iv) was also more efficacious with respect to survival than the free drug, NP(compound 1), and saline control groups, resulting in mice living a relatively healthy period of 20 d. However, regimen iv was less efficacious with respect to survival compared with the NP(siREV1, siREV3L, compound 1) group. Inclusion of scrambled siRNA in compound 1-loaded NP also decreased the survival rate compared with the NP(siREV1, siREV3L, compound 1) group (Fig. S6B). This study strongly suggests that the combinational effects of siRNA targeting TLS polymerases and an anticancer drug in our NPs can significantly improve survival in tumor-bearing mice.
Summary
In summary, we have reported the design and synthesis of a PLGA-PEG–based NP platform for codelivery of siRNA and Pt(IV) prodrug. The versatile composition of this NP allows for simultaneous encapsulation and sustained released of both payloads. We have demonstrated that the siRNA-containing NPs can successfully lower expression levels of target genes (reporter and both TLS genes) in vitro and in vivo without any evidence of associated toxicity. Furthermore, these NPs are capable of silencing target genes for at least 3 d after the administration of a single dose. Compared with the other formulations, the NP(siREV1, siREV3L, compound 1) shows a significantly lower EC50, suggesting a greater induced chemosensitization of cancer cells to platinum treatment. In an LNCaP xenograft mouse model of human prostate cancer, the NP(siREV1, siREV3L, compound 1) proved superior in the simultaneous delivery of two different payloads into tumor cells, which markedly inhibited tumor growth in a synergistic manner (2). As demonstrated in our previous studies, suppression of error-prone TLS polymerases could either render tumors more susceptible to DNA-damaging chemotherapy and/or delay acquired chemoresistance. In our prostate xenograft model, the first of these effects was clearly observed, whereas the characteristics of the xenograft model did not make it possible to examine acquired chemoresistance. This finding suggests that the unique design of our NP platform is optimized for siRNA therapy. In the future, this platform could be further modified and tested in many other cancer models. Although preliminary, the results of this present work demonstrate the tremendous potential of this simultaneous delivery system, providing a promising nanomedicine approach for cancer therapy.
Materials and Methods
Materials and procedures for the synthesis and characterization of all compounds and NPs can be found in SI Materials and Methods. The NMR analysis of G0-C14 is shown in Fig. S7., Platinum uptake by LNCaP cells, siRNA transfection, viability assays, bioluminescent assays, and animal experiments are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Akin Akinc (Alnylam Pharmaceuticals, Inc.) and Prof. Daniel G. Anderson (Massachusetts Institute of Technology) for providing Dual-Luc HeLa cells. G.C.W. is an American Cancer Society Professor. This work was supported by the David Koch-Prostate Cancer Foundation Award in Nanotherapeutics, the Centers of Cancer Nanotechnology Excellence, and the National Institutes of Health (NIH) (Grant CA151884). This work was also supported by National Institutes of Environmental Health Sciences Grant ES015818 (to G.C.W.). X.X. acknowledges postdoctoral support from an NIH National Research Service Award (Grant 1F32CA168163-01). J.S. received financial support from National Cancer Institute Grant K99CA160350.
Footnotes
Conflict of interest statement: O.C.F. and R.L. disclose their financial interest in BIND Biosciences, Selecta Biosciences, and Blend Therapeutics, three biotechnology companies developing nanoparticle technologies for medical applications. P.W.K. and S.J.L. disclose their financial interest in BIND Biosciences and Blend Therapeutics. BIND, Selecta, and Blend did not support the aforementioned research, and these companies currently have no rights to any technology or intellectual property developed as part of this research.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303958110/-/DCSupplemental.
References
- 1.Woodcock J, Griffin JP, Behrman RE. Development of novel combination therapies. N Engl J Med. 2011;364(11):985–987. doi: 10.1056/NEJMp1101548. [DOI] [PubMed] [Google Scholar]
- 2.Jia J, et al. Mechanisms of drug combinations: Interaction and network perspectives. Nat Rev Drug Discov. 2009;8(2):111–128. doi: 10.1038/nrd2683. [DOI] [PubMed] [Google Scholar]
- 3. Ryan CJ (2013) Clinical trial design strategies in the post-abiraterone/enzalutamide setting. 2013 Genitourinary Cancers Symposium Proceedings, ed Carducci MA (American Society of Clinical Oncology, Alexandria, VA), pp 26–28.
- 4.Ahmad S. Platinum-DNA interactions and subsequent cellular processes controlling sensitivity to anticancer platinum complexes. Chem Biodivers. 2010;7(3):543–566. doi: 10.1002/cbdv.200800340. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, et al. Structural basis of human DNA polymerase η-mediated chemoresistance to cisplatin. Proc Natl Acad Sci USA. 2012;109(19):7269–7274. doi: 10.1073/pnas.1202681109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie K, Doles J, Hemann MT, Walker GC. Error-prone translesion synthesis mediates acquired chemoresistance. Proc Natl Acad Sci USA. 2010;107(48):20792–20797. doi: 10.1073/pnas.1011412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doles J, et al. Suppression of Rev3, the catalytic subunit of Polζ, sensitizes drug-resistant lung tumors to chemotherapy. Proc Natl Acad Sci USA. 2010;107(48):20786–20791. doi: 10.1073/pnas.1011409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, et al. REV3L confers chemoresistance to cisplatin in human gliomas: The potential of its RNAi for synergistic therapy. Neuro Oncol. 2009;11(6):790–802. doi: 10.1215/15228517-2009-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 10.Ptasznik A, Nakata Y, Kalota A, Emerson SG, Gewirtz AM. Short interfering RNA (siRNA) targeting the Lyn kinase induces apoptosis in primary, and drug-resistant, BCR-ABL1(+) leukemia cells. Nat Med. 2004;10(11):1187–1189. doi: 10.1038/nm1127. [DOI] [PubMed] [Google Scholar]
- 11.Lage H. MDR1/P-glycoprotein (ABCB1) as target for RNA interference-mediated reversal of multidrug resistance. Curr Drug Targets. 2006;7(7):813–821. doi: 10.2174/138945006777709566. [DOI] [PubMed] [Google Scholar]
- 12.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: Advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng D, et al. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc Natl Acad Sci USA. 2012;109(30):11975–11980. doi: 10.1073/pnas.1118425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis ME, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodrow KA, et al. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8(6):526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang XQ, et al. Multimodal nanodiamond drug delivery carriers for selective targeting, imaging, and enhanced chemotherapeutic efficacy. Adv Mater. 2011;23(41):4770–4775. doi: 10.1002/adma.201102263. [DOI] [PubMed] [Google Scholar]
- 17.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Gao S, Ye WH, Yoon HS, Yang YY. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater. 2006;5(10):791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 19.Zhang XQ, et al. Interactions of nanomaterials and biological systems: Implications to personalized nanomedicine. Adv Drug Deliv Rev. 2012;64(13):1363–1384. doi: 10.1016/j.addr.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDiarmid JA, et al. Sequential treatment of drug-resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nat Biotechnol. 2009;27(7):643–651. doi: 10.1038/nbt.1547. [DOI] [PubMed] [Google Scholar]
- 21.Sun TM, et al. Simultaneous delivery of siRNA and paclitaxel via a “two-in-one” micelleplex promotes synergistic tumor suppression. ACS Nano. 2011;5(2):1483–1494. doi: 10.1021/nn103349h. [DOI] [PubMed] [Google Scholar]
- 22.Xiong XB, Lavasanifar A. Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. ACS Nano. 2011;5(6):5202–5213. doi: 10.1021/nn2013707. [DOI] [PubMed] [Google Scholar]
- 23.Farokhzad OC. Nanotechnology for drug delivery: The perfect partnership. Expert Opin Drug Deliv. 2008;5(9):927–929. doi: 10.1517/17425247.5.9.927. [DOI] [PubMed] [Google Scholar]
- 24.Hrkach J, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4(128):128ra139. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 25.Kolishetti N, et al. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc Natl Acad Sci USA. 2010;107(42):17939–17944. doi: 10.1073/pnas.1011368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci USA. 2011;108(5):1850–1855. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Love KT, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA. 2010;107(5):1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giandomenico CM, et al. Carboxylation of Kinetically Inert Platinum(IV) Hydroxy Complexes. An Entr.acte.ee into Orally Active Platinum(IV) Antitumor Agents. Inorg Chem. 1995;34(5):1015–1021. doi: 10.1021/ic00109a004. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Feng SS. In vitro investigation on poly(lactide)-Tween 80 copolymer nanoparticles fabricated by dialysis method for chemotherapy. Biomacromolecules. 2006;7(4):1139–1146. doi: 10.1021/bm050953v. [DOI] [PubMed] [Google Scholar]
- 30.Washington MT, Carlson KD, Freudenthal BD, Pryor JM. Variations on a theme: Eukaryotic Y-family DNA polymerases. Biochim Biophys Acta. 2010;1804(5):1113–1123. doi: 10.1016/j.bbapap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gan GN, Wittschieben JP, Wittschieben BO, Wood RD. DNA polymerase zeta (pol zeta) in higher eukaryotes. Cell Res. 2008;18(1):174–183. doi: 10.1038/cr.2007.117. [DOI] [PubMed] [Google Scholar]
- 32.Reedijk J. Why does Cisplatin reach Guanine-n7 with competing s-donor ligands available in the cell? Chem Rev. 1999;99(9):2499–2510. doi: 10.1021/cr980422f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.