Significance
Activation of NMDA-type glutamate receptors produces neuronal excitotoxicity, a primary cause of cell death in stroke and other neurological disorders. This cell death process requires superoxide release by neuronal NADPH oxidase. Results presented here show that small reductions in intracellular pH uncouple neuronal NADPH oxidase from NMDA receptor activation, and thereby prevent neuronal death. The findings establish a link between metabolic activity and excitotoxicity, and identify a mechanism by which mild acidosis improves outcome after excitotoxic and ischemic brain insults. The findings also suggest that variations in intracellular pH associated with physiological brain activity may likewise influence the cell-to-cell signaling normally mediated by neuronal superoxide release.
Keywords: NOX2, acidosis, Hv1, cariporide
Abstract
Sustained activation of N-methyl-d-aspartate (NMDA) -type glutamate receptors leads to excitotoxic neuronal death in stroke, brain trauma, and neurodegenerative disorders. Superoxide production by NADPH oxidase is a requisite event in the process leading from NMDA receptor activation to excitotoxic death. NADPH oxidase generates intracellular H+ along with extracellular superoxide, and the intracellular H+ must be released or neutralized to permit continued NADPH oxidase function. In cultured neurons, NMDA-induced superoxide production and neuronal death were prevented by intracellular acidification by as little as 0.2 pH units, induced by either lowered medium pH or by inhibiting Na+/H+ exchange. In mouse brain, superoxide production induced by NMDA injections or ischemia–reperfusion was likewise prevented by inhibiting Na+/H+ exchange and by reduced expression of the Na+/H+ exchanger-1 (NHE1). Neuronal intracellular pH and neuronal Na+/H+ exchange are thus potent regulators of excitotoxic superoxide production. These findings identify a mechanism by which cell metabolism can influence coupling between NMDA receptor activation and superoxide production.
Many metabolic processes generate hydrogen ions, and hydrogen ions in turn influence cell metabolism and survival (1). Cerebral ischemia in particular produces acidosis of variable degree, depending upon blood glucose levels, degree of blood flow reduction, and other factors. Severe acidosis, below pH 6.4, exacerbates ischemic injury (2) by mechanisms involving protein denaturation, acid-sensing calcium channels, and release of ferrous iron (3–5). Conversely, lesser degrees of acidosis, in the range of 7.0–6.5, reduce both ischemic injury (6) and glutamate-induced neuronal death (7). These neuroprotective effects have been attributed to an inhibitory effect of hydrogen ions on NMDA receptor activation (8–10), but a causal link has not been demonstrated.
Excessive activation of N-methyl-D-aspartate (NMDA) type glutamate receptors leads to excitotoxic cell death in stroke and other neurological disorders (11, 12). Superoxide production by NADPH oxidase is a requisite event in the process leading from NMDA receptor activation to excitotoxic cell death (13–19). NADPH oxidase exists as several isoforms, of which NOX2 is the one most abundantly expressed in CNS neurons. NOX2 is also the isoform most abundantly expressed in phagocytes, microglia, and other immune cells, in which its regulation and function have been extensively characterized (20). NOX2 is composed of three cytosolic subunits, p47phox, p67phox, and p40phox, which when phosphorylated bind with two membrane-bound subunits, p22phox and gp91phox (the catalytic unit), to form an active transmembrane enzyme complex. The transmembrane complex generates superoxide in the extracellular space and hydrogen ions in the intracellular space: 2O2 + NADPH → 2O2− + NADP+ + H+. In immune cells, H+ concentration influences the phosphorylation status of the NOX2 p47phox subunit, and the H+ generated by NOX2 must be transferred to the extracellular space to sustain NOX2 activity (21–24).
Together, the pH sensitivity of NOX2 and the role of NOX2 in NMDA receptor-mediated cell death suggest the possibility that reduced intracellular pH might limit neurotoxicity by dissociating NMDA receptor activation from superoxide production. Findings presented here confirm that both the superoxide production and cell death resulting from neuronal NMDA receptor activation are highly pH sensitive. We show that neurons use Na+/H+ exchange as a major route of proton efflux during NOX2 activation, and either genetic or pharmacologic inhibition of neuronal Na+/H+ exchange prevent both excitotoxic superoxide production and cell death.
Results
Mild Acidosis Blocks NMDA-Induced Superoxide Production and Cell Death.
We first performed cell culture studies at a physiological medium pH to confirm NOX2 as the primary source of NMDA-induced neuronal superoxide production. Normal brain extracellular pH is 7.20–7.30 (25). Mouse cortical neurons exposed to 100 µM NMDA at medium pH 7.2 showed an immediate intracellular calcium elevation, followed by superoxide formation and subsequent cell death (Fig. S1). By contrast, neurons pretreated with a peptide inhibitor of NOX2 assembly (gp91ds-Tat) showed no increased superoxide formation or cell death after NMDA exposure, despite comparable calcium elevations (Fig. S1). These findings agree with prior reports identifying NOX2 as the primary source of NMDA-induced superoxide production (13, 14, 17–19), and they establish that both of the indicators used to measure superoxide production are responsive to NOX2 inhibition.
Neurons were then exposed to NMDA in acidified culture medium to evaluate the effect of H+ on superoxide production. Surprisingly, reducing medium pH by just 0.2 pH units, from 7.2 to 7.0, nearly eliminated both NMDA-induced superoxide formation and cell death (Fig. 1). A direct effect of acidosis on the responsiveness of the superoxide indicators was excluded by the lack of a pH effect on signal induced by exogenous oxidant (Fig. S2). The effect of acidosis on superoxide production was evident in both bicarbonate-containing and bicarbonate-free media (Fig. S3), and all subsequent studies were performed in bicarbonate-free medium.
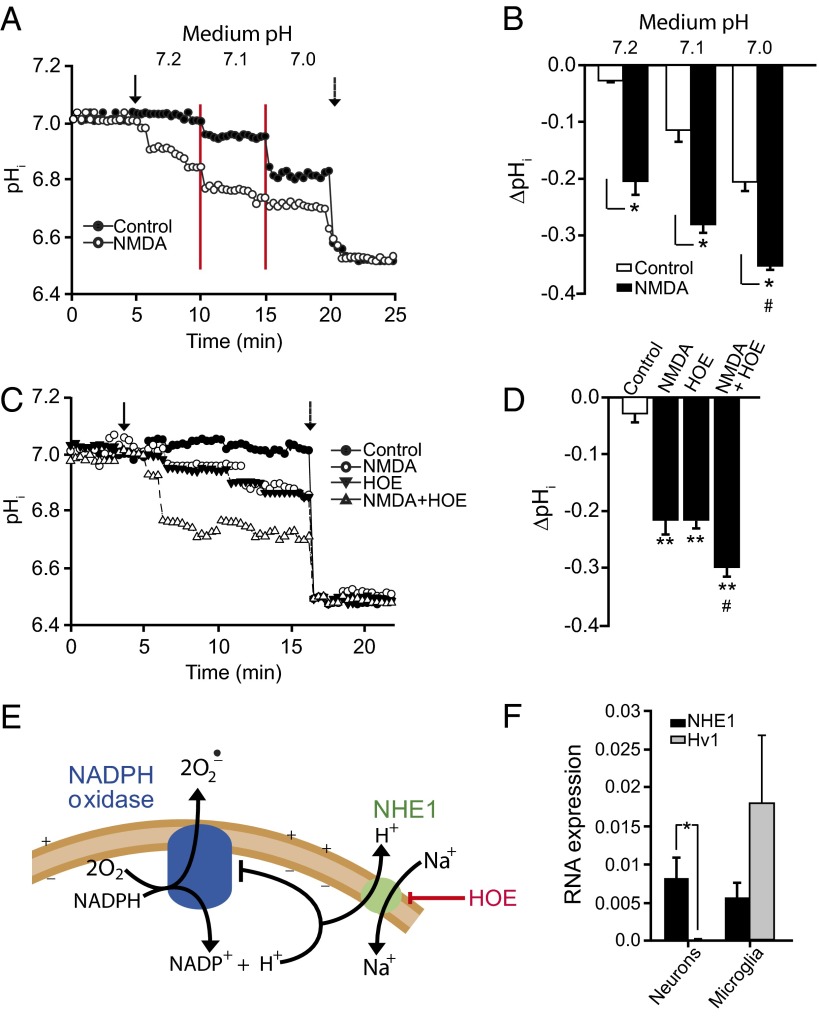
Fig. 1.
NMDA-induced superoxide formation and cell death are blocked by small reductions in medium pH. (A) Immunostaining for 4-hydroxynonenal (4HNE, red) shows superoxide formation produced by 20-min incubations in 100 µM NMDA at the designated medium pH. Staining for microtubule-associated protein 2 (MAP2; green) identifies neuronal processes, and DAPI (blue) identifies cell nuclei. (Scale bar: 25 µm.) (B) Quantified 4HNE immunofluorescence. Control wells received medium exchanges only. (n = 4; *P < 0.05 vs. NMDA, pH 7.2). (C) Superoxide formation assessed by formation of fluorescent ethidium species (Eth) from dihydroethidium, conditions as in A. (n = 4; *P < 0.05 vs. NMDA, pH 7.2). (D) Neuronal death evaluated by trypan blue staining in phase-contrast photographs of cultures 24 h after treatment as in A. (E) Quantified neuronal death (n = 3, *P < 0.05 vs. NMDA, pH 7.2). (F) Intracellular calcium elevations assessed by Fura-4F, conditions as in A. (n = 4, *P < 0.05 vs. corresponding pH control; #P < 0.05 vs. NMDA, pH 7.2).
The Effect of Mild Acidosis Is Not Attributable to NMDA Receptor Inhibition.
Calcium influx through the NMDA receptor is required for NMDA-induced NOX2 activation (13). This influx is sensitive to H+ binding to an extracellular site on NMDA receptors (8–10), suggesting a possible mechanism for the effect of medium acidification on NMDA-induced superoxide production. However, the effect of H+ on NMDA-induced current influx is relatively small between pH 7.2 and pH 6.8. Here, using the low-affinity dye Fura-4F, we confirmed a small but measurable attenuation of NMDA-induced calcium elevations over this pH range (Fig. 1F). To test the possibility that even small attenuations in calcium rise might prevent NOX2 activation, we increased the medium calcium concentration from the standard 1.26 mM to 2.0 mM, a value at which the Fura-4F signal induced by NMDA at pH 7.0 was at least equal to that induced by NMDA at pH 7.2 in 1.26 mM calcium. Under these conditions, NMDA-induced superoxide production was again prevented at the lower medium pH, despite comparable intracellular calcium elevations (Fig. S4).
Intracellular Acidification Is Sufficient to Block NMDA-Induced Superoxide Formation and Cell Death.
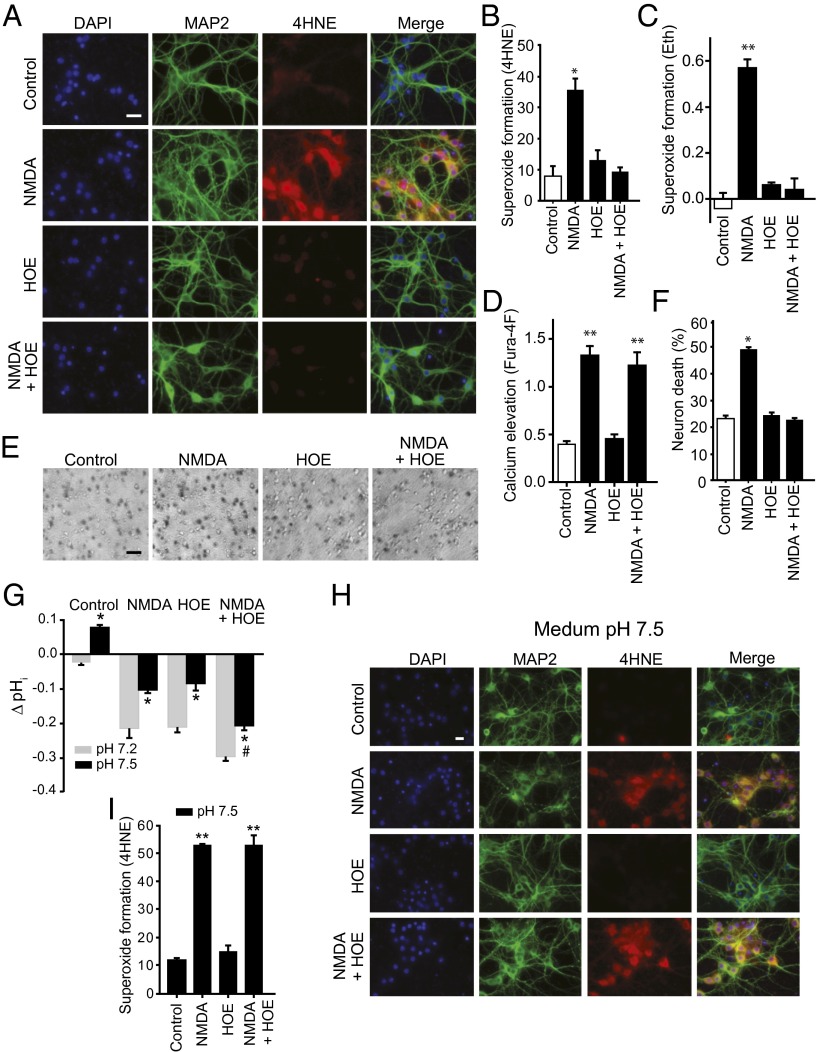
NOX2 is sensitive to small changes in intracellular pH (pHi) (24), and pHi in cultured cells varies with the medium pH. Using the pH-sensitive dye 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF), we found neuronal pHi to be at baseline about 0.2 pH units lower than the medium pH, and to drop further with reductions in medium pH (Fig. 2 A and B). NMDA receptor stimulation also reduces pHi (26), and acidification induced by NMDA was found to be additive with that induced by medium acidification (Fig. 2 A and B). We next evaluated the effect of selective acidification of the intracellular space using the Na+/H+ exchange inhibitor, 4-isopropyl-3-methylsulfonyl-benzoyl-guanidine-methanesulfonate (HOE). HOE produced an intracellular acidification comparable to that produced by reducing medium pH to 7.0, and this acidification was likewise additive to that induced by NMDA (Fig. 2 C and D). Importantly, neurons treated with 1 µM HOE showed a near-complete elimination of NMDA-induced superoxide formation and neuronal death, with no significant reduction in NMDA-induced calcium elevation (Fig. 3 A–F).
Fig. 2.
Effects of NMDA, medium pH, and Na+/H+ exchange inhibition on intracellular pH. (A) Representative single-cell BCECF traces show that both NMDA and medium acidification reduce intracellular pH (pHi), with additive effect. Arrow indicates NMDA addition, red lines delineate stepwise medium acidification, and dotted arrow shows pH 6.5 calibration. (B) Quantified aggregate results; n = 4, *P < 0.05, #P < 0.05 vs. pH 7.2. (C) Representative single-cell BCECF traces show that both NMDA and the Na+/H+ exchange inhibitor HOE (1 µM) reduce intracellular pH and have additive effects. Solid arrow indicates time of NMDA and HOE addition, dotted arrow shows pH 6.5 calibration. (D) Quantified aggregate results; n = 4, **P < 0.05 vs. control, #P < 0.05 vs. NMDA. (E) Intracellular H+ inhibits NADPH oxidase. H+ produced by NADPH oxidase and other sources may exit neurons via the Na+/H+ exchanger NHE1. HOE inhibits Na+/H+ exchange and thereby produces intracellular acidification. (F) RT-PCR assessment of cultured neurons and microglial shows that, in neurons, gene expression of NHE1 is high relative to the voltage-dependent H+ channel Hv1. (n = 4, P < 0.01).
Fig. 3.
Na+/H+ exchange inhibition blocks NMDA-induced superoxide formation and neuronal cell death. (A) Superoxide formation assessed by immunostaining for 4HNE (red). MAP2 (green) identifies neuronal processes, and DAPI (blue) identifies cell nuclei. Neurons were exposed to 100 µM NMDA for 20 min with or without the Na+/H+ exchange inhibitor HOE (1 µM). (Scale bar: 25 µm.) (B) Quantified 4HNE immunofluorescence (n = 4; *P < 0.05 vs. all other groups). (C) Superoxide formation assessed by Eth formation, conditions as in A; n = 4; **P < 0.01 vs. all other groups. (D) Calcium elevation assessed by Fura-4F, conditions as in A; n = 4; **P < 0.01 vs. control. (E) Neuronal death evaluated by trypan blue staining in phase-contrast photographs of cultures 24 h after treatment as in A. (Scale bar: 60 µm.) (F) Quantified neuronal death (n = 3, *P < 0.05 vs. all other groups). (G) Change in intracellular pH induced by 20-min exposure to 100 µM NMDA and 1 µM HOE, with medium pH set to either pH 7.2 or 7.5. (n = 4; *P < 0.05 vs. control, #P < 0.05 vs. NMDA). (H) Alkalization reverses the effect of HOE on NMDA-induced superoxide formation. Formation of 4HNE (red) is not suppressed by HOE at medium pH 7.5. MAP2 (green) identifies neuronal processes. (Scale bar: 25 µm.) (I) Quantification of 4HNE formation (n = 4, **P < 0.01 vs. control).
H+ ions produced by NOX2 must be transferred to the extracellular space to prevent intracellular acidification and resultant inhibition of NOX2 function (Fig. 2E). In immune cells, in which NOX2 function has been extensively characterized, H+ translocation occurs through both the voltage-sensitive proton channel Hv1 and the Na+/H+ exchanger NHE1 (21–23). A comparison of Hv1 and 4-hydroxynonenal (4HNE) gene expression in cultured neurons and microglia showed relatively greater NHE1 gene expression in neurons (Fig. 2F), consistent with the robust effect of HOE.
HOE blocks Na+ entry as well H+ efflux, and consequently the observed effects of HOE on superoxide production could alternatively stem from reduced Na+ entry. If HOE blocks superoxide production by reducing pHi, then normalizing pHi should reverse the effect of HOE. This prediction was confirmed: pHi in neurons treated with NMDA plus HOE was normalized by raising medium pH to 7.5 and, under this condition, HOE failed to prevent NMDA-induced superoxide formation (Fig. S5 and Fig. 3 E–H).
Reduced NHE1 Function Prevents NMDA- and Ischemia-Induced Superoxide Formation in Brain.
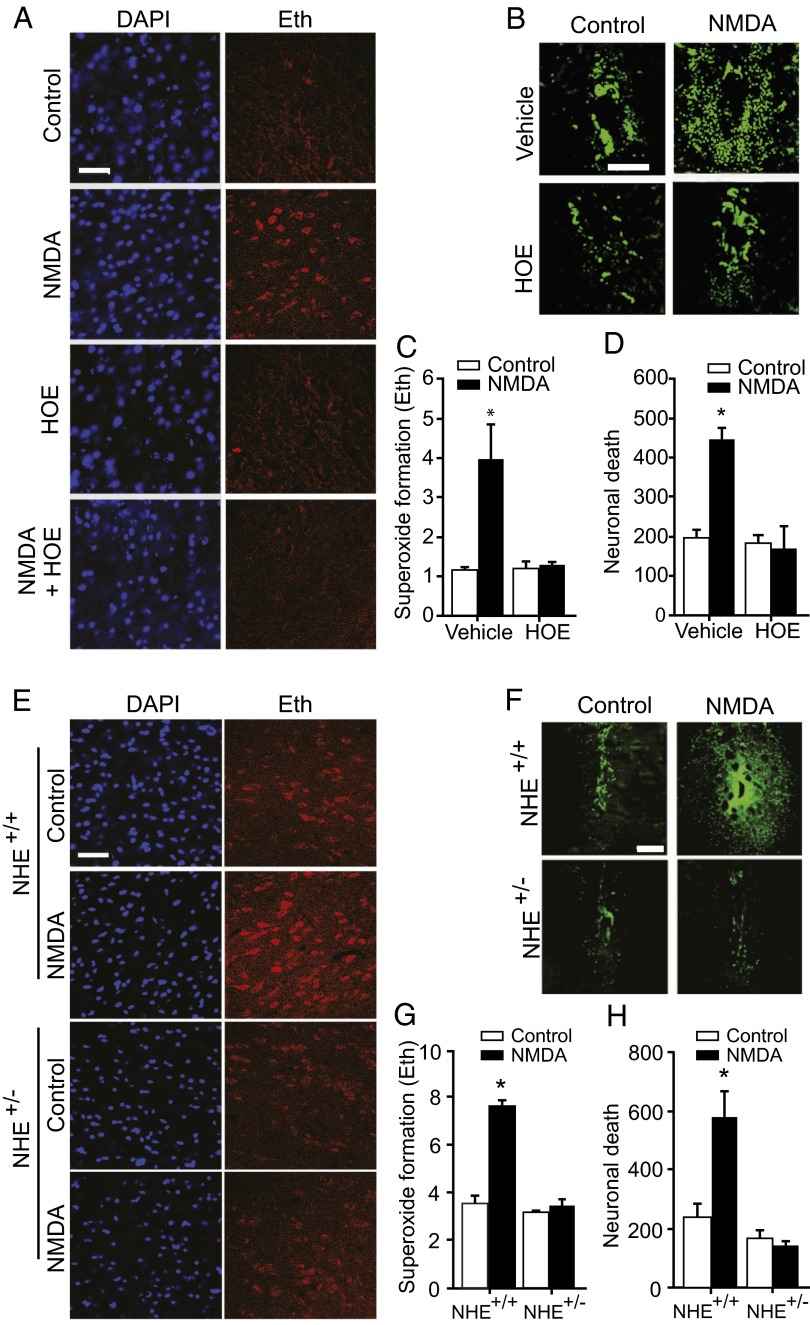
We next used HOE to evaluate the effect of intracellular acidosis on superoxide formation and neuronal death induced by NMDA injection into mouse striatum. NMDA induced a neuronal superoxide signal (ethidium; Eth) in neurons around the injection site within 1 h, and produced neuronal death within 24 h (Fig. 4 A–D). By contrast, mice coinjected with NMDA and HOE showed no increase in superoxide formation or cell death over that produced by vehicle injection alone.
Fig. 4.
Reduced Na+/H+ exchange prevents neuronal superoxide production after NMDA injections into brain. (A) Eth fluorescence (red) in representative striatal sections harvested 30 min after 6 nmol NMDA injections shows reduced superoxide formation in mice coinjected 6 nmol HOE. DAPI provides nuclear counterstaining. (B) Degenerating neurons identified by Fluoro-Jade B (green) in striatal sections harvested 1 d after NMDA injections. There is scattered neuronal death adjacent to the needle track in vehicle-injected brains. The more extensive neuronal death in NMDA-injected brains was reduced by HOE. (C and D) Quantified data; n = 4, *P < 0.05 vs. all other groups. (E) Eth fluorescence (red) in striatal sections harvested 30 min after 6 nmol NMDA injections shows reduced superoxide formation in mice with reduced expression of the NHE1 Na+/H+ exchanger (NHE1+/− mice). (F) Degenerating neurons identified by Fluoro-Jade B (green) in striatal sections harvested 1 d after NMDA injections. (G and H) Quantified data; n = 4, *P < 0.05 vs. all other groups. (Scale bars: 30 µm for A and E, 100 µm for B and F.)
NHE1 is the major Na+/H+ exchanger isoform expressed in brain (27, 28), and HOE is a relatively specific inhibitor of this isoform (29). To confirm the role of NHE1 in this process, we performed NMDA injections in NHE1+/− mice, which have a 70% reduction in brain expression of NHE1 relative to wild-type, NHE1+/+ mice (29). NHE1+/− mice, like HOE-treated wild-type mice, showed no increased superoxide formation or neuronal death following striatal NMDA injections (Fig. 4 E–H). Immunostaining for 4HNE formation was limited by technical issues in the NMDA-injected brains.
The release and impaired reuptake of endogenous glutamate during brain ischemia–reperfusion activates NMDA receptors, and the resulting production of superoxide by neuronal NOX2 is a primary cause of ischemic neuronal death (30–34). Therefore, we also compared superoxide formation after ischemia–reperfusion in wild-type and NHE1+/− mouse hippocampus. Wild-type mice showed a robust and rapid increase in superoxide production in CA1 pyramidal neurons, consistent with prior results (31, 32). By contrast, NHE1+/− mice showed no significant increase in neuronal superoxide production, as measured by either Eth or 4HNE formation (Fig. 5). NHE1+/− mice have previously been shown to have reduced neuronal death after ischemia–reperfusion (29, 35).
Fig. 5.
Reduced Na+/H+ exchange prevents neuronal superoxide production after ischemia–reperfusion. (A and B) Eth fluorescence (red) and 4HNE (green) in representative hippocampal sections harvested 30 min after ischemia–reperfusion show reduced superoxide formation in NHE1+/− mice. (Scale bar: 60 µm in A, 15 µm in B.) (C and D) show quantified data; n = 4, *P < 0.05 vs. all other groups.
Discussion
Superoxide contributes to neuronal injury in stroke and other conditions that lead to NMDA excitotoxicity. The present findings show that NMDA-induced superoxide production is highly sensitive to intracellular pH. Intracellular acidification in cultured neurons by as little as 0.2 pH units, induced by either lowered medium pH or with an NHE1 inhibitor (HOE), prevented NMDA-induced superoxide production and neuronal death. In mouse brain, both HOE and reduced NHE1 expression blocked superoxide production induced by NMDA or ischemia–reperfusion. Intracellular pH is thus a potent regulator of excitotoxic superoxide production.
Although superoxide can originate from multiple sources including mitochondria, suppression of NOX2 activity by pharmacologic agents, peptide inhibitors, or genetic deletion eliminates both the superoxide production and neuronal death caused by either NMDA receptor activation (Fig. S1; refs. 13–19) and reduce that caused by ischemia–reperfusion (30–32, 34, 36). Eth and 4HNE formation can be induced by factors other than superoxide (37), but the results presented here (Fig. S1) and elsewhere (13, 14, 19) confirm that the Eth and 4HNE formation induced by neuronal NMDA receptor activation are blocked by NOX2 inhibition, and thereby establish their specificity for superoxide under the conditions of these studies. However, both Eth and 4HNE formation are likely insensitive to superoxide production below levels that are normally scavenged by neurons, and superoxide-induced cell death likewise requires a minimum threshold of superoxide production. These threshold effects may partly explain why the relationships between pH and Eth formation, 4HNE formation, and cell death are not identical (Fig. 1).
A neuroprotective effect of mild acidosis in excitotoxicity and ischemia–reperfusion is well established (6, 7). This effect has been postulated to result from the inhibitory effect of extracellular H+ on NMDA-gated calcium influx (8, 9), but a causal relationship has not been demonstrated. Here we show instead that, in neuronal cultures, both NMDA-induced cell death and NOX2 activation are eliminated by small reductions in medium pH that have minimal effect on NMDA-induced Ca2+ influx. Moreover, selective acidification of the intracellular space by the NHE1 inhibitor HOE likewise prevented NMDA-induced cell death and NOX2 inactivation, despite no reduction in NMDA-induced Ca2+ influx. These findings argue that the neuroprotective effect of mild acidosis is not mediated by extracellular H+ on the NMDA receptor, but instead by intracellular acidification. NOX2 activity in nonneuronal cell types has previously been shown to be sensitive to modest intracellular pH reductions (24). The mechanism of this effect is unlikely to be simple mass action, given its steep pH dependence; instead, evidence suggests that H+ concentration influences the phosphorylation status of p47phox, a cytosolic “organizer” subunit of the NOX2 complex (20, 23).
NOX2 produces extracellular superoxide by transferring electrons from NADPH to O2, and in the process generates intracellular H+ (Fig. 2E). In microglia and other immune cells, the H+ generated by NOX2 activity is released to the extracellular space through both the voltage-sensitive proton channel Hv1 and the Na+/H+ exchanger NHE1, and inhibition of either process causes intracellular acidification and resultant cessation of NOX2 activity (21–23). A distinction between Hv1 and NHE1 is that protons released through Hv1 relieve the plasma membrane depolarization caused by intracellular H+ accumulation, whereas Na+/H+ exchange is electroneutral. The present results identified a much higher ratio of NHE1:Hv1 gene expression in neurons than microglia. The reason for this difference is not evident, but it may be that NMDA-induced neuronal acidification results largely from processes other than NOX2 activation, such as Ca2+–ATPase activity, glycolytic activity, and mitochondrial depolarization (26, 28), that require electroneutral H+ disposition to avoid a net charge transfer across the cell membrane.
Neurons express bicarbonate-coupled mechanisms of pH regulation in addition to NHE1 (38). However, studies comparing bicarbonate-containing and bicarbonate-free media showed no difference in the effect of medium pH on superoxide formation (Fig. S3), and studies performed in vivo support a dominant role for NHE1 (Fig. 4). These findings are consistent with reports that NHE1 is crucial for both bicarbonate-independent and bicarbonate-independent regulation of intracellular pH under acidosis conditions (39).
HOE (also known as cariporide) is a relatively selective inhibitor of NHE1, with an IC50 of 0.08 µM (29). The structure of HOE predicts that it does not cross an intact blood–brain barrier (40), necessitating delivery by stereotactic injection in the studies of its effect on acute excitotoxicity in vivo. Homozygous NHE1−/− mice cannot be used because they have seizures, spontaneous mortality, and compensatory changes in gene expression (29); however, NHE1+/− mice provide a useful complement to the HOE studies because these mice have reduced NHE1 activity. NHE1+/− mice have previously been shown to have reduced neuronal death after brain ischemia (29). It should be noted, however, that NHE1 deficiency has additional effects that can influence outcome from brain ischemia. Reduced sodium accumulation in NHE1+/− neurons may promote neuronal survival (29), and reduced sodium accumulation in nonneuronal cells limits brain edema (40). Moreover, suppression of NOX2 activation by NHE1 or Hv1 inhibition in microglia inhibits the innate immune response, and may thereby promote neuronal survival in the postischemic interval (21–23).
It is possible that intracellular acidosis could function in a teleologically advantageous way to limit superoxide production during ischemia. Neurons acidify during ischemia as a result of anaerobic lactic acidosis, in addition to processes induced by NMDA receptor activation. Impaired blood flow prevents removal of accumulated H+, and reperfusion clears accumulated H+. The present findings suggest that this pH-normalizing effect of reperfusion may explain, in part, why superoxide production is greater in reperfused than nonreperfused ischemic brain, and why both NOX2 inhibitors and NMDA receptor antagonists are better neuroprotective agents in reperfused brain than nonreperfused brain (41–43). The degree of intracellular acidification shown here to influence neuronal superoxide production is within the range induced by physiological brain activity (28, 44). Intracellular pH changes may thereby also influence the normal, physiological intercellular signaling mediated by neuronal superoxide production (45).
Materials and Methods
Studies were performed in accordance with protocols approved by the San Francisco Veterans Affairs Medical Center animal studies subcommittee. NHE1+/− and NHE1+/+ littermate controls on SV129/Black Swiss background were bred as described (29). Both males and females were used, equally proportioned between control and experimental groups. All other studies used wild-type Black Swiss mice (Simonsen). N-(diaminomethylidene)-3-methanesulfonyl-4-(propan-2-yl)benzamide (HOE642; abbreviated as “HOE”) was obtained from Sanofi-Aventis (Frankfurt). Cell culture reagents were obtained from Mediatech, and all other reagents were obtained from Sigma-Aldrich except where noted. Data analyses for all studies were performed by observers blinded to the experimental conditions.
Neuronal Cultures.
Primary neuron cultures were prepared as described (46) and used at 10–14 d in vitro, at which time greater than 95% of the cells are neurons (46). Primary microglia were isolated from primary mouse astrocyte/microglial cocultures as described (46). Except where otherwise noted, experiments were initiated by placing the cultures in balanced salt solution (BSS) containing 1.2 mM CaCl2, 0.8 mM MgSO4, 5.3 mM KCl, 0.4 mM KH2PO4, 137 mM NaCl2, 0.3 mM NaHPO4, 5 mM glucose, and 10 mM 1,4 piperazinediethanesulfonate (PIPES), adjusted to the designated pH. Exposures to 100 µM NMDA were performed in BSS with reduced Mg2+ (0.4 mM MgSO4). Where used, drugs were added from concentrated stocks in BSS 10 min before the addition of NMDA. HOE was used at 1 µM, a concentration at which it has high selectivity for the NHE1 isoform of Na+/H+ exchanger (29).
Statistical Analysis.
For in vivo studies, the n denotes the number of mice. For cell cultures studies, the n denotes the number of independent experiments, each using neurons prepared from different embryos. Each independent experiment contained triplicate culture wells or coverslips of each study condition, with measurements obtained from at least 200 neurons in each well from a 24-well plate or an average of 15 neurons per each coverslip. All data are expressed as means ± SEM and assessed using one-way ANOVA followed by the Tukey–Kramer test, where multiple groups are compared against one another, or by Dunnett’s test, where multiple groups are compared against a common control group.
Additional details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Stephen Massa for helpful discussions. This work was supported by the Department of Veterans Affairs (R.A.S.) and by National Institutes of Health Grants NS081149 (to R.A.S.), R01NS48216 (to D.S.), and R01NS38118 (to D.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313029110/-/DCSupplemental.
References
- 1.Boron WF. Regulation of intracellular pH. Adv Physiol Educ. 2004;28(1-4):160–179. doi: 10.1152/advan.00045.2004. [DOI] [PubMed] [Google Scholar]
- 2.Plum F. What causes infarction in ischemic brain?: The Robert Wartenberg Lecture. Neurology. 1983;33(2):222–233. doi: 10.1212/wnl.33.2.222. [DOI] [PubMed] [Google Scholar]
- 3.Aoyama K, et al. Acidosis causes endoplasmic reticulum stress and caspase-12-mediated astrocyte death. J Cereb Blood Flow Metab. 2005;25(3):358–370. doi: 10.1038/sj.jcbfm.9600043. [DOI] [PubMed] [Google Scholar]
- 4.Ying W, Han SK, Miller JW, Swanson RA. Acidosis potentiates oxidative neuronal death by multiple mechanisms. J Neurochem. 1999;73(4):1549–1556. doi: 10.1046/j.1471-4159.1999.0731549.x. [DOI] [PubMed] [Google Scholar]
- 5.Xiong ZG, et al. Neuroprotection in ischemia: Blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118(6):687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Simon RP, Niro M, Gwinn R. Brain acidosis induced by hypercarbic ventilation attenuates focal ischemic injury. J Pharmacol Exp Ther. 1993;267(3):1428–1431. [PubMed] [Google Scholar]
- 7.Tombaugh GC, Sapolsky RM. Mild acidosis protects hippocampal neurons from injury induced by oxygen and glucose deprivation. Brain Res. 1990;506(2):343–345. doi: 10.1016/0006-8993(90)91277-n. [DOI] [PubMed] [Google Scholar]
- 8.Mott DD, et al. Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nat Neurosci. 1998;1(8):659–667. doi: 10.1038/3661. [DOI] [PubMed] [Google Scholar]
- 9.Vyklický L, Jr, Vlachová V, Krůsek J. The effect of external pH changes on responses to excitatory amino acids in mouse hippocampal neurones. J Physiol. 1990;430:497–517. doi: 10.1113/jphysiol.1990.sp018304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang CM, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+ Proc Natl Acad Sci USA. 1990;87(16):6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 12.Lipton SA. Failures and successes of NMDA receptor antagonists: Molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004;1(1):101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan AM, et al. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12(7):857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan-Minnella AM, Shen Y, El-Benna J, Swanson RA. Phosphoinositide 3-kinase couples NMDA receptors to superoxide release in excitotoxic neuronal death. Cell Death Dis. 2013;4:e580. doi: 10.1038/cddis.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364(6437):535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- 16.Patel M, Day BJ, Crapo JD, Fridovich I, McNamara JO. Requirement for superoxide in excitotoxic cell death. Neuron. 1996;16(2):345–355. doi: 10.1016/s0896-6273(00)80052-5. [DOI] [PubMed] [Google Scholar]
- 17.Guemez-Gamboa A, et al. Activation of NOX2 by the stimulation of ionotropic and metabotropic glutamate receptors contributes to glutamate neurotoxicity in vivo through the production of reactive oxygen species and calpain activation. J Neuropathol Exp Neurol. 2011;70(11):1020–1035. doi: 10.1097/NEN.0b013e3182358e4e. [DOI] [PubMed] [Google Scholar]
- 18.Girouard H, et al. NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci. 2009;29(8):2545–2552. doi: 10.1523/JNEUROSCI.0133-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes RC, Brennan AM, Shen Y, Baldwin Y, Swanson RA. Activation of neuronal NMDA receptors induces superoxide-mediated oxidative stress in neighboring neurons and astrocytes. J Neurosci. 2012;32(37):12973–12978. doi: 10.1523/JNEUROSCI.1597-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, et al. Activation of microglia depends on Na+/H+ exchange-mediated H+ homeostasis. J Neurosci. 2010;30(45):15210–15220. doi: 10.1523/JNEUROSCI.3950-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci USA. 2009;106(18):7642–7647. doi: 10.1073/pnas.0902761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, Chanana V, Watters JJ, Ferrazzano P, Sun D. Role of sodium/hydrogen exchanger isoform 1 in microglial activation and proinflammatory responses in ischemic brains. J Neurochem. 2011;119(1):124–135. doi: 10.1111/j.1471-4159.2011.07403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan D, Cherny VV, Murphy R, Katz BZ, DeCoursey TE. The pH dependence of NADPH oxidase in human eosinophils. J Physiol. 2005;569(Pt 2):419–431. doi: 10.1113/jphysiol.2005.094748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siemkowicz E, Hansen AJ. Brain extracellular ion composition and EEG activity following 10 minutes ischemia in normo- and hyperglycemic rats. Stroke. 1981;12(2):236–240. doi: 10.1161/01.str.12.2.236. [DOI] [PubMed] [Google Scholar]
- 26.Irwin RP, Lin SZ, Long RT, Paul SM. N-methyl-D-aspartate induces a rapid, reversible, and calcium-dependent intracellular acidosis in cultured fetal rat hippocampal neurons. J Neurosci. 1994;14(3 Pt 1):1352–1357. doi: 10.1523/JNEUROSCI.14-03-01352.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douglas RM, et al. Sodium-hydrogen exchangers and sodium-bicarbonate co-transporters: Ontogeny of protein expression in the rat brain. Neuroscience. 2001;102(1):217–228. doi: 10.1016/s0306-4522(00)00473-5. [DOI] [PubMed] [Google Scholar]
- 28.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83(4):1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 29.Luo J, Chen H, Kintner DB, Shull GE, Sun D. Decreased neuronal death in Na+/H+ exchanger isoform 1-null mice after in vitro and in vivo ischemia. J Neurosci. 2005;25(49):11256–11268. doi: 10.1523/JNEUROSCI.3271-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walder CE, et al. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28(11):2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- 31.Yoshioka H, et al. NADPH oxidase mediates striatal neuronal injury after transient global cerebral ischemia. J Cereb Blood Flow Metab. 2011;31(3):868–880. doi: 10.1038/jcbfm.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suh SW, et al. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64(6):654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahles T, Brandes RP. NADPH oxidases as therapeutic targets in ischemic stroke. Cell Mol Life Sci. 2012;69(14):2345–2363. doi: 10.1007/s00018-012-1011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang QG, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29(44):13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, et al. Gene inactivation of Na+/H+ exchanger isoform 1 attenuates apoptosis and mitochondrial damage following transient focal cerebral ischemia. Eur J Neurosci. 2008;28(1):51–61. doi: 10.1111/j.1460-9568.2008.06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Q, et al. Increased NADPH oxidase-derived superoxide is involved in the neuronal cell death induced by hypoxia-ischemia in neonatal hippocampal slice cultures. Free Radic Biol Med. 2012;53(5):1139–1151. doi: 10.1016/j.freeradbiomed.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radic Biol Med. 2010;48(8):983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majumdar D, Bevensee MO. Na-coupled bicarbonate transporters of the solute carrier 4 family in the nervous system: Function, localization, and relevance to neurologic function. Neuroscience. 2010;171(4):951–972. doi: 10.1016/j.neuroscience.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao H, Ma E, Gu XQ, Haddad GG. Intracellular pH regulation of CA1 neurons in Na(+)/H(+) isoform 1 mutant mice. J Clin Invest. 1999;104(5):637–645. doi: 10.1172/JCI6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Donnell ME, et al. Intravenous HOE-642 reduces brain edema and Na uptake in the rat permanent middle cerebral artery occlusion model of stroke: Evidence for participation of the blood-brain barrier Na/H exchanger. J Cereb Blood Flow Metab. 2013;33(2):225–234. doi: 10.1038/jcbfm.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HA, et al. Brain infarct volume after permanent focal ischemia is not dependent on Nox2 expression. Brain Res. 2012;1483:105–111. doi: 10.1016/j.brainres.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 42.Dawson DA, Graham DI, McCulloch J, Macrae IM. Anti-ischaemic efficacy of a nitric oxide synthase inhibitor and a N-methyl-D-aspartate receptor antagonist in models of transient and permanent focal cerebral ischaemia. Br J Pharmacol. 1994;113(1):247–253. doi: 10.1111/j.1476-5381.1994.tb16201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchan A, Pulsinelli WA. Hypothermia but not the N-methyl-D-aspartate antagonist, MK-801, attenuates neuronal damage in gerbils subjected to transient global ischemia. J Neurosci. 1990;10(1):311–316. doi: 10.1523/JNEUROSCI.10-01-00311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magnotta VA, et al. Detecting activity-evoked pH changes in human brain. Proc Natl Acad Sci USA. 2012;109(21):8270–8273. doi: 10.1073/pnas.1205902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 2011;14(10):2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kauppinen TM, Swanson RA. Poly(ADP-ribose) polymerase-1 promotes microglial activation, proliferation, and matrix metalloproteinase-9-mediated neuron death. J Immunol. 2005;174(4):2288–2296. doi: 10.4049/jimmunol.174.4.2288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.