Significance
Vascular normalization and stress-alleviation treatments are promising strategies to improve tumor perfusion and delivery of drugs. Vascular normalization is more effective for tumors with hyperpermeable but uncompressed vessels, whereas stress alleviation is more beneficial for compressed but low-permeable vessels. In the case of tumors with hyperpermeable and compressed vessels, the two strategies need to be combined for improved treatment.
Keywords: tumor microenvironment, vessel decompression, vessel permeability, mechanical forces, mathematical modeling
Abstract
Blood perfusion in tumors can be significantly lower than that in the surrounding normal tissue owing to the leakiness and/or compression of tumor blood vessels. Impaired perfusion reduces oxygen supply and results in a hypoxic microenvironment. Hypoxia promotes tumor progression and immunosuppression, and enhances the invasive and metastatic potential of cancer cells. Furthermore, poor perfusion lowers the delivery of systemically administered drugs. Therapeutic strategies to improve perfusion include reduction in vascular permeability by vascular normalization and vascular decompression by alleviating physical forces (solid stress) inside tumors. Both strategies have shown promise, but guidelines on how to use these strategies optimally are lacking. To this end, we developed a mathematical model to guide the optimal use of these strategies. The model accounts for vascular, transvascular, and interstitial fluid and drug transport as well as the diameter and permeability of tumor vessels. Model simulations reveal an optimal perfusion region when vessels are uncompressed, but not very leaky. Within this region, intratumoral distribution of drugs is optimized, particularly for drugs 10 nm in diameter or smaller and of low binding affinity. Therefore, treatments should modify vessel diameter and/or permeability such that perfusion is optimal. Vascular normalization is more effective for hyperpermeable but largely uncompressed vessels (e.g., glioblastomas), whereas solid stress alleviation is more beneficial for compressed but less-permeable vessels (e.g., pancreatic ductal adenocarcinomas). In the case of tumors with hyperpermeable and compressed vessels (e.g., subset of mammary carcinomas), the two strategies need to be combined for improved treatment outcomes.
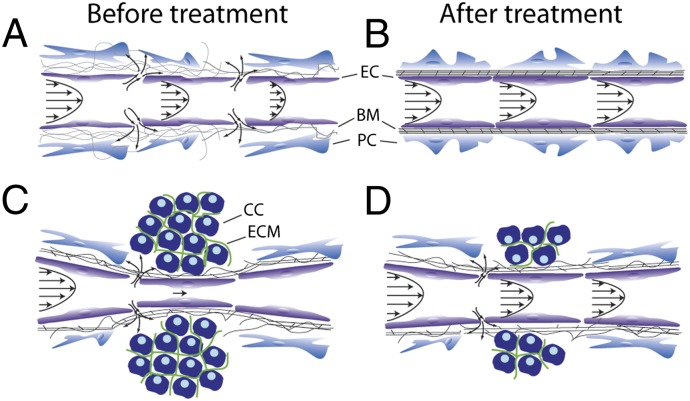
Perfused vessels are necessary for enabling adequate oxygenation and distribution of systemically administered drugs in solid tumors. However, perfusion rates in some regions of a tumor can be significantly lower than that in the peritumor normal tissue, leading to hypoxia, low pH, and inadequate drug delivery. Impaired blood perfusion in tumors could result from (i) excessive fluid loss from the vasculature to the interstitial space owing to vessel hyperpermeability (Fig. 1A), (ii) increased resistance to fluid flow caused by vessel tortuosity, and (iii) reduced effective cross-sectional area for blood flow due to vessel compression (Fig. 1C) (1, 2). Vessel hyperpermeability and tortuosity can be lowered using judicious doses of antiangiogenic agents (Table 1), which increases pericyte coverage and fortifies the leaky vessels without excessive pruning of vessels. This strategy—referred to as “vascular normalization”—can improve tumor perfusion and thereby increase oxygen and drug delivery as well as treatment efficacy (1, 3–6). Fig. 1 A and B shows a schematic of how vascular normalization can improve perfusion by improving the structure and composition of the vessel wall, and Table 1 summarizes evidence in various preclinical tumor models and in cancer patients.
Fig. 1.
Schematic of therapeutic strategies to improve tumor perfusion. (A) Abnormalities in interendothelial junctions, pericyte coverage, and/or basement membrane lead to hyperpermeability of tumor blood vessels and excessive fluid leakage that slows down blood flow. (B) Vascular normalization fortifies the vessel wall, resulting in smaller interendothelial gaps (“pores”) and improved perfusion. (C) Structural components of the tumor microenvironment exert forces on blood vessels, resulting in vessel compression and reduced blood flow. (D) Alleviation of these forces by selective depletion of tumor constituents (e.g., cells or extracellular matrix) can decompress the vessels and improve vessel perfusion. BM, basement membrane; CC, cancer and/or stromal cell; EC, endothelial cell; ECM, extracellular matrix; PC, pericyte.
Table 1.
Studies showing vascular normalization improves perfusion measured as improved oxygenation
| Therapeutic agent | Tumor model | Effect on oxygenation (refs.) |
| Antibody | ||
| Bevacizumab | Melanoma, breast and ovarian carcinomas, GBM | ↑ (7, 8) |
| DC101 | GBM, mammary carcinoma | ↑ (9, 10) |
| TKI | ||
| Cediranib | GBM | ↑ (11–13)* |
| Sunitinib | Squamous carcinoma | ↑ (14) |
| Semaxanib | Melanoma | ↑ (15) |
| Other therapies | ||
| PI-103 (PI3K inhibitor) | Fibrosarcoma, squamous carcinoma | ↑ (16) |
| FTIs (Ras inhibitors) | Prostate carcinoma, bladder carcinoma, glioma, fibrosarcoma, squamous carcinoma | ↑ (16–18) |
| Nelfinavir (AKT inhibitor) | Fibrosarcoma, squamous carcinoma | ↑ (16) |
| PHD2 down-regulation | Lung carcinomas | ↑ (19) |
| R-Ras up-regulation | Melanomas and lung carcinomas | ↑ (20) |
| Cancer cells nitric oxide synthesis inhibition | Glioblastomas | ↑ (21) |
| VE-PTP inhibition | Breast carcinomas | ↑ (22) |
Information given in the table is updated from ref. 5. FTI, farnesyltransferase inhibitors; GBM, glioblastoma multiforme; PHD2, prolyl hydroxylase domain protein 2; TKI, tyrosine kinase inhibitor; VE-PTP, vascular endothelial protein tyrosine phosphatase.
Clinical evidence.
Vessel compression is a result of physical forces—referred to as solid stress—accumulated within solid components of tumors (cancer and stromal cells, collagen, and hyaluronan) (23–25). Stress alleviation can be achieved by depletion of any or all of these components, which can reopen compressed vessels and improve perfusion and delivery of drugs (25, 26). The schematic in Fig. 1 C and D shows how depletion of cancer or stromal cells can decompress blood vessels, and Table 2 presents therapeutic agents that have been used to deplete components of tumors and decompress tumor vessels.
Table 2.
Studies showing stress alleviation decompresses vessels and improves perfusion
| Therapeutic agent | Target | Tumor model | Effect on vessel diameter/density (refs.) | Effect on perfusion (refs.) |
| Diptheria toxin | Cancer cells | Soft tissue sarcoma | ↑ (24) | Not reported |
| Taxane | Cancer cells | Soft tissue sarcoma | ↑ (23) | Not reported |
| Saridegib | Stromal cells | Pancreatic ductal carcinoma | ↑ (25, 27) | ↑ (25, 27) |
| PEGPH20 | Hyaluronan | Pancreatic ductal carcinoma | ↑ (28, 29) | ↑ (28, 29) |
| 1D11 | Collagen | Mammary carcinoma | ↑ (30) | ↑ (30) |
| Losartan | Stromal cells, hyaluronan, collagen | Pancreatic ductal carcinoma | ↑ (26) | ↑ (26) |
Diphtheria toxin is preferentially toxic to human cancer cells and thus preferentially kills cancer cells in a human tumor xenograft model. Taxane is a chemotherapeutic agent. Saridegib is an inhibitor of the Hedgehog cellular signaling pathway. PEGPH20 is a PEGylated human recombinant hyaluronidase. 1D11 is a TGF-β neutralizing antibody. Losartan is an angiotensin receptor blocker.
One challenge now is to better understand under what conditions vascular normalization and vessel decompression improve perfusion in solid tumors and to develop guidelines for optimal use alone and in combination. To this end, we use a mathematical model for fluid flow and drug transport in tumors that accounts explicitly for the geometry and leakiness of the blood vessels. Our model accounts for preclinical and clinical data and suggests guidelines for effective use of these two therapeutic strategies. The tumor vasculature is represented as a percolation network with one inlet and one outlet (31, 32) (Fig. S1). The baseline value for the diameter of the vessels is set at 15 μm (1), whereas the leakiness is defined by the size (i.e., diameter) of the pores of the vessel wall. The pore size in our analysis varies from 50 nm (poorly permeable tumor vessels) to 400 nm (hyperpermeable vessels) (33). To quantify vascular efficiency we calculate the fraction of perfused vessels and the effective vascular density. Perfused vessels are considered to be those with velocities greater than 0.1 mm/s (34), whereas the effective vascular density is the density of the perfused vessels. Perfusion is considered optimal when these two parameters are maximal.
Results
Vessel Decompression Improves Perfusion in Tumors with Abundant Compressed but Poorly or Moderately Permeable Vessels.
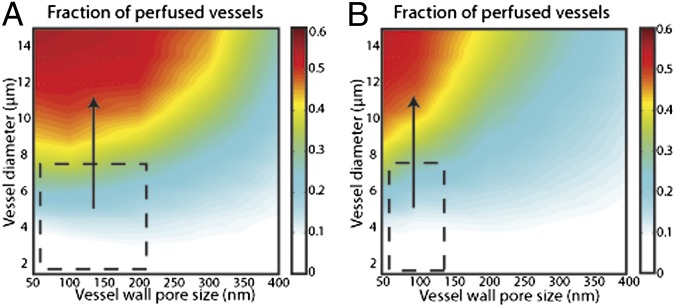
We first investigated under what conditions decompression of blood vessels with stress-alleviation treatment can improve tumor perfusion. We modeled vessel decompression by increasing the diameter of the vessels to the baseline value. The effects for different vessel wall pore sizes are shown in Fig. 2, and in Figs. S2 and S3 for compressed central regions occupying 80% and 30% of the tumor, respectively. The figures show that our results are qualitatively independent from the region of vessel compression. For pore sizes less than 200 nm (low or moderately permeable tumors), there is a strong dependence of the fraction of perfused vessels and the effective vascular density on increases in vessel diameter. Recently, we found that stress-alleviation therapy in murine pancreatic ductal adenocarcinomas increased vessel diameter by 20% (from 10 to 12 μm) and the perfused vessel fraction by 47% (from 0.32 to 0.47) (25). Our model predictions are within this range and, moreover, they show that perfusion is optimal for uncompressed vessels with low permeability. Therefore, vessel decompression would be most beneficial for tumors with abundant compressed but poorly or moderately permeable vessels (Fig. 2, dashed line), such as pancreatic ductal adenocarcinomas. However, decompression of tumor vessels is reversible and vessels can revert to their compressed state unless stress-alleviation treatment is continued.
Fig. 2.
Results for the fraction of perfused vessels as a function of the vessel diameter and the vessel wall pore size, a determinant of vascular permeability. Two values of the hydraulic conductivity of the interstitial space were used: (A) 1 × 10−7 cm2/mmHg-s and (B) 1 × 10−6 cm2/mmHg-s. The compressed central region occupies 80% of the tumor. The colors represent the values of the fraction of perfused vessels and the dashed line depicts the region within which vessel decompression is beneficial. The fraction of the well-perfused vessels becomes optimal for uncompressed (larger diameter) and low-permeable (smaller vessel wall pore size) vessels. The fraction of perfused vessels decreases if the tumor vessels are compressed or hyperpermeable. Vessel decompression is beneficial for compressed and low-permeable vessels.
The ease with which the interstitial fluid percolates through the extravascular space of a tissue is referred to as its hydraulic conductivity. Hydraulic conductivity affects blood velocity because of the communication between vascular and interstitial fluid (35). High hydraulic conductivity allows fluid to rapidly flow in the interstitial space and be drained by peripheral lymphatic vessels. This prevents accumulation of interstitial fluid and thus more fluid can leak from the vessel wall, which in turn decreases perfusion. Results shown in Fig. 2 and Fig. S2 suggest that tumors with a relatively high hydraulic conductivity, usually tumors with low collagen and hyaluronan levels such as some melanomas, would be less responsive to stress-alleviation treatment.
Vascular Normalization Improves Perfusion of Tumors with Hyperpermeable and Uncompressed Vessels.
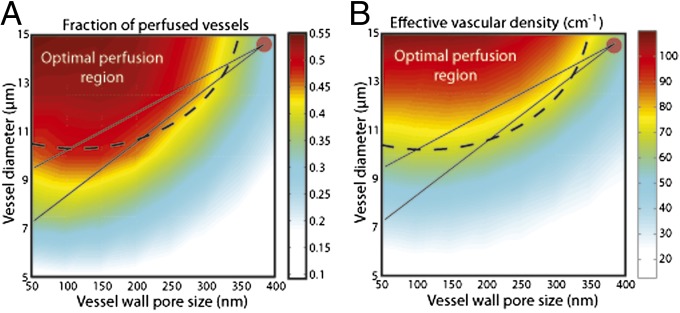
In parallel, we tested under what conditions vascular normalization can improve perfusion (Fig. 3). The tumor vessel was assigned an initial diameter of 15 μm and a vascular pore size of 400 nm (circle in the top right corner of Fig. 3). An ideal treatment to optimize perfusion in such a vessel would aim at decreasing its permeability while keeping the vessel open. In our previous animal study (36), the vessel diameter changed from 14.5 μm to 7.3, 9.5, and 9.5 μm at days 1, 3, and 5 after anti-VEGF treatment, a predominant angiogenic growth factor in many tumors. The solid lines in the figure show the potential paths of the treatment as the diameter decreased from 14.5 μm to 7.3 μm (day 1) or 9.5 μm (days 3 and 5). Even though the decrease in pore size was not measured, there is a large range of pore sizes for which treatment falls into the optimal perfusion region. One should note that vascular normalization is reversible and if anti-VEGF treatment is stopped the vascular structure will return to an abnormal, less-functional state (37). Indeed, in our clinical trial in recurrent glioblastoma patients we saw a similar reversal in tumor vessel structure and function (38).
Fig. 3.
Results for (A) fraction of perfused vessels and (B) effective vascular density as a function of vessel diameter and vessel wall pore size. The dashed line depicts the region within which vascular normalization is beneficial (referred to as optimal perfusion region), the circle shows the initial values, and the solid lines show potential paths of the treatment. The fraction of the well-perfused vessels and the effective vascular density become optimal for uncompressed (larger diameter) and low-permeable (smaller vessel wall pore size) vessels. The fraction of perfused vessels decreases if the tumor vessels are compressed or hyperpermeable. Vascular normalization is beneficial for compressed and hyperpermeable vessels.
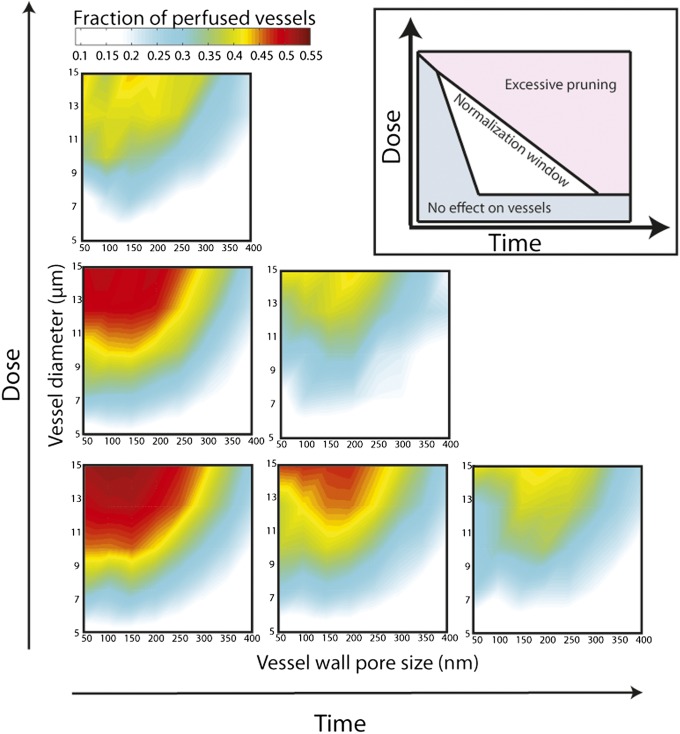
In another study (10), we found that the increase in tumor perfusion was dependent on the dose of anti-VEGF receptor 2 (VEGFR2) antibody used. The lowest dose of anti-VEGFR2 antibody caused an increase in the perfused vessel fraction from 0.2 to 0.45, whereas the highest dose did not increase perfusion. These values of perfused vessel fraction are within the range of our study—the highest dose of the antibody led to a significant decrease in vessel diameter and/or pruning of tumor vessels, yielding compromised perfusion (Figs. 3 and 4). Fig. 4 shows changes in perfusion as a function of time and dose. The region of improved perfusion is related to the window of normalization, and thus the higher the dose of treatment, the shorter the window of normalization, and excessive pruning would lead to hypoxia.
Fig. 4.
Normalization window as a function of dose and time. Higher doses of antiangiogenic treatment over time increase vessel pruning, which in turn decreases the effective vascular density and impairs vascular efficiency. As a result the fraction of perfused vessels and the region of optimal perfusion decrease. These dose- and time-dependent effects create a normalization window within which drug delivery is optimized. (Inset) Schematic presents the effects of dose and time on the normalization window (adapted from ref. 2).
Other parameters that affect vascular normalization are the degree of vessel compression and the hydraulic conductivity. Our results suggest that effective anti-VEGF treatment requires tumor vessels to be open (Fig. 3). In cases where tumor vessels are compressed normalization of abnormal vessels and the resulting decrease in vascular permeability will not be sufficient to improve perfusion. Fig. S4 depicts the fraction of perfused vessels for three different values of the hydraulic conductivity. As for vessel decompression, a high conductivity compromises the therapeutic outcome. Taken all together, vascular normalization is expected to be more effective for tumors with hyperpermeable and uncompressed vessels with low or moderate values of hydraulic conductivity, such as a subset of glioblastomas, melanomas, and ovarian carcinomas.
Combined Treatment Improves Perfusion in Tumors with Compressed Yet Hyperpermeable Vessels.
Our analysis thus far suggests that neither vessel decompression nor vascular normalization would be effective in treating tumors with compressed and hyperpermeable vessels, such as those seen in a subset of mammary carcinomas. In such a case, a combined treatment might be beneficial. A treatment to alleviate solid stress (depletion of cells or extracellular matrix) to decompress blood vessels would increase the effective vascular density without affecting significantly the fraction of perfused vessels (Fig. 2 and Fig. S2). Then, a subsequent or concurrent vascular normalization treatment would have the potential to improve perfusion (Fig. 3). Attention should be given, however, to the fact that stress-alleviation therapies, which aim to selectively deplete structural components of tumors, might increase the hydraulic conductivity of the interstitial space and thus reduce the therapeutic potential of vascular normalization. Indeed, the hydraulic conductivity depends on the concentration of collagen and hyaluronan, and empirical correlations to describe this relationship exist (39–41). In Fig. S5, we used these methodologies to calculate the hydraulic conductivity of tumors. Assuming that the optimal perfusion region is severely affected by conductivity values higher than 5 × 10−7 cm2/mmHg-s (Fig. S4), data in Fig. S5 suggest that collagen or hyaluronan can be significantly decreased and still the tumors be responsive to anti-VEGF treatment. The schematic in Fig. 5 summarizes the guidelines for improving perfusion with these two strategies based on our baseline values. Fig. S6 provides examples from preclinical studies.
Fig. 5.
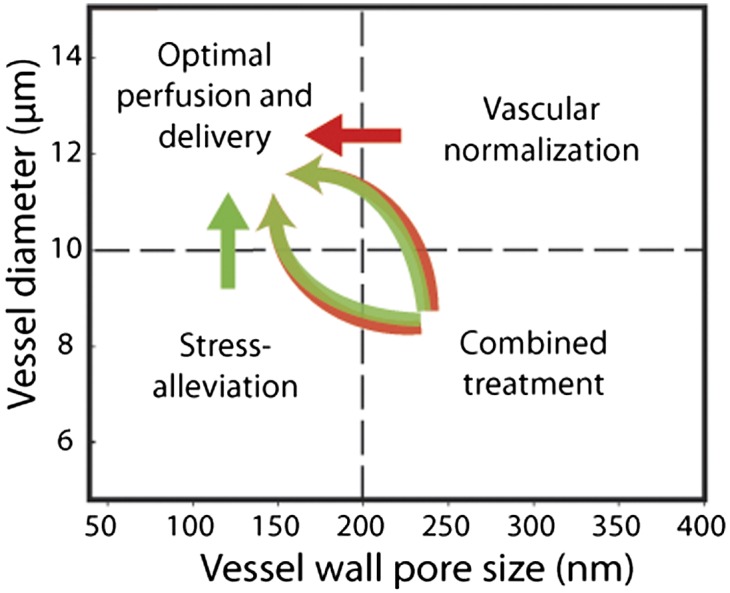
Schematic of the proposed therapeutic strategies to improve perfusion and drug delivery. Tumors with hyperpermeable and uncompressed vessels benefit from vascular normalization strategy (red arrow). Tumors with compressed and low or moderately permeable vessels benefit from vessel decompression/stress-alleviation strategy (green arrow). Tumors with compressed and hyperpermeable vessels benefit from the application of stress-alleviation treatment to decompress vessels along with vascular normalization treatment to decrease permeability (two-color arrows).
Improved Perfusion Enhances Delivery of Drugs.
To elucidate the relationship between perfusion and drug delivery, we calculated the transport of drugs with sizes ranging from 1 nm (e.g., chemotherapeutic agents) to 10 nm (e.g., antibodies, nab-paclitaxel following disintegration in plasma) to 60 nm (e.g., liposomes and micelles). The model accounts for the transport of the drug from the vessels to the tumor interstitial space and for drug binding to and internalized by cancer cells (details are given in Supporting Information) (42–44). Fig. 6 shows the intratumoral distribution of the drug with low or high binding affinity as a function of the perfused vessel fraction. Intratumoral distribution is defined as the fraction of the tumor in which the drug is bound to cancer cells in amounts higher than 1% of the concentration at the inlet of the vascular network. Delivery is improved with improved perfusion for drugs of all sizes, and the effect is more prominent for small drugs with low binding affinity. Small drugs diffuse quickly in the tumor extracellular matrix and thus their transport is perfusion-limited. By contrast, large-size particles cannot effectively penetrate into the tumor tissue and a rate-limiting step is the interstitial transport. Binding to cells or matrix is an additional barrier to interstitial transport. The higher the binding affinity, the more difficult it is for the drug to spread into the tumor. Interestingly, for drugs binding with a high affinity, the distribution of the 10-nm and 60-nm drugs is the same. This suggests that drug binding to cells dominates interstitial diffusion, whereas for low binding affinity interstitial diffusion is important and for that reason 10-nm particles distribute better than 60-nm particles. Our results are in agreement with previous studies showing that delivery of 10-nm particles is superior compared with that of 60-nm and 120-nm particles in tumors following vascular normalization (32). In general, the smaller the drug, the better it will distribute into the tumor, unless binding dominates. This may explain why nab-paclitaxel (10 nm in size) improves overall survival of metastatic breast and pancreatic adenocarcinoma patients, whereas pegylated liposomal doxorubicin (100 nm in size) does not robustly improve survival over conventional chemotherapy (45–47).
Fig. 6.
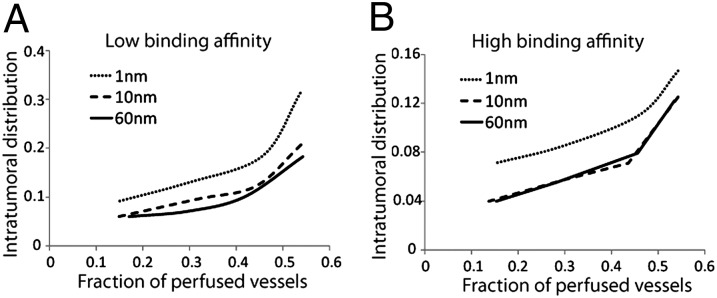
Intratumoral distribution of therapeutic agents as a function of perfused vessel fraction for drugs with (A) low binding affinity and (B) high binding affinity (details in Supporting Information). Three curves in each panel show the intratumoral distribution of drugs of three sizes (1, 10, and 60 nm) versus the fraction of perfused vessels. The curves demonstrate that the intratumoral distribution of drugs of all three sizes increases with the fraction of perfused vessels, but in a nonlinear manner. The increase is more substantial for smaller drugs than for larger drugs. Intratumoral distribution is defined as the fraction of the tumor extravascular space that the drug is bound to cancer cells in amounts higher than 1% of the concentration at the inlet of the vascular network.
Discussion
In this article, we used a mathematical framework for blood perfusion in solid tumors focusing on the effects of vessel leakiness and compression. In agreement with our model predictions, vascular normalization has been shown to improve perfusion and therapeutic outcomes in both animal and human studies (9–13, 22, 32, 48) (Table 1). It is, however, effective mainly in the setting of leaky tumor vessels with open lumen (e.g., glioblastomas) and might not benefit compressed vessels. This would explain why antiangiogenic agents have failed thus far in desmoplastic tumors such as pancreatic ductal adenocarcinomas, with abundant compressed vessels (49, 50). Furthermore, anti-VEGF treatment has a dose-dependent effect, and a high dose or long-term treatment with anti-VEGF agents can cause excessive pruning of vessels and impairment of vascular efficiency (Fig. 4 and refs. 2 and 10). This could explain why in many studies antiangiogenic strategies reduce drug delivery (51–55). Monitoring of blood perfusion or oxygenation in tumors during treatment could be used to identify the right dose. This could be done, for instance, with a novel functional MRI approach termed “vessel architecture imaging” (13). Additionally, anti-VEGF treatment has a size-dependent effect because the decrease in vessel wall pore size might exclude the delivery of nanoscale drugs larger than the pore size. Indeed, we have recently shown that normalization treatment improved the delivery of small particles with a size of 10 nm but not the delivery of 60-nm and 120-nm particles (32). In our analysis anti-VEGF agents were considered to cause a decrease in vessel diameter and vascular density, which compromises the benefit of reducing vessel leakiness on perfusion. One notable exception is when vessels are normalized with restoring nitric oxide gradients, which increases both vessel density and diameter (21, 22, 56). Finally, vessel leakiness is a hallmark of inflammation as well. In inflammation, however, plasma extravasation and leukocyte adhesion occur in a coordinated manner to enable the immune response and to maintain tissue perfusion.
Our model suggests that treatment to alleviate solid stress in tumors has the potential to enhance blood flow. Many studies, indeed, support the idea that decompression of blood vessels by alleviating solid stress improves perfusion and treatment efficacy (Table 2). We have recently shown that depletion of hyaluronan or collagen can lower solid stress levels in a variety of tumors, including pancreatic cancers (25, 26). This reduction in solid stress may explain how enzymatic degradation of hyaluronan combined with cytotoxic agents can improve perfusion and the overall survival of mice bearing pancreatic ductal adenocarcinomas (28, 29). Furthermore, depletion of collagen using losartan, an angiotensin receptor blocker, or an anti–TGF-β antibody improved the in vivo delivery of therapeutics in a variety of tumors in mice (26, 30, 57). In addition, pharmacological depletion of stromal cells with saridegib, an inhibitor of the Sonic Hedgehog pathway, has been shown to decrease solid stress (25) and improve drug delivery and overall survival of mice bearing pancreatic tumors (27). This drug, however, recently failed in a phase-II clinical trial for pancreatic cancer patients when combined with gemcitabine (58), presumably owing to intrinsic resistance to gemcitabine by these tumors. Increased delivery of a drug might not benefit patients if cancer cells are resistant to that drug.
Retrospective analysis of clinical data has shown that patients with pancreatic ductal adenocarcinomas receiving angiotensin receptor blockers survive approximately 6 mo longer than those who do not (59). Similar retrospective analyses have shown increased survival in patients with lung or renal cancers treated with such drugs (60, 61). Indeed, based on our preclinical findings (26), our Massachusetts General Hospital colleagues have initiated a phase-II trial with losartan and FOLFIRINOX in pancreatic ductal adenocarcinoma patients (clinicaltrials.gov identifier NCT01821729). Finally, depletion of cells with chemotherapeutics (or fractionated radiation) is another way to alleviate stresses and decompress vessels, which might explain the greater tumor response to chemotherapy in a subset of pancreatic cancer patients who are able to tolerate a combination of highly toxic drugs (62).
Our current study is of particular importance because it proposes new considerations for the use of treatments that modify the tumor microenvironment. Vessel compression is caused by physical forces exerted by the cells and extracellular matrix (25). Therefore, desmoplastic tumors should have a large amount of compressed vessels and thus anti-VEGF drugs (Table 1) most likely would not work, unless these drugs also alleviate solid stress by depleting stromal elements. If not, stress-alleviation drugs (Table 2) to decompress vessels should be considered either alone or in combination with anti-VEGF agents (Table 1). Less-desmoplastic tumors are expected to have a low fraction of compressed vessels, and thus stress-alleviation treatment would not be required. In this case, the use of anti-VEGF treatment with agents that target cancer cells should be considered (Table 3). To turn our theoretical predictions into practice, one needs to identify which tumors are leaky, compressed, both, or neither. This is a challenging task. Although we can make some broad statements (e.g., pancreatic ductal adenocarcinomas are desmoplastic), there are many tumors such as breast cancers in which the degree of desmoplasia is highly variable from one tumor to the next and potentially from the primary site to the metastatic site, and thus it could be hard to choose an appropriate strategy until the state of that individual tumor is known. Emerging imaging approaches have the potential to help in this selection (13).
Table 3.
Summary of barriers to and strategies for improved drug delivery
| Tumor microenvironment | Strategy |
| Desmoplastic, moderately permeable tumors (e.g., pancreatic ductal adenocarcinomas) | Stress alleviation |
| Hyperpermeable, nondesmoplastic tumors (e.g., subset of glioblastomas and ovarian carcinomas) | Vascular normalization |
| Hyperpermeable, desmoplastic tumors (e.g., a subset of mammary carcinomas) | Combined treatment |
| Drug | Delivery barrier |
| Small size (≤ 10 nm), low binding affinity | Perfusion-limited transport. Improved perfusion increases delivery. |
| Large size (> 10 nm), low binding affinity | Perfusion, transvascular, and interstitial limited transport. Improved perfusion increases delivery. |
| Any size, high binding affinity | Additional barrier to interstitial transport. Improved perfusion increases delivery but benefit may be compromised. |
It is conceivable that opening blood vessels could bring more nutrients to the tumor and increase its growth rate. Also, the opened vessels could allow more metastatic cells to leave the primary tumor and increase metastases. Indeed, this has been shown in a number of preclinical studies (63, 64). Therefore, drugs that open vessels should be given only with concurrent cytotoxic treatments, such as chemotherapy, radiation therapy, immune therapy, or another cancer-cell-targeted treatment. Finally, apart from vessel compression and hyperpermeability, other causes of decreased blood flow in tumors include heterogeneous distribution of blood vessels, intravascular coagulation/thrombosis, and formation of vascular shunts (65, 66). Our model accounts for vascular heterogeneity with the use of an irregular network structure and by permitting vessel pruning. Mathematically, thrombosis is similar to vascular compression because both phenomena reduce the effective cross-sectional area of tumor blood vessels. Vascular shunts are short, high-flow vascular pathways that bypass long downstream pathways and thus exclude downstream regions from blood supply (66). Our model does not recapitulate the high heterogeneity of path lengths of the vessels as well as the enlargement of short flow pathways.
Methods
The current model builds on our recently published framework (32, 67). The baseline parameters of the model are summarized in Table S1. Description of model equations, methodology, and limitations (Figs. S7 and S8) can be found in Supporting Information.
Supplementary Material
Acknowledgments
We thank J. Baish, V. Chauhan, D. Duda, K. Emblem, D. Fukumura, E. Gerstner, S. Goel, L. Munn, T. Padera, and M. Datta. This work was supported by National Cancer Institute Grants P01-CA080124, R01-CA126642, R01-CA115767, R01-CA096915, R01-CA085140, and R01-CA098706; Federal Share Income Grant T32-CA073479; Department of Defense Breast Cancer Research Innovator Award W81XWH-10-1-0016 (to R.K.J.); and European Commission Grants FP7-PIRG08-GA-2010-276894 and ERC-2013-StG-336839 (to T.S.).
Footnotes
Conflict of interest statement: R.K.J. received research grants from MedImmune and Roche; received consultant fees from Enlight, Noxxon, and Zyngenia; owns equity in Enlight, SynDevRx, and XTuit; and serves on the Board of Directors of XTuit and Boards of Trustees of H&Q Healthcare Investors and H&Q Life Sciences Investors. No reagents or funds from these organizations were used in this study.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318415110/-/DCSupplemental.
References
- 1.Jain RK. Determinants of tumor blood flow: A review. Cancer Res. 1988;48(10):2641–2658. [PubMed] [Google Scholar]
- 2.Jain RK. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med. 2001;7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 4.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 5.Goel S, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goel S, Fukumura D, Jain RK. Normalization of the tumor vasculature through oncogenic inhibition: An emerging paradigm in tumor biology. Proc Natl Acad Sci USA. 2012;109(20):E1214. doi: 10.1073/pnas.1203794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dings RP, et al. Scheduling of radiation with angiogenesis inhibitors anginex and Avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res. 2007;13(11):3395–3402. doi: 10.1158/1078-0432.CCR-06-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGee MC, et al. Improved intratumoral oxygenation through vascular normalization increases glioma sensitivity to ionizing radiation. Int J Radiat Oncol Biol Phys. 2010;76(5):1537–1545. doi: 10.1016/j.ijrobp.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA. 2012;109(43):17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batchelor TT, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1318022110. 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorensen AG, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72(2):402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emblem KE, et al. Vascular architecture imaging identifies patient responders to anti-angiogenic therapy. Nat Med. 2013;19(9):1178–1183. doi: 10.1038/nm.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto S, et al. Antiangiogenic agent sunitinib transiently increases tumor oxygenation and suppresses cycling hypoxia. Cancer Res. 2011;71(20):6350–6359. doi: 10.1158/0008-5472.CAN-11-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichhorn ME, et al. Contrast enhanced MRI and intravital fluorescence microscopy indicate improved tumor microcirculation in highly vascularized melanomas upon short-term anti-VEGFR treatment. Cancer Biol Ther. 2008;7(7):1006–1013. doi: 10.4161/cbt.7.7.5997. [DOI] [PubMed] [Google Scholar]
- 16.Qayum N, et al. Tumor vascular changes mediated by inhibition of oncogenic signaling. Cancer Res. 2009;69(15):6347–6354. doi: 10.1158/0008-5472.CAN-09-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen-Jonathan E, et al. The farnesyltransferase inhibitor L744,832 reduces hypoxia in tumors expressing activated H-ras. Cancer Res. 2001;61(5):2289–2293. [PubMed] [Google Scholar]
- 18.Delmas C, et al. The farnesyltransferase inhibitor R115777 reduces hypoxia and matrix metalloproteinase 2 expression in human glioma xenograft. Clin Cancer Res. 2003;9(16 Pt 1):6062–6068. [PubMed] [Google Scholar]
- 19.Leite de Oliveira R, et al. Gene-targeting of Phd2 improves tumor response to chemotherapy and prevents side-toxicity. Cancer Cell. 2012;22(2):263–277. doi: 10.1016/j.ccr.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Sawada J, et al. Small GTPase R-Ras regulates integrity and functionality of tumor blood vessels. Cancer Cell. 2012;22(2):235–249. doi: 10.1016/j.ccr.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashiwagi S, et al. Perivascular nitric oxide gradients normalize tumor vasculature. Nat Med. 2008;14(3):255–257. doi: 10.1038/nm1730. [DOI] [PubMed] [Google Scholar]
- 22.Goel S, et al. Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J Natl Cancer Inst. 2013;105(16):1188–1201. doi: 10.1093/jnci/djt164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 1999;59(15):3776–3782. [PubMed] [Google Scholar]
- 24.Padera TP, et al. Pathology: Cancer cells compress intratumour vessels. Nature. 2004;427(6976):695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 25.Stylianopoulos T, et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci USA. 2012;109(38):15101–15108. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chauhan VP, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. doi: 10.1038/ncomms.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobetz MA, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62(1):112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Provenzano PP, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, et al. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc Natl Acad Sci USA. 2012;109(41):16618–16623. doi: 10.1073/pnas.1117610109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baish JW, et al. Role of tumor vascular architecture in nutrient and drug delivery: An invasion percolation-based network model. Microvasc Res. 1996;51(3):327–346. doi: 10.1006/mvre.1996.0031. [DOI] [PubMed] [Google Scholar]
- 32.Chauhan VP, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7(6):383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hobbs SK, et al. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95(8):4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamoun WS, et al. Simultaneous measurement of RBC velocity, flux, hematocrit and shear rate in vascular networks. Nat Methods. 2010;7(8):655–660. doi: 10.1038/nmeth.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Netti PA, Roberge S, Boucher Y, Baxter LT, Jain RK. Effect of transvascular fluid exchange on pressure-flow relationship in tumors: A proposed mechanism for tumor blood flow heterogeneity. Microvasc Res. 1996;52(1):27–46. doi: 10.1006/mvre.1996.0041. [DOI] [PubMed] [Google Scholar]
- 36.Yuan F, et al. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA. 1996;93(25):14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mancuso MR, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116(10):2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swabb EA, Wei J, Gullino PM. Diffusion and convection in normal and neoplastic tissues. Cancer Res. 1974;34(10):2814–2822. [PubMed] [Google Scholar]
- 40.Jain RK. Transport of molecules in the tumor interstitium: A review. Cancer Res. 1987;47(12):3039–3051. [PubMed] [Google Scholar]
- 41.Levick JR. Flow through interstitium and other fibrous matrices. Q J Exp Physiol. 1987;72(4):409–437. doi: 10.1113/expphysiol.1987.sp003085. [DOI] [PubMed] [Google Scholar]
- 42.Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. III. Role of binding and metabolism. Microvasc Res. 1991;41(1):5–23. doi: 10.1016/0026-2862(91)90003-t. [DOI] [PubMed] [Google Scholar]
- 43.Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008;60(12):1421–1434. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mok W, Stylianopoulos T, Boucher Y, Jain RK. Mathematical modeling of herpes simplex virus distribution in solid tumors: Implications for cancer gene therapy. Clin Cancer Res. 2009;15(7):2352–2360. doi: 10.1158/1078-0432.CCR-08-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien ME, et al. CAELYX Breast Cancer Study Group Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 46.Gradishar WJ, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 47.Von Hoff DD, et al. Results of a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic adenocarcinoma of the pancreas with PET and CA19-9 correlates. J Clin Oncol Suppl. 2013;31(abstr 4005) [Google Scholar]
- 48.Tong RT, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 49.Kindler H, et al. A double-blind, placebocontrolled, randomized phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo (P) in patients (pts) with advanced pancreatic cancer (PC): A preliminary analysis of cancer and leukemia group B (CALGB) J Clin Oncol. 2007;25(18) Suppl:4508. [Google Scholar]
- 50.Philip PA. Targeting angiogenesis in pancreatic cancer. Lancet. 2008;371(9630):2062–2064. doi: 10.1016/S0140-6736(08)60770-9. [DOI] [PubMed] [Google Scholar]
- 51.Daldrup-Link HE, et al. Decrease in tumor apparent permeability-surface area product to a MRI macromolecular contrast medium following angiogenesis inhibition with correlations to cytotoxic drug accumulation. Microcirculation. 2004;11(5):387–396. doi: 10.1080/10739680490457665. [DOI] [PubMed] [Google Scholar]
- 52.Pastuskovas CV, et al. Effects of anti-VEGF on pharmacokinetics, biodistribution, and tumor penetration of trastuzumab in a preclinical breast cancer model. Mol Cancer Ther. 2012;11(3):752–762. doi: 10.1158/1535-7163.MCT-11-0742-T. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, Balthasar JP. Evaluation of a catenary PBPK model for predicting the in vivo disposition of mAbs engineered for high-affinity binding to FcRn. AAPS J. 2012;14(4):850–859. doi: 10.1208/s12248-012-9395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van der Veldt AA, et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: Implications for scheduling of anti-angiogenic drugs. Cancer Cell. 2012;21(1):82–91. doi: 10.1016/j.ccr.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 55.Arjaans M, et al. Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake. Cancer Res. 2013;73(11):3347–3355. doi: 10.1158/0008-5472.CAN-12-3518. [DOI] [PubMed] [Google Scholar]
- 56.Fukumura D, Yuan F, Endo M, Jain RK. Role of nitric oxide in tumor microcirculation. Blood flow, vascular permeability, and leukocyte-endothelial interactions. Am J Pathol. 1997;150(2):713–725. [PMC free article] [PubMed] [Google Scholar]
- 57.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA. 2011;108(7):2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hidalgo M, Von Hoff DD. Translational therapeutic opportunities in ductal adenocarcinoma of the pancreas. Clin Cancer Res. 2012;18(16):4249–4256. doi: 10.1158/1078-0432.CCR-12-1327. [DOI] [PubMed] [Google Scholar]
- 59.Nakai Y, et al. Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer. 2010;103(11):1644–1648. doi: 10.1038/sj.bjc.6605955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilop S, et al. Impact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non-small-cell lung cancer undergoing first-line platinum-based chemotherapy. J Cancer Res Clin Oncol. 2009;135(10):1429–1435. doi: 10.1007/s00432-009-0587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keizman D, et al. Angiotensin system inhibitors and outcome of sunitinib treatment in patients with metastatic renal cell carcinoma: A retrospective examination. Eur J Cancer. 2011;47(13):1955–1961. doi: 10.1016/j.ejca.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conroy T, et al. Groupe Tumeurs Digestives of Unicancer PRODIGE Intergroup FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 63.Stockmann C, et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456(7223):814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rhim AD, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1–2):349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: Biological and clinical aspects. J Thromb Haemost. 2013;11(2):223–233. doi: 10.1111/jth.12075. [DOI] [PubMed] [Google Scholar]
- 66.Pries AR, Höpfner M, le Noble F, Dewhirst MW, Secomb TW. The shunt problem: Control of functional shunting in normal and tumour vasculature. Nat Rev Cancer. 2010;10(8):587–593. doi: 10.1038/nrc2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stylianopoulos T, Soteriou K, Fukumura D, Jain RK. Cationic nanoparticles have superior transvascular flux into solid tumors: Insights from a mathematical model. Ann Biomed Eng. 2013;41(1):68–77. doi: 10.1007/s10439-012-0630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.