Significance
GATA-binding protein 3 (Gata3) controls the differentiation of naive CD4 T cells into T helper 2 (Th2) cells by induction of chromatin remodeling at the Th2 cytokine gene loci. Gata3 also facilitates Th2 cell proliferation via unknown mechanisms. We have identified a functional Gata3/RuvB-like protein 2 (Ruvbl2) complex that regulates the proliferation of differentiating Th2 cells through the repression of a CDK inhibitor, cyclin-dependent kinase inhibitor 2c (Cdkn2c). Gata3 directly bound to the Cdkn2c locus in an Ruvbl2-dependent manner, and Cdkn2c-knockdown experiments indicated an important role for this molecule in the Gata3-mediated induction of Th2-cell proliferation. Ruvbl2-knockdown Th2 cells showed decreased antigen-induced expansion and caused less airway inflammation in vivo, indicating an important role for Ruvbl2 in Th2 cells in allergic reactions.
Keywords: master transcription factor, transcriptional regulation, polycomb group complex
Abstract
GATA-binding protein 3 (Gata3) controls the differentiation of naive CD4 T cells into T helper 2 (Th2) cells by induction of chromatin remodeling of the Th2 cytokine gene loci, direct transactivation of Il5 and Il13 genes, and inhibition of Ifng. Gata3 also facilitates Th2 cell proliferation via additional mechanisms that are far less well understood. We herein found that Gata3 associates with RuvB-like protein 2 (Ruvbl2) and represses the expression of a CDK inhibitor, cyclin-dependent kinase inhibitor 2c (Cdkn2c) to facilitate the proliferation of Th2 cells. Gata3 directly bound to the Cdkn2c locus in an Ruvbl2-dependent manner. The defect in the proliferation of Gata3-deficient Th2 cells is rescued by the knockdown of Cdkn2c, indicating that Cdkn2c is a key molecule involved in the Gata3-mediated induction of Th2 cell proliferation. Ruvbl2-knockdown Th2 cells showed decreased antigen-induced expansion and caused less airway inflammation in vivo. We therefore have identified a functional Gata3/Ruvbl2 complex that regulates the proliferation of differentiating Th2 cells through the repression of a CDK inhibitor, Cdkn2c.
After antigenic stimulation in a particular cytokine milieu, naive CD4 T cells differentiate into various T helper (Th) cell subsets including Th1, Th2, and Th17 cells (1, 2). The differentiation of Th2 cells requires IL-4 stimulation, which leads to Stat6 activation and the induction of GATA-binding protein 3 (Gata3) transcription (3, 4). In addition, the Ras-ERK MAPK cascade controls Gata3 stability through the ubiquitin/proteasome-dependent pathway (5, 6). Gata3 is expressed in T lymphocytes, and its expression is required for the CD4 versus CD8 lineage choice and at the β-selection checkpoint in the thymus (7, 8), as well as for Th2 cell differentiation in the periphery (9–12).
It has been known that activated CD4 T cells proliferate more vigorously under the Th2 culture conditions where IL-4 is present compared with the Th1 conditions (13, 14). Gata3-deficient Th2 cells show a substantially reduced BrdU incorporation, indicating that Gata3 is involved in the regulation of Th2 cell expansion (15). However, no definitive analysis has yet been reported regarding the molecular mechanisms underlying the Gata3-mediated induction of Th2 cell proliferation.
T-cell proliferation following TCR signaling is stimulated by the increase in the expression levels of CDK4/6 and cyclin D (16). CDK inhibitors including the Ink4 family members Cdkn2a (p16, ink4a), Cdkn2b (p15, ink4b), Cdkn2c (p18, ink4c), and Cdkn2d (p19, ink4d) negatively regulate the activity of the cyclin D–CDK4/6 complex and block the G1-S phase transition, halting cellular proliferation in nonimmune cells (17). Cdkn2c has been implicated in the regulation of T-cell proliferation, supported by the observation that T cells from Cdkn2c-deficient mice exhibit a hyperproliferative phenotype in response to TCR stimulation (18). Although expression of Cdkn2c is restrained by GATA3 in mammary luminal progenitor cells, the transcriptional regulation of this gene in Th2 cells is yet to be fully elucidated (19).
We herein identified a Gata3/RuvB-like protein 2 (Ruvbl2) complex as a key regulatory mechanism of Th2 cell proliferation via repression of Cdkn2c. Such a regulatory mechanism differs from other cell types and might be uniquely specific for Th2-cell proliferation. Ruvbl2 regulates the recruitment of Gata3 to the Cdkn2c locus, and, together, they repress the expression of the Cdkn2c. Moreover, the defect in the proliferation of Gata3-deficient Th2 cells is rescued by knockdown of Cdkn2c, indicating that Cdkn2c is a key molecule involved in the Gata3-mediated induction of Th2 cells. Therefore, the Gata3/Ruvbl2 complex plays a pivotal role in the proliferation of differentiating Th2 cells via the repression of Cdkn2c.
Results
Identification of Ruvbl2 as a Molecule That Interacts with Gata3 in Th2 Cells.
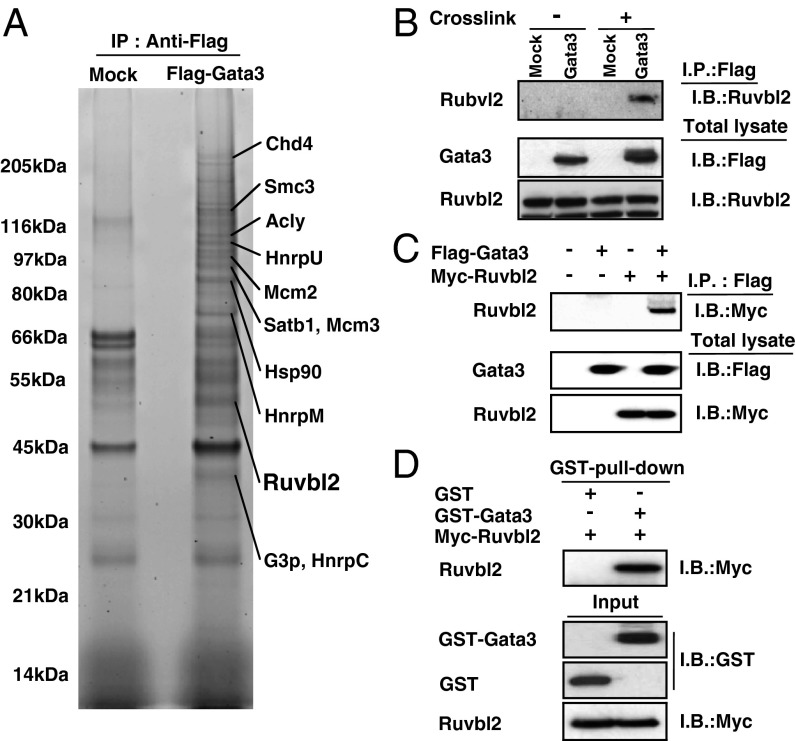
Gata3 is well-established as a key transcription factor involved in Th2-cell differentiation, but the nature of the functional Gata3 complexes that control the various processes required for Th2-cell generation, including their proliferation, has been unclear. To identity the functional components of the Gata3 complexes, we adopted a unique proteomics approach using affinity purification from the material obtained from the formaldehyde cross-linked Gata3 complex, in which associated molecules with low affinity can be identified (20). The 3xFlag–Gata3 complexes were precipitated efficiently by an anti-Flag mAb from the 3xFlag-tagged Gata3-expressing Th2 cell line, D10G4.1, and a mass-spectrometry analysis identified several polypeptides, including Ruvbl2 (also known as reptin or Tip49b) (Fig. 1A). To confirm the association of Gata3 with Ruvbl2, formaldehyde cross-linled cell extracts from the same cells were subjected to immunoprecipitation with an anti-Flag mAb. Ruvbl2 was easily detected in the cross-linked Gata3 complexes, but not in the non–cross-linked Gata3 complexes, thus indicating that there is a low-affinity association of these two molecules in D10G4.1 cells (Fig. 1B). The interaction between Gata3 and Ruvbl2 was also detected in non–cross-linked 293T cells in which Flag-tagged Gata3 and Myc-tagged Ruvbl2 were overexpressed (Fig. 1C). To address the direct molecular association of these two molecules, a GST–pull-down assay with purified recombinant Gata3 and Ruvbl2 was performed (Fig. 1D). The association of Gata3 with Ruvbl2 persisted in the presence of ethidium bromide, suggesting that the association is DNA-independent (Fig. S1). These results indicate that Ruvbl2 is a bona fide Gata3-interacting molecule in Th2 cells.
Fig. 1.
Identification of Ruvbl2 as a molecule that interacts with Gata3 in Th2 cells. (A) Total extracts from 3xFlag–Gata3-expressing cross-linked D10G4.1 cells were subjected to affinity purification using a Flag mAb, followed by SDS/PAGE and SYPRO Ruby staining. Several specific polypeptides were identified by mass spectrometry as described in Materials and Methods. (B) The 3xFlag–Gata3-expressing D10G4.1 cells were treated with or without formaldehyde as indicated (Cross-link + or −) before extraction. The extracts were then immunoprecipitated (I.P.) with a Flag mAb, followed by immunoblotting (I.B.) with an Ruvbl2 Ab (Upper). Total lysates were also subjected to I.B. in parallel (Lower). (C) The 293T cells were transfected with expression plasmids encoding Flag-tagged Gata3 and Myc-tagged Ruvbl2. Two days later, the extracts were I.P. with a Flag mAb, followed by I.B. with a Myc mAb (Upper). The total lysates were also subjected to I.B. in parallel (Lower). (D) GST or GST–Gata3 proteins were bound to glutathione Sepharose 4B and incubated with purified Myc-tagged Ruvbl2. The bound proteins were subjected to I.B. with an anti-Myc mAb (Upper). The inputs represent 10% of the amount of protein used in the pull-down sample (Lower). Three (C) and two (B and D) independent experiments were performed, and similar results were obtained.
Ruvbl2 Regulates the Proliferation of Th2 Cells.
To identify the function of Ruvbl2 in differentiating Th2 cells, carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled naive CD4 T cells were transfected with Ruvbl2 siRNA and were cultured under Th2 conditions in vitro. The cell division was significantly inhibited in the Ruvbl2 knockdown (KD) cells, which was accompanied by decreased generation of IL-4–producing Th2 cells (Fig. 2A). Ruvbl2 KD had no impact on the mRNA or protein expression of Gata3 although efficient silencing of Ruvbl2 mRNA expression was detected (Fig. S2 A and B). OX40-Cre–driven conditional Gata3 knockout (Gata3-deficient) Th2 cells showed a similar phenotype as the Ruvbl2 KD Th2 cells (Fig. 2B). A substantial reduction in BrdU incorporation was observed in the Ruvbl2 KD Th2 cells (42.8% versus 21.6%) and also in Gata3-deficient Th2 cells (49.6% versus 34.5%) (Fig. 2C). These results indicate that Ruvbl2 and Gata3 positively regulate the proliferation of Th2 cells.
Fig. 2.
Ruvbl2 regulates the proliferation of Th2 cells. (A) A control or Ruvbl2 siRNA was transfected into naive CD4 T cells, and the cells were labeled with CFSE. Then, the cells were stimulated with an immobilized anti-TCRβ mAb and anti-CD28 mAb under Th2 conditions for 3 d. The cells were then restimulated and subjected to intracellular staining with an APC-conjugated anti–IL-4 mAb (Left). The percentages of the cells in the gates representing the number of cell divisions (nos. 1 to 7) are shown (Right). (B) Naive CD4 T cells from WT or Gata3-deficient mice were labeled with CFSE and cultured under Th2 conditions for 3 d. The cells were then restimulated and subjected to IL-4 staining (Left). The percentages of the cells in the gates are shown (Right). (C) A control or Ruvbl2 siRNA was transfected into naive CD4 T cells, and the cells were stimulated with an immobilized anti-TCRβ mAb and anti-CD28 mAb under Th2 conditions for 4 d (Left). Naive CD4 T cells from WT or Gata3-deficient mice were cultured under Th2 conditions for 5 d (Right). Representative intracellular staining profiles for BrdU are shown with the percentages of cells in the gate. Three independent experiments were performed and similar results were obtained (A, B, and C).
The Expression of Cdkn2c Is Repressed in a Gata3- and Ruvbl2-Dependent Manner.
Earlier reports demonstrated that Gata3 regulates cell cycle in luminal progenitor cells and neuroblastoma cell via control of Cdkn2c and Ccnd1 expression, respectively (19, 21). Thus, we next assessed the expression of Cdkn2c and Ccnd1 in primary Th1 and Th2 cells from wild-type or Gata3-deficient mice. Although Ccnd1 expression was not detected in primary Th1 and Th2 cells, the expression of Cdkn2c was lower in Th2 cells compared with Th1 cells, and the depletion of Gata3 in Th2 cells resulted in increased expression of Cdkn2c (Fig. 3A). Moreover, the Cdkn2c expression was up-regulated in primary Th2 cells when Ruvbl2 was silenced by siRNA (Fig. 3B). These results indicate that the expression of Cdkn2c is repressed in primary Th2 cells in a Gata3- and Ruvbl2-dependent manner.
Fig. 3.
The expression of Cdkn2c controls the Gata3-dependent proliferation of Th2 cells. (A) Naive CD4 T cells from either WT or Gata3-deficient mice were cultured under Th1 or Th2 conditions for 5 d, and the expression levels of Cdkn2c mRNA in Th1 WT, Th2 WT, or Th2 Gata3 KO cells were determined by RT-qPCR. The relative expression (/Hprt) is shown with SDs. **P < 0.01 by Student t test. (B) Naive CD4 T cells were transfected with control or Ruvbl2 siRNA, and cultured under Th2 conditions for 4 d; then, the expression levels of Cdkn2c mRNA were determined by RT-qPCR. (C) Naive CD4 T cells from WT or Gata3-deficient mice were cultured under Th1 or Th2 conditions for 3 d. The binding of Gata3 to the Cdkn2c locus was determined by a ChIP assay with a qPCR analysis. The relative intensities (/input) are shown with SDs. (D) D10G4.1 cells were transfected with the indicated reporter constructs. Two days after transfection, the cells were assayed for luciferase activity. The data indicate the mean values of three independent experiments with SDs. (E) Naive CD4 T cells from WT or Gata3-deficient mice were cultured under Th2 conditions for 2 d and then infected with a retroviral vector encoding a control shRNA or shCdkn2c bicistronically with a GFP gene. Four days later, the retrovirus-infected GFP-expressing cells were purified, and the levels of Cdkn2c mRNA were measured by RT-qPCR. (F) The BrdU incorporation in a portion of the same cultured cells used in E was determined. (G) The same cultured cells used in E were labeled with Cell Proliferation Dye eFluor 670 on day 3 after stimulation. Two days later, cell division was assessed by FACS. Numbers represent mean fluorescent units. (H) The same cultured cells used in E were stimulated with immobilized TCRβ mAb and subjected to IL-4 staining, followed by FACS analysis. The percentages of IL-4-producing cells are shown. Four (A), three (B, C, E, and F), and two (D, G, and H) independent experiments were performed, and similar results were obtained.
To identify Gata3-bindng sites around the Cdkn2c locus, we performed a chromatin immunoprecipitation assay, followed by a massive parallel sequencing (ChIP-Seq) analysis using 3xFlag–Gata3-expressing Th2 clone cells (D10G4.1). Statistics of the tags generated for the experiment are summarized in Fig. S3A. The binding of 3xFlag-Gata3 at the previously validated Gata3-binding sites (Th2 cytokine and Ccr8 loci) was confirmed (Fig. S3 B and C) (10, 11). From our ChIP-seq dataset, we identified two Gata3-binding sites around the Cdkn2c locus (Intron2 and +7.5-kb regions) (Fig. S3D, Upper). In the differentiating Th2 cells, Gata3 binding was detected only at the downstream region (+7.5 kb) of the Cdkn2c locus (Fig. 3C). Consistent with our results, the strongest binding of Gata3 at the +7.5-kb region of Cdkn2c was observed in primary Th2 cells compared with Th1 and Th17 cells in the previously reported ChIP-seq analysis for endogenous Gata3 (Fig. S3D, Lower) (11). We named the +7.5-kb region the Gata3 binding site (G3BS). To assess whether the G3BS plays a functional role in the Gata3-dependent transcriptional repression of Cdkn2c, a 500-bp fragment spanning the Cdkn2c G3BS (+7,261 ∼ +7,760) (Fig. S4) was placed at the 5′-end of the Cdkn2c promoter (−500), and luciferase reporter assays were performed (Fig. 3D). The introduction of the G3BS substantially repressed the transcriptional activity of the Cdkn2c promoter whereas insertion of a G3BS with three mutations at the GATA consensus binding sequence did not show any effects (Fig. 3D and Fig. S4). These results indicate that Gata3 binds directly to the Cdkn2c locus and represses the mRNA expression of Cdkn2c.
Knockdown of Cdkn2c Expression Rescued the Impaired Proliferation of Gata3-Deficient Th2 Cells.
Next, we examined whether Cdkn2c plays an important role in the Gata3-mediated induction of Th2 cell proliferation. Naive CD4 T cells from Gata3-deficient mice were stimulated under Th2 conditions for 2 d; then, the cells were infected with a retroviral vector containing shRNA specific for Cdkn2c. Four days after infection, the increased Cdkn2c mRNA expression in Gata3-deficient Th2 cells was abrogated in the cells in which shCdkn2c had been introduced (Fig. 3E). We also found that the decreased BrdU incorporation and cell division in the Gata3-deficient Th2 cells compared with WT cells was restored by the introduction of shCdkn2c (Fig. 3 F and G). Interestingly, the restoration in IL-4 production was not observed by introduction of shCdkn2c in Gata3-deficient Th2 cells (Fig. 3H). These results indicate that Cdkn2c is specifically critical for the Gata3-mediated induction of the proliferation of Th2 cells.
The Transactivation Domain of Gata3 Is Required for the Association with Ruvbl2 and the Repression of Cdkn2c Expression.
The GATA family transcriptional factors (Gata1 to -6) typically bind to a consensus motif (A/T)GATA(A/G) and regulate the specification and differentiation of numerous tissues. All GATA family members share two highly conserved C2H2-type zinc fingers, both of which are involved in DNA binding and protein–protein interactions (22, 23). Two transactivation domains are also known to be important for the function of Gata3 (24).
We examined which domains of Gata3 were important for the binding to Ruvbl2. Flag-tagged wild-type or deletion mutants of Gata3 (as depicted in Fig. S5 A, Upper and B, Upper) were cotransfected with Myc-tagged Ruvbl2 into 293T cells; then, the interaction was assessed by immunoprecipitation with a Flag mAb and subsequent immunoblotting with a Myc mAb. The association with Ruvbl2 was almost completely abrogated by the deletion of amino acids 65–178 (dTA), which is the region around the two transactivation domains of Gata3 (Fig. S5 A and B), thus indicating that the transactivation domains of Gata3 are important for binding to Rubvl2. To further examine whether these regions of Gata3 are important for the expression of Cdkn2c, we generated a stable Gata3 knockdown T-cell line. shRNAs against Gata3 or Ruvbl2 were introduced into a mouse T-cell line 68–41 using a lentivirus system. As expected, the expression of Cdkn2c was up-regulated in the Gata3 or Ruvbl2 knockdown 68–41 cells (Fig. S5C). We then expressed WT or dTA mutant Gata3 in these Gata3 knockdown 68–41 cells using a retrovirus system. Both the WT and dTA mutant Gata3 were substantially expressed in the Gata3 knockdown 68–41 cells (Fig. S5D, Upper). As shown in Fig. S5D, Lower, WT Gata3 repressed the Cdkn2c expression whereas the dTA mutant did not repress the expression of Cdkn2c. These results indicate that the transactivation domain of Gata3 is required for the repression of Cdkn2c expression.
Ruvbl2 Is Necessary for the Recruitment of Gata3 to the Cdkn2c Locus in Th2 Cells.
To further investigate the molecular requirements for the Gata3-mediated repression of Cdkn2c expression in primary T cells, we used differentiating Th2 cells from Gata3-deficient mice. The introduction of WT Gata3 reduced the expression of Cdkn2c whereas the dTA mutant did not show any effect in the Gata3-deficient Th2 cells (Fig. 4A). The binding of the Gata3 dTA mutant to the Cdkn2c G3BS region was significantly compromised (Fig. 4B, Left). In contrast, the binding of the Gata3 dTA to the Th2 cytokine gene loci at the CGRE region was not compromised (Fig. 4B, Right), thus indicating that the Gata3 dTA mutant had a preserved DNA binding activity. In addition, a ChIP assay for endogenous Gata3 revealed that the Gata3 binding at the Cdkn2c G3BS region was impaired in Ruvbl2 KD Th2 cells (Fig. 4C). Thus, Ruvbl2 appears to regulate the binding of Gata3 to the Cdnk2c G3BS region in Th2 cells. Taken together, these results suggest that the association of Ruvbl2 with Gata3 is required for the binding of Gata3 to the Cdkn2c G3BS region.
Fig. 4.
Ruvbl2 is necessary for the recruitment of Gata3 at the Cdkn2c locus in developing Th2 cells. (A) Naive CD4 T cells from Gata3-deficient mice were stimulated under Th2 conditions for 2 d and then infected with a retroviral vector carrying WT or mutant (dTA) Gata3 cDNAs. Three days later, the retrovirus-infected GFP-expressing cells were purified, and the levels of mRNA of Cdkn2c were measured by RT-qPCR. **P < 0.01 by Student t test. (B) The binding of Gata3 WT or dTA to the Cdkn2c G3BS and Th2 cytokine loci (CGRE region) were determined by the ChIP assay with a qPCR analysis using the same cells shown in A. (C) A control or Ruvbl2 siRNA was transfected into naive CD4 T cells, and the cells were stimulated under Th1 or Th2 conditions for 3 d. The binding of Gata3 to the Cdkn2c locus was determined. (D) A schematic representation of the Cdkn2c locus. The locations of primers and exons are indicated. (E) Naive CD4 T cells from WT or Gata3-deficient mice were cultured under Th1 or Th2 conditions for 5 d. The status of H3-K27 Me3 and H2A-K119 Ub at the Cdkn2c and Hprt loci was determined by a ChIP assay, using specific primers to detect the indicated regions. **P < 0.01, *P < 0.05 by Student t test. (F) A control or Ruvbl2 siRNA was transfected into naive CD4 T cells, and the cells were stimulated under Th2 conditions for 4 d. The status of H3-K27 Me3 and H2A-K119 Ub at the Cdkn2c and Hprt promoters was determined by a ChIP assay. Four (A), three (B and C), and two (E and F) independent experiments were performed, and similar results were obtained.
Repressive Histone Modifications at the Cdkn2c Locus Induced by the Expression of Gata3 and Ruvbl2.
We previously reported that the polycomb group (PcG) gene product, Bmi1, associates with Gata3 and controls the stability of the Gata3 protein in Th2 cells (25). In addition, Ruvbl2 was previously shown to be a component of the PcG complex in Drosophila (26, 27). Thus, we next investigated the histone modifications at the Cdkn2c locus, particularly histone H3-K27 trimethylation (H3-K27 Me3) and H2A-K119 monoubiquitination (H2A-K119 Ub), which are repressive marks known to be induced by PcG complexes. Interestingly, the H3-K27 Me3 and H2A-K119 Ub signals at the Cdkn2c locus, including the promoter, intron2, and G3BS regions, were higher in Th2 cells compared with Th1 and Gata3-deficient Th2 cells (Fig. 4 D and E). In addition, as shown in Fig. 4F, these histone modifications at the Cdkn2c promoter were significantly reduced in Ruvbl2 KD Th2 cells. Thus, repressive histone modifications at the Cdkn2c locus correlate strongly with both Gata3 and Ruvbl2 expression.
The Role of Ruvbl2 in Antigen-Induced Expansion of Th2 cells and Subsequent Induction of Allergic Airway Inflammation in Vivo.
Finally, we investigated the in vivo physiological role of Ruvbl2 using a Th2 cell-dependent allergic airway inflammation model (28). Ruvbl2 KD Th2 cells or control cells generated by in vitro culture from DO11.10 Tg mice were i.v. injected into normal BALB/c mice. The mice were challenged twice by inhalation with 1% (wt/vol) OVA (Fig. S6). Before inhalation, control and Ruvbl2 KD Th2 cells were similarly detectable in the lung (Fig. 5A). A dramatic increase in the number of transferred Th2 cells (KJ1+ cells) was induced by OVA inhalation (Fig. 5A, Right, black bars), and the increase was significantly impaired when Ruvbl2 was knocked down (Fig. 5A, Right). A significant decrease in the infiltration of inflammatory cells, including eosinophils, in the bronchioalveolar lavage (BAL) fluid was observed in the Ruvbl2 KD group in comparison with the control group (Fig. 5B). Histological analysis showed that mononuclear cell infiltration into the peribronchiolar regions of the lung was also modest in the Ruvbl2 KD animals (Fig. 5C, Upper). The levels of mucus hyperproduction and Goblet-cell metaplasia assessed by PAS staining were lower in the bronchioles of the Ruvbl2 KD group (Fig. 5C, Lower). These results indicate that Ruvbl2 regulates antigen-induced Th2 cell expansion and subsequent induction of allergic airway inflammation in vivo.
Fig. 5.
Ruvbl2 regulates OVA-induced Th2 cell expansion and allergic airway inflammation in vivo. (A) A control or Ruvbl2 siRNA was transfected into naive CD4 T cells from DO11.10 Tg mice, and the cells were stimulated under Th2 conditions. On day 4, cells were i.v. transferred into BALB/c mice, and the mice were challenged with aerosolized OVA on days 5 and 7. Cells from lung were stained with antibodies against CD4 and KJ1.26 and assessed by FACS on days 5 (before inhalation) and 9 (after inhalation). A summary of the numbers of KJ1+ cells in the lung is presented (Right graph). Four mice per group were used. **P < 0.01 by Student t test. (B) The number of inflammatory cells in the bronchioalveolar lavage (BAL) fluid was counted. The absolute cell numbers of eosinophils (Eos.), neutrophils (Neu.), lymphocytes (Lym.) and macrophages (Mac.) are shown with SDs. Four mice per group were used. (C) The lungs were fixed and stained with hematoxylin/eosin (H&E) or with PAS. Representative staining patterns are shown. (Scale bar: 100 μm.) Two independent experiments were performed, and similar results were obtained (A, B, and C).
Discussion
We have herein identified a mechanism by which Gata3 facilitates the proliferation of differentiating Th2 cells. Gata3 associates with Ruvbl2 to form a unique repressive complex and represses the expression of a CDK inhibitor, Cdkn2c, via direct binding to the downstream region of the Cdkn2c gene (Fig. S7B). The discovery of the unique repressive Gata3/Ruvbl2 complex and the critical role of Ruvbl2 in the repression of Cdkn2c expression allow for mechanistic insight into the process of Th2-cell differentiation and proliferation.
Cdkn2c is a gene encoding p18 (ink4c), a member of the Ink4 family of cyclin-dependent kinase inhibitors. Ink4 family proteins bind to Cdk4 and Cdk6 to prevent the assembly of catalytically active cyclin D–CDK4/6 complexes and block the G1-S phase transition (17). Our results are consistent with the observation that Cdkn2c-deficient mice showed a hyperproliferative phenotype in response to TCR stimulation (18). In luminal progenitor cells, Gata3 was shown to directly bind to the promoter and intron of Cdkn2c and repress its expression to regulate the cell cycle (19). In addition, the involvement of Gata3 in the activation of Cyclin D1 (encoded by Ccnd1) in neuroblastoma cell lines and cell cycle entry in long-term repopulating hematopoietic stem cells were reported in previous studies (21, 29). Therefore, Gata3 appears to regulate the proliferation of various cell types through several distinct mechanisms.
Ruvbl2, a paralogue of Ruvbl1, was identified independently in multiple organisms and is implicated in many cellular pathways. Ruvbl1 and Ruvbl2 were both initially found as proteins that interacted with the TATA-box binding protein (Tbp) in Drosophila, yeast, and humans (30, 31). Ruvbl1 and Ruvbl2 belong to the AAA+ (ATPase associated with multiple activities) ATPase family, have an ATPase domain with Walker A and Walker B motifs, and are conserved in different species. Ruvbl1 and Ruvbl2 are involved in the transcription of over 5% of yeast genes, many of which are directly involved in cell-cycle regulation (32). In addition to Tbp, the Ruvbl1 and Ruvbl2 have also been found to interact with various transcription factors, including c-Myc, β-catenin, and E2f, all of which are critical in the regulation of cell growth, proliferation, and apoptosis (30, 31). Previous reports have identified Ruvbl2 in various chromatin remodeling complexes, such as Tip60, Swr1, Baf, Ino80, and PcG molecule complexes. Moreover, mice heterozygous for a Ruvbl2 mutation showed impaired T-cell development and maximal T-dependent antibody responses (33). The expression of Ruvbl2 thus appears to play a critical role in the expansion of T cells in vivo.
Because Gata3 binding to the Cdkn2c locus is significantly lower in the absence of Ruvbl2, Gata3-mediated repression of Cdkn2c expression is dependent on Ruvbl2–Gata3 interaction (Fig. 4 and Fig. S5). With regard to the mechanisms by which Ruvbl2 controls Gata3 binding to the Cdkn2c locus, two possibilities should be considered. One is that the association of Gata3 with Ruvbl2 has an effect on the DNA binding activity of Gata3, and the other is that Ruvbl2 recruits chromatin-remodeling complexes altering the accessibility of the Cdkn2c locus to Gata3 (31). The binding of the Ruvbl2 to the Cdkn2c locus was comparable in Th1 and Th2 cells (Fig. S7A), indicating that the binding of Ruvbl2 to the Cdkn2c locus is Gata3-independent. Thus, our findings may support the latter hypothesis, Ruvbl2 might associate with Gata3 at the Cdkn2c locus to facilitate and stabilize the DNA binding of Gata3 (Fig. S7B).
The recent genome-wide analyses indicated that GATA family transcription factors mediate both activating and repressive gene regulation (10, 11, 34–36). Interestingly, most of the GATA binding sites are localized at intragenic or intergenic regions where they can function as distal enhancers or silencers (11, 34–36). We have recently reported that Gata3 organizes a Gata3/Chd4/p300 activating complex at the enhancer regions of Th2 cytokine loci and a Gata3/Chd4-NuRD repressive complex at the intron 1 of Tbx21 locus in Th2 cells and, thus, simultaneously positively regulates the expression of the Th2 cytokine genes and negatively regulates the Tbx21 locus (37). In the current study, we found that Gata3 binds to the downstream region of the Cdkn2c locus (+7.5 kb from the transcriptional start site) and down-regulates the expression of Cdkn2c (Fig. 3). The introduction of a fragment spanning the Cdkn2c G3BS specifically repressed the transcriptional activity of the Cdkn2c promoter (Fig. 3D). Thus, the G3BS downstream of the Cdkn2c may have a Gata3-dependent intergenic silencer activity that affects the expression of Cdkn2c.
In summary, our results indicate that the Gata3/Ruvbl2 complex plays a crucial role in the regulation of Th2-cell proliferation by repressing the expression of a CDK inhibitor, Cdkn2c. This finding highlights a unique molecular mechanism that controls the process of the proliferation during Th-cell differentiation.
Materials and methods
Mice.
C57BL/6 and BALB/c mice were purchased from CLEA Co. Conditional Gata3-deficient mice and OX40-Cre transgenic mice were kindly provided by Dr. William E. Paul (National Institutes of Health, Bethesda, MD) and Dr. Nigel Killeen (University of California, San Franciso, CA), respectively. Anti-ovalbumin (OVA)-specific TCR-αβ (DO11.10) transgenic (Tg) mice were provided by Dr. Dennis Loh (Washington University, St. Louis, MO). All mice were maintained under specific pathogen-free conditions and were used at 6–8 wk of age. All animal experiments were approved by the Chiba University Review Board for Animal Care.
The Generation of Th1 and Th2 Cells.
Th1 and Th2 cells were generated as described previously (38). In brief, CD4 T cells with a naive phenotype (CD44low) were purified using a FACSAria instrument (Becton Dickinson) yielding a purity of >98% and were stimulated with 3 μg/mL immobilized anti-TCRβ mAb plus 1 μg/mL anti-CD28 mAb under the Th1 or Th2 conditions in vitro.
Detailed descriptions of all materials and methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Kaoru Sugaya, Hikari Kato, Terumi Horiuchi, Kayo Suzuki, and Toshihiro Ito for expert technical assistance. We also thank Dr. Yuka Kanno for critical suggestions on the manuscript. The lentivirus system was kindly provided by Dr. Hiroyuki Miyoshi (RIKEN BioResource Center). This work was supported by Global Center for Education and Research in Immune System Regulation and Treatment (Ministry of Education, Culture, Sports, Science and Technology, Japan) Grants-in-Aid Innovative Areas “Genome Science” 221S0002, “Crosstalk Between Transcription Control and Energy Pathway” 24116506, Scientific Research (B) 21390147, 22300325, and 24390239, (C) 19659121, and Exploratory Research and Young Scientists (B) 20790367 and 23790522; the Takeda Science Foundation; the Sagawa Cancer Foundation; the Astellas Foundation for Research on Metabolic Disorders; the Naito Foundation Natural Science Scholarship; the Princess Takamatsu Cancer Research Foundation; NOVARTIS Foundation for Gerontologic Research; NOVARTIS Foundation for the Promotion of Science; the Uehara Memorial Foundation; and the Research Fund of the Mitsukoshi Health and Welfare Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The ChIP-seq data have been deposited in the DNA Data Bank of Japan, www.ddbj.nig.ac.jp (accession no. DRA001102).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311100110/-/DCSupplemental.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiner SL. Development in motion: Helper T cells at work. Cell. 2007;129(1):33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Kurata H, Lee HJ, O’Garra A, Arai N. Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity. 1999;11(6):677–688. doi: 10.1016/s1074-7613(00)80142-9. [DOI] [PubMed] [Google Scholar]
- 4.Onodera A, et al. STAT6-mediated displacement of polycomb by trithorax complex establishes long-term maintenance of GATA3 expression in T helper type 2 cells. J Exp Med. 2010;207(11):2493–2506. doi: 10.1084/jem.20100760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashita M, et al. Ras-ERK MAPK cascade regulates GATA3 stability and Th2 differentiation through ubiquitin-proteasome pathway. J Biol Chem. 2005;280(33):29409–29419. doi: 10.1074/jbc.M502333200. [DOI] [PubMed] [Google Scholar]
- 6.Shinnakasu R, et al. Gfi1-mediated stabilization of GATA3 protein is required for Th2 cell differentiation. J Biol Chem. 2008;283(42):28216–28225. doi: 10.1074/jbc.M804174200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosoya T, Maillard I, Engel JD. From the cradle to the grave: Activities of GATA-3 throughout T-cell development and differentiation. Immunol Rev. 2010;238(1):110–125. doi: 10.1111/j.1600-065X.2010.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothenberg EV, Scripture-Adams DD. Competition and collaboration: GATA-3, PU.1, and Notch signaling in early T-cell fate determination. Semin Immunol. 2008;20(4):236–246. doi: 10.1016/j.smim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 10.Horiuchi S, et al. Genome-wide analysis reveals unique regulation of transcription of Th2-specific genes by GATA3. J Immunol. 2011;186(11):6378–6389. doi: 10.4049/jimmunol.1100179. [DOI] [PubMed] [Google Scholar]
- 11.Wei G, et al. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity. 2011;35(2):299–311. doi: 10.1016/j.immuni.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayama T, Yamashita M. Initiation and maintenance of Th2 cell identity. Curr Opin Immunol. 2008;20(3):265–271. doi: 10.1016/j.coi.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Grogan JL, et al. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14(3):205–215. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J, et al. Growth factor independent-1 induced by IL-4 regulates Th2 cell proliferation. Immunity. 2002;16(5):733–744. doi: 10.1016/s1074-7613(02)00317-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5(11):1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 16.Nagasawa M, Melamed I, Kupfer A, Gelfand EW, Lucas JJ. Rapid nuclear translocation and increased activity of cyclin-dependent kinase 6 after T cell activation. J Immunol. 1997;158(11):5146–5154. [PubMed] [Google Scholar]
- 17.Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta. 2002;1602(1):73–87. doi: 10.1016/s0304-419x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 18.Kovalev GI, Franklin DS, Coffield VM, Xiong Y, Su L. An important role of CDK inhibitor p18(INK4c) in modulating antigen receptor-mediated T cell proliferation. J Immunol. 2001;167(6):3285–3292. doi: 10.4049/jimmunol.167.6.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pei XH, et al. CDK inhibitor p18(INK4c) is a downstream target of GATA3 and restrains mammary luminal progenitor cell proliferation and tumorigenesis. Cancer Cell. 2009;15(5):389–401. doi: 10.1016/j.ccr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka T, Ohkubo S, Tatsuno I, Prives C. hCAS/CSE1L associates with chromatin and regulates expression of select p53 target genes. Cell. 2007;130(4):638–650. doi: 10.1016/j.cell.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Molenaar JJ, et al. Cyclin D1 is a direct transcriptional target of GATA3 in neuroblastoma tumor cells. Oncogene. 2010;29(18):2739–2745. doi: 10.1038/onc.2010.21. [DOI] [PubMed] [Google Scholar]
- 22.Bates DL, Chen Y, Kim G, Guo L, Chen L. Crystal structures of multiple GATA zinc fingers bound to DNA reveal new insights into DNA recognition and self-association by GATA. J Mol Biol. 2008;381(5):1292–1306. doi: 10.1016/j.jmb.2008.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowry JA, Atchley WR. Molecular evolution of the GATA family of transcription factors: conservation within the DNA-binding domain. J Mol Evol. 2000;50(2):103–115. doi: 10.1007/s002399910012. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, et al. Human GATA-3 trans-activation, DNA-binding, and nuclear localization activities are organized into distinct structural domains. Mol Cell Biol. 1994;14(3):2201–2212. doi: 10.1128/mcb.14.3.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosokawa H, et al. Regulation of Th2 cell development by Polycomb group gene bmi-1 through the stabilization of GATA3. J Immunol. 2006;177(11):7656–7664. doi: 10.4049/jimmunol.177.11.7656. [DOI] [PubMed] [Google Scholar]
- 26.Qi D, Jin H, Lilja T, Mannervik M. Drosophila Reptin and other TIP60 complex components promote generation of silent chromatin. Genetics. 2006;174(1):241–251. doi: 10.1534/genetics.106.059980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diop SB, et al. Reptin and Pontin function antagonistically with PcG and TrxG complexes to mediate Hox gene control. EMBO Rep. 2008;9(3):260–266. doi: 10.1038/embor.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miki-Hosokawa T, et al. CD69 controls the pathogenesis of allergic airway inflammation. J Immunol. 2009;183(12):8203–8215. doi: 10.4049/jimmunol.0900646. [DOI] [PubMed] [Google Scholar]
- 29.Ku CJ, Hosoya T, Maillard I, Engel JD. GATA-3 regulates hematopoietic stem cell maintenance and cell-cycle entry. Blood. 2012;119(10):2242–2251. doi: 10.1182/blood-2011-07-366070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huen J, et al. Rvb1-Rvb2: Essential ATP-dependent helicases for critical complexes. Biochem Cell Biol. 2010;88(1):29–40. doi: 10.1139/o09-122. [DOI] [PubMed] [Google Scholar]
- 31.Jha S, Dutta A. RVB1/RVB2: Running rings around molecular biology. Mol Cell. 2009;34(5):521–533. doi: 10.1016/j.molcel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jónsson ZO, et al. Rvb1p and Rvb2p are essential components of a chromatin remodeling complex that regulates transcription of over 5% of yeast genes. J Biol Chem. 2001;276(19):16279–16288. doi: 10.1074/jbc.M011523200. [DOI] [PubMed] [Google Scholar]
- 33.Arnold CN, et al. A forward genetic screen reveals roles for Nfkbid, Zeb1, and Ruvbl2 in humoral immunity. Proc Natl Acad Sci USA. 2012;109(31):12286–12293. doi: 10.1073/pnas.1209134109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu M, et al. Insights into GATA-1-mediated gene activation versus repression via genome-wide chromatin occupancy analysis. Mol Cell. 2009;36(4):682–695. doi: 10.1016/j.molcel.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujiwara T, et al. Discovering hematopoietic mechanisms through genome-wide analysis of GATA factor chromatin occupancy. Mol Cell. 2009;36(4):667–681. doi: 10.1016/j.molcel.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He A, Kong SW, Ma Q, Pu WT. Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proc Natl Acad Sci USA. 2011;108(14):5632–5637. doi: 10.1073/pnas.1016959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosokawa H, et al. Functionally distinct Gata3/Chd4 complexes coordinately establish T helper 2 (Th2) cell identity. Proc Natl Acad Sci USA. 2013;110(12):4691–4696. doi: 10.1073/pnas.1220865110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamashita M, et al. Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity. 2006;24(5):611–622. doi: 10.1016/j.immuni.2006.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.