Significance
The canonical mechanism of GroEL/GroES protein folding rests on at least three untested assumptions: (i) symmetric GroEL-GroES2 “football” particles have no role; (ii) because of negative cooperativity, ATP binds to GroEL, one ring at a time; and (iii) the turnover of the chaperonin system occurs at the same rate in the presence of substrate protein (SP) as in its absence. Each of these assumptions is shown to be incorrect. (i) Because of an SP-induced change in the kinetic mechanism, the predominant species under protein folding conditions is the symmetric football; (ii) simultaneous occupancy of both GroEL rings by ATP and GroES occurs; and (iii) the residence time of encapsulated SP is much shorter than believed.
Abstract
Using calibrated FRET, we show that the simultaneous occupancy of both rings of GroEL by ATP and GroES occurs, leading to the rapid formation of symmetric GroEL:GroES2 “football” particles regardless of the presence or absence of substrate protein (SP). In the absence of SP, these symmetric particles revert to asymmetric GroEL:GroES1 “bullet” particles. The breakage of GroES symmetry requires the stochastic hydrolysis of ATP and the breakage of nucleotide symmetry. These asymmetric particles are both persistent and dynamic; they turnover via the asymmetric cycle. When challenged with SP, however, they revert to symmetric particles within a second. In the presence of SP, the symmetric particles are also persistent and dynamic. They turn over via the symmetric cycle. Under these conditions, the stochastic hydrolysis of ATP and the breakage of nucleotide symmetry also occur within the ensemble of particles. However, on account of SP-catalyzed ADP/ATP exchange, GroES symmetry is rapidly restored. The residence time of both GroES and SP on functional GroEL is reduced to ∼1 s, enabling many more iterations than was previously believed possible, consistent with the iterative annealing mechanism. This result is inconsistent with currently accepted models. Using a foldable SP, we show that as the SP folds to the native state and the population of unfolded SP declines, the population of symmetric particles reverts to asymmetric particles in parallel, a result that is consistent with the former being the folding functional form.
GroEL plays a central role in the function of the GroEL/GroES nanomachine. It binds unfolded proteins, transiently encapsulates them under the GroES “lid,” and then releases them to the external medium, this cycle of events being driven by the hydrolysis of ATP (1, 2). GroEL, however, consists of two heptameric rings, and some controversy has arisen as to whether each ring operates alternately via an asymmetric cycle or simultaneously via a symmetric cycle. In the former case, GroEL engages one GroES at a time and the predominant species is the asymmetric GroEL:GroES1 “bullet” complex whereas, in the latter, symmetric GroEL:GroES2 “football” particles predominate in the symmetric cycle (figure 9 in ref. 4) (3, 4). Nevertheless, the asymmetric cycle, championed by leading authorities (5, 6), has become the widely accepted model of chaperonin function, promulgated in recent textbooks (7, 8) despite much evidence (9–21) that assigns a role to the symmetric football particles. However, the involvement of these symmetric particles in chaperonin function has been questioned on the basis of three items of chaperonin dogma: (i) that the formation of symmetric GroEL:GroES2 particles and polypeptide binding are mutually exclusive (22, 23); (ii) the belief that, because of negative cooperativity, “when one GroEL ring binds ATP, the other ring cannot also do so” (5); and (iii) that the chaperonin ATPase cycle turns over at the same rate in the presence of substrate protein as it does in its absence. Here, we report results that refute these dogmas. In the presence of GroES, the biologically relevant state, simultaneous occupancy of both rings of GroEL by ATP can readily be demonstrated and leads to the rapid (within a second) formation of symmetric GroEL:GroES2 particles both in the presence and in the absence of substrate protein (SP), a time at which only ∼3 of the 14 ATPs have undergone hydrolysis. In the absence of SP, however, these symmetric particles revert to asymmetric GroEL:GroES1 particles, a reaction that requires the stochastic hydrolysis of ATP and the breakage of nucleotide symmetry. These asymmetric particles are both persistent and dynamic; they turnover via the asymmetric cycle (3, 4). When challenged with SP, however, they revert to symmetric particles within a second. In the presence of SP, the symmetric particles are also persistent and dynamic. They turn over via the symmetric cycle (figure 9 in ref. 4) (3, 4). Under these conditions, the stochastic hydrolysis of ATP and the breakage of nucleotide symmetry also occur within the ensemble of particles. However, on account of SP-catalyzed ADP/ATP exchange (4), GroES symmetry is rapidly restored. Using a foldable SP, we show that as the SP folds to the native state and the population of unfolded SP declines, the population of symmetric particles reverts to asymmetric particles in parallel, a result that is consistent with the former being the folding functional form.
Results
Calibration of a FRET System to Monitor the Population of GroEL:GroES1 and GroEL:GroES2 in Real Time.
A FRET-based system calibrated in principle as previously described (18, 20) allowed us to measure the populations of asymmetric GroEL:GroES1 particles and symmetric GroEL:GroES2 particles (Fig. S1). This method was adapted for pre–steady-state analyses using a stopped-flow device, enabling us to make measurements in real time (4). Additionally, we followed the hydrolysis of ATP by use of the fluorescent inorganic phosphate binding protein (24). The stopped flow calibration using either ATP plus BeFx (for symmetric GroEL:GroES2 particles) or ADP plus BeFx (for asymmetric GroEL:GroES particles) is shown in Fig. S1E.
The Formation and Fate of Symmetric GroEL:GroES2 Particles in the Absence of SP.
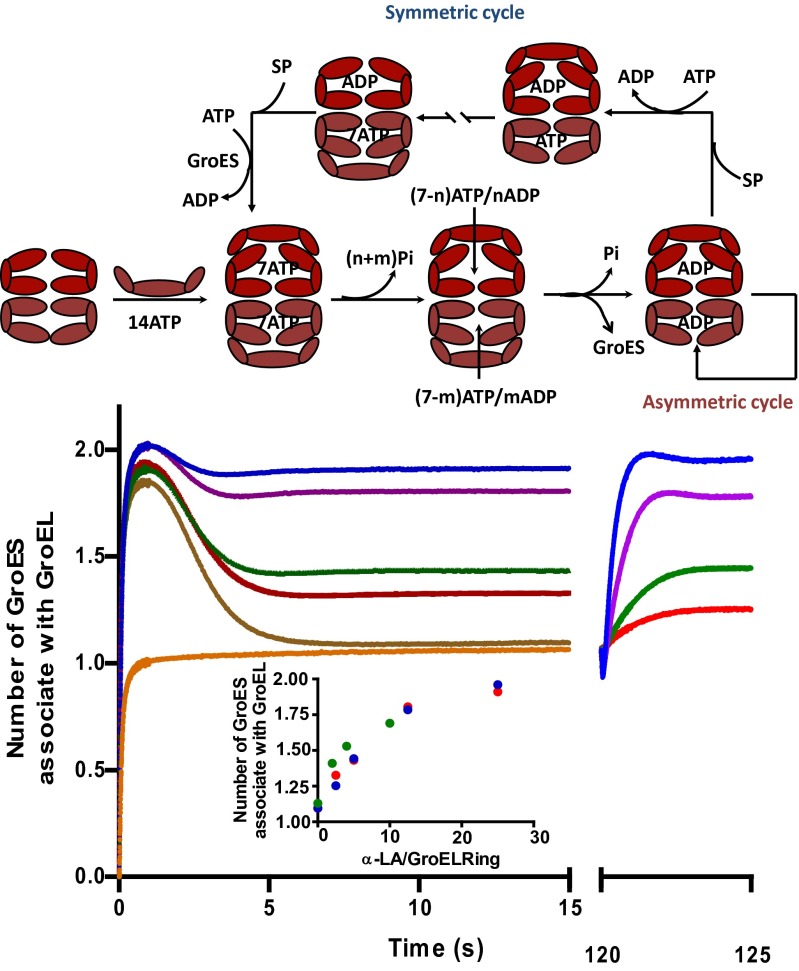
Upon mixing apo-FRET GroEL, FRET-GroES, and ATP in the absence of SP, an ensemble of particles, substantially enriched with symmetric GroEL:GroES2, was formed within 1 s, a time at which only ∼3 of the 14 ATPs have undergone hydrolysis (Fig. 1A). It is clear that both GroEL rings are simultaneously populated with ATP, contrary to accepted chaperonin dogma (5). In the absence of SP, ATP hydrolysis by both rings continues, leading to the breakage of nucleotide symmetry, the dissociation of one or the other FRET GroES and the formation of asymmetric GroEL:GroES1 particles. This relaxation is complete when one cycle of ATP hydrolysis has occurred. Although these asymmetric particles persist for tens of minutes, they are dynamic, as revealed by the continuing steady-state hydrolysis of ATP. They do so in the absence of SP by operating the asymmetric cycle (3, 4).
Fig. 1.

Formation and decay of GroEL:GroES2 complexes. (A) Formation of symmetric GroEL:GroES2 and asymmetric GroEL:GroES1 complexes measured by FRET (red) overlaid with Pi release trace (blue). Both experiments were started with a mixture of apo-GroEL and GroES at 100 mM K+, which was rapidly mixed with 0.5 mM ATP (final) in a stopped-flow apparatus. The FRET measurement was performed with 2 μM GroELE315C-IAEDAN (∼four dye molecules per ring) and 5 μM GroES98C-F5M (∼three dye molecules per ring) (both are subunit concentrations). Pi release was measured with 1 μM GroEL and 2.5 μM GroES, respectively, as previously described (4). The FRET signal (left axis) was converted to the number of GroES associated with GroEL based on the calibration in Fig. S1E, whereas the Pi release data (right axis) were converted to the Pi released per GroEL subunit. The cartoon above the graph depicts the formation and fate of the GroEL:GroES2 symmetric complex in such measurement. (B) GroEL:GroES complex formation using either FRET GroELD398A/E315C (green trace) or FRET GroELE315C (blue trace). FRET measurements for both cases were performed in the same manner as described in A. (C) The symmetric GroEL:ES2 complex formed by GroELD398A eventually reverts back to the asymmetric GroEL:ES1 complex as ATP, initially trapped in the active site, slowly hydrolyzes to ADP. FRET measurements show relaxation of the symmetric GroELD398A:GroES2 complex to the asymmetric GroELD398A:GroES1 complex over the time course of 30–40 min (blue trace). Ten units of hexokinase plus 10 mM glucose were included to deplete free ATP and, thus, prevent symmetric complex from reforming. If the quenching system was omitted, very little breakage of symmetric complex was observed on this time scale (green trace). The red trace and the purple trace are calibrations performed with ADP + BeFx (GroEL:ES1 complex) and ATP + BeFx (GroEL:ES2 complex), respectively. All FRET measurements were conducted in the same manner as those for A and B, except in a Perkin-Elmer fluorometer and the reaction was initiated by manually adding 0.5 mM ATP. The first data point was collected 60 s after the reaction was initiated. (D) The decay of the population of symmetric GroEL:GroES2 complex was plotted against average number of ATP that was hydrolyzed during the initial turnover for both GroELWT (red) and GroELD398A (blue). The change in the population of symmetric and asymmetric complexes was followed by FRET as in A and B. Pi release by GroELWT was monitored by PBP-MDCC, whereas Pi release by GroELD398A was measured by a continuous assay using MESG and PNPase. The reaction was initiated with addition of 20 μM GroELD398A (subunits) to a solution containing 10 mM glucose, 0.2 mM MESG, 2 units per milliliter PNPase, 50 μM GroES (subunits), and 0.5 mM ATP. Shortly thereafter, free ATP was removed by the addition of 10 units per milliliter hexokinase. The absorption change at 360 nm was followed in a Cary UV-Vis spectrometer.
Breakage of GroES Symmetry Requires the Hydrolysis of ATP and the Breakage of Nucleotide Symmetry.
When a similar experiment was conducted using the ATPase-defective mutant GroELD398A (Fig. 1B), the symmetric GroEL:GroES2 particles formed rapidly as before. However, no relaxation to the asymmetric GroEL:GroES1 particles occurred, at least not on the time scale of seconds, confirming the requirement for ATP hydrolysis for GroES symmetry to be broken. However, GroELD398A hydrolyzes ATP, albeit at rate that is ∼0.1% of the wild type. In that case, one round of ATP hydrolysis requires ∼40 min to complete (Fig. 1C). However, the breakage of nucleotide symmetry and the relaxation to the asymmetric state begins to occur after only 4–5 of the 14 ATP have been hydrolyzed. To show that the same breakage of nucleotide symmetry and the consequential breakage of GroES symmetry is occurring in both wild type and GroELD398A, we have plotted both sets of data against the number of ATP that have undergone hydrolysis (Fig. 1D). Despite the ∼150-fold difference in time scales, the same fundamental process occurs in the wild type and mutant. Note that in the case of GroELD398A, we have included an ATP-quench system (glucose plus hexokinase) that prevents the reestablishment of sufficient nucleotide symmetry to permit GroES symmetry to occur. For GroELD398A, nucleotide exchange is much faster than ATP hydrolysis and in the absence of the ATP quench, GroES symmetry is restored and the steady-state population of footballs is maintained (Fig. 1D).
The Formation and Fate of Symmetric GroEL:GroES2 Particles in the Presence of SP.
When the same experiment was conducted in the presence of varying concentrations of SP (unfolded α-lactalbumin), an altogether different scenario developed (Fig. 2). As in the absence of SP, symmetrical particles formed within one second of mixing. What transpired thereafter depended on the concentration of SP. With a saturated concentration of SP, only a little breakage of GroES symmetry was evident, even as ATP hydrolysis occurred; the population of symmetric GroEL:GroES2 particles persisted for tens of minutes provided an ATP-regenerating system was present. They do so in the presence of SP by operating the symmetric cycle (3, 4). At intermediate concentrations of SP, the system relaxed to a steady-state mixture of symmetric and asymmetric particles, consistent with the central role played by SP in partitioning the system between the symmetric and the asymmetric cycles (figure 9 in ref. 4) (3, 4).
Fig. 2.
Kinetic partitioning of GroEL/ES complex into symmetric cycle mediated by SP. Two sets of experiments are presented: the darker colored traces were generated with reactions initiated as in Fig. 1 by mixing 2 μM FRET apo-GroELE315C, 5 μM FRET GroES98C mixture with 0.5 mM ATP (final) plus the indicated concentrations of SP (denatured α-lactalbumin). The set of traces on the right was acquired by introducing the indicated concentration of SP into an actively turning-over system comprising 2 μM FRET GroEL, 5 μM FRET GroES, and 0.5 mM ATP plus an ATP-regenerating system (0.4 mM PEP, 5 U/mL pyruvate kinase), 2 min after the experiment was initiated. Concentrations of SP applied in both cases: 0 μM (brown trace), 0.71 μM (red), 1.42 μM (green), 3.6 μM (violet), 7.1 μM (blue). The orange trace was acquired by mixing GroEL and GroES mixture with 0.5 mM ADP and 1 mM BeFx to generate a base line showing the signal level of asymmetric complex upon which the second set of SP-jump trajectories were based. (Inset) the level of symmetric GroEL:GroES2 complex was plotted against the ratio of α-lactalbumin (α-LA) over GroEL7 rings. Three different sets of experiments with different starting states were combined and plotted together: red, apo-GroEL + apo-GroES mixture; green, synchronized acceptor state GroEL:GroES1 complex; blue, asynchronous GroEL:GroES1 complexes turning over via the asymmetric cycle. The cartoon above the figure illustrates the kinetic partitioning of GroEL:GroES complexes between the symmetric and asymmetric cycles under the influence of SP.
Unfolded α-lactalbumin is a rather weakly bound SP, although it is experimentally convenient because it cannot fold. In the experiment shown in Fig. 2, it was necessary to use a 24-fold molar excess of unfolded α-lactalbumin (7.1 μM) over GroEL rings (0.3 μM) to maximally populate the symmetric GroEL:GroES2 particles in the steady state (blue traces in Fig. 2). The weak affinity of α-lactalbumin for GroEL, plus the limited solubility of 5-((((2-iodoacetyl)amino)ethyl)amino)naphthalene-1-sulfonic acid (IAEDANS)-labeled α-lactalbumin, set constraints on the design of the SP “wash-off” experiment shown below, such that the GroEL rings were less than maximally populated with GroES at the onset of the experiment. SPs such as unfolded malate dehydrogenase (MDH) or Rubisco are much more tightly bound so that it is possible to maximally populate the symmetric GroEL:GroES2 particles in the steady state with a molar equivalent of unfolded MDH (or Rubisco) subunits: GroEL rings. Indeed, the population of symmetric GroEL:GroES2 particles is initially a linear function of the [MDH subunits]:[GroEL rings]. The wash-off experiments with MDH and Rubisco were thus initiated in the steady state with a close-to maximal population of symmetric GroEL:GroES2 particles.
The SP-Jump Experiments.
The partitioning of the system between the two cycles is very rapid as can be seen from the experiment shown in Fig. 2. Here, apo-FRET GroEL was first mixed with FRET GroES and ATP (plus an ATP-regenerating system) in the absence of SP, and the system was permitted to relax to asymmetric particles. Two minutes afterward, the asymmetric particles were then challenged with SP at the same concentrations as used before. The system reaches a new equilibrium position within a second or two. The equilibrium position depends on the concentration of SP. At saturating concentrations, the ensemble consists predominantly of symmetric particles, whereas at intermediate concentrations, equilibrium mixtures of both asymmetric and symmetric particles are established. As shown in Fig. 2, the equilibrium position is the same regardless of whether it is approached from symmetric particles (with SP present initially) or from asymmetric particles (with SP added after 2 min).
These “SP-jump” experiments provide a clear-cut challenge to the widely accepted model for chaperonin assisted protein folding (5, 6) that (i) proposes that the two rings of GroEL are alternatively active and (ii) assigns no role to symmetric GroEL:GroES2 particles, aside, perhaps, from their involvement as a transient, sparsely populated intermediate. According to this model, the steady-state population of asymmetric particles remains unperturbed by the addition of SP. Indeed, the involvement of symmetric particles in chaperonin-assisted protein folding has been categorically and unequivocally dismissed (22, 23). On the contrary, the results of the SP-jump experiments demonstrate how rapidly the system responds to the presence of SP by the formation of symmetric particles in which both rings of GroEL are simultaneously functional.
Both Symmetric GroEL:GroES2 Footballs and Asymmetric GroEL:GroES1 Bullets are Dynamic.
The turnover of GroES in the asymmetric cycle.
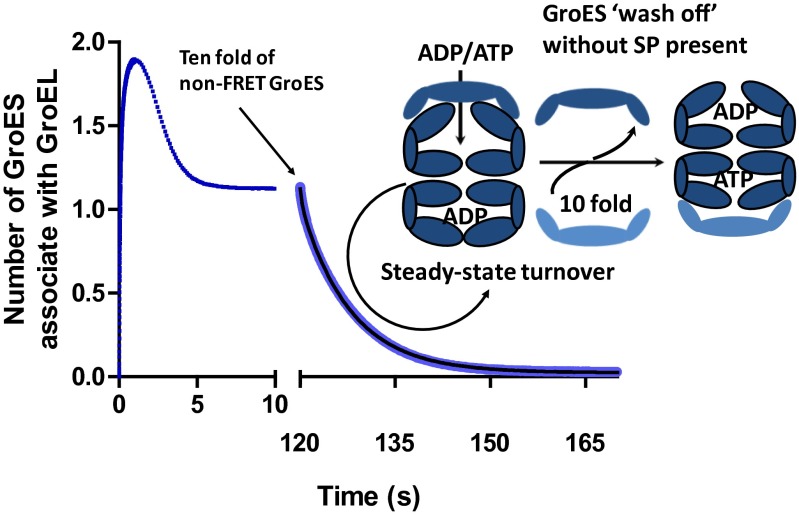
In the absence of SP, GroES populates the two rings of GroEL alternately as the chaperonin cycles in the asymmetric mode. The asymmetric GroEL:GroES1 particles are dynamic, the rate-determining step being the dissociation of the product ADP from the trans ring, ∼0.1 s−1 (4). Here we mixed FRET-GroEL and FRET-GroES with ATP in the absence of SP allowing them to turnover in the asymmetric cycle. The actively turning-over particles, almost entirely asymmetric in nature, were next loaded into the stopped-flow device at 37 °C. After turning over for 2 min, the asymmetric complex was challenged with a 10-fold molar excess of non-FRET GroES. The FRET signal decayed, as the non-FRET GroES displaced the FRET-GroES (Fig. 3), with a half time of ∼7 s, consistent with the rate-determining step being the dissociation of ADP from the trans ring, ∼0.1 s−1 (4).
Fig. 3.
Dynamics of the GroEL:GroES1 asymmetric and GroEL:ES2 symmetric complexes under turnover conditions. GroES wash out from the asymmetric GroEL:GroES1 complex. The plot combines two related FRET measurements. Both measurements include 2 μM FRET apo-GroEL and 3 μM FRET GroES mixture. The trajectory in the left section (blue) was initiated by mixing with 0.5 mM ATP and shows a similar transient as in Figs. 1 and 2. The right portion of the trajectory (light blue), however, was generated as follows: after addition of 0.5 mM ATP, the system was left turning over for 2 min (marked by the arrow) before 30 μM non-FRET GroES (dark blue) was added, displacing the FRET GroES and thus showing the kinetics of the turnover of the asymmetric complex. An ATP-regenerating system (0.4 mM PEP plus 10 U/mL pyruvate kinase) was included in both measurements. The GroES wash-off trace can be fitted with double-exponential equations: y = Amp1 × exp(−k1 × t) + Amp2 × exp(−k2 * t) + B, yielding two rate constants: 2.49 ± 0.06 s−1 (Amp, ∼7%) and 0.1265 ± 0.0003 s−1 (Amp, ∼93%). The latter is very close to the rate constant for the dissociation of ADP trans ring from the resting state asymmetric complex (4), whereas the former can be attributed to a small fraction of asymmetric complex in the acceptor state (no ADP release required before ATP-induced dissociation of the cis-bound GroES).
The turnover of GroES in the symmetric cycle.
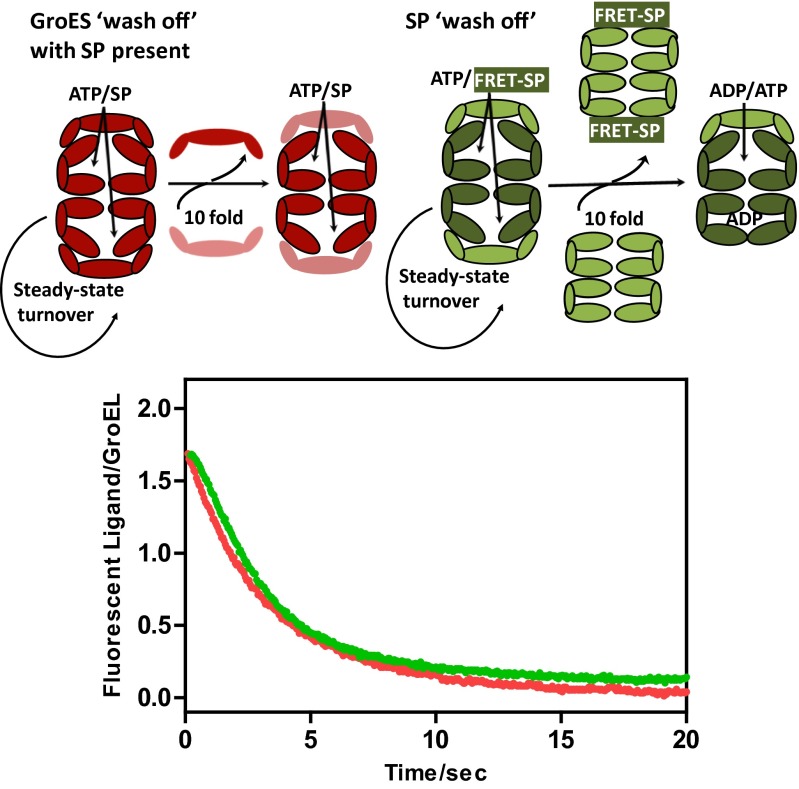
The belief that the formation of symmetric GroEL:GroES2 particles and polypeptide binding are mutually exclusive (22, 23) rests on the assumption that these particles are static entities, not subject to turnover. We show here in the following manner that this assumption is mistaken. Symmetric particles were first created by mixing FRET-GroEL, FRET-GroES, and ATP (plus a regenerating system) in the presence of a saturating concentration of SP. Five minutes later, a 10-fold molar excess of non-FRET GroES was introduced, enabling us to follow the dissociation of the FRET-GroES from both rings of FRET GroEL (Fig. 4). The reaction leading to the dissociation of the FRET GroES from both rings of GroEL proceeded with a half time of ∼1.7 s, even though the population of symmetric particles was maintained throughout. Because the release of the FRET-GroES from both GroEL rings involves minimally two hemicycles, we may approximate the half-time for the release of GroES from a single GroEL ring as 0.85 s. As shown in Figs. 1 and 2, the dissociation of GroES from symmetric particles requires the hydrolysis of ATP and the breakage of nucleotide symmetry. The breakage of nucleotide symmetry in the symmetric particles occurs independent of SP and begins after as few as 5 of the 14 ATPs have been hydrolyzed (Fig. 1C). This process is almost certainly stochastic and leads to the dissociation of one or the other GroES from the “leading” GroEL ring (defined as the GroEL ring with fewer ATPs), from which the GroES dissociates. Thereafter, SP catalyzed nucleotide exchange on, and GroES binding to, the transGroEL leading ring restores the symmetric complex. Although a substantial population of symmetrical particles are reformed, that merely requires that there be insufficient nucleotide asymmetry to break GroES symmetry without additional ATP hydrolysis. Further ATP hydrolysis establishes the minimal nucleotide asymmetry necessary for the dissociation of GroES from one ring or the other. This result calls into question another item of chaperonin dogma, namely that ATP hydrolysis in a GroEL ring must be complete before the GroES to which it is attached can be induced to dissociate (25). This cannot be so because in the time needed to release half of the GroES (∼0.85 s), fewer than three of the seven ATPs will have been hydrolyzed.
Fig. 4.
GroES and SP wash off from the symmetric GroEL:GroES2 complex. GroES wash off: a solution of predominantly symmetric GroEL:GroES2 particles was prepared by mixing 2µM FRET apo-GroELE315C, 2.5µM FRET-GroES98C, 10 mM DTT, 2.8 µM denatured α-lactalbumin (α-LA), 0.5 mM ATP, 5 mM PEP, and 20 U/mL pyruvate kinase. The solution was quickly loaded into one syringe of the stopped-flow device where it equilibrated at 37 °C. The other syringe contained 25µM unlabeled GroES and 2.8 µM denatured α-lactalbumin. Upon mixing, as the symmetric GroEL:GroES2 particles turned over via the symmetric cycle the wash-out of the FRET-GroES was observed (red trace). The system was calibrated by mixing 2 µM FRET apo-GroELE315C, 2.5µM FRET-GroES98C with the presence of ATP· BeFx or ADP· BeFx as standards. SP wash off: to monitor the release of SP from the symmetric GroEL:GroES2 complex, a different FRET system was used using IAEDANS-labeled α-lactalbumin and fluorescein labeled GroELE315C (SI Materials and Methods). A solution of buffer A [0.05 M Tris⋅HCl (pH 7.5), 10 mM MgCl2, 0.2 M KCl, 1 mM DTT] containing 4 µM fluorescein-labeled GroELE315C, 5.7 μM IAEDANS-labeled α-lactalbumin, 8 µM unlabeled GroES, 1 mM PEP, and 20 U/mL pyruvate kinase was first mixed with 1 mM ATP and quickly were loaded in one syringe of the stopped-flow device. This permits the formation of actively turning over GroEL:GroES2 complexes loaded with an unfolded (and unfoldable) SP. The other syringe contained 57.1µM unlabeled GroEL in buffer A. The system was equilibrated at 37 °C and ∼5 min after the addition of ATP, the contents of the two syringes were mixed and the decrease in the FRET signal (green trace) (λexcit = 336 nm; λemis > 530 nm) monitored as the IAEDANS-labeled SP is transferred from the fluorescein-labeled GroEL to the unlabeled GroEL as the GroEL:GroES2 complexes turnover. The stoichiometry of IAEDANS-labeled α-lactalbumin to fluorescein-labeled GroEL was determined independently under the same conditions fluorescence spectroscopy. In the cartoons, components of the FRET system are shown in darker shades, whereas unlabeled components are shown with lighter shades.
The turnover of SP in the symmetric cycle.
The rapid exchange of GroES from a steady-state population of symmetric GroEL:GroES2 particles, dependent as it was on the breakage of nucleotide symmetry and SP catalyzed nucleotide exchange, suggested that a similar exchange process might occur with SP. To study this, we labeled the SP (unfolded α-lactalbumin) with IAEDANS and GroEL with fluorescein and observed the FRET signal (λexcit = 336 nm; cutoff filter at 530 nm) that developed upon the formation of a binary GroEL-SP complex or upon encapsulation of SP that accompanies the formation of symmetric, ternary GroEL-SP-GroES2 complex. Symmetric particles were created by mixing FRET-GroEL, non–FRET-GroES and ATP (plus a regenerating system) with a limiting concentration of FRET-SP as before. The actively turning-over particles (∼70% of them symmetric) were next loaded into the stopped-flow device at 37 °C. After turning over for 5 min, the asymmetric complex was challenged with either a 10-fold molar excess of non-FRET GroEL, enabling us to follow the transfer of the FRET-SP from both rings of FRET GroEL to the non-FRET GroEL (Fig. 4). The reaction leading to the dissociation of the FRET-SP from both rings of GroEL proceeded with a half time of ∼2.1 s, even though the population of symmetric particles was maintained throughout (Fig. 4). Note that the kinetics of GroES and SP release from the actively turning over particles track one another closely, as is to be expected because it has long been known that the release of both are coupled (9). Because the dissociation of FRET-SP from both GroEL rings involves minimally two hemicycles, we may approximate the half-time for the release of SP from a single GroEL ring to be ∼1.0 s.
Two important points that challenge the prevailing chaperonin dogma may be gleaned from the results of these “wash-out” experiments. Firstly, symmetric GroEL:GroES2 footballs are not static entities as has been assumed (22, 23) but rather highly dynamic particles. Secondly, the residence time of GroES (and in turn of SP) on a GroEL ring of the symmetric GroEL:GroES2 complex in the presence of SP is much shorter than the residence time of GroES on a GroEL ring of the asymmetric GroEL:GroES1 complex in the absence of SP.
Symmetric GroEL:GroES2 “Footballs” as the Folding Functional Form.
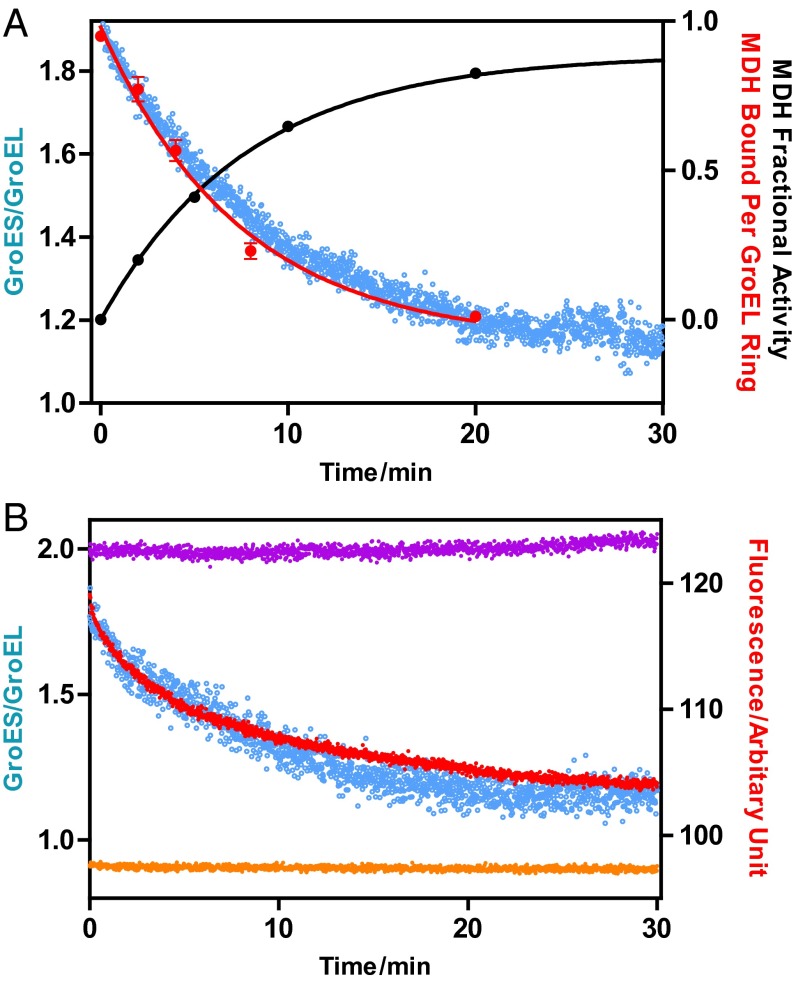
The symmetric GroEL:GroES2 particles persist for tens of minutes, provided they are supplied with an ATP-regenerating system and a source of unfolded SP. We have provided the latter in the form of unfolded (and unfoldable) α-lactalbumin. However, what happens to the population of symmetric particles if we provide an unfolded SP that is foldable, such as MDH or Rubisco? We explore this question with the experiments shown in Fig. 5, using the calibrated FRET system to continuously monitor the population of symmetric GroEL:GroES2 and asymmetric GroEL:GroES1. Binary complexes containing 1 mol of either MDH (Fig. 5A) or Rubisco (Fig. 5B) per FRET-GroELE315C ring were first prepared. These were then mixed with a slight excess of FRET-GroES98C and folding initiated by the addition of ATP. Initially, 80–90% of the particles are symmetric under these conditions. However, as time progresses and the SP folds, the population of symmetric particles declines. In separate studies using nearly identical conditions, the quantity of MDH or Rubisco that remained bound to the GroEL:GroES2 complexes was found to decline in parallel (Fig. 5). The correlations are not perfect; there are small deviations that might be attributable to the slightly different conditions used. Nevertheless, when considered together with the SP-dependent equilibrium between the symmetric and asymmetric GroEL:GroES complexes (Fig. 2), the results of this set of experiments strongly suggest that the symmetric GroEL:GroES2 particles are the folding functional form.
Fig. 5.
Symmetric GroEL:GroES2 particles decay to asymmetric GroEL:GroES1 particles coincident with the folding of foldable substrate proteins. (A) Mitochondrial malate dehydrogenase (MDH). Three parameters were measured: (i) the increase in MDH activity, indicative of the folded, native state; (ii) the decrease in the quantity of MDH that remained bound to GroEL; and (iii) the relaxation of the calibrated FRET-labeled symmetric GroEL:GroES2 complex to the asymmetric GroEL:GroES1 complex accompanying the folding of MDH. (i) MDH folding: unfolded MDH (23.5 μM in 5 M urea) was diluted 88-fold into a solution of buffer A containing 2 μM GroEL subunits, 4 μM GroES subunits and equilibrated at 30 °C. Folding was initiated by adding 2 mM ATP. Native MDH (not unfolded in urea) but otherwise treated identically served as a control. MDH activity was determined essentially as previously described (for additional information, see SI Materials and Methods). The MDH folded (black) is normalized with respect to the native control. (ii) MDH bound to GroEL: unfolded MDH (23.2 μM in 5 M urea) was diluted to a final concentration of 3.0 μM into a solution of buffer A, containing 21.5 μM GroEL subunits, 42 μM GroEShis subunits, 5 mM PEP, and 20 U/mL pyruvate kinase and equilibrated at 30 °C. The reaction was started by the addition of 0.5 mM ATP. At various times, 100-µL aliquots were quenched with 1 mM BeCl2 and 0.01 M NaF as previously reported (18) and applied to a small column of Ni-NTA agarose, equilibrated with buffer A containing 1 mM BeCl2 and 0.01 M NaF. Following a 0.6 mL wash with the same solution to remove unbound MDH, the GroEL:GroEShis2 complex containing bound MDH was eluted with 0.25 M imidazole in buffer A. Aliquots of the eluate were subjected to SDS/PAGE, along with standard solutions containing known amounts of GroEL and MDH. The resultant gel was stained with Coomassie blue to visualize GroEL and MDH. The gels were scanned with a densitometer and the quantity of each protein determined by reference to the standards. This analysis yielded an initial value of 0.95 MDH per GroEL ring before the addition of ATP. As MDH folded, progressively less MDH remained bound to GroEL (red circles). (iii) Relaxation of GroEL:GroES2 was conducted in a Perkin-Elmer fluorimeter (λexcit = 336 nm; λemis = 520 nm). Unfolded MDH (23.5 μM in 5 M urea) was diluted 88-fold into a solution of buffer A containing 2 μM FRET-GroELE315C subunits labeled with IAEDANS, 2.5 μM FRET-GroES subunits labeled with fluorescein, 1 mM DTT, 5 mM PEP, 20 units per milliliter pyruvate kinase, and 1 mg/mL BSA. After equilibration at 30 °C the reaction was initiated with 0.5 mM ATP. To calibrate the number of GroES on GroEL, two control experiments were performed under the same conditions. In one control experiment, 10 mM BeCl2 and 100 mM NaF was added at the presence of ATP to establish the FRET signal corresponding to the symmetric GroEL:GroES2 complex, whereas in the other, the ATP was replaced with ADP, so as to establish the FRET signal corresponding to the asymmetric GroEL:GroES1 complex. The decay in the FRET signal concomitant with the folding of MDH is shown in blue. (B) Rhodospirillum rubrum Rubisco. (i) Rubisco bound to GroEL: we exploited the absence of tryptophan in GroEL and GroES and its presence in Rubisco to develop a tryptophan-IAEDANS FRET system to monitor the association of Rubisco with GroEL. This was conducted in a Perkin-Elmer fluorimeter (λexcit = 295 nm; λemis = 470 nm). Acid denatured Rubisco was incubated in 50 mM glycine⋅HCl (pH 2.72) for ∼3 min on ice; 2.5 μL of acid-denatured 23 µM Rubisco was added to a 0.2 mL of solution of buffer A containing 2 µM GroELE315C-IAEDANS, 5 µM unlabeled GroES, 1 mM DTT, 5 mM PEP, 20 units of pyruvate kinase, 1 mg/mL of BSA. After 5 min equilibration at 25 °C, the reaction was initiated by the addition of 0.5 mM ATP. The decrease in the quantity of Rubisco bound to GroEL is shown in red. Two controls were performed for this experiment: in one control, no ATP was added in the solution (purple), demonstrating the stability of the GroEL-Rubisco complex. In the other control, native Rubisco was used instead of denatured Rubisco (orange). (ii) Relaxation of GroEL:GroES2 was performed essentially as above, at 25 °C instead of 30 °C and with denatured Rubisco replacing denatured MDH. The decay in the FRET signal concomitant with the folding of Rubisco is shown in blue.
Discussion
Both Rings of GroEL Can Be Simultaneously Occupied by ATP.
The allosteric behavior of GroEL (2) involves positively cooperative interactions between the subunits of one ring and negatively cooperative interactions between the subunits of different rings. Simply stated the negative cooperativity of GroEL means that binding of a ligand (e.g., ATP) to one ring decreases the affinity of the other ring for that ligand. Some have taken this to an improbable extreme, claiming that the binding of ATP to one ring absolutely precludes the binding of ATP to the other ring (5). Others have suggested more thermodynamically plausible scenarios in which the hydrolysis of ATP by one ring occurs only after the dissociation of the product ADP from the other ring (26). This kinetic mechanism would ensure that only one of the rings of GroEL is occupied by ATP at any given time. However, addition of ATP to the trans ring of the acceptor state complex triggers a burst of Pi release of ∼0.5 mol/mol GroEL subunits arising from the nascent cis ring that clearly precedes the dissociation of ADP from the nascent trans ring (4). Additionally, three independent experiments reported here are contrary to the idea that ATP binds only to one GroEL ring at a time. (i) Mixing GroEL, GroES, and ATP in the presence of BeF2 leads to the formation of symmetrical GroEL:GroES2 particles and the hydrolysis of 1 mol of ATP per GroEL subunit, implying the occupancy of both rings by ATP (Fig. S1E). (ii) Mixing GroEL, GroES, and ATP in the absence of SP leads to the transient formation of symmetric GroEL:GroES2 particles at which point only ∼3 of the 14 ATPs have been hydrolyzed. The remaining 11 ATPs must be distributed between both rings. The subsequent reversion to asymmetric GroEL:GroES1 particles is accompanied by the hydrolysis of the remaining ATP yielding an equivalent to one ADP per GroEL subunit (Fig. 1A). (iii) When the ATPase-defective mutant D398A is mixed with ATP and GroES, symmetric GroEL:GroES2 particles are again formed (Fig. 1B). These particles initially contain 1 mol of ATP per GroEL subunit, that slowly yields 1 mol of Pi per GroEL subunit as the symmetric particles slowly revert to asymmetric GroEL:GroES1 particles (Fig. 1C). We conclude that ATP can be bound simultaneously to both rings of GroEL; that in turn enables GroES to populate both rings.
Symmetric GroEL:GroES2 and Asymmetric GroEL:GroES1 Particles Exist in a Dynamic, Substrate Protein-Dependent Equilibrium.
The steady-state population of GroEL:GroES1 particles that predominate in the absence of SP are consistently slightly less than the population established with the (ADP plus BeFx) standard (Fig. 1A). This is to be expected, because in the operation of the asymmetric cycle (figure 9 in ref. 4) (3, 4), a small population of symmetric GroEL:GroES2 particles exist as a transiently populated intermediate. Similarly, in the presence of a saturating concentration of SP, the population of symmetric GroEL:GroES2 particles is consistently slightly less than the population established with the (ATP plus BeFx) standard (Fig. 2A) or with the D398A mutant (Fig. 1B).
Between these two extremes, a dynamic equilibrium exists between symmetric and asymmetric particles that is dependent upon the prevailing concentration of SP (Fig. 2). This equilibrium can be approached from either direction. It rapidly (within a few seconds) adjusts to changes in the concentration of SP, increasing the population of symmetric particles at the expense of asymmetric particles, suggesting that the symmetric particles are the folding-functional species. This implies that SP can simultaneously populate both rings of GroEL and evidence that this is so has been reported (15, 21). Our finding of one unfolded MDH per GroEL ring in a population of predominantly symmetric particles (Fig. 5) confirms this. Once again, the experimental evidence is at odds with the prevailing chaperonin dogma that proposes that the two rings of GroEL are alternately populated with SP.
On the Lifetime of Anfinsen’s Cage in the Presence of Substrate Protein.
Currently, the most widely accepted mechanism of chaperonin assisted SP folding (5, 6) is based upon an analysis of the chaperonin ATPase cycle performed in the absence of SP (9, 26). In this scenario, the two rings of GroEL are alternately functional, the predominant species is an asymmetric GroEL:GroES1 complex, and no role whatsoever is assigned to symmetric GroEL:GroES2 particles, except perhaps as a very transient, sparsely populated intermediate. Following refs. 3 and 4, we refer to this as the asymmetric cycle upon which the encapsulation of SP is simply superimposed. The encapsulation of a single SP molecule by the chaperonin nanomachine creates a sequestered space, the so-called Anfinsen cage that is viewed as a space of infinite SP dilution in which SP folding to the native state can occur unimpeded by self-association events that would otherwise lead to aggregation. The signal for the dissociation of GroES from the cis GroEL ring of asymmetric complex is triggered by the binding of ATP to the trans ring of the complex (9). This is preceded by the rate-determining dissociation of ADP from the trans ring, the product of the previous hemiasymmetric cycle (4). Because the rate of ADP dissociation from the trans ring is ∼five times slower than the rate of ATP hydrolysis in the cis ring (4), it follows that all of the ATP in the cis ring will have been hydrolyzed before the completion of ADP dissociation from the trans ring. This ensures that, when ATP binds to the trans ring to initiate the next hemicycle, maximum nucleotide asymmetry exists between the two GroEL rings, the basis for alternate function in the asymmetric cycle. We show here (Fig. 3A) that the residence half-time of GroES on GroEL actively turning over via the asymmetric cycle is ∼7 s in the absence of SP, a value that agrees with the independently measured rate constant of ∼0.1 s−1 for the dissociation of the trans ring ADP in the absence of SP (4).
The currently accepted mechanism of chaperonin dependent protein folding makes several questionable assumptions. It is simply assumed, most often tacitly, that the lifetime of Anfinsen’s cage, the residence half-time of both SP and GroES on GroEL in the presence of SP, is the same as in the absence of SP (5, 6, 22, 23). This assumption is mistaken because we show here (Fig. 3B) that in the presence of SP, the residence half-time of both GroES and SP on GroEL is ∼10-fold reduced to ∼1 s.
SR1: A Case of Mistaking What Can Happen for What Does Happen.
The fact that the SP is at least transiently encapsulated in the central cavity of GroEL under the GroES cap is not disputed. There is evidence, involving the artful use of a single-ringed variant of GroEL, SR1, that the SP can fold to the native state while encapsulated. The residence time of the encapsulated SP and of GroES on the single GroEL ring of SR1 is effectively set to infinity. Because aggregation of the encapsulated SP through self-association is no longer possible, its folding to the most thermodynamically stable native state in the fullness of time is inevitable, and after Anfinsen, frankly unremarkable. However, this confuses what can happen with what does happen when, as we show here, in a system functioning naturally the residence half-time of the encapsulated SP is reduced to ∼1 s. That being so raises the following questions “where does the SP fold, inside the cage or outside? (27)”. The questions are moot. Suffice to note that most (>99%) of the SP molecules that emerge from the cage with each turnover remain unfolded, still recognizable by GroEL. More importantly the rapid turnover of the SP by the symmetric GroEL:GroES2 complex creates repeated opportunities for work to be done by the chaperonin machine by unfolding the SP (28–30) or its surrogates (31). As we have proposed in the iterative annealing mechanism, the rate of chaperonin-assisted protein folding is related to the number of iterations per unit time (32), in contrast to the Anfinsen cage model, which places a premium on extending the residence time of the encapsulated SP (5, 6, 22, 23).
Materials and Methods
Purification and Labeling of Phosphate-Binding Protein A197C.
Phosphate-binding protein A197C (PBP) was purified and labeled with N-[2-(1-maleimidyl)ethyl]-7-(diethylamino)coumarin-3-carboxamide (MDCC) as previously described (4, 24).
Purification of GroELWT, GroELE315C, GroELD398A, GroELK315C/D398A, GroESWT, GroES98C, and GroEShis.
These proteins were prepared, purified, and labeled as previously described (33). Typically, GroEL preparations contained <0.2 mol of contaminating SP per GroEL14 (i.e., <10% of the rings may be contaminated with an ensemble of SPs). The concentrations of purified GroEL and GroES were measured at 280 nm using the extinction coefficients of 9,600 and 1,200 cm−1⋅M−1, respectively.
Release of Pi Following ATP Hydrolysis.
The PBP-MDCC method.
These measurements were made by a modified version of a previously described method (4); 10 μM labeled PBP-MDCC was used throughout, all of the measurements were conducted at 37 °C, and asymmetric complexes containing 1 μM GroEL subunits were used. Whenever different concentrations of K+ were used, Na+ was used to balance the ionic strength such that ([K+] + [Na+]) = 0.1 M. Compounds such as ATP and phosphoenolpyruvate (PEP) were treated with the “Pi mop” to lower background [Pi] (24). The fluorescence change of PBP-MDCC was calibrated against known concentrations of Pi standard as before (4).
The pre–steady-state measurements of Pi release were carried out on an Applied Photophysics SX18MV-R stopped-flow apparatus in fluorescence mode at 37 °C as described in the figure legends. Data from >five kinetic traces were collected and averaged.
The 2-amino-6-mercapto-7-methylpurine riboside–purine nucleoside phosphorylase method.
Pi released by the slow hydrolysis of ATP by the mutant GroELD398A was measured using 2-amino-6-mercapto-7-methylpurine riboside (MESG) and purine nucleoside phosphorylase (PNPase) as previously described (34).
Labeling GroELE315C and GroES98C.
A slight modification of the method described by Rye (35) was used to label used to label GroELE315C with IAEDANS, with fluorescein-5-maleimide and GroES98C with fluorescein-5-maleimide. About three dye molecules were incorporated per ring.
Preparation and Labeling of Unfolded α-Lactalbumin.
Unfolded α-lactalbumin was prepared as described (36). The unfolded α-lactalbumin was labeled with IAEDANS (0.85 mol of dye per monomer) as above.
Malate Dehydrogenase Activity.
This was measured essentially as previously described (37).
Other Methods.
Additional methods are described in SI Materials and Methods.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318862110/-/DCSupplemental.
References
- 1.Lin Z, Rye HS. GroEL-mediated protein folding: Making the impossible, possible. Crit Rev Biochem Mol Biol. 2006;41(4):211–239. doi: 10.1080/10409230600760382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horovitz A, Willison KR. Allosteric regulation of chaperonins. Curr Opin Struct Biol. 2005;15(6):646–651. doi: 10.1016/j.sbi.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Sameshima T, Iizuka R, Ueno T, Funatsu T. Denatured proteins facilitate the formation of the football-shaped GroEL-(GroES)2 complex. Biochem J. 2010;427(2):247–254. doi: 10.1042/BJ20091845. [DOI] [PubMed] [Google Scholar]
- 4.Ye X, Lorimer GH. Substrate protein switches GroE chaperonins from asymmetric to symmetric cycling by catalyzing nucleotide exchange. Proc Natl Acad Sci USA. 2013;110:E4289–E4297. doi: 10.1073/pnas.1317702110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horwich AL. Protein folding in the cell: An inside story. Nat Med. 2011;17(10):1211–1216. doi: 10.1038/nm.2468. [DOI] [PubMed] [Google Scholar]
- 6.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 7.Nelson DL, Cox MM. Principles of Biochemistry. 6th Ed. New York: Freeman; 2013. p. 148. [Google Scholar]
- 8.Voet D, Voet JG. Biochemistry. 4th Ed. Hoboken, NJ: John Wiley & Sons; 2011. pp. 293–300. [Google Scholar]
- 9.Todd MJ, Viitanen PV, Lorimer GH. Dynamics of the chaperonin ATPase cycle: Implications for facilitated protein folding. Science. 1994;265(5172):659–666. doi: 10.1126/science.7913555. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt M, et al. Symmetric complexes of GroE chaperonins as part of the functional cycle. Science. 1994;265(5172):656–659. doi: 10.1126/science.7913554. [DOI] [PubMed] [Google Scholar]
- 11.Azem A, Diamant S, Kessel M, Weiss C, Goloubinoff P. The protein-folding activity of chaperonins correlates with the symmetric GroEL14(GroES7)2 heterooligomer. Proc Natl Acad Sci USA. 1995;92(26):12021–12025. doi: 10.1073/pnas.92.26.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llorca O, Marco S, Carrascosa JL, Valpuesta JM. The formation of symmetrical GroEL-GroES complexes in the presence of ATP. FEBS Lett. 1994;345(2-3):181–186. doi: 10.1016/0014-5793(94)00432-3. [DOI] [PubMed] [Google Scholar]
- 13.Llorca O, Marco S, Carrascosa JL, Valpuesta JM. Symmetric GroEL-GroES complexes can contain substrate simultaneously in both GroEL rings. FEBS Lett. 1997;405(2):195–199. doi: 10.1016/s0014-5793(97)00186-5. [DOI] [PubMed] [Google Scholar]
- 14.Corrales FJ, Fersht AR. Kinetic significance of GroEL14(GroES7)2 complexes in molecular chaperone activity. Fold Des. 1996;1(4):265–273. doi: 10.1016/s1359-0278(96)00040-5. [DOI] [PubMed] [Google Scholar]
- 15.Sparrer H, Rutkat K, Buchner J. Catalysis of protein folding by symmetric chaperone complexes. Proc Natl Acad Sci USA. 1997;94(4):1096–1100. doi: 10.1073/pnas.94.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grallert H, Rutkat K, Buchner J. GroEL traps dimeric and monomeric unfolding intermediates of citrate synthase. J Biol Chem. 1998;273(50):33305–33310. doi: 10.1074/jbc.273.50.33305. [DOI] [PubMed] [Google Scholar]
- 17.Beissinger M, Rutkat K, Buchner J. Catalysis, commitment and encapsulation during GroE-mediated folding. J Mol Biol. 1999;289(4):1075–1092. doi: 10.1006/jmbi.1999.2780. [DOI] [PubMed] [Google Scholar]
- 18.Taguchi H, Tsukuda K, Motojima F, Koike-Takeshita A, Yoshida M. BeF(x) stops the chaperonin cycle of GroEL-GroES and generates a complex with double folding chambers. J Biol Chem. 2004;279(44):45737–45743. doi: 10.1074/jbc.M406795200. [DOI] [PubMed] [Google Scholar]
- 19.Koike-Takeshita A, Yoshida M, Taguchi H. Revisiting the GroEL-GroES reaction cycle via the symmetric intermediate implied by novel aspects of the GroEL(D398A) mutant. J Biol Chem. 2008;283(35):23774–23781. doi: 10.1074/jbc.M802542200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sameshima T, et al. Football- and bullet-shaped GroEL-GroES complexes coexist during the reaction cycle. J Biol Chem. 2008;283(35):23765–23773. doi: 10.1074/jbc.M802541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takei Y, Iizuka R, Ueno T, Funatsu T. Single-molecule observation of protein folding in symmetric GroEL-(GroES)2 complexes. J Biol Chem. 2012;287(49):41118–41125. doi: 10.1074/jbc.M112.398628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayer-Hartl MK, Martin J, Hartl FU. Asymmetrical interaction of GroEL and GroES in the ATPase cycle of assisted protein folding. Science. 1995;269(5225):836–841. doi: 10.1126/science.7638601. [DOI] [PubMed] [Google Scholar]
- 23.Hayer-Hartl MK, Ewalt KL, Hartl FU. On the role of symmetrical and asymmetrical chaperonin complexes in assisted protein folding. Biol Chem. 1999;380(5):531–540. doi: 10.1515/BC.1999.068. [DOI] [PubMed] [Google Scholar]
- 24.Brune M, Hunter JL, Corrie JET, Webb MR. Direct, real-time measurement of rapid inorganic phosphate release using a novel fluorescent probe and its application to actomyosin subfragment 1 ATPase. Biochemistry. 1994;33(27):8262–8271. doi: 10.1021/bi00193a013. [DOI] [PubMed] [Google Scholar]
- 25.Rye HS, et al. GroEL-GroES cycling: ATP and nonnative polypeptide direct alternation of folding-active rings. Cell. 1999;97(3):325–338. doi: 10.1016/s0092-8674(00)80742-4. [DOI] [PubMed] [Google Scholar]
- 26.Kad NM, Ranson NA, Cliff MJ, Clarke AR. Asymmetry, commitment and inhibition in the GroE ATPase cycle impose alternating functions on the two GroEL rings. J Mol Biol. 1998;278(1):267–278. doi: 10.1006/jmbi.1998.1704. [DOI] [PubMed] [Google Scholar]
- 27.Motojima F, Yoshida M. Polypeptide in the chaperonin cage partly protrudes out and then folds inside or escapes outside. EMBO J. 2010;29(23):4008–4019. doi: 10.1038/emboj.2010.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Z, Madan D, Rye HS. GroEL stimulates protein folding through forced unfolding. Nat Struct Mol Biol. 2008;15(3):303–311. doi: 10.1038/nsmb.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madan D, Lin Z, Rye HS. Triggering protein folding within the GroEL-GroES complex. J Biol Chem. 2008;283(46):32003–32013. doi: 10.1074/jbc.M802898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Z, Puchalla J, Shoup D, Rye HS. Repetitive protein unfolding by the trans ring of the GroEL-GroES chaperonin complex stimulates folding. J Biol Chem. 2013 doi: 10.1074/jbc.M113.480178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corsepius NC, Lorimer GH. Measuring how much work the chaperone GroEL can do. Proc Natl Acad Sci USA. 2013;110(27):E2451–E2459. doi: 10.1073/pnas.1307837110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todd MJ, Lorimer GH, Thirumalai DT. Chaperonin-facilitated protein folding: Optimization of rate and yield by an iterative annealing mechanism. Proc Natl Acad Sci USA. 1996;93(9):4030–4035. doi: 10.1073/pnas.93.9.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grason JP, Gresham JS, Widjaja L, Wehri SC, Lorimer GH. Setting the chaperonin timer: The effects of K+ and substrate protein on ATP hydrolysis. Proc Natl Acad Sci USA. 2008;105(45):17334–17338. doi: 10.1073/pnas.0807429105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webb MR. A continuous spectrophotometric assay for inorganic phosphate and for measuring phosphate release kinetics in biological systems. Proc Natl Acad Sci USA. 1992;89(11):4884–4887. doi: 10.1073/pnas.89.11.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rye HS. Application of fluorescence resonance energy transfer to the GroEL-GroES chaperonin reaction. Methods. 2001;24(3):278–288. doi: 10.1006/meth.2001.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yifrach O, Horovitz A. Allosteric control by ATP of non-folded protein binding to GroEL. J Mol Biol. 1996;255(3):356–361. doi: 10.1006/jmbi.1996.0028. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt M, Buchner J, Todd MJ, Lorimer GH, Viitanen PV. On the role of groES in the chaperonin-assisted folding reaction. Three case studies. J Biol Chem. 1994;269(14):10304–10311. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.