Significance
The mind easily wanders away from mundane tasks, but pain is presumed to automatically capture attention. We demonstrate that individuals differ in how often their minds spontaneously wander away from pain and that these differences are associated with the disruptive effect of pain on cognitive performance. Brain–behavior relationships underscore these individual differences. When people’s minds wander away from pain, there are increased activations of the default mode network (DMN) and strong interactions between the DMN and periaqueductal gray (PAG), an opiate-rich region mediating pain suppression. Individuals with greater tendencies to mind wander from pain have stronger anatomical links and dynamic functional communication between PAG and DMN. These findings provide clinically important clues about why some individuals cannot disengage from pain.
Keywords: pain modulation, salience network, stimulus-independent thought, ventral attention network, experience sampling
Abstract
Human minds often wander away from their immediate sensory environment. It remains unknown whether such mind wandering is unsystematic or whether it lawfully relates to an individual’s tendency to attend to salient stimuli such as pain and their associated brain structure/function. Studies of pain–cognition interactions typically examine explicit manipulation of attention rather than spontaneous mind wandering. Here we sought to better represent natural fluctuations in pain in daily life, so we assessed behavioral and neural aspects of spontaneous disengagement of attention from pain. We found that an individual’s tendency to attend to pain related to the disruptive effect of pain on his or her cognitive task performance. Next, we linked behavioral findings to neural networks with strikingly convergent evidence from functional magnetic resonance imaging during pain coupled with thought probes of mind wandering, dynamic resting state activity fluctuations, and diffusion MRI. We found that (i) pain-induced default mode network (DMN) deactivations were attenuated during mind wandering away from pain; (ii) functional connectivity fluctuations between the DMN and periaqueductal gray (PAG) dynamically tracked spontaneous attention away from pain; and (iii) across individuals, stronger PAG–DMN structural connectivity and more dynamic resting state PAG–DMN functional connectivity were associated with the tendency to mind wander away from pain. These data demonstrate that individual tendencies to mind wander away from pain, in the absence of explicit manipulation, are subserved by functional and structural connectivity within and between default mode and antinociceptive descending modulation networks.
Humans spend nearly half their time on thoughts unrelated to their present sensory environment (1), a phenomenon referred to as “mind wandering.” These thoughts can persist even when engaged in salient and challenging everyday activities (1, 2), such as driving a car through traffic. In such situations, mind wandering can be deleterious. However, in some situations, mind wandering may be beneficial, such as when an individual needs to cope with pain.
Cognitive manipulations, such as alterations of attention/distraction (3–5), placebo effects (6–9), changing expectations, and other strategies (10), have shown some efficacy in altering perceptions and neural responses elicited by painful stimuli. It is generally assumed that these effects involve enhanced endogenous analgesic activity within the descending pain modulatory system [e.g., prefrontal cortex, perigenual cingulate cortex, periaqueductal gray (PAG), and rostroventral medulla] and decreased activity in regions that support the salience of pain [e.g., insula and midcingulate cortex (MCC)] (10).
A crucial assumption in previous studies of explicit pain manipulation is that there is a static, invariant neurocognitive state during incoming nociceptive activity. However, the experience of pain can vary significantly, depending on trial-to-trial fluctuations in the prestimulus brain state (11–14). This may be due to intrinsic activity that fluctuates on multiple time scales, a hallmark of brain function related to ongoing cognitive, behavioral, and perceptual dynamics (15, 16). Pain modulation may therefore be considered as an ongoing, intrinsically dynamic process, rather than a binary (on/off) phenomenon triggered by explicit manipulation. The study of mind wandering during pain is thus well suited to examine the dynamic nature of pain modulation. The brain’s default mode network (DMN) has been implicated in mind wandering and self-oriented attention (17–20), but its interactions with pain-related brain networks in the disengagement of attention from pain are poorly understood. Furthermore, it remains unknown whether spontaneous disengagement of attention from pain and associated network activity is stable within individuals and predictive of behavior.
Thus, we developed an experience sampling approach (17, 19, 21) to examine the frequency and cognitive aspects of spontaneous attentional fluctuations during painful stimulation in 51 healthy volunteers. We established test–retest reproducibility of an individual’s tendency to attend away from pain, which we term “intrinsic attention to pain” (IAP), and tested whether IAP relates to behavioral performance on a cognitive task. Using functional magnetic resonance imaging (fMRI), we tested the hypothesis that the interplay between the DMN, salience-/attention-related regions, and the descending pain modulatory system reflects interactions between mind wandering and ongoing pain. We then used diffusion-weighted MRI (DWI) and resting state fMRI to determine whether intrinsic structural/functional connections between key regions that interact during attentional fluctuations away from pain are related to interindividual differences in IAP.
Results
Unique Protocol to Evaluate Mind Wandering Away from Pain.
Subject testing was carried out in two sessions. In session 1, subjects completed experience sampling and cognitive interference tasks including epochs of painful transcutaneous electrical nerve stimulation (TENS) of the left median nerve (Methods). In session 2, subjects completed experience sampling during fMRI, DWI, and resting state fMRI scans.
For the experience sampling task, the TENS level was maintained to consistently elicit pain intensity rated as 4–5 out of 10. Participants were instructed to avoid actively attending either toward or away from pain (SI Methods). On each trial, participants viewed a fixation cross while receiving painful TENS which was interrupted after 20 s with a thought probe in which subjects indicated whether their attention had just been “only on pain,” “mostly on pain,” “mostly on something else,” or “only on something else” (Fig. 1A). An IAP score was calculated for each subject based on the proportions of trials in the task with reports of attention to pain vs. attention to something else and could range from +2 (always attending to pain) to −2 (always attending to something else) (Methods). At the end of trials, participants were asked to rate the degree to which their reports of “something else” belonged to the categories of external sensory distractions (EDs) (e.g., auditory/visual events), task-related interferences (TRIs) (e.g., considering response to upcoming probe), or mind wandering (MW) (i.e., thoughts completely unrelated to present environment) (19).
Fig. 1.
Experience sampling during painful stimulation reveals frequency and sensory/cognitive aspects of attentional fluctuations away from pain. (A) Task trial design (Upper) and example of fluctuations in attentional state during the psychophysics session (Lower). (B) Distribution of the incidence of trials in session 1 (n = 51) (Upper) and session 2 (n = 50) (Lower) in which subjects experienced pain and/or something else. (C) Group averages (±SD) for ratings of something else being MW, TRIs, and EDs for session 1 and session 2. (D) Consistency of responses between session 1 and session 2 in IAP scores (ICC = 0.83; P = 4.7 × 10−10). (E) IAP scores from session 1 positively correlated with pain catastrophizing scale scores (r = 0.30; P = 0.03). ISI, interstimulus interval.
The cognitive interference task was one we used previously (22) to characterize the degree to which individuals prioritize cognitive task performance versus pain (Fig. 2A). The difference between mean reaction time (RT) across pain vs. no-pain trials was used to quantify the effect of pain on performance as done previously (22). Subjects were classified as P type if their RTs were slower during concomitant pain compared with “no pain” trials (i.e., pain dominates) or A type if they had faster RTs during concomitant pain (i.e., attention dominates).
Fig. 2.

Tendency to attend to pain relates to the disruptive effect of pain on cognitive task performance. (A) The task required subjects to choose the box with the greatest number of digits (highest count). Green-outlined boxes show the correct response in this example. Subjects performed the task with pain (P) and without pain (NP). Subjects with mean reaction time (RT) for P faster than for NP trials were classified as A type (attention dominates). Subjects with slower RTs for P compared with NP trials were P-type (pain dominates) (3, 22). (B) Positive correlation between ΔRT [P − NP] in the cognitive interference task and IAP from experience sampling (n = 48) (r = 0.42; P = 0.003). Quadrants show classification of subjects of A/P type and low/high IAP.
Behavioral Results.
The IAP scores for sessions 1 and 2 ranged from, respectively, −1.1 to +1.9 (mean ± SD = 0.17 ± 0.67) and −1.3 to +1.6 (mean ± SD = 0.01 ± 6.8). Individual subjects showed marked fluctuations in their attention to pain from trial to trial (e.g., Fig. 1A) but did so with remarkably consistent frequency between the two sessions (see below). The group data indicate an almost equal split of trials in which subjects were experiencing “only or mostly pain” vs. trials in which the experience was “only or mostly something else” (Fig. 1B): trials were rated as “only pain” (session 1: 13.8%; session 2: 11.0%), “mostly pain” (session 1: 41.4%; session 2: 39.3%), “mostly something else” (session 1: 38.1%; session 2: 39.4%), or “only something else” (session 1: 6.7%; session 2: 10.3%) (Fig. 1B). There was a strong, significant intraclass correlation (ICC) between IAP scores during session 1 vs. session 2 (ICC = 0.83; P = 4.7 × 10−10) (Fig. 1D), suggesting that IAP is a trait-like quality.
Participants reported that they were confident in their abilities to accurately indicate attentional state during the task (average confidence rating ± SD out of 7 = 5.7 ± 0.82; 1 indicates not confident at all, and 7 indicates very confident). Participants rated the degree to which their reports of Something Else belonged to the categories of EDs, TRIs, and MW (Likert scale: 1 indicates never, 7 indicates always) as follows: EDs (session 1: 2.6 ± 1.63; session 2: 3.8 ± 1.61), TRIs (session 1: 3.9 ± 1.60; session 2: 3.5 ± 1.15), and MW (session 1: 4.8 ± 1.58; session 2: 4.1 ± 1.52) (Fig. 1C).
Correlations between session 1 and session 2 of the frequencies of categories that prompted Something Else reports also revealed significant positive correspondence (EDs: Spearman’s ρ = 0.44, P = 0.002; TRIs: ρ = 0.39, P = 0.005; MW: ρ = 0.61, P = 2 × 10−6) (Table S1). This suggests that the sensory/cognitive contents of attentional fluctuations away from pain were also stable within individuals. The correspondences between rating categories were as follows: EDs vs. TRIs (session 1: ρ = 0.28, P = 0.051; session 2: ρ = 0.33, P = 0.018), EDs vs. MW (session 1: ρ = −0.29, P = 0.037; session 2: ρ = −0.41, P = 0.003), and TRIs vs. MW (session 1: ρ = −0.65, P = 2.7 × 10−7; session 2: ρ = −0.22, P = 0.13). Therefore, participants who were high in MW generally tended to be low in TRIs and EDs.
We next tested whether individual factors impact IAP and found a modest trend toward a positive correlation between IAP and pain catastrophizing scale (PCS) scores (r = 0.30; P = 0.03; not significant after Bonferroni correction) (Fig. 1E). There was no correlation between IAP and daydreaming frequency scale (DDF) scores (r = −0.085; P = 0.55), indicating that the tendency to attend away from pain is unrelated to the tendency to daydream in general.
Finally, we tested our hypothesis that individuals who frequently reported that they attended to pain during experience sampling would be more likely to show P-type behavior (i.e., slower RTs during pain), whereas those who frequently attended away from pain would be more likely to show A-type behavior (i.e., faster RTs during pain). We found a significant positive correlation (r = 0.42, P = 0.003) between mean RT across pain vs. no-pain trials and IAP that supported this hypothesis and provides behavioral validation for the experience sampling task (Fig. 2B). There was no correlation between mean RT across pain vs. no-pain trials and PCS (r = 0.086; P = 0.55).
Neural Correlates of Ongoing Fluctuations in Attention to Pain.
We next analyzed trial-to-trial brain activity fluctuations occurring during experience sampling with fMRI. When subjects reported attention to pain, activations occurred in regions previously reported as being pain- and salience-related [e.g., insula, MCC, thalamus, contralateral primary somatosensory cortex and secondary somatosensory cortex, and temporoparietal junction (TPJ)] (23–25), and deactivations occurred in nodes of the DMN [medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC)/precuneus, and temporooccicipital junction (TOJ)] (Fig. S1; full list in Table S2). In contrast, there were no significant deactivations in Something Else trials wherein subjects reported that their thoughts/feelings were on something other than pain. However, the regions activated in “Pain” trials were also activated in “Something Else” trials (Fig. S1; full list in Table S3). Crucially, Pain compared with Something Else trials were associated with greater activation of a predominantly right-lateralized network (e.g., right TPJ/S2, right IFG, right dorsolateral prefrontal cortex, and bilateral insula), including regions that are consistent with previous definitions of the “ventral attention” and “salience” networks (26–28) (Fig. 3A; full list in Table S4). Something Else compared with Pain trials were associated with greater activation of the DMN (e.g., mPFC, PCC/precuneus, lateral parietal areas, and medial temporal lobe) and regions implicated in executive control (superior parietal lobule, superior/middle frontal gyrus, and supplementary motor area) (Fig. 3B; full list in Table S5).
Fig. 3.
Salience network and DMN activations relate to fluctuations in attention to pain (n = 32). (A) Regions with greater activation during periods preceding reports of attention to pain compared with attention to something else. Bar graphs show mean % signal change (±SD), extracted from 3-mm-radius spheres at peak coordinates. (B) Regions with greater activation during periods preceding reports of attention to something else compared with attention to pain. Bar graphs show mean % signal change (±SD), extracted from 3-mm-radius spheres at peak coordinates. Statistical images are thresholded at FWE-corrected Z > 2.3; cluster P < 0.05.
Given the purported role of the DMN in MW and our observed anticorrelations of MW with EDs and TRIs, we next tested how individual differences in the change of activation within the DMN core (mPFC and PCC/precuneus) (18) between Something Else compared with Pain trials [Δ DMN activation (Else > Pain)] related to MW or other distractions from pain. For EDs, TRIs, and MW, respectively, there was a significant negative correlation (ρ = −0.61, P = 0.0002), no significant correlation (ρ = −0.24, P = 0.19), and a significant positive correlation (ρ = 0.45, p = 0.011) with Δ DMN activation (Else > Pain) (Fig. 4). Therefore, individuals distracted because of EDs were unlikely to engage the DMN, whereas high-MW individuals were likely to engage the DMN when their attention fluctuated away from pain.
Fig. 4.

Mean change of activation within the DMN core, defined as the medial prefrontal cortex and posterior cingulate cortex/precuneus, between attention to something else vs. attention to pain [ΔDMN activation (Else > Pain)], correlated with postscan ratings of the degree to which Something Else reports were due to external sensory distractions (ρ = −0.61, P = 0.0002) (Left), task-related interferences (ρ = −0.24, P = 0.19) (Center), and mind-wandering (ρ = −0.45, P = 0.011) (Right).
Functional Coupling Between PAG and DMN.
We next tested whether activity in the descending pain modulatory system interacts with attention networks, potentially to suppress ascending nociceptive input during attentional fluctuations away from pain. To do this, we used psychophysiological interaction analysis (PPI) (29) to determine whether functional connectivity of the descending pain modulatory system related to attention toward vs. away from pain. We focused on the PAG because it has a high concentration of opiate-containing neurons with descending projections (30) and has functional interactions with the cingulate/prefrontal cortex implicated in cognitive modulation of pain (5, 7).
During Something Else compared with Pain trials, we found enhanced functional coupling of the PAG with DMN regions (mPFC, PCC/precuneus/retrosplenial cortex, and medial temporal lobe) and left middle frontal gyrus (Fig. 5A; full list in Table S6). No regions had significantly greater PAG functional connectivity for periods associated with attention to vs. away from pain. However, PPI analysis for a PCC seed revealed several regions (angular gyrus, inferior/middle temporal gyrus, lingual gyrus, and cerebellar regions) with greater functional connectivity for attention toward compared with away from pain (Fig. S2).
Fig. 5.
(A) Functional connectivity of the PAG relates to fluctuations in attention to pain (n = 32). Statistical image shows greater functional connectivity of the PAG with areas of the DMN during periods preceding reports of attention to something else compared with attention to pain (FWE-corrected Z > 2.3; cluster P < 0.05). Bar graph shows % mean change (±SD) of functional connectivity between the PAG and mPFC for the two contrasted conditions, extracted from a 6-mm-radius sphere surrounding peak mPFC coordinates. (B) Structural connectivity between PAG and mPFC relates to individual differences in IAP. Image shows the across-subject aligned white matter mPFC–PAG skeleton (yellow) overlaid on the common pathway between PAG and mPFC identified with probabilistic tractography (Methods). Plot shows a negative correlation of IAP with mean FA in the mPFC–PAG skeleton (n = 51) (r = −0.36, P = 0.009).
Structural Connectivity Between PAG and mPFC.
White matter connections have been identified between the mPFC and PAG in humans (31, 32). Thus, our finding of increased PAG–DMN functional coupling within individuals during attentional fluctuations away from pain raises the possibility that individuals with stronger PAG–DMN anatomical connections more easily disengage attention from pain. We therefore tested the hypothesis that there is stronger structural connectivity in the mPFC–PAG pathway in individuals who tend to attend away from pain than in individuals who have greater IAP.
To evaluate structural connectivity, we first used probabilistic tractography (33) to define the pathway between the PAG and the mPFC region that was identified in our PPI analysis (Fig. 5). We then applied tract-based spatial statistics (34) to calculate mean fractional anisotropy (FA) in the mPFC–PAG white matter “skeleton” pathway. We found a significant negative correlation between mPFC–PAG tract FA and IAP score (r = −0.36, P = 0.009), supporting the hypothesis that individuals who frequently attend away from pain have stronger descending structural connections between mPFC and PAG (Fig. 5B). This link was even stronger when controlling for PCS score and sex (r = −0.45, P = 0.001).
Dynamic mPFC–PAG Resting State Functional Connectivity.
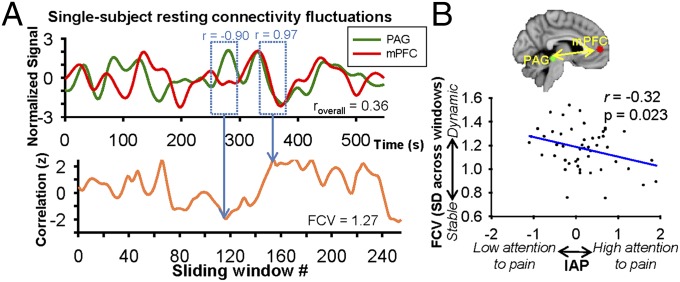
We further probed the involvement of mPFC–PAG communication in IAP using static and dynamic “resting state” functional connectivity (FC) analysis. We found that IAP was not significantly associated with mPFC–PAG FC strength based on correlated signals over the course of a ∼9 min resting state scan (i.e., static FC) (r = 0.064, P = 0.655). However, this conventional analysis of static FC does not capture dynamic FC fluctuations on shorter time scales (35) that may better reflect an individual’s capacity for flexible FC and/or spontaneous changes in vigilance/attention (36) related to the tendency to spontaneously fluctuate attention away from pain. We therefore applied a unique analysis of a metric to quantify the FC variability (FCV) as the SD of FC values within 254 sliding time windows (40 s each) in the resting state scan. Dynamic FC analysis revealed a significant negative correlation between mPFC–PAG FCV and IAP (r = −0.32, P = 0.023) (Fig. 6), suggesting that individuals with more dynamic/flexible mPFC–PAG FC spontaneously disengage attention from pain more frequently than those with stable mPFC–PAG FC. This correlation remained significant when controlling for PCS score and sex (r = −0.31, P = 0.029), overall mPFC–PAG FC strength (r = −0.31, P = 0.027), and mPFC–PAG tract FA (r = −0.28, P = 0.047).
Fig. 6.
Dynamic resting state functional connectivity between the PAG and mPFC relates to individual differences in IAP. (A) Single-subject example of PAG and mPFC signals during a resting state scan (Upper) and fluctuations in functional connectivity between PAG and mPFC across 40-s sliding windows (each window progressively sliding every 2 s) (Lower). (B) Group-level significant negative correlation between IAP and mPFC–PAG functional connectivity variability (SD of correlation values across sliding time windows) (r = −0.32, P = 0.023).
Discussion
These data demonstrate that routine, spontaneous disengagement of attention to pain (i.e., mind wandering) occurs consistently within an individual but varies considerably across individuals in concert with the effect of pain on their individual cognitive task performance. Furthermore, we show that these behavioral and perceptual outcomes are linked with function and structure of pain- and attention-related brain networks. Crucially, we reveal a key role of the antinociceptive system in both intraindividual and interindividual variability in spontaneous attentional fluctuations away from pain. These data support the notion that cognitive modulation of pain is an ongoing, intrinsically dynamic process that can occur without explicit manipulation.
Mind wandering has been defined as a state of “perceptual decoupling,” or disengagement of attention from perception (37). Our study describes the relationship between mind wandering and perceptual decoupling from pain. Pain is inherently salient compared with other sensory modalities, so diverting attention away from it likely requires a different or more robust mechanism than those previously identified. Our finding of increased functional connectivity between the DMN and PAG during attention away from pain could represent such a mechanism. The mPFC and retrosplenial cortex, identified here as DMN regions with enhanced PAG functional connectivity during attentional disengagement from pain, both have efferent connections to the PAG identified in monkeys (38, 39). Our finding of a negative correlation between FA in the descending mPFC–PAG pathway and IAP directly implicates this connection in perceptual decoupling of pain. The PAG sends antinociceptive signals to the rostroventral medulla, which projects to the spinal cord dorsal horn to inhibit incoming nociceptive information (30). fMRI studies suggest that explicit cognitive manipulation of pain engages this pathway (4, 7, 9, 10). Furthermore, the pain-modulatory action of this pathway during placebo manipulations and attentional tasks is inhibited when opiate activity is blocked (4, 7). We therefore propose that during pain, interactions between the DMN and descending pain modulatory system fluctuate continuously, reflecting cognitive modulation that results in neural activity underlying perceptual decoupling of pain. In the absence of pain, structural and dynamic functional connectivity between the antinociceptive system and DMN may maintain an individual’s predisposition for spontaneously attending toward/away from pain.
For nonpain modalities, neural activity for perceptual decoupling has been associated with increased DMN and executive control network activation and decreased activation in sensory cortices (37). DMN activation has been linked to mind wandering/internal mentation in experience sampling studies (17, 19, 21). Our results reveal a similar role of the DMN in the context of pain. Notably, the role of the DMN in pain perception has remained under debate. Deactivations of the DMN during pain were reported in early imaging studies (40, 41), but recent studies suggest a more nuanced view in which the DMN responds nonlinearly or even activates during pain (42, 43). In any pain study, attention likely fluctuates on a trial-to-trial basis variably in different individuals. Our findings indicate that DMN activity levels are virtually at baseline level when attention fluctuates away from pain. Thus, analysis of averaged responses within and/or between individuals would not adequately delineate the effect of pain on DMN activity. Our findings of trial-to-trial variability in pain-evoked DMN activity suggest that DMN activity varies with how pain captures attention and whether it does so consistently. This view reconciles previous disparate findings.
In contrast to the DMN, the ventral attention network (e.g., anterior insula (aINS) and TPJ) has been linked to salience detection, such as pain (25), and is also known as the salience network (27). We found that pain activates this network regardless of attentional state, but the magnitude of activation was greatest during attention to pain. This extends our previous work showing that this network activates during salient changes in the sensory environment (23), exhibits prolonged activation during tonic pain (24), and exhibits right-lateralized structural and intrinsic functional connectivity (28, 44). In particular, the present work demonstrates that this network, particularly in right-lateralized regions, encodes ongoing fluctuations in the salience of pain.
The ongoing dynamics of attention to pain appear to involve similar as well as unique neural processes compared with those identified in studies of active pain suppression and explicit pain manipulation. Engagement of the descending pain modulatory system and decreased activation in regions supporting the salience of pain (e.g., aINS) are in agreement with explicit manipulation studies (10). Fluctuations in DMN activity and functional connectivity have not generally been emphasized previously as part of the pain modulation process. However, studies of placebo analgesia demonstrate enhanced functional connectivity between the PAG and medial cingulofrontal regions, which are possibly part of the DMN, during endogenous pain modulation (7, 8). In a DWI study, individual differences in FA of the white matter pathway between the PAG and a rostral portion of the cingulate positively correlated with the magnitude of placebo analgesia (32). Furthermore, elevated activation in DMN regions and enhanced cingulofrontal–PAG functional connectivity has been found during distraction from pain with a Stroop task compared with pain without distraction (5), comparable to our results here. Our findings build on this previous work and demonstrate links of attention to pain with PAG–DMN structural connectivity and functional connectivity during both pain and rest.
Notably, we did not ask subjects to provide pain ratings during our experience sampling paradigm because such ratings would have explicitly drawn attention, interfering with spontaneous fluctuations in attention. It therefore remains uncertain whether mind wandering away from pain and associated PAG–DMN interactions reflects an antinociceptive process. Future work is needed to examine the relationship between antinociception and attentional fluctuations.
Chronic pain patients report that their attention to ongoing pain often varies (45) and the intensity of their pain can fluctuate on short time scales (seconds/minutes) (46). Our data suggest that such fluctuations (characterized as IAP) are stable within individuals and involve interactions between the brain’s DMN and antinociceptive system. These findings raise an intriguing possibility, namely, that intrinsic attention to pain might interact with ongoing nociceptive input in chronic pain to determine the course of pain-related structural brain reorganization and disease prognosis. Supporting this notion are findings of aberrant DMN function in chronic pain disorders (47, 48). Of particular note, longitudinal studies point to a potential causal role of DMN–insula interactions in pain reduction in fibromyalgia (49) and of mPFC–nucleus accumbens interactions in the transition from subacute to chronic back pain (50). Long-term changes in DMN communication with other brain systems are likely mediated by attention to pain in daily life, involving fluctuations in neural activity identified here. Thus, further study of intrinsic attention to pain could provide critical insight into behavioral and neural mechanisms underlying individual differences in recovery from chronic pain.
Methods
General Procedures.
Fifty-one healthy right-handed adults (26 female, 25 male; mean age ± SD = 25.02 ± 2.68) provided informed consent to procedures approved by the University Health Network Research Ethics Board. TENS was used to stimulate the median nerve of the left forearm. A stimulation level that evoked a pain rating of 4–5 out of 10 (0 indicates no pain; 10 indicates the most intense pain imaginable) was used during performance of an experience sampling and a cognitive interference task on day 1 (described below). Participants returned on day 2 for neuroimaging with a 3T GE MRI. Scans included one run of resting state fMRI followed by four runs of fMRI with the experience sampling task and DWI (SI Methods).
Experience Sampling and Cognitive Interference Tasks.
During both psychophysics and imaging (fMRI) sessions, an experience sampling task was performed. The participant received instructions to minimize active efforts to attend toward or away from pain (SI Methods). Each trial consisted of painful TENS that was interrupted after 20 s with an attentional state probe displayed for 8 s, followed by a 22-s interstimulus interval (Fig. 1). Participants fixated on a white cross on a black background throughout. During the attentional state probe, the screen displayed, “At the end of this last trial, to what degree were your thoughts/feelings about pain or something else?” The participant indicated with a button press one of four possible responses (only pain, mostly pain, mostly something else, or only something else). Each run began with a 30-s fixation period followed by 10 repeated trials. After all runs in session 1, the participant rated how confident he or she were in his or her ability to accurately indicate attentional state during the task (Likert scale: 1 indicates not confident at all; 7 indicates very confident). After all runs in sessions 1 and 2, participants were asked to classify (Likert scale: 1 indicates never; 7 indicates always) the frequency with which their reports of something else related to each of three categories (19): (i) EDs, (ii) TRIs, and (iii) MW. A cognitive interference task (“number task”) was performed with and without concomitant painful TENS during the psychophysics session (Fig. 2) as done previously (22) (SI Methods).
Behavioral Analysis.
For the experience sampling task, an IAP score was calculated for each participant for sessions 1 and 2 separately, with the following formula:
where n = number of trials
The maximum and minimum scores (+2 and −2) correspond to only pain and only something else reports on every trial, respectively. A two-way mixed intraclass correlation coefficient (absolute agreement) was calculated for session 1 vs. session 2 scores to evaluate the trait-like quality of IAP. The intersession consistency of ratings for EDs, TRIs, and MW and correspondences between these categories were evaluated with Spearman’s ρ because session 1 values for EDs, TRIs, and MW and session 2 values for TRIs and MW were not normally distributed (Shapiro–Wilk and Kolmogorov–Smirnov, P < 0.05). Pearson’s correlation coefficient was calculated between IAP (session 1) and both PCS and DDF scores (results were Bonferroni-corrected for two comparisons). Cognitive interference task mean reaction times and performance accuracies for individual subjects are reported in Table S7. Pearson’s correlation coefficient was calculated to evaluate the relationship between IAP (session 1) and the difference in reaction times between pain and no pain trials (ΔRT [P − NP]).
Task fMRI Analysis.
Thirty-two subjects were included in analyses of contrasts of fMRI activity during pain vs. “else” reports (see SI Methods for inclusion criteria). All image processing and statistical analyses were performed with FMRIB Software Library (FSL) v5.0, MATLAB v7.12.0, fMRISTAT (51), and statistical package for the social sciences (SPSS) v21.0 (IBM Corp.). Contrasts were performed to identify activation/deactivation during stimulation before pain and else reports as well as pain > else and else > pain differences (SI Methods). Group-level analysis was performed with FMRIB's local analysis of mixed effects (FLAME) 1 + 2 (whole-brain family-wise error (FWE)-corrected Z > 2.3; cluster P < 0.05).
We performed a PPI analysis (29) with a seed region defined in the PAG as was done previously (7) (SI Methods). Contrasts were performed between two interaction regressors (PAG time course × stimulation before pain reports and PAG time course × stimulation before else reports) to identify regions with pain > else and else > pain PAG functional connectivity (FLAME 1 + 2 thresholding: whole-brain FWE-corrected Z > 2.3; cluster P < 0.05).
Diffusion MRI Analysis.
Probabilistic tractrography was used to define the mPFC–PAG pathway for analysis with tract-based spatial statistics (TBSS) in all 51 participants (SI Methods). A white matter skeleton obtained from TBSS was masked with the PAG–mPFC pathway derived from probabilistic tractography. Pearson’s correlation coefficient was then calculated between mean PAG–mPFC pathway FA values and IAP (session 1) scores.
Resting State fMRI Analysis.
Mean time series across voxels were calculated from preprocessed data (SI Methods) within PAG and within mPFC. Overall FC strength was calculated as the Fisher-transformed correlation between PAG and mPFC mean time series. For dynamic FC analysis, both time series were split into 40-s sliding time windows, with each window shifted 2 s forward from the previous window (i.e., 1 volume [i.e., 1 TR (repetition time)]). Fisher-transformed correlations between PAG and mPFC within each of the 254 obtained windows were then computed, and FCV was calculated as the SD of these correlation values. Pearson’s correlation coefficient was then calculated for PAG–mPFC FC and FCV vs. IAP (session 1) scores (data were normally distributed). To test the effect of sliding window duration on the correlation between FCV and IAP, we redid the FCV analysis using 30-, 50-, and 60-s windows and demonstrated converging results (Table S8).
Supplementary Material
Acknowledgments
We thank Adrian Crawley, Geoff Pope, Nathalie Erpelding, Gang Wang, Eugen Hlasny, and Keith Ta for technical assistance. We also thank Adrian Crawley, Eve De Rosa, Lucia Gagliese, Mojgan Hodaie, and Mary Pat McAndrews for critical review of the study design. This work was performed at Toronto Western Research Institute, University Health Network and funded by the Canadian Institutes of Health Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312902110/-/DCSupplemental.
References
- 1.Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330(6006):932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- 2.Kane MJ, et al. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychol Sci. 2007;18(7):614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- 3.Seminowicz DA, Mikulis DJ, Davis KD. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain. 2004;112(1–2):48–58. doi: 10.1016/j.pain.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Sprenger C, et al. Attention modulates spinal cord responses to pain. Curr Biol. 2012;22(11):1019–1022. doi: 10.1016/j.cub.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Valet M, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—An fMRI analysis. Pain. 2004;109(3):399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 6.Wager TD, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 7.Eippert F, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104(26):11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eippert F, Finsterbusch J, Bingel U, Büchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326(5951):404. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- 10.Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci. 2008;12(8):306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Boly M, et al. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA. 2007;104(29):12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohara S, Crone NE, Weiss N, Kim JH, Lenz FA. Analysis of synchrony demonstrates that the presence of “pain networks” prior to a noxious stimulus can enable the perception of pain in response to that stimulus. Exp Brain Res. 2008;185(2):353–358. doi: 10.1007/s00221-008-1284-1. [DOI] [PubMed] [Google Scholar]
- 13.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci USA. 2010;107(1):355–360. doi: 10.1073/pnas.0906186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayhew SD, Hylands-White N, Porcaro C, Derbyshire SW, Bagshaw AP. Intrinsic variability in the human response to pain is assembled from multiple, dynamic brain processes. Neuroimage. 2013;75:68–78. doi: 10.1016/j.neuroimage.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12(1):43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- 16.Palva JM, Palva S. Roles of multiscale brain activity fluctuations in shaping the variability and dynamics of psychophysical performance. Prog Brain Res. 2011;193:335–350. doi: 10.1016/B978-0-444-53839-0.00022-3. [DOI] [PubMed] [Google Scholar]
- 17.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA. 2009;106(21):8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stawarczyk D, Majerus S, Maquet P, D’Argembeau A. Neural correlates of ongoing conscious experience: Both task-unrelatedness and stimulus-independence are related to default network activity. PLoS ONE. 2011;6(2):e16997. doi: 10.1371/journal.pone.0016997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanhaudenhuyse A, et al. Two distinct neuronal networks mediate the awareness of environment and of self. J Cogn Neurosci. 2011;23(3):570–578. doi: 10.1162/jocn.2010.21488. [DOI] [PubMed] [Google Scholar]
- 22.Erpelding N, Davis KD. Neural underpinnings of behavioural strategies that prioritize either cognitive task performance or pain. Pain. 2013;154(10):2060–2071. doi: 10.1016/j.pain.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3(3):277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- 24.Downar J, Mikulis DJ, Davis KD. Neural correlates of the prolonged salience of painful stimulation. Neuroimage. 2003;20(3):1540–1551. doi: 10.1016/s1053-8119(03)00407-5. [DOI] [PubMed] [Google Scholar]
- 25.Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix”. Neuroimage. 2011;54(3):2237–2249. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- 26.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kucyi A, Hodaie M, Davis KD. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J Neurophysiol. 2012;108(12):3382–3392. doi: 10.1152/jn.00674.2012. [DOI] [PubMed] [Google Scholar]
- 29.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 30.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 31.Hadjipavlou G, Dunckley P, Behrens TE, Tracey I. Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: A diffusion tensor imaging study in healthy controls. Pain. 2006;123(1–2):169–178. doi: 10.1016/j.pain.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 32.Stein N, Sprenger C, Scholz J, Wiech K, Bingel U. White matter integrity of the descending pain modulatory system is associated with interindividual differences in placebo analgesia. Pain. 2012;153(11):2210–2217. doi: 10.1016/j.pain.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SM, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 35.Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010;50(1):81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hutchison RM, et al. Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schooler JW, et al. Meta-awareness, perceptual decoupling and the wandering mind. Trends Cogn Sci. 2011;15(7):319–326. doi: 10.1016/j.tics.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 38.An X, Bandler R, Ongür D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401(4):455–479. [PubMed] [Google Scholar]
- 39.Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci USA. 2006;103(5):1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coghill RC, et al. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14(7):4095–4108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porro CA, Cettolo V, Francescato MP, Baraldi P. Temporal and intensity coding of pain in human cortex. J Neurophysiol. 1998;80(6):3312–3320. doi: 10.1152/jn.1998.80.6.3312. [DOI] [PubMed] [Google Scholar]
- 42.Ter Minassian A, et al. Dissociating anticipation from perception: Acute pain activates default mode network. Hum Brain Mapp. 2013;34(9):2228–2243. doi: 10.1002/hbm.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loggia ML, et al. Disentangling linear and nonlinear brain responses to evoked deep tissue pain. Pain. 2012;153(10):2140–2151. doi: 10.1016/j.pain.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kucyi A, Moayedi M, Weissman-Fogel I, Hodaie M, Davis KD. Hemispheric asymmetry in white matter connectivity of the temporoparietal junction with the insula and prefrontal cortex. PLoS ONE. 2012;7(4):e35589. doi: 10.1371/journal.pone.0035589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viane I, Crombez G, Eccleston C, Devulder J, De Corte W. Acceptance of the unpleasant reality of chronic pain: Effects upon attention to pain and engagement with daily activities. Pain. 2004;112(3):282–288. doi: 10.1016/j.pain.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Foss JM, Apkarian AV, Chialvo DR. Dynamics of pain: Fractal dimension of temporal variability of spontaneous pain differentiates between pain States. J Neurophysiol. 2006;95(2):730–736. doi: 10.1152/jn.00768.2005. [DOI] [PubMed] [Google Scholar]
- 47.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: Chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weissman-Fogel I, et al. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain. 2011;152(2):384–396. doi: 10.1016/j.pain.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 49.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012;64(7):2398–2403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baliki MN, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15(8):1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Worsley KJ, et al. A general statistical analysis for fMRI data. Neuroimage. 2002;15(1):1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.