Abstract
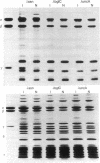
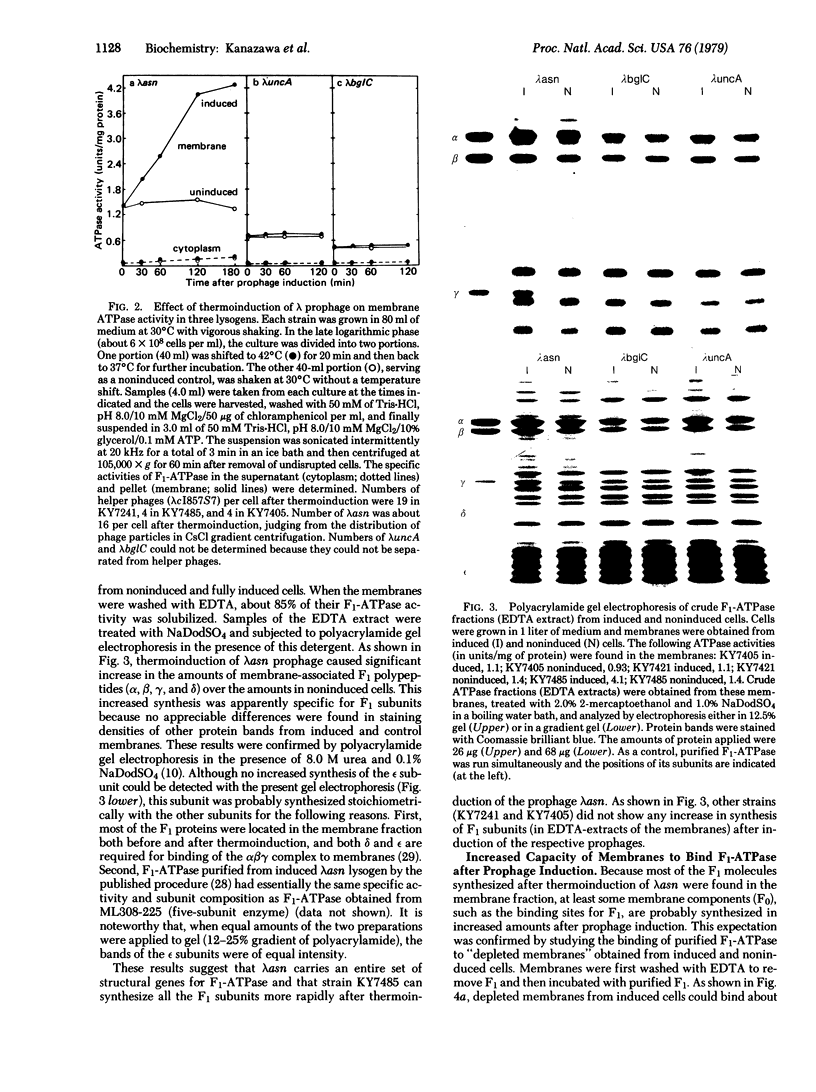
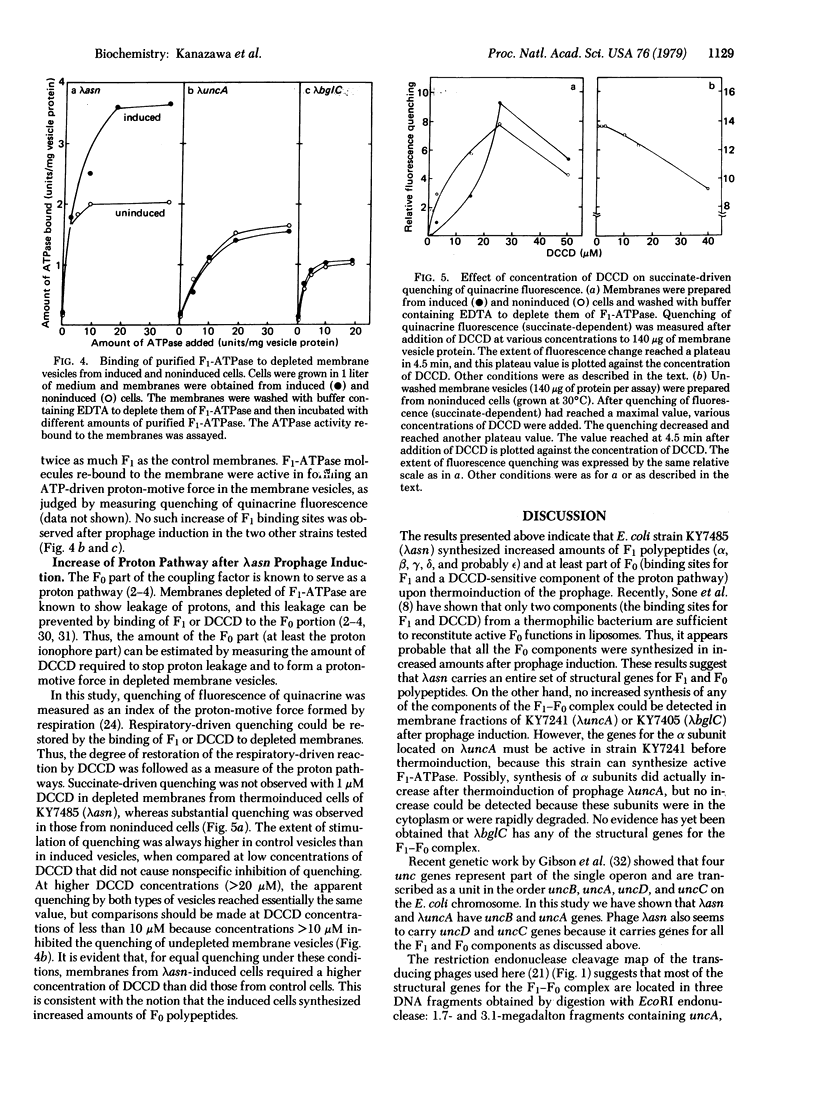
Studies were made of the synthesis of the coupling factor complex (F1--F0) of oxidative phosphorylation after prophage induction of a set of Escherichia coli strains lysogenic for defective transducing phage lambda asn, lambda uncA, or lambda bglC. The transducing phages had been isolated from a strain of E. coli carrying prophage lambda cI857 S7 within the bglB gene located near the unc gene cluster [Miki, T., Hiraga, S., Nagata, T. & Yura, T. (1978) Proc. Natl. Acad. Sci. USA 75, 5099--5103]. When lysogenic cells carrying lambda asn and lambda cI857 S7 were induced at high temperature, synthesis of the F1-ATPase portion of the complex increased to severalfold that of the noninduced cells. In contrast, no increase was observed upon thermoinduction of cells carrying lambda uncA or lambda bglC. The number of membrane sites that could bind purified F1-ATPase also increased significantly upon induction by lambda asn but not by lambda uncA or lambda bglC. In addition, F1-depleted membranes prepared from lambda asn-induced bacteria required more dicyclohexylcarbodiimide to seal the proton pathway than did those from noninduced bacteria. These results strongly suggest that lambda asn carries a set of bacterial genes coding for all the F1 polypeptides (the alpha, beta, gamma, delta, and probably the epsilon subunits) and at least some of the genes involved in formation of F0 polypeptides. Although lambda uncA carries the structural gene (uncA) for the alpha subunit of F1-ATPase, it apparently does not carry the whole set of F1--F0 genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K., Harold F. M., Simoni R. D. Impairment and restoration of the energized state in membrane vesicles of a mutant of Escherichia coli lacking adenosine triphosphatase. J Biol Chem. 1974 Jul 25;249(14):4587–4593. [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell K. J., Lindop C. R., Knight I. G., Beechey R. B. The identification of the site of action of NN'-dicyclohexylcarbodi-imide as a proteolipid in mitochondrial membranes. Biochem J. 1971 Nov;125(1):169–177. doi: 10.1042/bj1250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Downie J. A., Gibson F., Radik J. Genetic complementation between two mutant unc alleles (unc A401 and unc D409) affecting the Fl portion of the magnesium ion-stimulated adenosine triphosphatase of Escherichia coli K12. Biochem J. 1978 Mar 15;170(3):593–598. doi: 10.1042/bj1700593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. D. Identification of the altered subunit in the inactive F1ATPase of an Escherichia coli uncA mutant. Biochem Biophys Res Commun. 1978 May 30;82(2):596–602. doi: 10.1016/0006-291x(78)90916-6. [DOI] [PubMed] [Google Scholar]
- Fayle D. R., Downie J. A., Cox G. B., Gibson F., Radik J. Characterization of the mutant-unc D-gene product in a strain of Escherichia coli K12. An altered beta-subunit of the magnesium ion-stimulated adenosine triphosphatase. Biochem J. 1978 Jun 15;172(3):523–531. doi: 10.1042/bj1720523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H. Identification of the dicyclohexylcarbodiimide-reactive protein component of the adenosine 5'-triphosphate energy-transducing system of Escherichia coli. J Bacteriol. 1975 Nov;124(2):870–883. doi: 10.1128/jb.124.2.870-883.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H. Purification of the carbodiimide-reactive protein component of the ATP energy-transducing system of Escherichia coli. J Biol Chem. 1976 Nov 10;251(21):6630–6637. [PubMed] [Google Scholar]
- Futai M. Reconstitution of ATPase activity from the isolated alpha, beta, and gamma subunits of the coupling factor, F1, of Escherichia coli. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1231–1237. doi: 10.1016/0006-291x(77)91138-x. [DOI] [PubMed] [Google Scholar]
- Futai M., Sternweis P. C., Heppel L. A. Purification and properties of reconstitutively active and inactive adenosinetriphosphatase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2725–2729. doi: 10.1073/pnas.71.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Cox G. B., Downie J. A., Radik J. A mutation affecting a second component of the F0 portion of the magnesium ion-stimulated adenosine triphosphatase of Escherichia coli K12. The uncC424 allele. Biochem J. 1977 Apr 15;164(1):193–198. doi: 10.1042/bj1640193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Downie J. A., Cox G. B., Radik J. Mu-induced polarity in the unc operon of Escherichia coli. J Bacteriol. 1978 Jun;134(3):728–736. doi: 10.1128/jb.134.3.728-736.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H., Saito S., Futai M. Coupling factor ATPase from Escherichia coli. An uncA mutant (uncA401) with defective alpha subunit. J Biochem. 1978 Dec;84(6):1513–1517. doi: 10.1093/oxfordjournals.jbchem.a132276. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marsh R. C., Worcel A. A DNA fragment containing the origin of replication of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2720–2724. doi: 10.1073/pnas.74.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Hiraga S., Nagata T., Yura T. Bacteriophage lambda carrying the Escherichia coli chromosomal region of the replication origin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5099–5103. doi: 10.1073/pnas.75.10.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Sone N., Hirata H., Yoshida M., Kagawa Y. Purified proton conductor in proton translocating adenosine triphosphatase of a thermophilic bacterium. J Biol Chem. 1977 Sep 10;252(17):6125–6131. [PubMed] [Google Scholar]
- Rosen B. P. Beta-galactoside transport and proton movements in an adenosine triphosphatase-deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1289–1296. doi: 10.1016/0006-291x(73)90605-0. [DOI] [PubMed] [Google Scholar]
- Senior A. E. The structure of mitochondrial ATPase. Biochim Biophys Acta. 1973 Dec 31;301(3):249–277. doi: 10.1016/0304-4173(73)90006-2. [DOI] [PubMed] [Google Scholar]
- Serrano R., Kanner B. I., Racker E. Purification and properties of the proton-translocating adenosine triphosphatase complex of bovine heart mitochondria. J Biol Chem. 1976 Apr 25;251(8):2453–2461. [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Kagawa Y. Resolution of the membrane moiety of the H+-ATPase complex into two kinds of subunits. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4219–4223. doi: 10.1073/pnas.75.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stekhovan F. S., Waitkus R. F., Van Moerkerk H. T. Identification of the dicyclohexylcarbodiimide-binding protein in the oligomycin-sensitive adenosine triphosphatase from bovine heart mitochondria. Biochemistry. 1972 Mar 28;11(7):1144–1150. doi: 10.1021/bi00757a005. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C. The epsilon subunit of Escherichia coli coupling factor 1 is required for its binding to the cytoplasmic membrane. J Biol Chem. 1978 May 10;253(9):3123–3128. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Rosen B. P. Characterization of an active transport system for calcium in inverted membrane vesicles of Escherichia coli. J Biol Chem. 1975 Oct 10;250(19):7687–7692. [PubMed] [Google Scholar]
- Tsuchiya T., Rosen B. P. Energy transduction in Escherichia coli. The role of the Mg2+ATPase. J Biol Chem. 1975 Nov 10;250(21):8409–8415. [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assembly of the mitochondrial membrane system. V. Properties of a dispersed preparation of the rutamycin-sensitive adenosine triphosphatase of yeast mitochondria. J Biol Chem. 1971 Dec 10;246(23):7328–7336. [PubMed] [Google Scholar]