Abstract
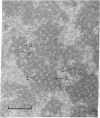
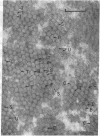
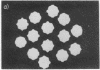
Fibers of deoxyHb S have been investigated by thin-section electron microscopy, utilizing a tannic acid embedding procedure. On the basis of numerous measurements of cross-sectional center-to-center distances for adjacent fibers in pairs or arrays, fiber diameters (mean +/- SD) of 205 +/- 5 A in embedded cells and 212 +/- 8 A in embedded hemolysates were obtained. This is an agreement with values obtained by conventional embedding procedures [Crepeau, R. H., Dykes, G., Garrell, R. L. & Edelstein, S. J. (1978) Nature (London) 274, 616--617]. The use of tannic acid has resulted in improved resolution of fiber cross sections, revealing individual strands of Hb S molecules. Because the section thickness corresponds to approximately one-fifth of the fiber helical repeat distance, the strands in projection superimpose to form characteristic image patterns. Additional superposition patterns arise in sections taken at small deviations from perpendicularity to the longitudinal fiber axis. These patterns are consistent with the 14-strand structure for hemoglobin S fibers [Dykes, G., Crepeau, R. H. & Edelstein, S. J. (1978) Nature (London) 272, 506--510], as indicated by computer models of cross-sectional patterns for various thicknesses and angular deviations of sections.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crepeau R. H., Dykes G., Garrell R., Edelstein S. J. Diameter of haemoglobin S fibres in sickled cells. Nature. 1978 Aug 10;274(5671):616–617. doi: 10.1038/274616a0. [DOI] [PubMed] [Google Scholar]
- Dykes G., Crepeau R. H., Edelstein S. J. Three-dimensional reconstruction of the fibres of sickle cell haemoglobin. Nature. 1978 Apr 6;272(5653):506–510. doi: 10.1038/272506a0. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Perutz M. F., Bertles J. F., Döbler J. Structure of sickled erythrocytes and of sickle-cell hemoglobin fibers. Proc Natl Acad Sci U S A. 1973 Mar;70(3):718–722. doi: 10.1073/pnas.70.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Tilney L. G. Substructural analysis of the microtubule and its polymorphic forms. Ann N Y Acad Sci. 1975 Jun 30;253:27–50. doi: 10.1111/j.1749-6632.1975.tb19190.x. [DOI] [PubMed] [Google Scholar]
- Ohtsuki M., White S. L., Zeitler E., Wellems T. E., Fuller S. D., Zwick M., Makinen M. W., Sigler P. B. Electron microscopy of fibers and discs of hemoglobin S having sixfold symmetry. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5538–5542. doi: 10.1073/pnas.74.12.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumphrey J. G., Steinhardt J. Formation of needle-like aggregates in stirred solutions of hemoglobin S1. Biochem Biophys Res Commun. 1976 Mar 8;69(1):99–105. doi: 10.1016/s0006-291x(76)80278-1. [DOI] [PubMed] [Google Scholar]