SUMMARY
Acquired chromosomal instability and copy number alterations are hallmarks of cancer. Enzymes capable of promoting site-specific copy number changes have yet to be identified. Here, we demonstrate that H3K9/36me3 lysine demethylase KDM4A/JMJD2A overexpression leads to localized copy gain of 1q12, 1q21, and Xq13.1 without global chromosome instability. KDM4A amplified tumors have increased copy gains for these same regions. 1q12h copy gain occurs within a single cell cycle, requires S phase and is not stable but regenerated each cell division. Sites with increased copy number are re-replicated and have increased KDM4A, MCM and DNA polymerase occupancy. Suv39h1/KMT1A or HP1γ overexpression suppresses the copy gain, while H3K9/K36 methylation interference promotes gain. Our results demonstrate that overexpression of a chromatin modifier results in site-specific copy gains. This begins to establish how copy number changes could originate during tumorigenesis and demonstrates that transient overexpression of specific chromatin modulators could promote these events.
INTRODUCTION
Genomic instability is a major contributing factor to the development and onset of age-related diseases such as cancer (Maslov and Vijg, 2009; Negrini et al., 2010). Cancer cells are often characterized by copy number alterations: gains or losses of chromosome arms and/or whole chromosomes as well as amplifications of smaller genomic fragments (Beroukhim et al., 2010; Hook et al., 2007; Stratton et al., 2009). Genome wide analysis of copy number changes in cancer has identified chromosomal regions with higher frequencies of amplification, which often contain putative oncogenes (Beroukhim et al., 2010). In some cases, the oncogenes have been shown to impact cellular behavior (e.g., cMyc and Mcl1), while other genes within these regions do not have clear connections with tumorigenesis. The lack of obvious connection does not preclude the gene’s involvement. For example, cellular stresses can select for gene amplification that will promote cancer cell survival, as exemplified by the amplification of dihydrofolate reductase when cells are treated with methotrexate (Schimke, 1984). Even though cancer genomes frequently have altered chromosomal regions, there is little knowledge about the regulatory mechanisms or factors that are involved in promoting copy number alterations at specific regions of the genome.
Several mechanisms have been proposed for generating copy number variation (CNV). For example, many models for DNA amplification incorporate stalled replication forks and DNA double-strand breaks that are generated during replication. It is proposed that these stalled/collapsed replication forks are associated with, and can cause, tandem duplications. A second mechanism proposed to contribute to CNV involves the use of breaks or repair intermediates as primers for re-replication of specific stretches of DNA, which can re-incorporate into the genome, resulting in gene duplications or deletions. Alternatively, it is also possible that these events will not integrate in the genome (Hastings et al., 2009). A third mechanism which could generate re-replicated fragments and copy number alteration is the head to tail collision of elongating DNA polymerases (Davidson et al., 2006; Hook et al., 2007). Since chromatin structure impacts replication initiation and elongation efficiency as well as DNA damage response and repair (Alabert and Groth, 2012; Papamichos-Chronakis and Peterson, 2013), the chromatin state or modifying enzyme(s) could have a significant impact on each of these possible mechanisms.
Recently, Kiang and colleagues demonstrated that local DNA fragment amplification occurs during S phase (Kiang et al., 2010) and that the chromatin context or chromosome microenvironments play a major role in this process. Consistent with an important role for the chromatin context, mis-regulation of the histone 4 lysine 20 mono-methyltransferase KMT5A (H4K20me1, PR-Set7/Set8) promotes re-replication, at least in part, by increasing H4K20me2/3 levels and promoting ORC recruitment through binding of H4K20me2 (Beck et al., 2012; Kuo et al., 2012; Tardat et al., 2010). However, the role of methylation in modulating replication is not limited to the direct recruitment of DNA replication factors. For example, we have previously demonstrated that the H3K9me3 demethylase KDM4A/JMJD2A was able to increase accessibility and alter the replication timing at specific heterochromatic regions (Black et al., 2010). The regulation of KDM4A protein levels are also important in modulating its chromatin occupancy, replication initiation and S phase progression (Van Rechem et al., 2011). Furthermore, Mallette and colleagues demonstrate that increased KDM4A expression abrogates 53BP1 recruitment to DNA damage sites, suggesting a role for KDM4A in DNA damage response (Mallette et al., 2012). Therefore, we hypothesize that overexpression of catalytically active KDM4A may provide a potential enzymatic link to the proposed methods for generating copy number alterations through replication abnormalities, which may contribute to copy number changes in cancer.
In this study, we analyzed The Cancer Genome Atlas (TCGA) data and observed that KDM4A is amplified and overexpressed in several tumor types. KDM4A overexpression in transgenic cells was sufficient to promote copy gain of specific chromosomal domains (e.g., 1q12). KDM4A-dependent copy gain was induced in less than 24 hours and required S phase. These copy gains were not stably inherited, but generated transiently in each subsequent S phase and cleared by late G2. KDM4A was the only KDM4 family member that generated the gains in a catalytically-dependent manner. These copy gains were antagonized by co-expression of Suv39h1/KMT1A or HP1γ, while H3K9 or H3K36 methylation interference promoted gain. Furthermore, KDM4A associated with replication machinery and promoted re-replication of regions exhibiting copy gain. KDM4A overexpression increased KDM4A, MCM and DNA polymerase association as well as decreased HP1γ occupancy at regions that undergo KDM4A-dependent re-replication. Interestingly, focal amplifications of 1q21 and Xq13.1 were correlated with KDM4A amplification in tumors, which was recapitulated in KDM4A overexpressing cell lines. Our findings demonstrate that KDM4A overexpression results in site-specific copy gain of regions amplified in human tumors.
RESULTS
KDM4A is Amplified and Overexpressed in Cancer
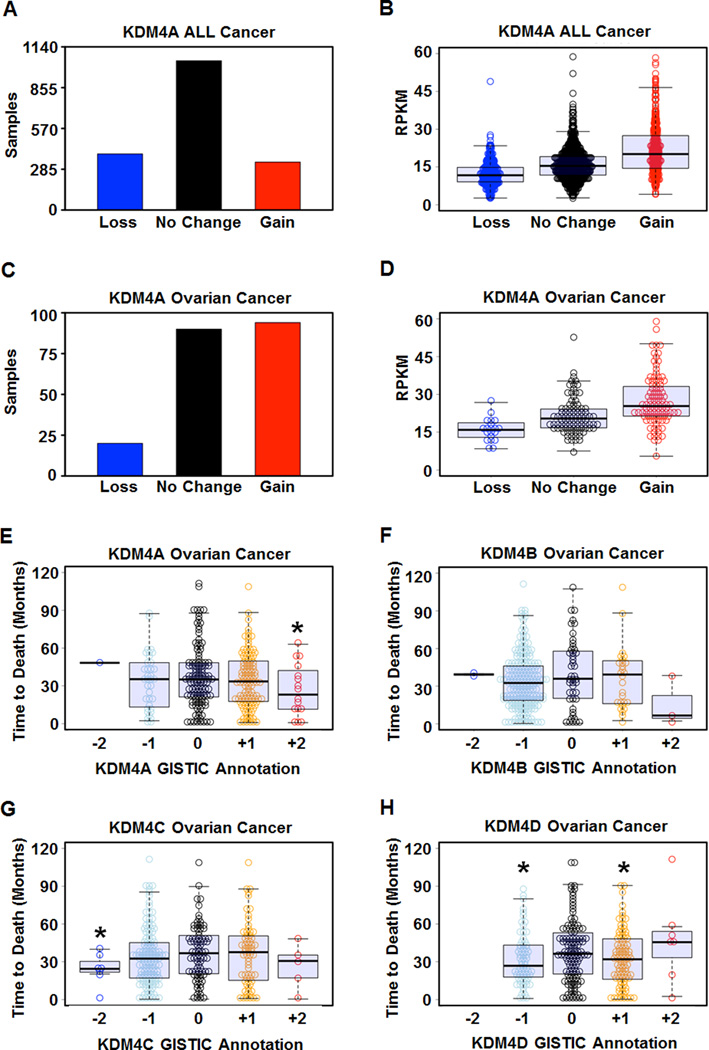
KDM4A has previously been demonstrated to be overexpressed in breast and lung cancer (Berry et al., 2012; Mallette and Richard, 2012). However, a comprehensive profile of primary tumors for alterations in KDM4A expression levels has yet to be established. There are few insights into the mechanisms that promote increased expression in tumors. Therefore, we conducted a comprehensive analysis of KDM4A copy number and expression level in 1,770 primary tumor samples from 8 different cancer types (Figure S1) represented in TCGA (Beroukhim et al., 2010). We found evidence of increased KDM4A copy number (GISTIC annotation of +1 or +2; (Mermel et al., 2011)) in 18.9% of tumors (335 out of 1,770 samples, Figure 1A) and copy loss in 22.1% of tumors (392 out of 1,770 samples, Figure 1A). Furthermore, amplification or deletion of KDM4A resulted in increased or decreased KDM4A expression, respectively (Figure 1B, Figure S1A). KDM4A was both amplified and deleted across many disparate cancer types, and KDM4A expression correlated with copy number in these samples (Figure S1E–L). We also observed amplification and deletion of KDM4B-D in cancer, which correlated with expression (Figure S1B–D). These data provide a molecular basis for the elevated KDM4A levels observed in different tumor samples.
Figure 1. KDM4A is amplified and overexpressed in cancer and correlates with poor outcome in ovarian cancer.
(A) Distribution of gain (GISTIC annotation +1 or +2) or loss (GISTIC annotation −1 or −2) of copy of KDM4A in 1770 cancer samples. (B) Amplification of KDM4A correlates with increased expression of KDM4A in TCGA. (C) KDM4A is frequently amplified in ovarian cancer (P=1.4×10−21 for Gain vs No change or Loss by Fisher’s exact test). (D) Amplification of KDM4A in ovarian cancer correlates with increased expression of KDM4A. (E) Focal amplification of KDM4A in ovarian cancer correlates with poor outcome in 285 deceased ovarian cancer samples (P = 0.02 by one-tailed Student’s t-test and 0.048 by one-tailed, Wilcoxon rank sum test for +2 vs 0). (F) Copy number of KDM4B does not correlate with outcome in ovarian cancer. (G) Deletion of KDM4C in ovarian cancer correlates with outcome (P=0.014 for Loss vs None). (H) Copy number loss and gain of KDM4D correlate with outcome in ovarian cancer (P=0.018 for Gain vs None and 0.013 for Loss vs None by Student’s t-test). * indicates significant difference from No Change samples (P< 0.05). RPKM denotes Reads per kilobase exon model per million reads of RNA seq data (see supplemental experimental procedures). See also Figure S1.
Ovarian cancer was significantly enriched for KDM4A amplification, which was amplified in 46% of the tumors (94 out of 204, P=1.4×10−21 for Gain vs No change or Loss by Fisher’s exact test), with relatively few examples of deletion (9.8%; 20 out of 204, samples) (Figure 1C and Figure S1I). The amplification of KDM4A in ovarian cancer also correlated with increased expression (Figure 1D and Figure S1I). In addition, KDM4A focal amplification (GISTIC +2) was significantly associated with the time to death in the ovarian cancer patient data set with a median time to death of 691 days compared to 1052 days without KDM4A amplification (Figure 1E, P=0.02); however, KDM4A loss (GISTIC −2 or −1) was not significantly different from patients without changes in KDM4A copy number (P=0.85). In sharp contrast to KDM4A, few cases of focal amplifications were observed for KDM4B-D and no statistical significance was associated with their focal amplifications and time to death (Figure 1F–H). However, focal deletion of KDM4C (GISTIC −2) and broad loss or gain (GISTIC −1 or +1) of KDM4D modestly associated with poor outcome (P=0.014, P=0.013 and P=0.018, respectively). These data highlight the differences between the KDM4 family and cancer outcome, which suggests non-overlapping functions in certain cancer types. These data also suggest that KDM4A levels could function as a biomarker in ovarian cancer.
KDM4A Overexpression Promotes Copy Gain of 1q12
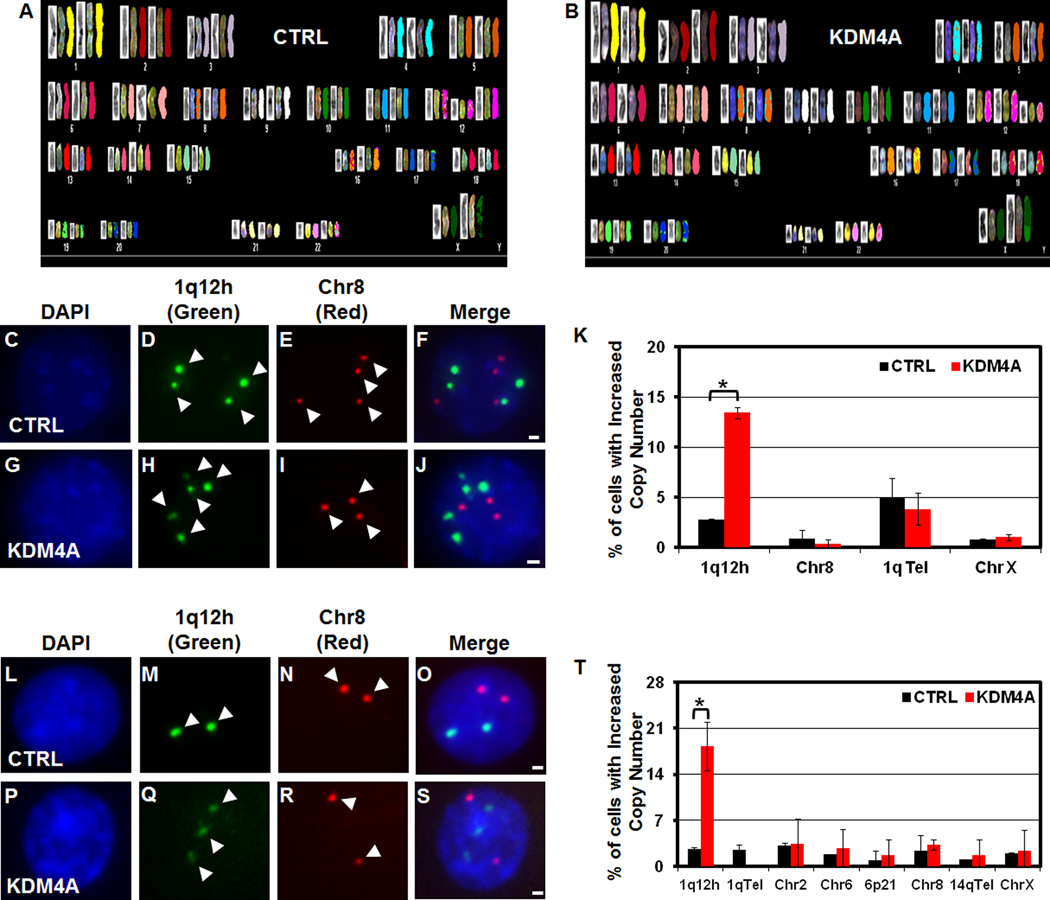
We previously demonstrated that KDM4A overexpression promoted faster S phase progression, increased chromatin accessibility and altered replication timing (Black et al., 2010). KDM4A was also shown to impact the DNA damage response (Mallette et al., 2012). For these reasons, we hypothesized that KDM4A overexpression may promote genomic instability, which is a hallmark of cancer (Luo et al., 2009). Therefore, we stably overexpressed KDM4A in the karyotypically stable, immortalized, but not transformed, RPE1-hTERT (RPE) cell line (GFP: referred to as control or CTRL; GFP-KDM4A: referred to as KDM4A) (Jiang et al., 1999). Similar to our previously reported 293T stable cells (Figure S2A), RPE stable cell lines expressed KDM4A about 2–3 fold over endogenous level (Figure S2B). Upon spectral karyotyping these cell lines, we did not observe major genomic events that were specific to the KDM4A cell lines when compared to CTRL cell lines (SKY; Figure 2A and 2B; Table S1). Similarly, G-band analysis of a 293T KDM4A overexpressing stable cell line did not document any amplification, deletion or translocation specific to GFP-KDM4A cells (data not shown). These data support the notion that modest KDM4A overexpression does not promote large scale genomic instability.
Figure 2. KDM4A overexpression results in 1q12h copy gain.
(A–B) SKY analysis of RPE GFP-CTRL and GFP-KDM4A cells. (C–J) FISH of stable 293T cells overexpressing GFP-CTRL or GFP-KDM4A, respectively: (DAPI- C, G); 1q12h (Green- D, H); Chr 8 centromere (Red- E, I); merged images in F, J. (K) Quantification of FISH experiments in stable 293T GFP-CTRL (Black bars) and GFP-KDM4A cells (Red bars) with the indicated FISH probes. (L–S) FISH of stable RPE cells overexpressing GFP-CTRL or GFP-KDM4A, respectively: (DAPI- L, P); 1q12h (Green- M, Q); Chr 8 (Red- N, R); merged images in O, S. (T) Quantitation of RPE FISH experiments. Arrowheads indicate foci in FISH images. Error bars represent the S.E.M. * indicates significant difference from GFP-CTRL (P<0.05) by two-tailed students t-test. Scale bars represent 2µm. See also Figure S2.
We reasoned that KDM4A might promote instability at specific genomic loci that could be below the detection threshold of SKY. In order to identify these candidate regions, we re-analyzed our chromatin immunoprecipitation (ChIP) on chip analysis of KDM4A binding in 293T cells (Figure S2C) (Van Rechem et al., 2011) for KDM4A enrichment by cytogenetic band. Of the top 10 enriched cytogenetic bands, only 1q12 was specifically enriched in KDM4A overexpressing cells when compared to control cells (Figure S2C). 1q12/21 is a region with frequent CNV in lung cancer, multiple myeloma, congenital heart abnormalities and has been described as a susceptibility locus for schizophrenia and autism (Brunet et al., 2009; Brzustowicz et al., 2000; Inoue et al., 2004; Yakut et al., 2006). To determine if the copy number of 1q12 was altered following manipulation of KDM4A protein levels, we performed fluorescent in situ hybridization (FISH) in CTRL and KDM4A overexpressing 293T cells (Figure 2C–K, Figure S2D). KDM4A overexpression resulted in increased copy number of 1q12h in 14% of cells (Figure 2K), which was not due to a gain of the entire 1q chromosome arm as the 1q telomere did not have an increase in copy number (Figure 2K; 1qTel). Furthermore, no other gains occurred at additional pericentric regions (Figure 2K). We further validated these results in the RPE stable cell lines used in the SKY analysis (Figure 2A, B and Figure S2B). Similar to the 293T cells, 17% of KDM4A overexpressing RPE cells showed an increase in copy of 1q12h, while no significant changes in copy number were observed for the 1q telomere or other centromeres on chromosomes 2, 6, 8, or X (Figure 2L–2T; Chr 2, 6, 8, X).
To eliminate the possibility that KDM4A promoted chromatin accessibility so that there was increased 1q12h detection, Condensin 1 (CapD2) or Condensin 2 (CapD3) were depleted from cells (Figure S2F). Depletion of either condensin did not increase detection of 1q12h amplification in CTRL or KDM4A cells (Figure S2G). Therefore, the increased copy number of 1q12h in KDM4A cells is most likely not an artifact of increased chromatin accessibility. Furthermore, 1q12h copy gain was not due to alterations in p53 activity since doxorubicin treatment resulted in p53 stabilization and target gene activation in CTRL and KDM4A cells (Figure S2H and S2I).
KDM4A-dependent 1q12h Copy Gain is Dose-dependent, Requires Catalytic Activity and Tudor Domains
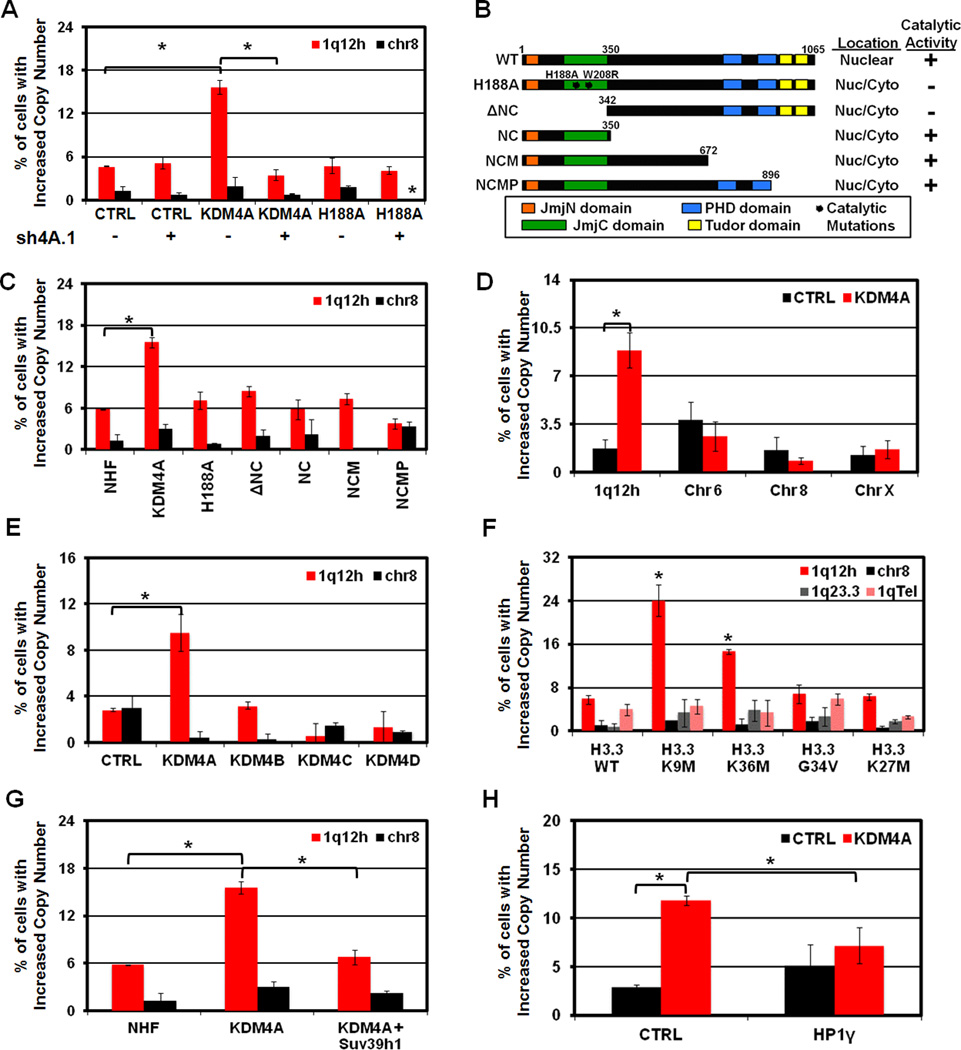
In order to determine if the expression level and catalytic activity of KDM4A are required for 1q12h copy gain, we overexpressed catalytically active and inactive (H188A; (Whetstine et al., 2006)) KDM4A with and without KDM4A depletion by shRNA (Figure 3A, S3A). Transient overexpression of KDM4A was sufficient to promote 1q12h copy gain, but not alter copy number of Chr 8. However, concomitant depletion of KDM4A suppressed 1q12h gain, which demonstrates the importance of increasing KDM4A levels in order to observe 1q12h gain (Figure 3A, S3A). Importantly, neither catalytically dead KDM4A overexpression (Figure 3A, B; H188A) nor KDM4A depletion promoted 1q12h copy gain, which emphasizes the gain is not a dominant negative effect due to KDM4A overexpression.
Figure 3. 1q12h copy gain can be induced transiently, depends on KDM4A catalytic activity, and can be antagonized by Suv39h1 and HP1γ.
(A) Quantification of FISH experiments in 293T cells overexpressing CTRL, KDM4A or catalytically inactive KDM4A (H188A) with (+) and without (-) depletion of endogenous KDM4A (sh4A.1) with the indicated FISH probes. (B) Schematic of NHF-tagged KDM4A constructs. The subcellular localization (“Location”- occurred in greater than 80% of assayed cells) and catalytic activity (“+” indicates strong reduction in total nuclear H3K36me3). (C,D) Quantification of FISH experiments with indicated probes in RPE cells transfected for 24 hours with the indicated constructs: (C) NHF-KDM4A constructs; (D) GFP-CTRL (Black bars) or GFP-KDM4A (Red bars). (E) 1q12h copy gain is specific to KDM4A overexpression and not other KDM4 family members. (F) Overexpression of H3.3 histone variants for H3K9 or H3K36 promotes 1q12h gain. (G,H) Co-expression of Suv39h1 (G) or HP1γ (H) abrogates KDM4A-dependent 1q12h gain. Error bars represent the S.E.M. * indicates significant difference from GFP-CTRL or NHF-CTRL (P<0.05) by two-tailed students t-test. See also Figure S3.
We further demonstrated that 1q12h gain occurred in less than 24 hours of KDM4A transient overexpression in RPE cells (Figure 3C, D, Figure S3B,C), while not altering copy gain at other regions (Figure 3D). Interestingly, KDM4B, KDM4C, or KDM4D overexpression for 24 hours did not alter 1q12h copy number (Figure 3E and Figure S3C). The copy gain required KDM4A catalytic activity (H188A) and enzymatic domains (JmjC and JmjN; referred to as ΔNC) in RPE cells (Figure 3B,C and Figure S3B). However, the KDM4A catalytic domain alone was insufficient to generate 1q12h gain (Figure 3B,C; referred to as NC). Interestingly, the loss of the Tudor domains alone was sufficient to block the 1q12h gain (Figure 3B,C; referred to as NCMP). Taken together, these data emphasize that transient exposure to increased KDM4A levels is sufficient to promote 1q12h copy gain, but this can only occur with a catalytically active enzyme and functional Tudor domains.
Interfering with H3K9 or H3K36 Methylation Promotes 1q12 Copy Gain
The requirement for KDM4A catalytic activity to promote 1q12 copy gain suggests that demethylation of chromatin or a non-histone target is important for proper regulation of 1q12h ploidy. Recently, Lewis and colleagues demonstrated that H3.3 variants with a methionine in place of the lysine (i.e., H3K27M, H3K9M and H3K36M) can inhibit EZH2 (K27M), G9a (K9M), Suv39h1 (K9M) as well as reduce H3K36me3 levels (K36M) (Lewis et al., 2013). Therefore, these H3.3 variants were used to ascertain if interfering with methylation at any one of these lysines could promote 1q12h copy gain. Each variant was expressed and successfully incorporated into chromatin in 24 hours and reduced the corresponding tri-methylation (Figure S3D,E). Expression of H3.3 WT, G34V and H3K27M failed to promote 1q12h copy gain; however, expression of either H3.3K9M or H3.3K36M was sufficient to promote 1q12h gain (Figure 3F; P=0.026 for K9M and P=0.006 for K36M). Furthermore, the 1q12h gain was not caused by a gain of chromosome 1 since there was not an increase in 1q Tel or in the 1q23.3 cytogenetic band midway down the 1q arm (Figure 3F). Since H3.3K9M promoted copy gain at 1q12h and inhibits Suv39h1 (Lewis et al., 2013), we reasoned that overexpression of the H3K9me3 methyltransferase Suv39h1 may suppress KDM4A-dependent copy gain. Consistent with this prediction, co-expression of Halo-Suv39h1 was sufficient to abrogate KDM4A-dependent 1q12h copy gain (P=.0003 for KDM4A and P=0.47 for KDM4A+Suv39h1) (Figure 3G, S3F). These results highlight the importance of methylation in modulating site-specific copy gain, especially the lysines that are substrates for KDM4A.
HP1γ Antagonizes KDM4A-dependent Increased 1q12 Copy Number
KDM4A-dependent changes in cell cycle progression and replication timing at Chr1 sat2 are antagonized by HP1γ overexpression (Black et al., 2010). Therefore, we hypothesized that HP1γ overexpression could antagonize the increased copy number of 1q12 in KDM4A overexpressing cells. Co-transfection of HP1γ reduced the 1q12h copy gain to levels comparable to that seen in control cells (P=0.29) (Figure 3H, Figure S3G). Surprisingly, transfection of HP1γ into RPE cells stably overexpressing KDM4A or stably co-overexpressing HP1γ and KDM4A did not reverse the increased copy number of 1q12h (data not shown). These results imply that once an altered chromatin conformation is established, HP1γ is insufficient to restore proper regulation of 1q12 copy number in KDM4A overexpressing cells.
1q12 Copy Gain Is Not Stably Inherited and Requires S Phase
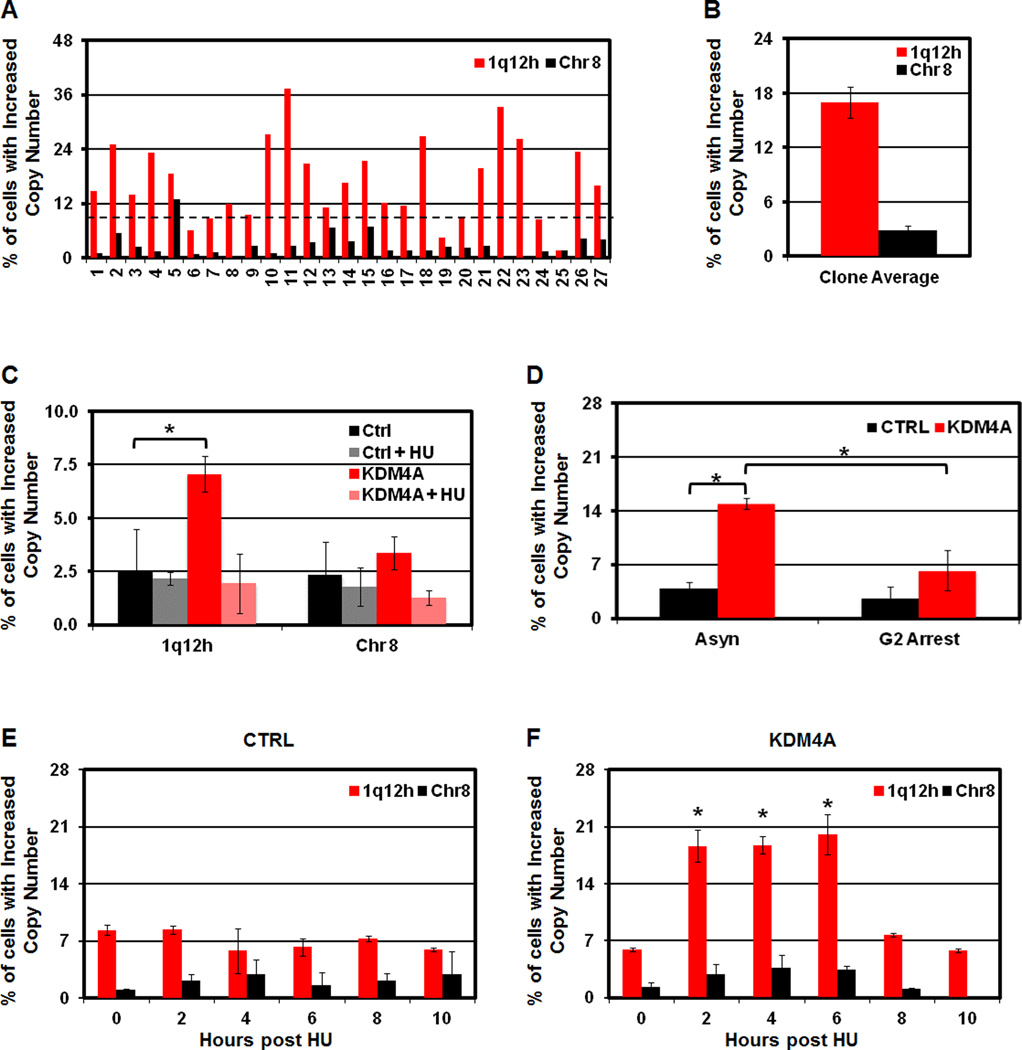
Since KDM4A overexpression promotes 1q12 gain, we asked whether the increased copy number was stably inherited or was regenerated during subsequent cell cycles. First, single cell clones were established from our stably overexpressing KDM4A RPE cell line and assayed by 1q12h FISH (Figure 4A). If the copy number of 1q12h was stably inherited, some clones should have 100% of cells with 1q12h copy gain. Instead, we observed a distribution of copy gain between 1.5–37%, which supports the model that 1q12 gain is most likely not stably inherited (Figure 4A, Figure S4A). The clones lacking increased copy of 1q12h (below black dashed line) no longer overexpressed KDM4A (Figure S4A). Furthermore, the average of all clones assayed was 17.0%, agreeing with our analysis of the starting stable population (Figure 4B, 2T, respectively).
Figure 4. 1q12h copy gain is not stably inherited and requires S phase each cell cycle.
(A) Increased 1q12h copy number in GFP-KDM4A RPE cells is not inherited. FISH of single cell clones derived from RPE KDM4A cells. (B) Average copy gain for 27 single cell clones from A is graphically depicted. (C) Increased copy number of 1q12h requires S phase. (D) 1q12h gain is lost by the end of G2. (E, F) 1q12h copy gain is generated in S phase. Stable GFP-CTRL (E) and GFP-KDM4A (F) RPE cells were arrested in hydroxyurea (HU) for 20 hours and released for the time indicated prior to FISH analysis. Error bars represent the S.E.M. * indicates significant difference from GFP-CTRL (P<0.05) by two-tailed students t-test. For the HU release, P values are based on the comparison of KDM4A to CTRL at each individual time point (F, E, respectively). See also Figure S4.
We next investigated whether stably overexpressing KDM4A RPE cells gain extra copies of 1q12h during each cell division. The 1q12h copy gain was eliminated in KDM4A overexpressing RPE cells arrested in G1/S with HU (Figure 4C, Figure S4B). Since apoptosis was not increased in asynchronous or HU arrested KDM4A cells, the lack of copy gain was not due to increased apoptosis removing cells with extra 1q12h (Figure S4C). However, doxorubicin treatment could induce apoptosis in both CTRL and KDM4A cells, which was significantly reduced in KDM4A overexpressing cells (Figure S4C). We then tested whether 1q12h gain persisted through G2 by arresting cells in late G2 with the CDK1 inhibitor R03306 [Figure S4B; (Vassilev, 2006)]. KDM4A cells arrested in late G2 and did not display 1q12h copy gain (Figure 4D).
Given that KDM4A-dependent 1q12h copy gain did not occur during G1/S or G2 arrest, we hypothesized that KDM4A promotes copy gain during S phase. In agreement with this hypothesis, GFP-KDM4A cells, but not GFP-CTRL cells, were able to promote additional copies of 1q12h, but not Chr 8 centromere following HU release (Figure 4E,F). The additional copies occurred between 2 and 6 hours post HU release and were lost between 8 and 10 hours following release. Taken together, our data support a model whereby KDM4A promotes copy gain of specific chromosomal regions during S phase, which are then eliminated by the end of the G2 phase of cell cycle.
KDM4A Associates with Replication Machinery and Promotes Re-replication of 1q12
In order to gain molecular insight into how KDM4A is involved in generating 1q12h copy gain, we identified KDM4A interacting proteins by performing mass spectrometry analysis of proteins interacting with Halo-KDM4A. We observed a significant enrichment for proteins involved in replication using IPA (Figure 5A; P=0.00000795). Interestingly, many of these proteins are required for re-replication [e.g., MCMs and DNA polymerases; (Arias and Walter, 2007; Snaith and Forsburg, 1999)].
Figure 5. KDM4A interacts with replication machinery and KDM4A overexpression promotes re-replication.
(A) Table depicting mass spectrometry analysis of KDM4A interacting proteins related to replication. (B) Western blots of co-immunoprecipitation of endogenous KDM4A and the indicated licensing and replication machinery in RPE Cells. (C) KDM4A overexpression in RPE cells leads to re-replication of Chr1 sat2. (D) KDM4A is enriched at Chr1 sat2 (1q12) in HU arrested KDM4A-overexpressing RPE cells. (E) H3K9me3, but not H3K36me3 decreases at Chr1 sat2 in HU arrested cells. (F) HP1γ enrichment decreases at Chr1 sat2 (1q12) in HU arrested KDM4A-overexpressing cells. (G, H) MCM7 and DNA polymerase α (Pol α) are enriched at Chr1 sat2 (1q12) in HU arrested KDM4A-overexpressing cells. Error bars represent the S.E.M. * indicates significant difference from GFP-CTRL (P<0.05) by two-tailed students t-test. See also Figure S5.
Previous work verified KDM4A associations with cullin 1 (Van Rechem et al., 2011) and p53 (Kim et al., 2012). We further validated additional interactions by conducting endogenous KDM4A co-immunoprecipitation with MCM2, MCM3, and MCM7 or Halo-tagged DNA polymerase subunits (Figure 5B and S5A). While MCM2 was not identified in our mass spectrometry analysis, it associated with KDM4A, suggesting that the entire MCM complex interacts with KDM4A.
Since KDM4A overexpression promoted copy gain in a replication-dependent manner and interacted with DNA polymerases and the replication licensing machinery, we hypothesized that KDM4A overexpression was promoting re-replication within 1q12. To test this hypothesis, we utilized cesium chloride density gradient centrifugation (Figure S5B). Our labeling procedure was performed for less than one complete cell cycle, producing an enrichment in heavy-light (H:L) replicated DNA, while still maintaining an un-replicated light-light fraction (L:L). We did not detect a peak of enrichment of H:H DNA, indicating that KDM4A overexpression does not promote widespread re-replication as seen with other chromatin regulators [KMT5A;(Tardat et al., 2010)]. We pooled and purified the fractions where the H:H DNA should separate and then assayed the re-replicated DNA for specific regions. Since Chr1 sat2 resides in 1q12 (Wong et al., 2001), is bound and modulated by KDM4A, we reasoned it could be a re-replicated target. We observed a seven fold enrichment of Chr1 sat2 in the re-replicated fraction from KDM4A overexpressing cells, while the β-actin locus and a region near the X centromere, which we previously reported as a KDM4A target (Black et al., 2010), were not enriched (Figure 5C, S5C). The enrichment in Chr1 sat2 re-replication represented a small amount of the input DNA and was consistent with a subpopulation of cells generating and losing the 1q12h copy gain (Figure S5C). Taken together, our data demonstrate that KDM4A associates with replication proteins and promotes re-replication at a specific locus that exhibits copy gains.
KDM4A Overexpression Promotes Chromatin State Changes and Recruitment of Replication Machinery
Our data support a model whereby KDM4A overexpression promotes methylation changes, displacement of HP1γ and recruitment of replication machinery to specific genomic regions resulting in re-replication. To test this model, we performed ChIP experiments to evaluate methylation levels, HP1γ enrichment and replication machinery occupancy at Chr1 sat2. As a negative control, we identified an intergenic region on chromosome 10 (Chr10) that is acetylated at H3K9 in numerous cell types (according to UCSC browser; data not shown) that should not be enriched for H3K9me3 or KDM4A. KDM4A overexpression increased KDM4A recruitment to Chr1 sat2 but not Chr10 (Figure 5D), which corresponded to a loss of H3K9me3 and HP1γ depletion (Figure 5E,F). We did not observe any change in H3K36me3 at Chr1 sat2, which was consistent with our previous findings in 293T cells (Black et al., 2010). Finally, both MCM7 and Polα were enriched at Chr1 sat2, but not at Chr10, upon KDM4A overexpression (Figure 5G,H, respectively). Our data demonstrate that KDM4A overexpression promotes H3K9me3 and HP1γ loss, increased replication machinery recruitment and re-replication.
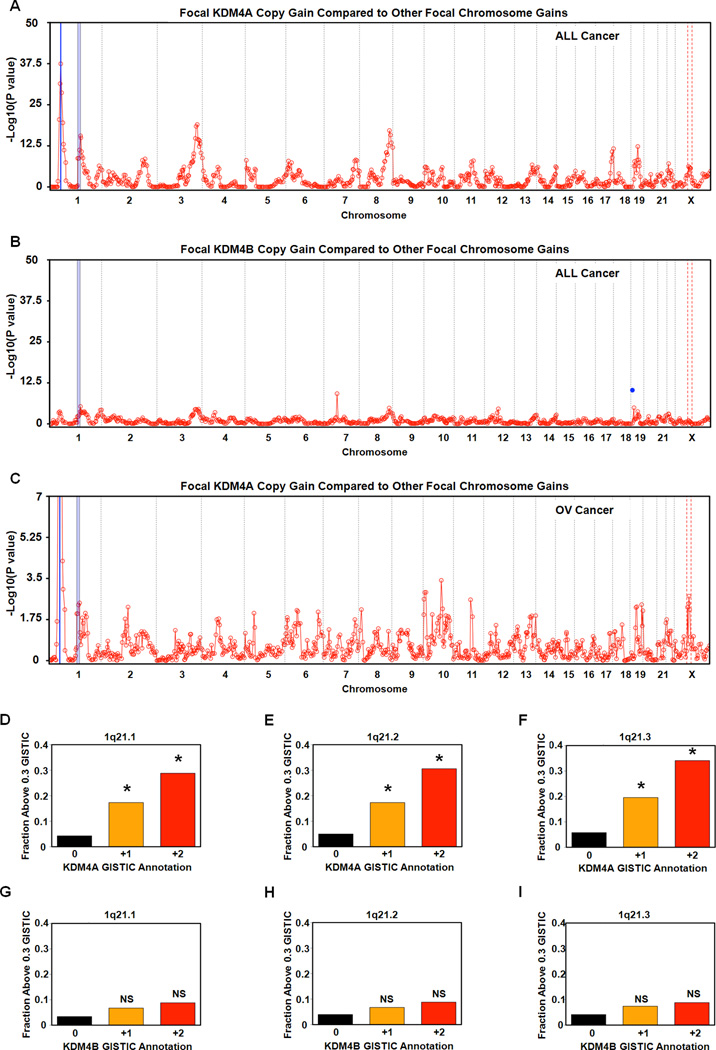
Identification of Regions Co-Amplified with KDM4A in Cancer
To identify additional regions that have copy gains upon KDM4A overexpression, we determined whether KDM4A focal amplification (1p34.2) in primary tumors was correlated with copy gains of any of the 807 cytogenetic bands in 4,420 tumor samples (Figure 6A, which represents 19 tumor types including the 8 analyzed in Figure S1). We observed correlated copy gains from 1p11.2 through 1q21.3 on chromosome 1 in two independent statistical tests (Figure 6A and S6A, blue shading; See Experimental Procedures). Due to the minimal sequence annotation and repetitive nature of 1q12, we did not calculate a correlation for this cytogenetic band. However, the cytogenetic bands immediately flanking 1q12 (1p11.2 and 1q21.1) exhibited co-gain with KDM4A amplification, which suggests that 1q12 is likely co-amplified in these tumors. These co-amplified regions were specific to KDM4A because there was not a strong correlation when the identical analysis was performed with respect to KDM4B co-amplification (Figure 6B and S6B). We observed that individual co-amplified regions could universally be observed, while others could be differentially regulated in a tumor and/or tissue-specific manner. For example, the ovarian cancer data sets demonstrated co-amplification with 1p11.2-1q21.3, while some KDM4A co-amplified regions were lost (e.g., the region on 17q- position 17q24.2 to 17q25.3) and others were enhanced (e.g., the region on X chromosome- position Xp11.2 to Xq13.2) in the ovarian cancer profile (Figure 6C and S6C).
Figure 6. Identification of cytogenetic bands co-amplified with KDM4A in cancer.
(A) Focal amplification of cytogenetic bands correlates with amplification of KDM4A in cancer. The blue line represents the locus of KDM4A and its gene-specific significance is P=1.5×10−37. (B) Focal amplification of cytogenetic bands with amplification of KDM4B. The blue dot represents the gene-specific significance of KDM4B. (C) Focal amplification of cytogenetic bands correlates with amplification of KDM4A in 547 ovarian cancer samples. The blue line represents the locus of KDM4A and its gene-specific significance is P=1.1×10−19. For each co-amplification plot, blue shaded regions indicates 1p11.2 through 1q21.3 and red dashed lines indicates Xp11.2 through Xq13.2. (D) Increased copy of KDM4A is associated with increased mean focal copy of 1q21.1 P=2×10−9 for +2 vs 0 and P= 2.04×10−25 for +1 vs 0 by Fisher’s exact test. (E) Increased copy of KDM4A is associated with increased copy of 1q21.2 (P = 1.9×10−9 for +2 vs 0 and P=6.28×10−22 for +1 vs 0). (F) Increased copy of KDM4A is associated with increased copy of 1q21.3 (P=1.02×10−10 for +2 vs 0 and 3×10−24 for +1 vs 0). (G) Increased copy number of KDM4B is not associated with increased copy of 1q21.1 (P=0.18 for +2 vs 0). (H) Increased copy number of KDM4B is not associated with increased copy of 1q21.2 (P=0.22 for +2 vs 0). (I) Increased copy number of KDM4B is not associated with increased copy number of 1q21.3 (P=0.24 for +2 vs 0). * indicates significant difference of +1 or +2 vs 0 by Fisher’s exact test. NS indicates not significantly different from 0. See also Figure S6.
Next, we tested whether gains in 1q21.1-1q21.3 were affected by the amplification level of KDM4A (Fig 6D–F). Indeed, when KDM4A had a high-level focal amplification (GISTIC +2), a significantly greater fraction of samples were amplified in 1q21.1-1q21.3 compared to cases in which KDM4A was not amplified (GISTIC 0; Fisher’s exact test; P= 2*10−9 in 1q21.1, 1.9*10−9 in 1q21.2, and 1.02*10−10 in 1q21.3) (Figure S6D–I). When comparing lower-level amplification of KDM4A (GISTIC +1) to the KDM4A-unamplified cases, we observed a reduced sample fraction, but still highly significant amplification of 1q21.1-1q21.3 (P= 2.04*10−25 in 1q21.1, 6.28*10−22 in 1q21.2, and 3*10−24 in 1q21.3). In contrast, when stratifying the samples based on KDM4B amplification status (Fig 6G–I), the differences are not significant (all P-values > 0.1 by Fisher’s exact test), although a minimal trend in the same direction is observed. These results demonstrate that KDM4A amplification in tumors correlated with amplification of specific cytogenetic bands, which suggests that KDM4A may promote site-specific copy gains in vivo.
Copy Gain and Re-replication Occurs in Regions Co-Amplified in Tumors
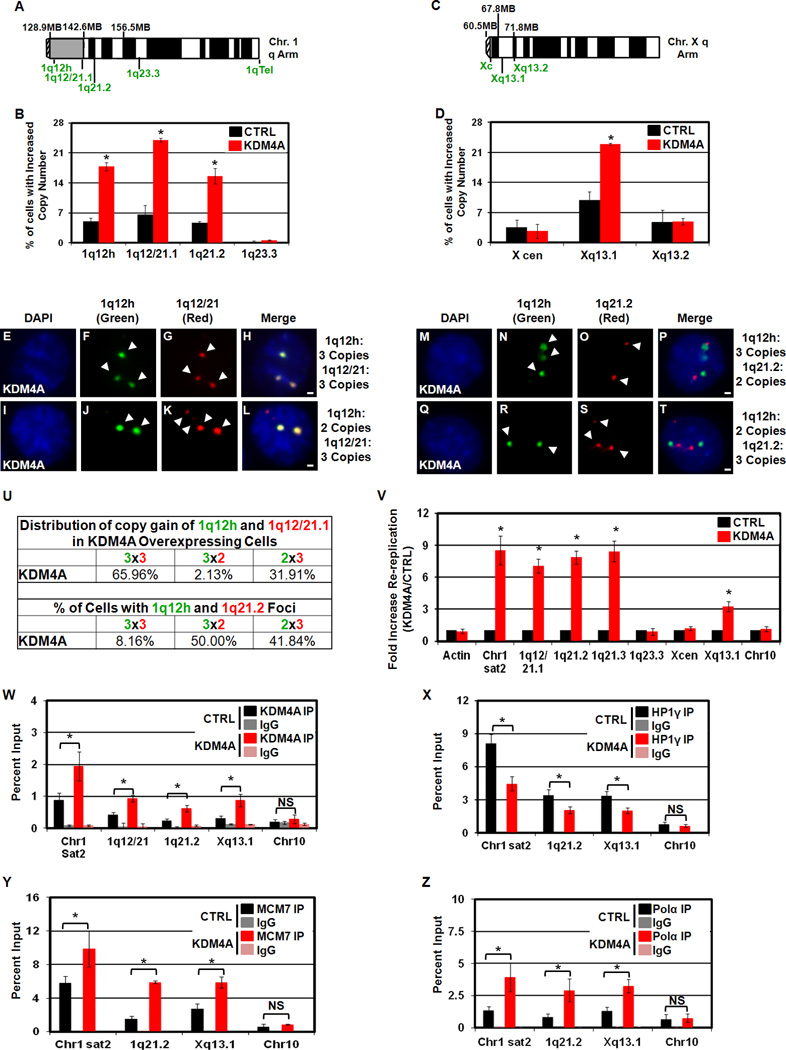
Since 1q21.1-1q21.3 amplification correlated with KDM4A amplification, we assessed whether these regions were gained by FISH and re-replicated by density gradient centrifugation in transgenic cell lines. We observed increased copy number of 1q12 through 1q21.2 in our KDM4A overexpressing cells, but not in control cells (Figure 7A,B). Our co-amplification analysis of all tumors indicated that the correlation with KDM4A copy number diminished at 1q23.3, suggesting that 1q23.3 is not amplified in KDM4A overexpressing cells, which was in fact the case in KDM4A overexpressing RPE cells (Figure 7A,B).
Figure 7. KDM4A overexpression leads to copy gains and re-replication of regions co-amplified in tumors.
(A) Chromosome arm schematic depicting location of FISH probes used on chromosome 1. (B) KDM4A overexpression increased copy number of 1q12h, 1q12/21.1 and 1q21.2 but not 1q23.3. (C) Chromosome arm schematic depicting location of FISH probes used on chromosome X. (D) KDM4A overexpression increases copy number of Xq13.1 but not X cen or Xq13.2 in RPE cells. (E–T) FISH of stable RPE cells overexpressing GFP-CTRL or GFP-KDM4A with indicated FISH probes. (U) Table summarizing co-amplification of 1q12h, 1q12/21.1 and 1q21.2 in panels E–T. Data are presented as % of amplified cells having 2 or 3+ copies of the indicated FISH probes. (V) KDM4A-dependent re-replication of chromosomal domains. (W) KDM4A ChIP in HU arrested cells. KDM4A is enriched in re-replicated regions in KDM4A-overexpressing RPE cells. (X) HP1γ enrichment decreases at re-replicated regions in KDM4A-overexpressing cells following 1 hour release from HU arrest. (Y,Z) MCM7 and DNA polymerase α (Pol α) are enriched at re-replicated regions in HU arrested KDM4A-overexpressing cells, respectively. Error bars represent the S.E.M. * indicates significant difference from GFP-CTRL (P<0.05) by two-tailed students t-test. For Re-replication (V) and ChIP experiments (W, Y, Z) Chr1 sat2, and Chr10 are the data presented in Figure 5 for reference. Scale bars represent 2µm. See also Figure S6,S7.
We then evaluated copy gain of other chromosomal domains identified through our co-amplification analysis. We chose to examine the small focal peak on the X-chromosome (Xp11.2-Xq13.2), which was specific to co-amplification with KDM4A across all cancers and even more enriched in ovarian cancer (Figure 6A,C, respectively). KDM4A overexpressing RPE cells exhibited Xq13.1 copy gains, but not the X centromere or Xq13.2 (Figure 7C,D). Xq13.1 as well as 1q12/21 and 1q21.2 copy gains were also observed in 293T cells overexpressing KDM4A (Figure S6J). These data demonstrate that KDM4A co-amplified regions in primary tumors are generated by KDM4A overexpression.
The co-amplification analysis of tumor data sets does not distinguish whether the entire intervening sequence between 1q12h and 1q21.3 was amplified in the same tumor cells. Therefore, we scored the 1q12/21 or 1q21.2 FISH probes with the 1q12h probe in the same KDM4A overexpressing cells (Figure 7A,E–U). We observed that approximately 2/3 of the KDM4A overexpressing cells with 1q12h amplification also had 1q12/21.1 amplification, while approximately 1/3 had only 1q12/21.1 copy gain but lacked 1q12h gain (Figure 7U). In the case of 1q12h or 1q21.2 amplification, the majority of cells had gains in one or the other cytogenetic band (Figure 7M–U). Taken together, these data demonstrate that the entire region between 1q12h and 1q21.3 is not contiguously amplified, but that KDM4A is directing copy gain within these cytogenetic bands. The ability to detect non-overlapping foci also suggests that the additional copies may exist as extrachromosomal pieces. This possibility was further supported by the fact that 1q12/21 FISH marked the additional 1q12/21 copy adjacent to or physically distinct from the chromosome 1 painted territory (Figure S7A–L).
To address whether these gained regions were through re-replication, we assayed our CsCl gradient H:H fraction for regions under our FISH probes in the indicated cytogenetic bands (Figure 7V). We observed Re-replication at 1q12h (indicated by Chr1 sat2), 1q12/21.1, 1q21.2 and 1q21.3, but not in 1q23.3 or on Chr10. Re-replication was detected inside Xq13.1 but not near the X centromere. In addition, the re-replicated regions were bound by KDM4A, which was further enriched upon KDM4A overexpression (Figure 7W, Figure S6K). As with Chr1 sat2, KDM4A overexpression promoted loss of HP1γ (Figure 7X) and recruitment of MCM7 and Polα to 1q21.2 and Xq13.1 (Figure 7Y,Z, respectively). Taken together, our data are consistent with the model that KDM4A overexpression promotes copy gain and re-replication at specific sites within the genome in vivo (tumors) and in vitro (transgenic cell lines).
DISCUSSION
Genomic instability is a major contributing factor to the development and onset of age-related diseases. Cancer cells often contain alterations in copy number of genes, specific genomic regions, chromosome arms, and entire chromosomes. However, the underlying molecular mechanisms that lead to these copy number alterations are poorly understood. Here, we report that overexpression of a single chromatin modifying enzyme, KDM4A, is sufficient to promote re-replication and copy gain of specific chromosomal domains. Furthermore, KDM4A-dependent amplified regions are found co-amplified with KDM4A in primary tumors. Our results support a model where increased expression of KDM4A promotes recruitment of KDM4A to specific genomic regions and promotes re-replication (Figure S7M).
KDM4 Members and Cancer
Our results demonstrate that KDM4A is amplified in different tumor types and correlates with increased KDM4A expression. Intriguingly, when compared to other tumor types ovarian cancer is enriched for amplifications of KDM4A, which correlate with poor outcome in these cases. Levels of KDM4A may therefore represent a good biomarker for ovarian cancer, especially with respect to novel therapies that target this lysine demethylase. In addition, ovarian patients with increased levels of KDM4A may respond more poorly to S phase chemotherapeutics since KDM4A overexpression resulted in better recovery from HU (Black et al., 2010). Consistent with this possibility, ovarian cell lines with 1q12-21 amplification are often more resistant to cisplatin treatment (Kudoh et al., 1999; Takano et al., 2001). Interestingly, drug resistance in multiple myeloma is also associated with 1q12-21 amplification (Inoue et al., 2004). Addressing the relationship between KDM4A, 1q12-21 co-amplification and drug responses will be important in future studies.
Even though KDM4 family members have a high degree of homology, they have different distribution of genomic anomalies in cancer. We observed focal deletions in KDM4B-D, while none were observed in KDM4A across the 8 different tumor types (Figure S1A–D). We also found that KDM4A was the only family member that had a correlation between focal amplification and poor outcome for ovarian cancer patients. Thus, it is intriguing to speculate that KDM4 family enzymes may play different roles in ovarian cancer and other tumor types. Overall, our data highlights the idea that these family members are not created equal, but most likely, have their own specific roles in cancer and other diseases.
Chromatin Environment and KDM4A-dependent Copy Gain
Work in yeast demonstrates that specific genomic regions can generate extra DNA fragments in S phase, which depends on the chromatin environment (Kiang et al., 2010). These observations are consistent with our KDM4A-dependent re-replication of specific chromosomal regions. These regions are enriched for KDM4A binding upon overexpression of KDM4A, re-replicate, and have increased copy number during S phase. Interestingly, not all KDM4A occupied sites are re-replicating in the cell lines tested (Figure 7V). However, this could reflect the chromatin environment in these particular cells, or distinct chromatin states influenced by other chromatin modifiers. This would be consistent with our ability to see Xq13.1 and X centromere copy gain in KDM4A focally amplified tumors, while only seeing the copy gain and re-replication of Xq13.1 in our transgenic cell lines. Thus, different genomic regions may be susceptible to KDM4A-dependent re-replication in specific tissue types.
Consistent with the model that chromatin state impacts copy gain, we demonstrated that interfering with H3K9 or K36 methylation resulted in the site-specific gain of 1q12h in 24 hours; while, overexpression Suv39h1 or HP1γ was able to suppress the 1q12h gain. Surprisingly, we were unable to reverse KDM4A-dependent copy gains in cells stably overexpressing KDM4A by overexpressing HP1γ, even though these copy gains are regenerated each cell cycle. This observation supports a model whereby KDM4A may establish a chromatin state that promotes re-replication and increased copy number of specific chromosomal regions. Formation of this chromatin state could be antagonized by HP1γ, but not reversed once established. Taken together, these data strongly suggest that the reader, the lysine and the KDM/KMT balance are required to maintain regulation at these regions. Therefore, we hypothesize that the local chromatin environment will be an important determinant in designating whether certain regions are more susceptible to copy gains and re-replication (see Figure S7M). Based on our observations, we also believe that other methyltransferases or chromatin modulators (e.g., readers or remodelers) may be able to block or reset the established chromatin state in KDM4A overexpressing cells or that these modulators may have their own independent roles in regulating site-specific copy gains.
KDM4A and Re-replication
Increased KDM4A occupancy promotes a more open chromatin environment through decreasing H3K9me3 and HP1γ occupancy (Figure S7M; (Black et al., 2010)). KDM4A overexpression also promotes recruitment of the replication licensing machinery and DNA polymerases to sites of re-replication and copy gain (Figure S7M; model). Since copy gains require the KDM4A Tudor domains, we favor the model that KDM4A directly increased chromatin accessibility, and in turn, loading of the MCM and replication complex on chromatin (Figure S7M). This loading and re-replication could occur in the absence of CDT1, CDC6 and ORC since they are not necessary for replication after MCMs have been successfully loaded (Arias and Walter, 2007). However, the more open chromatin could independently promote inappropriate recruitment of MCMs and DNA polymerases to unused or reused origins, thus promoting re-replication (Figure S7M). This later model would be consistent with the observation that interfering with H3K9 or K36 methylation can promote the site-specific gain in cells with wild type KDM4A levels. Regardless of the exact details, the data presented in this study supports the model that alterations of heterochromatin and methylation at specific regions are more prone to re-replication, and in turn, copy gain.
KDM4A and Extrachromosomal DNA
Since the extra copies of 1q12 are not inherited, but removed prior to completion of G2, it is likely that such regions exist as extrachromosomal DNA (Figure S7H,L). As such, it is possible that KDM4A is promoting re-replication at regions that promote head-to-tail collision of one replication fork chasing another (Figure S7M). This model fits a previous study showing that deregulation of replication licensing promotes DNA fragmentation was consistent with fork collision (Davidson et al., 2006). The presence of these fragments could also explain why KDM4A overexpressing cells exhibit a moderate increase in p53 stabilization (Figure S2H); that remains below the threshold required to elicit the p53 checkpoint (Kracikova et al., 2013). It is also possible that site-specific re-replication may not be restricted to cancer cells. Transiently up regulating enzymes that direct site-specific re-replication to increase copy number of specific genes may be a general mechanism to allow cells the plasticity to respond to developmental, environmental, or stress conditions without altering their genetic makeup.
KDM4A-dependent Transient Copy Gain
Since transient KDM4A overexpression was sufficient to promote localized changes in copy number in a single cell cycle, the amplification of specific regions may precede genetic changes in tumors. For instance, transient misregulation of chromatin regulators by altered environmental factors, metabolic changes, hypoxia or miRNAs could lead to temporary changes in copy number of small genomic regions. If these regions contain oncogenes, this could create a feedback loop that promotes tumorigenesis while masking the originating event (e.g., transient up-regulation of KDM4A). Of note, several putative oncogenes reside in the 1q12 and 1q21 cytogenetic bands, including Bcl9 and Mcl1. In fact, KDM4A binds the Bcl9 locus and causes both copy gain and re-replication of this site (Figure 7W and 7V, respectively). However, we do not observe increased expression of Bcl9, which likely reflects the lack of additional stimulus, transcription factors or the low percentage of cells with this particular copy gain (data not shown).
It remains unclear how the re-replicated regions are removed as cells exit S phase and enter G2/M. It is possible that cells possess an active method for degradation or removal of these regions. Understanding the events leading to removal of inappropriately amplified regions could be critical to help identify pathways that may be mis-regulated in cancer, and lead to the accumulation and inheritance of copy number changes. We hypothesize that other events could then promote incorporation of these transiently amplified regions, and in turn, influence tumorigenesis.
Conclusion
It is clear that the chromatin context influences replication timing and initiation choices. However, chromatin may additionally play an important role in ensuring replication fidelity and preventing re-replication. Distinct chromatin domains may have increased propensity for re-replication under different circumstances and cell types. This is supported by work in Drosophila that demonstrates that heterochromatic regions re-replicate upon loss of geminin (Ding and MacAlpine, 2010). Additionally, some chromatin modifiers such as KMT5A/B/C regulate re-replication on a more global scale (Beck et al., 2012; Tardat et al., 2010). Taken together, these results suggest that proper regulation of chromatin state is critical for suppressing re-replication. Therefore, a “chromatin checkpoint” may be intimately associated with the timing of replication and the propensity to undergo re-replication and copy gain.
While we have uncovered a single enzyme that can regulate site-specific copy gain, several additional questions remain to be answered (Figure S7M): How does the chromatin environment regulate CNV? Is regulation of methylation of non-histone substrates involved in modulating copy number (e.g., replication machinery and/or HP1)? Do additional chromatin modifiers regulate CNV and contribute to tumor heterogeneity? What events could promote transient copy gains to be inherited? Future studies that identify additional factors and/or stimuli that address these questions will allow for a more complete picture surrounding copy number variation, re-replication, genome stability and cancer to emerge.
EXPERIMENTAL PROCEDURES
Extended Experimental Procedures are included in the Supplemental Information.
Cell Culture
Generation of stable cell lines, constructs, and antibodies used can be found in supplemental experimental procedures. Transient H3.3 variants RPE cells were transduced with lentiviral stocks provided from the Allis lab in the presence of 8µg/ml polybrene for 8 hours (Lewis et al., 2013).
Subcellular localization and Catalytic Activity of KDM4A deletion fragments
H3K36me3 and subcellular localization were assayed by examining transfected cells (positive for HA staining; HA.11 Covance) following fixation in 3.7% PFA in PBS (Whetstine et al., 2006).
Immunoprecipitation and Chromatin Immunoprecipitation
HaloTag purified KDM4A complexes were analyzed and processed by MS Bioworks, LLC (Ann Arbor, Michigan), with details in supplemental experimental procedures. Immunoprecipitations (IP) were performed essentially as described in (Van Rechem et al., 2011).
Chromatin IPs were performed as in (Black et al., 2010) with some minor changes. Sonication was performed using a Qsonica Q800R system with a constant chiller. RPE cells were arrested in 2mM HU for 20 hours prior to crosslinking to assess enrichment in KDM4A, HP1γ, H3K9me3, H3K36me3, and DNA polymerase α at G1/S transition. Data presented are averages from the two independently prepared polyclonal RPE cell lines from at least two independent chromatin preparations per cell line.
Fluorescent In Situ Hybridization (FISH)
FISH was performed as described in (Manning et al., 2010). Probes for 1q12h, 1q telomere, 6p21/chr 14 IGH translocation, and chromosome 2, 6, 8, and X alpha satellite were purchased from Rainbow Scientific. Probes for 1q12 (RP11-17L12), Xq13.2 (RP11-451A22), Xq13.1 (RP11-177A4) were purchased as BAC clones from Children’s Hospital Oakland Research Institute (CHORI BacPac) FISH verified clone repository. Probes for 1q21.2 (BCL9) and 1q23.3 were purchased from Agilent (SureFISH). For RPE cells, copy gain was scored as any cell with 3 or more distinct foci. For 293T cells, copy gain was scored for any cell with 5 or more distinct foci. Comprehensive methods can be found in supplemental experimental procedures.
Cesium Chloride Gradient Centrifugation
RPE cells were treated with 100µM BrdU 14 hours prior to harvest. DNA was purified, digested with RNAse A, EcoRI and BamHI (NEB) and resuspended in TE. 100 µg of DNA was mixed with CsCl in TE (refractive index of 1.4015–1.4031). The CsCl gradient was centrifuged at 44,400 RPM in a VTi-65 rotor for 72 hours at 25 degrees Celsius. Fractions were collected in ~200 µl aliquots and DNA concentration was measured by Nanodrop. Appropriate fractions were pooled, dialyzed, concentrated, and ethanol precipitated. Each re-replicated pool was diluted to 15ng/ul stock and 7.5ng of re-replicated DNA pool was analyzed by qPCR. Each sample was normalized to its own input prior to determination of fold-change in re-replication. Primers used in this study will be provided upon request.
Determination of Cytoband Copy Number and Correlation with KDM4A
Sample-specific mean focal copy numbers for 807 cytobands including X-chromosome were annotated by taking an average of GISTIC annotated focal copy numbers of every genes within the same cytoband. The p-values for the mean focal copy number changes between KDM4A copy-gained samples (GISTIC annotation = +1 or +2) and KDM4A copy-neutral samples (GISTIC annotation = 0) across 807 cytobands were annotated by two independent statistical tests (Figure 6A–C and S6A–C; see Supplemental Method D for details). As positive controls we also calculated the significance using the gene-specific copy-number for KDM4A and KDM4B. The empirical cumulative distribution functions (the fraction of samples below the given mean focal copy) were determined by enumerating samples having the mean focal copy number less than or equal to the value on the x-axis in Figure S6C–H for KDM4A and KDM4B amplified (+2), copy-gained (+1), and copy-neutral samples (0). Procedures for RNAseq and ovarian cancer outcome can be found in supplemental experimental procedures.
Supplementary Material
HIGHLIGHTS.
KDM4A is amplified and overexpressed in cancer.
KDM4A overexpression promotes site-specific copy gain and re-replication.
KDM4A transiently promotes site-specific copy gain within a cell cycle.
KDM4A co-amplified regions in tumors are generated in transgenic cell lines.
ACKNOWLEDGEMENTS
We are grateful to Ravi Mylvaganam and the MGH Flow Cytometry core for assistance with the cell sorting. We thank Mo Motamedi for helpful comments on the manuscript. This work was supported by funding to JRW from the Ellison Medical Foundation, CA059267 and R01GM097360. GG and JK are supported by NIH U24CA143845. JCB was a Fellow of The Jane Coffin Childs Memorial Fund for Medical Research. This investigation has been aided by a grant from The Jane Coffin Childs Memorial Fund for Medical Research. NJD is supported by R01CA155202. ALM is supported by MGH ECOR Tosteson Postdoctoral Fellowship. CM is supported by CCSG P30CA013330.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information contains Extended Experimental Procedures, 7 Figures and 1 Table.
REFERENCES
- Alabert C, Groth A. Chromatin replication and epigenome maintenance. Nat Rev Mol Cell Biol. 2012;13:153–167. doi: 10.1038/nrm3288. [DOI] [PubMed] [Google Scholar]
- Arias EE, Walter JC. Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells. Genes Dev. 2007;21:497–518. doi: 10.1101/gad.1508907. [DOI] [PubMed] [Google Scholar]
- Beck DB, Burton A, Oda H, Ziegler-Birling C, Torres-Padilla ME, Reinberg D. The role of PR-Set7 in replication licensing depends on Suv4-20h. Genes Dev. 2012;26:2580–2589. doi: 10.1101/gad.195636.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry WL, Shin S, Lightfoot SA, Janknecht R. Oncogenic features of the JMJD2A histone demethylase in breast cancer. Int J Oncol. 2012;41:1701–1706. doi: 10.3892/ijo.2012.1618. [DOI] [PubMed] [Google Scholar]
- Black JC, Allen A, Van Rechem C, Forbes E, Longworth M, Tschop K, Rinehart C, Quiton J, Walsh R, Smallwood A, et al. Conserved antagonism between JMJD2A/KDM4A and HP1gamma during cell cycle progression. Mol Cell. 2010;40:736–748. doi: 10.1016/j.molcel.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Brunet A, Armengol L, Heine D, Rosell J, Garcia-Aragones M, Gabau E, Estivill X, Guitart M. BAC array CGH in patients with Velocardiofacial syndrome-like features reveals genomic aberrations on chromosome region 1q21.1. BMC Med Genet. 2009;10:144. doi: 10.1186/1471-2350-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzustowicz LM, Hodgkinson KA, Chow EW, Honer WG, Bassett AS. Location of a major susceptibility locus for familial schizophrenia on chromosome 1q21-q22. Science. 2000;288:678–682. doi: 10.1126/science.288.5466.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson IF, Li A, Blow JJ. Deregulated replication licensing causes DNA fragmentation consistent with head-to-tail fork collision. Mol Cell. 2006;24:433–443. doi: 10.1016/j.molcel.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, MacAlpine DM. Preferential re-replication of Drosophila heterochromatin in the absence of geminin. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PJ, Lupski JR, Rosenberg SM, Ira G. Mechanisms of change in gene copy number. Nat Rev Genet. 2009;10:551–564. doi: 10.1038/nrg2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook SS, Lin JJ, Dutta A. Mechanisms to control rereplication and implications for cancer. Curr Opin Cell Biol. 2007;19:663–671. doi: 10.1016/j.ceb.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J, Otsuki T, Hirasawa A, Imoto I, Matsuo Y, Shimizu S, Taniwaki M, Inazawa J. Overexpression of PDZK1 within the 1q12-q22 amplicon is likely to be associated with drug-resistance phenotype in multiple myeloma. Am J Pathol. 2004;165:71–81. doi: 10.1016/S0002-9440(10)63276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XR, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, Beeche M, Bodnar AG, Wahl GM, Tlsty TD, Chiu CP. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat Genet. 1999;21:111–114. doi: 10.1038/5056. [DOI] [PubMed] [Google Scholar]
- Kiang L, Heichinger C, Watt S, Bahler J, Nurse P. Specific replication origins promote DNA amplification in fission yeast. J Cell Sci. 2010;123:3047–3051. doi: 10.1242/jcs.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TD, Shin S, Berry WL, Oh S, Janknecht R. The JMJD2A demethylase regulates apoptosis and proliferation in colon cancer cells. J Cell Biochem. 2012;113:1368–1376. doi: 10.1002/jcb.24009. [DOI] [PubMed] [Google Scholar]
- Kracikova M, Akiri G, George A, Sachidanandam R, Aaronson SA. A threshold mechanism mediates p53 cell fate decision between growth arrest and apoptosis. Cell Death Differ. 2013 doi: 10.1038/cdd.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh K, Takano M, Koshikawa T, Hirai M, Yoshida S, Mano Y, Yamamoto K, Ishii K, Kita T, Kikuchi Y, et al. Gains of 1q21-q22 and 13q12-q14 are potential indicators for resistance to cisplatin-based chemotherapy in ovarian cancer patients. Clin Cancer Res. 1999;5:2526–2531. [PubMed] [Google Scholar]
- Kuo AJ, Song J, Cheung P, Ishibe-Murakami S, Yamazoe S, Chen JK, Patel DJ, Gozani O. The BAH domain of ORC1 links H4K20me2 to DNA replication licensing and Meier-Gorlin syndrome. Nature. 2012;484:115–119. doi: 10.1038/nature10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PW, Muller MM, Koletsky MS, Cordero F, Lin S, Banaszynski LA, Garcia BA, Muir TW, Becher OJ, Allis CD. Inhibition of PRC2 Activity by a Gain-of-Function H3 Mutation Found in Pediatric Glioblastoma. Science. 2013 doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallette FA, Mattiroli F, Cui G, Young LC, Hendzel MJ, Mer G, Sixma TK, Richard S. RNF8- and RNF168-dependent degradation of KDM4A/JMJD2A triggers 53BP1 recruitment to DNA damage sites. Embo J. 2012 doi: 10.1038/emboj.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallette FA, Richard S. JMJD2A promotes cellular transformation by blocking cellular senescence through transcriptional repression of the tumor suppressor CHD5. Cell Rep. 2012;2:1233–1243. doi: 10.1016/j.celrep.2012.09.033. [DOI] [PubMed] [Google Scholar]
- Manning AL, Longworth MS, Dyson NJ. Loss of pRB causes centromere dysfunction and chromosomal instability. Genes Dev. 2010;24:1364–1376. doi: 10.1101/gad.1917310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov AY, Vijg J. Genome instability, cancer and aging. Biochim Biophys Acta. 2009;1790:963–969. doi: 10.1016/j.bbagen.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011;12:R41. doi: 10.1186/gb-2011-12-4-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- Papamichos-Chronakis M, Peterson CL. Chromatin and the genome integrity network. Nat Rev Genet. 2013;14:62–75. doi: 10.1038/nrg3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke RT. Gene amplification, drug resistance, and cancer. Cancer Res. 1984;44:1735–1742. [PubMed] [Google Scholar]
- Snaith HA, Forsburg SL. Rereplication phenomenon in fission yeast requires MCM proteins and other S phase genes. Genetics. 1999;152:839–851. doi: 10.1093/genetics/152.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M, Kudo K, Goto T, Yamamoto K, Kita T, Kikuchi Y. Analyses by comparative genomic hybridization of genes relating with cisplatin-resistance in ovarian cancer. Hum Cell. 2001;14:267–271. [PubMed] [Google Scholar]
- Tardat M, Brustel J, Kirsh O, Lefevbre C, Callanan M, Sardet C, Julien E. The histone H4 Lys 20 methyltransferase PR-Set7 regulates replication origins in mammalian cells. Nat Cell Biol. 2010;12:1086–1093. doi: 10.1038/ncb2113. [DOI] [PubMed] [Google Scholar]
- Van Rechem C, Black JC, Abbas T, Allen A, Rinehart CA, Yuan GC, Dutta A, Whetstine JR. The SKP1-Cul1-F-box and leucine-rich repeat protein 4 (SCF-FbxL4) ubiquitin ligase regulates lysine demethylase 4A (KDM4A)/Jumonji domain-containing 2A (JMJD2A) protein. J Biol Chem. 2011;286:30462–30470. doi: 10.1074/jbc.M111.273508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev LT. Cell cycle synchronization at the G2/M phase border by reversible inhibition of CDK1. Cell Cycle. 2006;5:2555–2556. doi: 10.4161/cc.5.22.3463. [DOI] [PubMed] [Google Scholar]
- Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, Spooner E, Li E, Zhang G, Colaiacovo M, Shi Y. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125:467–481. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Wong N, Lam WC, Lai PB, Pang E, Lau WY, Johnson PJ. Hypomethylation of chromosome 1 heterochromatin DNA correlates with q-arm copy gain in human hepatocellular carcinoma. Am J Pathol. 2001;159:465–471. doi: 10.1016/S0002-9440(10)61718-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakut T, Schulten HJ, Demir A, Frank D, Danner B, Egeli U, Gebitekin C, Kahler E, Gunawan B, Urer N, et al. Assessment of molecular events in squamous and non-squamous cell lung carcinoma. Lung Cancer. 2006;54:293–301. doi: 10.1016/j.lungcan.2006.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.