Abstract
Background
The actual in vivo tibiofemoral and patellofemoral kinematics of the posterior cruciate ligament (PCL)-reconstructed knee joint are unknown.
Hypothesis
Current single-bundle PCL reconstruction is unable to correct the abnormal tibiofemoral and patellofemoral kinematics caused by rupture of the ligament.
Study Design
Controlled laboratory study/case series; Level of evidence, 4.
Methods
Seven patients with an isolated PCL injury in 1 knee and the contralateral side intact were included in the study. Magnetic resonance and dual fluoroscopic imaging techniques were used to compare the tibiofemoral and patellofemoral kinematics between the intact contralateral (control group), PCL-deficient, and PCL-reconstructed knee during physiologic loading with a single-legged lunge. Data were collected preoperatively and 2 years after single-bundle reconstruction.
Results
The PCL reconstruction reduced the abnormal posterior tibial translation in PCL-deficient knees to levels not significantly different from those of the intact knee. Posterior cruciate ligament deficiency resulted in an increased lateral tibial translation between 75° and 120° of flexion, and reconstruction was unable to restore these values to normal. No differences were detected among the groups in varus-valgus and internal-external rotation. The PCL reconstruction reduced the increased patellar flexion of PCL-deficient knees between 90° and 120° of knee flexion and the lateral shift at 120°. The abnormal patellar rotation and tilt seen in PCL deficiency at flexion angles of 75° and greater persisted after reconstruction.
Conclusion
Single-bundle PCL reconstruction was successful in restoring normal anteroposterior translation of the tibia, as well as the patellar flexion and shift. However, single-bundle PCL reconstruction was unable to achieve the same success in mediolateral translation of the tibia or in the patellar rotation and tilt.
Clinical Relevance
The persistent abnormal mediolateral translation of the tibia, as well as decreased patellar rotation and tilt, provide a possible explanation for the development of cartilage degeneration after reconstruction of an isolated PCL injury.
Keywords: isolated posterior cruciate ligament (PCL) injury, posterior cruciate ligament (PCL) reconstruction, tibiofemoral kinematics, patellofemoral kinematics, patellar tracking, dual fluoroscopic imaging
The optimal treatment of isolated posterior cruciate ligament (PCL) injuries remains controversial. Whereas some authors have advocated nonoperative treatment of a torn PCL because many patients with PCL deficiency have good functional results,8,33 others have supported surgical reconstruction based on long-term follow-up studies revealing an association between posterior displacement of the tibia and joint degeneration.2,7,23,37 Unfortunately, osteoarthritis has been reported in 20% to 60% of patients after PCL reconstruction as well.1,7,20,36 The unpredictable outcomes of operative treatment reflect a gap in our present knowledge of the in vivo kinematics of the PCL-reconstructed knee.
The tibiofemoral kinematics of the PCL-deficient knee have been well documented. Both in vitro and in vivo studies have described an increased posterior tibial translation,†; as well as an increased external tibial rotation17,24,25 and lateral translation26 of the tibia after rupture of the PCL. These altered tibiofemoral kinematics in PCL deficiency result in a shift of the normal tibiofemoral contact location with a subsequent increase in cartilage deformation in the medial compartment, providing a possible explanation for the medial joint compartment cartilage degeneration.39 Less information is available on the patellofemoral joint after PCL injury. In vitro studies by Skyhar et al38 and Gill et al15 found that sectioning of the PCL in cadaveric studies resulted in elevated patellofemoral contact pressures. In our recent in vivo analysis of the patellofemoral joint of 10 PCL-deficient knees during a single-legged lunge, we found that the altered tibiofemoral kinematics in PCL deficiency were linked to changes in the patellofemoral joint function at flexion angles greater than 60°. The PCL deficiency caused an increased patellar flexion angle, and a decreased lateral shift, tilt, and valgus rotation from 75° to 120° of flexion, consequently causing a distal and medial shift of cartilage contact on the patellar surface.41
As persistent abnormal knee kinematics are a putative factor in the degeneration of cartilage after cruciate ligament injury, various surgical techniques have been recommended based on cadaveric experiments that would more closely replicate the native knee kinematics. Some authors have advocated a double-bundle reconstruction of the PCL, because this type of reconstruction might better approximate the anatomy and function of the PCL.19,35,43,45 Alternatively, it has recently been proposed that a sagittal osteotomy that increases the tibial slope and reduces the abnormal tibial sag in cadaveric knees may be beneficial for patients with PCL deficiency.14 However, before advocating promising surgical techniques that are potentially more efficient but unquestionably more complicated, obtaining a clear insight into the efficiency—or lack thereof—of the contemporary single-bundle PCL reconstruction technique to reproduce normal in vivo knee kinematics might be helpful in the present-day debate regarding optimal PCL injury treatment.
The purpose of the current study was to analyze the in vivo tibiofemoral and patellofemoral kinematics of PCL-deficient knees in patients who subsequently opted for surgical reconstruction of the ligament using an Achilles tendon allograft. The hypothesis of the present study was that single-bundle reconstruction of an isolated PCL injury, although efficient in the restoration of normal posterior stability of the knee, is unable to correct the abnormal tibiofemoral and patellofemoral kinematics caused by rupture of the ligament. The tibiofemoral and patellofemoral kinematics were analyzed at 2-year follow-up of a clinically successful PCL reconstruction under the identical protocol of previous studies,26,41 that is, during a quasistatic single-legged lunge using a combined magnetic resonance (MR) and dual fluoroscopic imaging technique.
MATERIAL AND METHODS
Patient Recruitment
Seven consecutive patients with an isolated PCL rupture documented by clinical examination (positive posterior drawer test, normal varus/valgus stress test, negative reversed pivot and dial test, measured by the senior orthopaedic surgeon [T.J.G.]), arthroscopic examination, and MR imaging, and who were scheduled for surgical reconstruction of the injured PCL were included in this study. The patients included 5 men and 2 women with an average age of 35 ± 15 years (range, 20–51 years), an average weight of 86 ± 9 kg (range, 77–97 kg), an average height of 175 ± 8 cm (range, 160–180 cm), with 4 right-injured and 3 left-injured knees, who were active on a minimal to moderate athletic level before injury, and with a primary complaint of persistent instability that limited their athletic participation or work. All subjects had healthy contralateral knees. Injury to other ligaments, menisci, capsule, noticeable cartilage lesions, and injury to the underlying bone were reasons for exclusion from the study. The purpose of the study was explained in detail to all of the patients at the time of recruitment. Each patient signed a consent form that had been approved by our institutional review board.
Preoperative Examination
Magnetic resonance and dual fluoroscopic imaging techniques have been described and validated in detail in previous publications.10,42 Before reconstruction of the ruptured PCL, both the intact and PCL-deficient knee were imaged by an MR scanner with a 3-Tesla magnet (Magnetom Trio, Siemens, Erlangen, Germany) and a fat-suppressed, 3-dimensional, spoiled gradient-recalled echo sequence to create 3-dimensional meshed models of the knees using a protocol established in our laboratory.10 Each anatomical knee model included the bony geometry of the femur, tibia, and fibula, as well as patella. Next, the intact contralateral and the PCL-deficient knees were imaged simultaneously with 2 orthogonally placed fluoroscopes (BV Pulsera, Philips, Eindhoven, The Netherlands) set to generate 8-ms width x-ray pulses (performed at 50–60 kV and 0.7–0.8 mA) with a dose rate of 13 µGy per scanning, as the patient performed a single-legged quasistatic lunge at 0°, 30°, 60°, 75°, 90°, 105°, and 120° of flexion while their upper body remained upright.
Next, the fluoroscopic images were imported into solid modeling software and placed in the orthogonal planes based on the position of the fluoroscopes during the imaging of the patient. In the next step, the MR image-based knee models were imported into the same software, viewed from the 2 orthogonal directions corresponding to the orthogonal fluoroscopic setup used to acquire the images, and independently manipulated in 6 degrees of freedom inside the software until the projections of the models matched the outlines of the fluoroscopic images. When the projections matched the outlines of the images taken during in vivo knee flexion, the models reproduced the in vivo position of the intact and PCL-deficient knee. The series of knee models reproducing the single-legged lunge performed by the intact contralateral knee were used as control group in the comparison with the PCL-deficient and PCLreconstructed knee kinematics.22
PCL Reconstruction Technique
After the initial analysis of the PCL-deficient knee function within a mean 4.4 ± 3 months of injury, the included patients underwent surgical reconstruction of the ruptured PCL. The same sports medicine fellowship-trained orthopaedic surgeon (T.J.G.) performed all surgeries. Reconstruction was performed using a 10-mm Achilles tendon allograft. An inferolateral portal was established and a diagnostic arthroscopic procedure was performed, documenting the status of each patient’s menisci and articular cartilage. The PCL remnants were then debrided and an accessory posteromedial portal was established. A 70° arthroscope was used to debride the posterior aspect of the proximal tibia under direct visualization. A PCL tibial guide (Arthrex, Naples, Florida) was inserted at a 70° angle and a 10-mm tibial tunnel drilled under direct visualization. The PCL femoral guide (Arthrex) was then placed approximately 6 mm posterior to the articular surface in the 11:30-o’clock position for left knees (12:30 position for right knees), a short longitudinal incision was made over the anteromedial aspect of the distal femur, and a 10-mm femoral tunnel was drilled. The Achilles tendon allograft was passed in antegrade fashion. A 25-mm titanium interference screw (Guardsman, Linvatec-Conmed, Largo, Florida) was used to secure the femoral tunnel. Screw diameter was determined based on graft-tunnel fit. Cycling of the knee revealed less than 1 mm of graft motion in all cases. The knee was then placed in 90° of flexion with an anterior drawer force. A 9 × 30 mm BioScrew (Linvatec) was placed posteriorly in the tibial tunnel. Fixation was augmented with a 9 × 30 mm titanium interference screw (Guardsman, Linvatec-Conmed) placed more anteriorly (Figure 1). Examination of the knee revealed negative Lachman and negative posterior drawer in all cases. The posterior laxity of the reconstructed knee as measured with the KT-1000 arthrometer (MEDmetric, San Diego, California) was similar to that of the intact contralateral knee.
Figure 1.
Postoperative fluoroscopic images of a right posterior cruciate ligament-reconstructed knee, taken by fluoroscope 1 (F1) and fluoroscope 2 (F2) during the lunge activity.
Postoperative Examination
A minimum of 24 months after PCL reconstruction (range, 24–28 months), the 7 patients were tested under physiologic loading by repeating the same single-legged lunge activity with the PCL-reconstructed knee while dual fluoroscopic images were recorded and processed in a manner similar to the initial preoperative examination,26,41 generating a series of knee models reproducing the in vivo position of the PCL-reconstructed knee at the designated flexion angles.
Tibiofemoral Kinematics
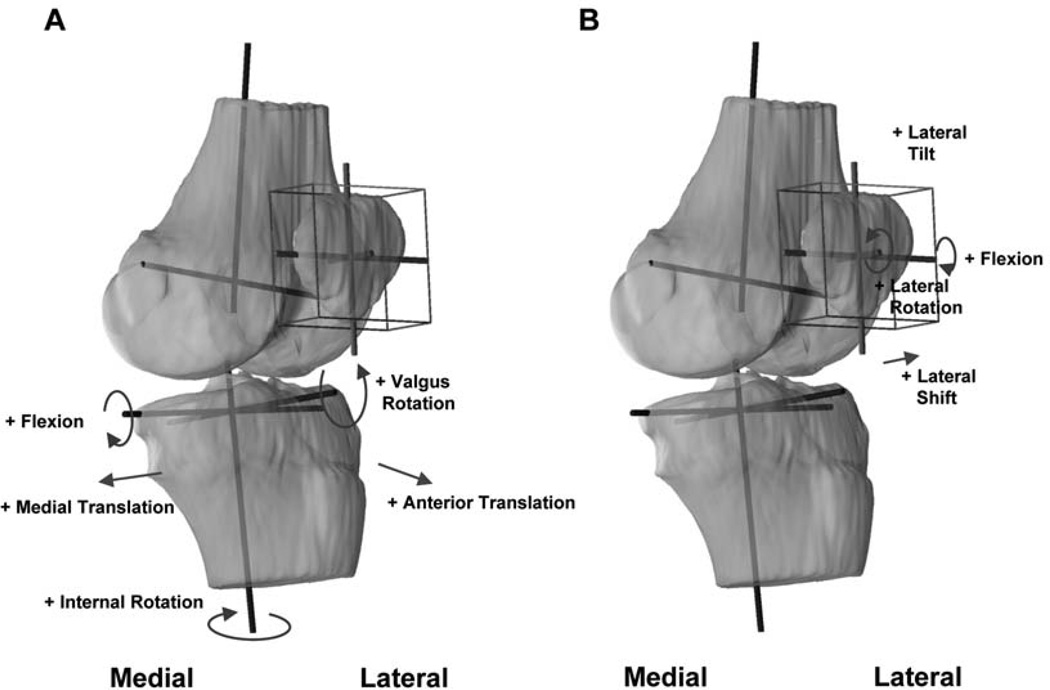
Coordinate systems were established to investigate the tibiofemoral kinematics of the series of knee models (Figure 2A).11,31,44 For the tibial coordinate system, a long axis of the tibial shaft was drawn through the middle of the tibial spines and parallel to the posterior edge of the tibial shaft in the sagittal view. The mediolateral axis was drawn parallel to the posterior edge of the tibial plateau. An anteroposterior axis was drawn perpendicular to the long axis and mediolateral axis. These axes formed a Cartesian coordinate system with the origin at a point between the tibial spines. For the femoral coordinate system, the flexion axis was constructed by fitting circles to the medial and lateral condyles and then connecting the centers of these circles with a line. The long axis was drawn parallel to the posterior wall of the femoral shaft in the sagittal plane. The middle point of the flexion axis was used as the origin of the femoral coordinate system. The anteroposterior axis was obtained as the cross product of the flexion and long axis.
Figure 2.
Coordinate systems used to quantify the tibiofemoral (A) and patellofemoral (B) kinematics.
With the tibial and femoral coordinate systems established, the in vivo knee motion could be described as the relative motion of the tibial coordinate system with respect to the femoral coordinate system. The flexion angle of the knee was defined as the angle between the long axes of the femur and tibia. The position of the tibial origin with respect to the femoral origin in the tibial coordinate system was used to describe the translation of the tibia during knee flexion. Rotation of the tibia around the long axis of the tibia was defined as internal-external rotation, and rotation around the anterior-posterior axis was defined as varus-valgus rotation.
Patellofemoral Kinematics
A joint coordinate system18 was established for each patella to describe the patellofemoral kinematics (Figure 2B). To reduce the variability in creating patellar coordinate systems, a cuboid was used to enclose the patella so that it touched the proximodistal, anteroposterior, and mediolateral borders of the patella.27,29,40,41 The center of the cuboid was defined as origin of the patella. The long axis of the patella was defined as the line along the superior-inferior direction.
Patellar flexion was defined as the rotation of the patella about the flexion axis of the femur.4 Patellar shift was defined as the medial or lateral movement of the center of the patella along the flexion axis of the femur. A positive shift corresponded to the lateral movement of the patellar center with respect to the knee center along the femoral flexion axis. Patellar rotation was the rotation of the patella about the anteroposterior axis of the femur, where valgus rotation followed the direction of valgus rotation in tibiofemoral motion, that is, an outward angulation of the distal segment of the patella. Patellar tilt was defined as the rotation of the patella about the long axis of the femur, where lateral tilt followed the direction of external femoral rotation. In this fashion, the patellofemoral kinematics were quantified for each subject as a function of flexion of the knee.
Statistical Methods
A 1-way repeated measures analysis of variance was used to determine differences in tibiofemoral kinematics (anteroposterior tibial translation, mediolateral tibial translation, internal-external tibial translation, and varus-valgus tibial translation) and patellofemoral kinematics (patellar flexion, shift, rotation, and tilt) of the intact contralateral, PCL-deficient, and PCL-reconstructed knees at every flexion angle. The Student-Newman-Keuls post hoc test was performed to isolate statistically significant differences between groups. The level of significance was set at P < .05.
RESULTS
Clinical Results
At 24 months after the PCL reconstruction, no differences between the intact contralateral and PCL-reconstructed knees were found in range of motion, rotational stability, or posterior laxity as measured with the KT-1000 arthrometer. Average postoperative International Knee Documentation Committee score was 83.5 ± 7.6, versus an average preoperative International Knee Documentation Committee score of 43.1 ± 15.5 (P < .05, determined with a paired Student t test).
Tibiofemoral Kinematics
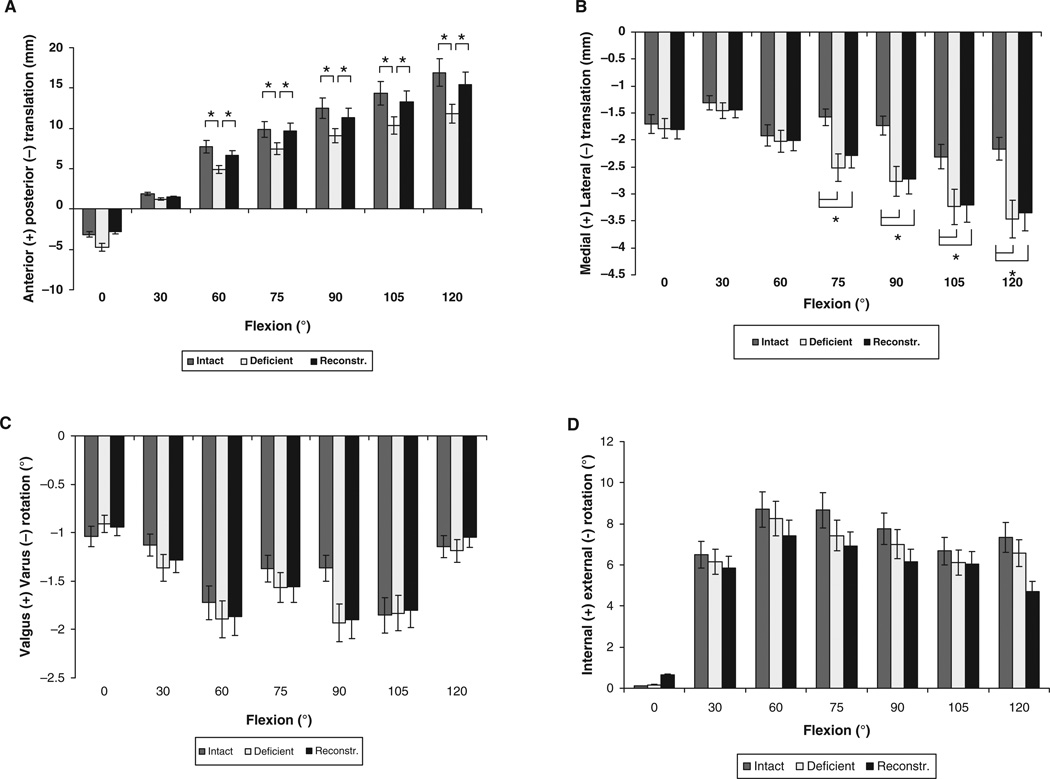
No significant differences in anteroposterior translation of the tibia between the 3 groups were found at 0° and 30° of knee flexion (Figure 3A). Between 60° and 120° of knee flexion, the PCL-deficient knees translated posteriorly on average 3.5 ± 1.1 mm, as compared with the intact contralateral knees (P < .05). Between 60° and 120° of knee flexion, the tibia of the PCL-reconstructed knee translated anteriorly from 6.6 ± 3.1 mm to 15.4 ± 4.2 mm, which was on average 2.5 mm more anterior than the PCL-deficient knee (P < .05). Between 60° and 120° of knee flexion, no significant differences in anteroposterior translation between the intact and PCL-reconstructed knees were found (P > .95) (Figure 3A).
Figure 3.
Tibiofemoral kinematics: Anteroposterior translation (A), mediolateral translation (B), varus-valgus rotation (C), and internal-external rotation (D) of the tibia of the intact, PCL-deficient, and PCL-reconstructed knees during weightbearing flexion. *P < .05 as determined with 1-way repeated measures analysis of variance.
No significant differences in mediolateral translation of the tibia between the 3 groups were found at 0°, 30°, and 60° of knee flexion (Figure 3B). On average, PCL deficiency resulted in a 1.1 mm increase in lateral tibial translation between 75° and 120° of flexion (P < .05), and PCL reconstruction was unable to restore the values to normal. The tibia of the PCL-reconstructed knee translated laterally from 2.3 ± 1.6 mm to 3.3 ± 1.5 mm from 75° to 120° of knee flexion, which was, on average, 1.0 mm more than the intact contralateral knee (P < .05) (Figure 3B).
No statistically significant differences were detected among the intact, PCL-deficient, and PCL-reconstructed groups in varus-valgus (Figure 3C) and internal-external rotation (Figure 3D).
Patellofemoral Kinematics
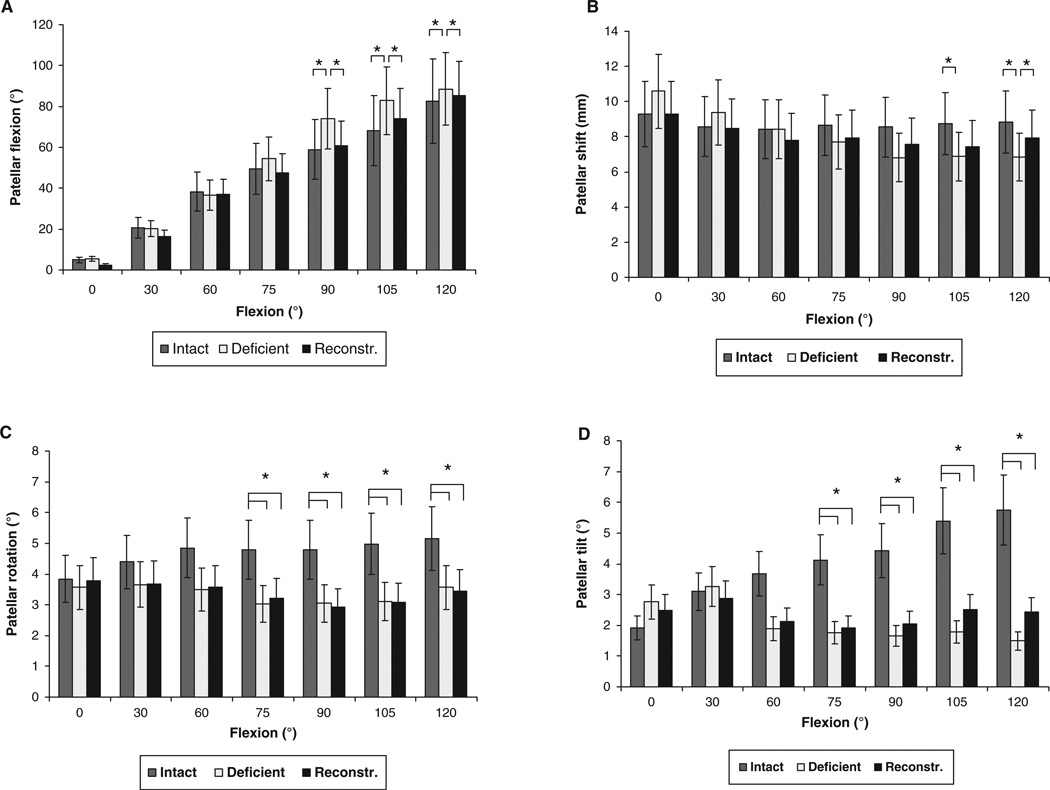
No statistically significant differences were detected among the intact and PCL-reconstructed knees when comparing patellar flexion (Figure 4A). The patella of the PCL-reconstructed knee flexed from 2.4° ± 7.4° at 0° of knee flexion to 85.2° ± 12.7° at 120° of knee flexion. The PCL reconstruction reduced the increased patellar flexion between 90° and 120° of knee flexion, which was observed in PCL deficiency, to levels not significantly different from those of the intact knee (Figure 4A).
Figure 4.
Patellofemoral kinematics: Patellar flexion (A), shift (B), rotation (C), and tilt (D) of the intact, PCL-deficient, and PCL-reconstructed knees during weightbearing flexion. *P < .05 as determined with 1-way repeated measures analysis of variance.
In the PCL-deficient knee of the present study sample, the lateral patellar shift significantly decreased with knee flexion at 105° and 120° of knee flexion (P < .05) (Figure 4B). At 120° of flexion, the value of the patellar shift in the PCL-reconstructed knee increased to a level significantly higher than that in the PCL-deficient knee, and not significantly different from that of the intact knee (intact, 8.8 ± 3.7 mm; PCL-deficient, 6.8 ± 2.8 mm; PCL-reconstructed, 7.9 ± 3.2 mm; P < .05) (Figure 4B).
A significant decrease in patellar rotation (Figure 4C) (P <.05) and tilt (Figure 4D) (P <.05) was observed in the PCL-reconstructed knee at flexion angles of 75° and greater, not significantly different from the patellar rotation and tilt values observed in PCL deficiency. The PCL-reconstructed knee demonstrated on average 1.8° less patellar rotation and 2.7° less patellar tilt, compared with the intact contralateral knee.
DISCUSSION
In the present study, the tibiofemoral and patellofemoral kinematics of 7 patients were investigated, before and 2 years after clinically successful reconstruction of an isolated rupture of the PCL. A combined MR and dual fluoroscopic imaging technique was used to compare the kinematics of the PCL-reconstructed knee with the PCL-deficient knee and intact contralateral knee (measured during the preoperative assessment) during a quasistatic single-legged lunge.
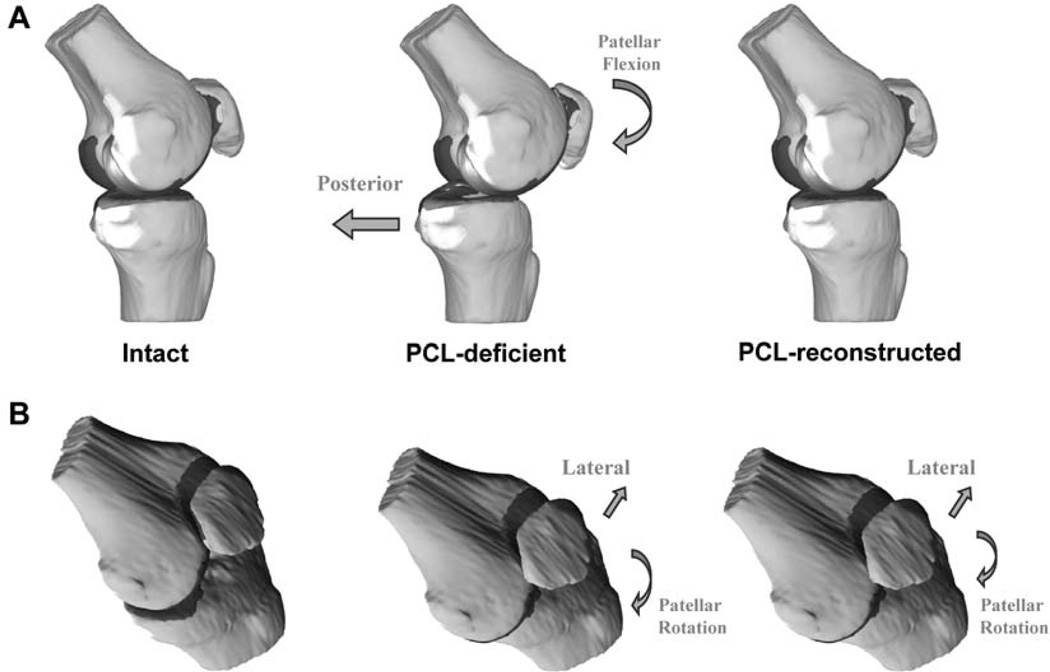
The PCL reconstruction reduced the abnormal posterior tibial translation between 60° and 120° of flexion, caused by PCL deficiency, to levels not significantly different from those of the intact knee. On the basis of these findings, it was not surprising to observe that PCL reconstruction restored the normal patellar flexion values as well, because the anteroposterior translation of the tibia and patellar flexion are intrinsically coupled in the sagittal knee plane (Figure 5A).27 However, the increased lateral tibial translation seen in PCL-deficient knees persisted after reconstruction of the PCL in the present study. The residual increased lateral translation of the tibia after PCL reconstruction would move the tibial attachment of the patellar tendon more laterally relative to its patellar attachment, effectively increasing the coronal plane angle of the patellar tendon,9 potentially explaining the abnormal patellar rotation and tilt in the PCL-reconstructed knee (Figure 5B).
Figure 5.
Overview of the kinematic changes observed in posterior cruciate ligament-deficient and posterior cruciate ligament-reconstructed knees in the sagittal plane (A) of the knee, and the coronal plane (B) of the knee. The block arrows illustrate tibial translation; curved arrows illustrate patellar motion.
The metabolic and structural changes of osteoarthritis are currently viewed as the adaptive response of synovial joints to a variety of genetic, constitutional, or biomechanical insults.3 Even so, the widely accepted assumption persists that abnormal kinematics and consequent abnormal cartilage deformation within the joint initiate knee osteoarthritis.12 When calculating the magnitude of cartilage deformation39 of the PCL-reconstructed knees in the present study (see Appendix, available in the online version of this article at http://ajs.sagepub.com/supplemental/), we found that—even though the normal anteroposterior tibial translation was restored—the persistent increase in abnormal lateral translation of the tibia resulted in a persistent increase in the magnitude of in vivo deformation of the tibiofemoral cartilage in the medial compartment. The increase in cartilage deformation provides a potential insight about the development of joint cartilage degeneration in patients after reconstruction of the PCL.
It is tempting to speculate on possible surgical solutions that might restore the knee joint in all degrees of freedom. However, it would be precarious to construct general clinical recommendations for the optimal treatment of PCL injury based on the present results, as the data were obtained during only 1 functional in vivo activity (ie, the single-legged lunge), with only 1 surgical technique investigated (ie, single-bundle reconstruction), and mostly because a nonoperative control group was not included. Notwithstanding these limitations, we believe the findings could be of use in the controversy regarding the optimal treatment of isolated PCL deficiency. The present study has obtained an insight in the efficiency—and lack thereof—of the contemporary single-bundle PCL reconstruction technique to reproduce normal in vivo knee kinematics. Surgical reconstruction of an isolated PCL injury could be considered a success, based on our findings, if the goal of the procedure were the restoration of the anteroposterior translation of the tibia. No statistically significant differences between intact and PCL-reconstructed knee in the sagittal plane were found in either the tibiofemoral or patellofemoral joint during weightbearing knee flexion. Subjectively, the patients all stated that they were very satisfied with the procedure, and would have the surgery again for the same problem.
However, PCL reconstruction was unable to achieve the same success in the mediolateral translation of the tibia, and subsequently the patellar rotation and tilt. These findings reinforce our hypothesis that re-creating the mediolateral stability when performing a PCL reconstruction may possibly be of comparable importance to surgically improving anteroposterior translation.39 Future studies should explore the optimal method for restoring the function of the PCL in both degrees of freedom, whether it is through, for example, alternate graft orientation, the addition of an extra bundle,43,45 or a tibial osteotomy.14
As previously mentioned, our study has several limitations. Although our data were collected under truly physiologic loading conditions, as opposed to previous in vitro studies in which such loading was not possible, only 1 activity, namely a single-legged lunge, was studied. Other in vivo activities such as walking, running, and specifically descending stairs,21 should be considered in future studies. Furthermore, the present study did not measure the ground-reaction force. Future studies should incorporate a load cell into the system, as well as a whole-body motion analysis system, to ensure that the performed functional activities are fully uniform among the tested limbs. The results of kinematic evaluation of the intact contralateral knee measured shortly after injury of the PCL were used as a control, because our earlier research did not find significant differences in knee kinematics between the intact knee of patients with acute PCL deficiency and the kinematics of patients with bilateral healthy knees.22 Although challenging to execute in the present clinical setting, it would be interesting in future studies to include a prospectively randomized nonoperative control group to isolate the role of the surgical reconstruction from the natural changes in kinematics that might occur independently from the intervention.
In summary, single-bundle reconstruction of an isolated PCL injury restored the normal anteroposterior translation of the tibia, as well as the patellar flexion and shift. However, the abnormal increased lateral tibial translation with subsequent increased cartilage deformation in the medial compartment, as well as decreased patellar tilt and valgus rotation that were previously described in PCL deficiency, persisted after reconstruction of the PCL. These findings reinforce the need for restoring the normal function of the injured ligament in both anteroposterior and mediolateral directions, if the goal of the surgical procedure is the prevention of osteoarthritis in the long term.
Supplementary Material
ACKNOWLEDGMENT
The authors gratefully acknowledge the financial support of the National Institutes of Health (grant R01 AR 052408) and the National Football League Charities Foundation.
We thank Jeffrey T. Bingham, Louis E. DeFrate, and Bijoy Thomas for their technical assistance with this study.
Footnotes
REFERENCES
- 1.Becker R, Ropke M, Nebelung W. Clinical outcome of arthroscopic posterior cruciate ligament-plasty. Unfallchirurg. 1999;102(5):354–358. doi: 10.1007/s001130050417. [DOI] [PubMed] [Google Scholar]
- 2.Boynton MD, Tietjens BR. Long-term followup of the untreated isolated posterior cruciate ligament-deficient knee. Am J Sports Med. 1996;24(3):306–310. doi: 10.1177/036354659602400310. [DOI] [PubMed] [Google Scholar]
- 3.Brandt K, Lohmander L, Doherty M. The concept of osteoarthritis as failure of the diarthrodial joint. In: Brandt K, Doherty M, Lohmander L, editors. Osteoarthritis. Oxford: Oxford University Press; 1998. pp. 70–74. [Google Scholar]
- 4.Bull AM, Katchburian MV, Shih YF, Amis AA. Standardisation of the description of patellofemoral motion and comparison between different techniques. Knee Surg Sports Traumatol Arthrosc. 2002;10(3):184–193. doi: 10.1007/s00167-001-0276-5. [DOI] [PubMed] [Google Scholar]
- 5.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee: a biomechanical study. J Bone Joint Surg Am. 1980;62(2):259–270. [PubMed] [Google Scholar]
- 6.Carlin GJ, Livesay GA, Harner CD, Ishibashi Y, Kim HS, Woo SL. In-situ forces in the human posterior cruciate ligament in response to posterior tibial loading. Ann Biomed Eng. 1996;24(2):193–197. doi: 10.1007/BF02667348. [DOI] [PubMed] [Google Scholar]
- 7.Cross MJ, Powell JF. Long-term followup of posterior cruciate ligament rupture: a study of 116 cases. Am J Sports Med. 1984;12(4):292–297. doi: 10.1177/036354658401200409. [DOI] [PubMed] [Google Scholar]
- 8.Dandy DJ, Pusey RJ. The long-term results of unrepaired tears of the posterior cruciate ligament. J Bone Joint Surg Br. 1982;64(1):92–94. doi: 10.1302/0301-620X.64B1.7068728. [DOI] [PubMed] [Google Scholar]
- 9.Defrate LE, Nha KW, Papannagari R, Moses JM, Gill TJ, Li G. The biomechanical function of the patellar tendon during in-vivo weightbearing flexion. J Biomech. 2007;40(8):1716–1722. doi: 10.1016/j.jbiomech.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Defrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sports Med. 2006;34(8):1240–1246. doi: 10.1177/0363546506287299. [DOI] [PubMed] [Google Scholar]
- 11.Eckhoff DG, Dwyer TF, Bach JM, Spitzer VM, Reinig KD. Three-dimensional morphology of the distal part of the femur viewed in virtual reality. J Bone Joint Surg Am. 2001;83(Suppl 2)(Pt 1):43–50. doi: 10.2106/00004623-200100021-00010. [DOI] [PubMed] [Google Scholar]
- 12.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 13.Fox RJ, Harner CD, Sakane M, Carlin GJ, Woo SL. Determination of the in situ forces in the human posterior cruciate ligament using robotic technology: a cadaveric study. Am J Sports Med. 1998;26(3):395–401. doi: 10.1177/03635465980260030901. [DOI] [PubMed] [Google Scholar]
- 14.Giffin JR, Stabile KJ, Zantop T, Vogrin TM, Woo SL, Harner CD. Importance of tibial slope for stability of the posterior cruciate ligament deficient knee. Am J Sports Med. 2007;35(9):1443–1449. doi: 10.1177/0363546507304665. [DOI] [PubMed] [Google Scholar]
- 15.Gill TJ, DeFrate LE, Wang C, et al. The biomechanical effect of posterior cruciate ligament reconstruction on knee joint function: kinematic response to simulated muscle loads. Am J Sports Med. 2003;31(4):530–536. doi: 10.1177/03635465030310040901. [DOI] [PubMed] [Google Scholar]
- 16.Girgis FG, Marshall JL, Monajem A. The cruciate ligaments of the knee joint: anatomical, functional and experimental analysis. Clin Orthop. 1975;106:216–231. doi: 10.1097/00003086-197501000-00033. [DOI] [PubMed] [Google Scholar]
- 17.Gollehon DL, Torzilli PA, Warren RF. The role of the posterolateral and cruciate ligaments in the stability of the human knee: a biomechanical study. J Bone Joint Surg Am. 1987;69(2):233–242. [PubMed] [Google Scholar]
- 18.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 19.Harner CD, Janaushek MA, Kanamori A, Yagi M, Vogrin TM, Woo SL. Biomechanical analysis of a double-bundle posterior cruciate ligament reconstruction. Am J Sports Med. 2000;28(2):144–151. doi: 10.1177/03635465000280020201. [DOI] [PubMed] [Google Scholar]
- 20.Hughston JC, Bowden JA, Andrews JR, Norwood LA. Acute tears of the posterior cruciate ligament: results of operative treatment. J Bone Joint Surg Am. 1980;62(3):438–450. [PubMed] [Google Scholar]
- 21.Iwata S, Suda Y, Nagura T, et al. Clinical disability in posterior cruciate ligament deficient patients does not relate to knee laxity, but relates to dynamic knee function during stair descending. Knee Surg Sports Traumatol Arthrosc. 2007;15(4):335–342. doi: 10.1007/s00167-006-0198-3. [DOI] [PubMed] [Google Scholar]
- 22.Kozanek M, Van de Velde SK, Gill TJ, Li G. The contralateral knee joint in cruciate ligament deficiency. Am J Sports Med. 2008;36(11):2151–2157. doi: 10.1177/0363546508319051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.L’Insalata JC, Harner CD. Treatment of acute and chronic posterior cruciate ligament deficiency: new approaches. Am J Knee Surg. 1996;9(4):185–193. [PubMed] [Google Scholar]
- 24.Li G, Gill TJ, DeFrate LE, Zayontz S, Glatt V, Zarins B. Biomechanical consequences of PCL deficiency in the knee under simulated muscle loads: an in vitro experimental study. J Orthop Res. 2002;20(4):887–892. doi: 10.1016/S0736-0266(01)00184-X. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Most E, DeFrate LE, Suggs JF, Gill TJ, Rubash HE. Effect of the posterior cruciate ligament on posterior stability of the knee in high flexion. J Biomech. 2004;37(5):779–783. doi: 10.1016/j.jbiomech.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Papannagari R, Li M, et al. Effect of posterior cruciate ligament deficiency on in vivo translation and rotation of the knee during weightbearing flexion. Am J Sports Med. 2008;36(3):474–479. doi: 10.1177/0363546507310075. [DOI] [PubMed] [Google Scholar]
- 27.Li G, Papannagari R, Nha KW, Defrate LE, Gill TJ, Rubash HE. The coupled motion of the femur and patella during in vivo weightbearing knee flexion. J Biomech Eng. 2007;129(6):937–943. doi: 10.1115/1.2803267. [DOI] [PubMed] [Google Scholar]
- 28.Markolf KL, Slauterbeck JR, Armstrong KL, Shapiro MS, Finerman GA. A biomechanical study of replacement of the posterior cruciate ligament with a graft. Part 1: isometry, pre-tension of the graft, and anterior-posterior laxity. J Bone Joint Surg Am. 1997;79(3):375–380. doi: 10.2106/00004623-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Nha KW, Papannagari R, Gill TJ, et al. In vivo patellar tracking: clinical motions and patellofemoral indices. J Orthop Res. 2008;26(8):1067–1074. doi: 10.1002/jor.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noyes FR, Stowers SF, Grood ES, Cummings J, VanGinkel LA. Posterior subluxations of the medial and lateral tibiofemoral compartments: an in vitro ligament sectioning study in cadaveric knees. Am J Sports Med. 1993;21(3):407–414. doi: 10.1177/036354659302100314. [DOI] [PubMed] [Google Scholar]
- 31.Olcott CW, Scott RD. The Ranawat Award. Femoral component rotation during total knee arthroplasty. Clin Orthop Relat Res. 1999;367:39–42. [PubMed] [Google Scholar]
- 32.Papannagari R, DeFrate LE, Nha KW, et al. Function of posterior cruciate ligament bundles during in vivo knee flexion. Am J Sports Med. 2007;35(9):1507–1512. doi: 10.1177/0363546507300061. [DOI] [PubMed] [Google Scholar]
- 33.Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137–146. doi: 10.1007/s11420-007-9058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Race A, Amis AA. Loading of the two bundles of the posterior cruciate ligament: an analysis of bundle function in A–P drawer. J Biomech. 1996;29(7):873–879. doi: 10.1016/0021-9290(95)00161-1. [DOI] [PubMed] [Google Scholar]
- 35.Race A, Amis AA. The mechanical properties of the two bundles of the human posterior cruciate ligament. J Biomech. 1994;27(1):13–24. doi: 10.1016/0021-9290(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 36.Richter M, Kiefer H, Hehl G, Kinzl L. Primary repair for posterior cruciate ligament injuries: an eight-year followup of fifty-three patients. Am J Sports Med. 1996;24(3):298–305. doi: 10.1177/036354659602400309. [DOI] [PubMed] [Google Scholar]
- 37.Schulte KR, Chu ET, Fu FH. Arthroscopic posterior cruciate ligament reconstruction. Clin Sports Med. 1997;16(1):145–156. doi: 10.1016/s0278-5919(05)70011-9. [DOI] [PubMed] [Google Scholar]
- 38.Skyhar MJ, Warren RF, Ortiz GJ, Schwartz E, Otis JC. The effects of sectioning of the posterior cruciate ligament and the posterolateral complex on the articular contact pressures within the knee. J Bone Joint Surg Am. 1993;75(5):694–699. doi: 10.2106/00004623-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Van de Velde SK, Bingham JT, Gill TJ, Li G. Analysis of tibiofemoral cartilage deformation in the posterior cruciate ligament-deficient knee. J Bone Joint Surg Am. 2009;91(1):167–175. doi: 10.2106/JBJS.H.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van de Velde SK, Gill TJ, DeFrate LE, Papannagari R, Li G. The effect of anterior cruciate ligament deficiency and reconstruction on the patellofemoral joint. Am J Sports Med. 2008;36(6):1150–1159. doi: 10.1177/0363546508314404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van de Velde SK, Gill TJ, Li G. Dual fluoroscopic analysis of the posterior cruciate ligament-deficient patellofemoral joint during lunge. Med Sci Sports Exerc. 2009;41(6):1198–1205. doi: 10.1249/MSS.0b013e3181981eb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van de Velde SK, Gill TJ, Li G. Evaluation of kinematics of anterior cruciate ligament-deficient knees with use of advanced imaging techniques, three-dimensional modeling techniques, and robotics. J Bone Joint Surg Am. 2009;91(Suppl 1):108–114. doi: 10.2106/JBJS.H.01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whiddon DR, Zehms CT, Miller MD, Quinby JS, Montgomery SL, Sekiya JK. Double compared with single-bundle open inlay posterior cruciate ligament reconstruction in a cadaver model. J Bone Joint Surg Am. 2008;90(9):1820–1829. doi: 10.2106/JBJS.G.01366. [DOI] [PubMed] [Google Scholar]
- 44.Yoshino N, Takai S, Ohtsuki Y, Hirasawa Y. Computed tomography measurement of the surgical and clinical transepicondylar axis of the distal femur in osteoarthritic knees. J Arthroplasty. 2001;16(4):493–497. doi: 10.1054/arth.2001.23621. [DOI] [PubMed] [Google Scholar]
- 45.Zehms CT, Whiddon DR, Miller MD, et al. Comparison of a double bundle arthroscopic inlay and open inlay posterior cruciate ligament reconstruction using clinically relevant tools: a cadaveric study. Arthroscopy. 2008;24(4):472–480. doi: 10.1016/j.arthro.2007.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.