Abstract
Retinal ganglion cells are the projection neurons that link the retina to the brain. Peptide immunoreactive cells in the ganglion cell layer (GCL) of the mammalian retina have been noted but their identity has not been determined1-3. We now report that, in the rabbit, 25–35% of all retinal ganglion cells contain substance P-like (SP) immunoreactivity. They were identified by either retrograde transport of fluorescent tracers injected into the superior colliculus, or by retrograde degeneration after optic nerve section. SP immunoreactive cells are present in all parts of the retina and have medium to large cell bodies with dendrites that ramify extensively in the proximal inner plexiform layer. Their axons terminate in the dorsal lateral geniculate nucleus, superior colliculus and accessory optic nuclei, and these terminals disappear completely after contralateral optic nerve section and/or eye enucleation. In the dorsal lateral geniculate nucleus large, beaded, it immunoreactive axons and varicosities make up a narrow plexus just below the optic tract, where they define a new geniculate lamina. The varicosities make multiple synaptic contacts with dendrites of dorsal lateral geniculate nucleus projection neurons and presumptive interneurons in complex glomerular neuropil. This is direct evidence that some mammalian retinal ganglion cells contain substance P-like peptides and strongly suggests that, in the rabbit, substance P (or related tachykinins) may be a transmitter or modulator in a specific population or populations of retinal ganglion cells.
New Zealand and Dutch Belted rabbits (not operated upon or surviving 3–143 days after unilateral optic nerve section or eye enucleation) were used. Most received an intraocular injection of colchicine, which is known to increase peptide levels in neuronal cell bodies4. Some received a unilateral injection of Fast Blue5 or rhodamine-labelled microspheres6 into the superficial layers of the superior colliculus (SC) to label ganglion cells, all or most of which are known to project to the SC in this species7. Wholemounts of retina or sections of retina or brain were processed by standard immunohistochemical techniques8,9, using a monoclonal antibody against substance P (see ref. 10, and Fig. 2 legend for technical details and specificity controls). Although control experiments cannot completely exclude the possibility of cross-reactivity with other substances, including related tachykinins11, the terms ‘SP-immunoreactive’ or ‘SP-containmg’ are used to describe stained tissue.
Fig. 2.
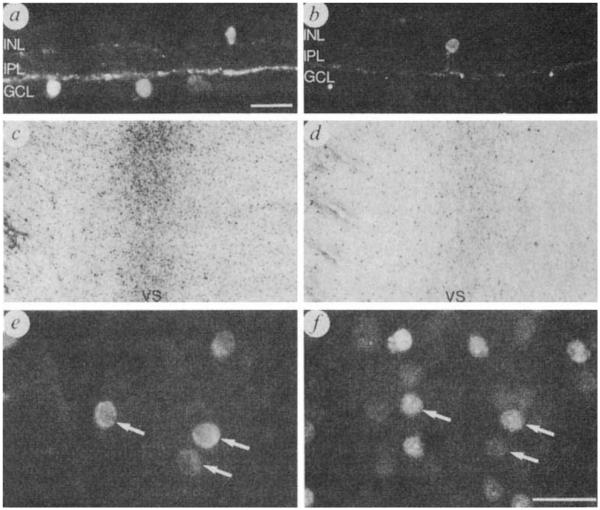
Localization of SP-immunoreactive somata and processes in the rabbit retina. Immunoreactive somata are present in the GCL as well as the inner nuclear layer (INL). Processes are distributed to laminae 1,3 and 5 of the inner plexiform layer (IPL) with the highest density in lamina 5 where they form a prominent continuous plexus, a, b, Transverse cryostat sections of the retina through the visual streak (retinal region with highest cell density) showing SP-immunoreactive cells in the GCL and INL. c, d, Wholemount preparations showing SP-immunoreactive somata in the GCL of the visual streak (VS) in a normal retina (c). and marked loss of immunoreactive cells 74 days after optic nerve section (d) e, f, Fast Blue retrogradely labelled SP immunoreactive ganglion cells near the visual streak in a wholemount. e, SP immunoreactive somata in the GCL. f, Same field as in e illustrating Fast Blue fluorescent somata. Arrows in e and f show the same cells containing both SP-immunoreactivity and Fast Blue fluorescence. Scale bars, 25 μm. Figs. 1c, d, ×20.
Methods. For intraocular colchicine treatment, rabbits (N = 15) were anaesthetized and 10–200 μg of colchicine (Sigma) in 50 μl of 0.9% NaCl was injected into the eye, just posterior to the ora serrata, 12–48 h before perfusion. Rabbits were subsequently anaesthetized and perfused transcardially with 0.9% NaCl in 0.1 M phosphate buffer (pH 7.4). For retinal sections, the NaCl perfusion was followed by 4% paraformaldehyde, the eyes were removed, the anterior segments cut away and the eyecups postfixed. Eyecups were subsequently transferred and washed in 20% sucrose, serial sections were cut perpendicular to the vitreal surface and processed for immunohistochemistry. For retinal wholemount preparations the retina was dissected from the pigment epithelium, fixed, washed in 20% sucrose and subsequently frozen, thawed and processed for immunohistochemistry. Wholemounts or sections were incubated for 12–96 h at 4 °C with a rat monoclonal antibody10. For immunofluorescence procedures, the tissue was subsequently washed, incubated in anti-rat immunoglobulin G (IgG) conjugated to either fluorescein isothiocyanate (FITC) or tetramethylrhodamine (TRITC) (Accurate), washed, mounted (GCL side up for retinal wholemounts) and covered with a cover slip. For peroxidase-antiperoxidase and avidin-biotin procedures, the tissue was washed, incubated in anti-rat IgG or biotinlyated IgG (Accurate or Vector), washed, incubated in peroxidase-antiperoxidase or avidin–biotin–peroxidase complex (Accurate or Vector), washed and then incubated in 3′,3′-diaminobenzidine solution with 0.05% H2O2 and washed. Tissue was mounted (GCL side up for wholemounts), incubated in 0.01% OsO4, washed, cleared and covered with a cover slip. Specificity was assessed by substituting the primary antibody with normal rat serum (1:10) or primary antibody which had been previously absorbed overnight at 4 °C with 10 μM synthetic substance P (Balchem). For optic nerve section, rabbits (N = 20) were deeply anaesthetized with a mixture of xylazine and ketamine and a 1-cm incision was made away from the lateral canthus, the eyelid gently retracted and the eye pulled slightly forward to expose the optic nerve. The optic nerve was sectioned, the skin sutured and the animal was allowed to recover and survive for 2–143 days. The retinas and brain were then fixed and processed as above for immunohistochemistry. For Fast Blue and rhodamine-labelled microsphere injections, rabbits (N = 8) were deeply anaesthetized, the skull and cortex overlying the SC was removed and an injection of 5 μl of 3% Fast Blue or 5 μl of rhodamine labelled microspheres (diameter 0.02–0.2 μm) in 0.9% NaCl was made into the superficial layers of the SC. After 24–96 h, the eye contralateral to the injection site was treated with colchicine and 24 h later the retina was fixed, checked for transport of Fast Blue or microspheres and then processed for immunohistochemistry. A Leitz Orthoplan microscope with a Ploem fluorescent attachment and ‘I2’, ‘N2’ and ‘A’ filter blocks was used to detect FITC, TRITC and Fast Blue fluorescence respectively. The brain was cut and examined to confirm the location and extent of the injection site.
SP immunoreactive cell bodies and processes were present in all regions of both colchicine-treated and untreated retinas but not in retinas incubated with antibodies absorbed with substance P. A minority of immunoreactive cells were in the inner nuclear layer (Fig. 2a, b). In keeping with previous reports1,2,12 some were unistratified amacrine cells which ramified in lamina 5 (ref. 13) whereas other slightly larger cells ramified in laminae 1, 3 and 5 of the inner plexiform layer and possibly also in the outer plexiform layer (multistratified amacrine and/or interplexiform cells).
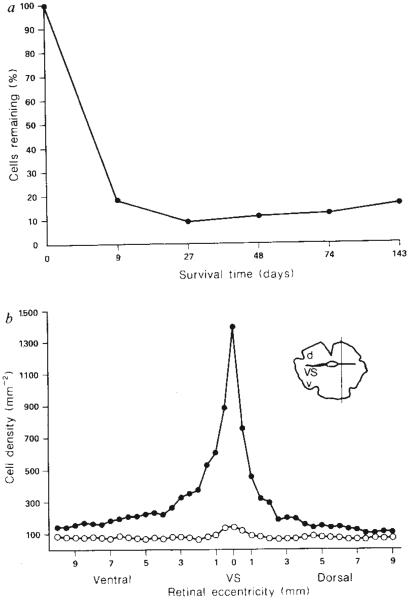
Most of the immunoreactive perikarya were, however, situated in the GCL (Fig. 2a, b), and typically gave rise to three or four denantes wmch branched extensively in the proximal inner plexiform layer (lamina 5), adjacent to the GCL. Primary and secondary dendrites could be followed for up to 100 μm from their parent cell bodies before they were lost among other stained processes. The absolute dimensions of the dendritic fields of individual cells could not therefore be determined. Dendrites of neighbouring cells overlapped extensively so that any given region of the retina was in the fields of several cells. Many of these cells also gave rise to an axon which could be followed through the nerve fibre layer towards the optic nerve head. Although there were some medium–large multipolar cell bodies, most immunoreactive cells in the GCL were ovoid in shape and had diameters between 9 and 12 μm (for central retina the mean diameter was 10.2 ± 1.3 (N = 257); for the peripheral retina, 11.6 ± 2.5 (N = 113)) which is within the reported range of diameters of ganglion cells and the largest displaced amacrine cells7,14-18. The immunoreactive cells of the GCL occur in all parts of the retina with a distribution density that parallels the reported density distribution of ganglion cells in different parts of the retina: thus there are 1,000–1,400 immunoreactive cells mm−2 in the visual streak, compared with 125–200 celis mm−2 in the peripheral retina (Fig. 1b).
Fig. 1.
a, Number of SP-immunoreactive cells remaining in the GCL of optic-nerve-sectioned compared to normal (contralateral) retina. Counts were made over the visual streak near the centre of the retina, b, Distribution and density of SP immunoreactive cells in optic-nerve-sectioned (open circles) compared to normal (contralateral) retina (solid circles; 74 day survival period). VS, visual streak.
Methods. The number of SP-immunoreactive somata was determined from wholemounts in two adjacent 0.5-mm2 fields at 0.5 mm intervals (final magnification ×272) along a dorso-ventral axis perpendicular to the visual streak and located about 3 mm from the optic nerve head (see inset; d, dorsal; v, ventral, VS visual streak). The average number of cells of the adjacent fields and cell density was calculated for each interval from these maps. Cell density is probably underestimated in the region of the myelinated fibre and retinal blood vessel band (retinal eccentricity about 2–4 mm dorsal to the visual streak) owing to difficulties in visualizing stained somata. Measurements were not corrected for retinal shrinkage.
By nine days after optic nerve section, and at all subsequent survival times, there was a marked loss of SP-immunoreactive cells in the GCL and processes in the proximal inner plexiform layer in all retinal regions (Figs 1a, and 2c, d). The number of SPimmunoreactive cells in the GCL was reduced by approximately 90% in central retina and by 45–60% in peripheral retina (Fig. 1b). Most SP-containing cells remaining in the GCL had diameters of 8–10 μm which is within the size range of the largest displaced amacrine cells7,15,18. These retrograde degeneration studies clearly suggest that most SP-immunoreactive cells in the GCL are indeed ganglion cells. Therefore on the basis of these experiments and comparisons with ganglion cell counts from Nissl stained preparations16-18, SP-immunoreactive cells probably constitute 25–35% of the ganglion cells in the GCL.
Following injection of either Fast Blue or rhodamine-labelled microspheres into the SC the distribution and density of retrogradely labelled ganglion cell bodies matched that reported previously7. In the central retina about 25% of Fast Blue labelled cells displayed SP-immunoreactivity (19–30% in several separate experiments) and 79% of the SP-immunoreactive cells in the GCL were retrogradely labelled (Fig. 2e, f). Immunoreactive cells in the GCL lacking retrograde tracer in double-labelling experiments could be either displaced amacrine cells or ganglion cells that failed to transport detectable quantities of the tracer. These studies also support the conclusion that a substantial number of ganglion cells contain SP-immunoreactivity.
SP-immunoreactive ganglion cells project upon the contralateral dorsal lateral geniculate nucleus (dLGN), SC and accessory optic nuclei. In the dLGN a prominent plexus of immunoreactive axons and varicosities formed a narrow band some 120–250 μm wide just below the optic tract (Fig. 3a, b). This plexus defines a concealed and previously undescribed lamina, which appears to correspond to the most superficial part of the outer layer of the alpha sector19. Immunoreactive axons, some of which could be traced into the dLGN from parent axons in the optic tract, gave rise to terminal arborizations characterized by spheroidal or sausage-shaped enlargements commonly 10 μm in diameter and up to 30 μm long (Fig. 3d). A similar immunoreactive plexus was present in the upper part of the stratum griseum superficiale of the SC and in the accessory optic nuclei. These plexuses were less prominent and disorganized between 3 and 6 days after, and absent 10 days or more after, contralateral optic nerve section or eye enucleation (Fig. 3a, c). However, such lesions had no detectable effect on the corresponding immunoreactive plexuses in the ipsilateral dLGN (Fig. 3a, b) or SC, or on the plexuses of finer axons and smaller varicosities in the intergeniculate leaflet, ventral lateral geniculate, thalamic reticular or pretectal nuclei. These plexuses are therefore probably not of retinal origin (Fig. 3a–c).
Fig. 3.
a, Frontal section of the diencephalon from a rabbit 16 days after unilateral optic nerve section showing immunoreactive fibres in the alpha sector of the ipsilateral dLGN just below the optic tract (OT), and loss of immunoreactive fibres from the contralateral dLGN. Note the plexuses of fine calibre immunoreactive processes in the intergeniculate leaflet (IGL) and the ventral lateral geniculate nucleus (vLGN) (×5.6). b, Enlargement of ipsilateral LGN from a, showing axon terminals and large varicosities in dLGN in a lamina just below the optic tract, and fine axons and varicosities in IGL and vLGN (×11.2). c, Enlargement of contralateral LGN from a, showing loss of immunoreactivity from the dLGN with preservation of the fine axonal plexuses in IGL and vLGN (×11.2). d, Immunoreactive axons and varicosities in the dLGN at higher magnification (×280).
Methods. For optic nerve section see Fig. 1 legend. For eye, enucleation, adult albino and Dutch belted rabbits (N = 9) were anaesthetized with Sagatal (30–40 mg per kg), one eye irrigated with 1% amethocaine and the optic nerve exposed in the orbit through a lateral approach. The nerve and extraocular muscles were cut, the eyeball removed, the eyelids sutured and sprinkled with Cicatrin and 0.5 ml Intramycetin given. Normal, eye-enucleated or optic-nerve-sectioned animals were anaesthetized, perfused with 0.9% NaCl in 0.1 M phosphate buffer followed by 4% paraformaldehyde (for light microscopy only) or with 4% paraformaldehyde and 0.1–0.5% glutaraldehyde followed by 4% paraformaldehyde (both containing 0.1 M lysine and 0.01 M sodium periodate) for light and electron microscopy. All perfusates were made up in 0.1 M phosphate buffer and were at room temperature. Brains were washed overnight in buffer (containing 20–30% sucrose in the case of brains to be cut on a freezing microtome) and sectioned in the frontal or horizontal plane at 25–30 μm (freezing microtome) or 50 μm (Vibroslice, Campden Instruments).
Immunoreactive profiles in the dLGN were identified by electron microscopy as large axonal boutons in areas of complex neuropil (synaptic glomeruli) (Fig. 4a, b). The boutons were apposed to and commonly deeply invaginated by other, nonimmunoreactive components of the glomeruli, which could be identified on the basis of previous ultrastructural studies of dLGN (for review see ref. 20) as the dendrites and dendritic appendages of presumptive projection cells and intrageniculate interneurons. Spherical synaptic vesicles and large, pale mitochondria, characteristic of retinal terminals in the mammalian dLGN20, were apparent in the immunoreactive boutons, the synaptic relations of which were similar to those of identified retinal terminals in non-immunoreacted tissue. Specifically, they were presynaptic both to the dendritic appendages of projection cells and to the vesicle-containing dendrites and dendritic appendates of interneurons: they also established filamentous contacts with projection cell dendritic shafts.
Fig. 4.
a, b, Electron micrographs of synaptic glomeruli in the dLGN containing SP-immunoreactive retinal terminals and other non-immunoreactive profiles identified as dendrites or dendritic appendages of presumptive projection cells (D) or intemeurons (P), which are postsynaptic to labelled profiles (arrows). The insets show synaptic contacts (arrows) between immunoreactive terminals and postsynaptic dendrites more clearly: the inset to b is an enlargement of part of the main panel; the inset to a is from a different glomerulus. Note the large, pale mitochondria, with swollen cristae, in the immunoreactive terminal profiles. Magnifications; in a, ×4,000 (inset ×31,500), in b, ×8,500 (inset ×30,500).
Methods. All tissue processed for electron microscopy was from rabbits perfused with a paraformaldehyde-glutaraldehyde mixture followed by paraformaldehyde alone and sectioned frontally at 50 μm on a Vibroslice (see legends to Figs 2 and 3). In some cases Triton X-100 was added to antibody-containing solutions to facilitate penetration. Reacted sections were osmicated (1% OsO4 in 0.1 M phosphate buffer) stained in 1% aqueous uranyl acetate and embedded in Araldite. Thin sections were viewed in a Philips EM 301 with or without lead citrate staining.
These studies clearly suggest that substance P (and/or closely related tachykinins) may function as transmitters or modulators of rabbit retinal ganglion cells and provide the first clear demonstration of any putative transmitter or modulator in the axon terminals of the central visual pathway of a mammal. The presence of a prominent population of SP-containing ganglion cells in the rabbit retina, the recent suggestion that there are several peptide-containing ganglion cell types in the frog retina21 and reports of peptide bioactivity and immunoreactivity in optic nerve extracts1, 22 raise the possibility that peptides are present in the retinal ganglion cells of other vertebrates including primates1,2,4.
The functional characteristics of SP-containing ganglion cells are unknown and it is not yet clear how they relate to the subclasses of retinal ganglion cells described in other studies23,24, although the distribution of their dendrites to the proximal inner plexiform layer suggests that they are likely to be involved in ‘on-centre’ pathways25. Because SP-containing ganglion cells are distributed throughout the retina and their terminals in dLGN form an uninterrupted band from dorsal to ventral in the frontal plane and from rostro-lateral to caudo-medial in the horizontal plane we conclude that this lamina may contain a representation of the entire monocular portion of the contralateral visual hemifield. Finally, note that the immunoreactive plexus in the dLGN is partially overlapped by the terminal field of the projection to dLGN from the SC26 and this region of dLGN may thus be comparable to the C-laminae in the cat, which also receive both retinal and collicular inputs27.
Acknowledgments
We thank Ms M. Cilluffo and E. Franke for technical assistance, Mr P. Marx for help with the morphometric programmes, Drs L. Katz and B. Burge for rhodamine labelled microspheres and Drs C. Sternini and L. Kruger for helpful discussions. This work was partially supported by a grant from NIH and an Alfred P. Sloan Fellowship to N.B.
References
- 1.Brecha N. In: Chemical Neuroanatomy. Emson PC, editor. Raven; New York: 1983. pp. 85–129. [Google Scholar]
- 2.Brecha NC, Eldred W, Kuljis RO, Karten HJ. Prog. retinal Res. 1984;3:185–226. [Google Scholar]
- 3.Brecha N, Hendrickson A, Floren I, Karten HJ. Invest. Ophthalmol. Vis. Sci. 1982;23:147–153. [PubMed] [Google Scholar]
- 4.Hökfelt T, Dahlstrom A. Z. Zellforsch. mikrosk. Anat. 1971;119:460–482. doi: 10.1007/BF00455243. [DOI] [PubMed] [Google Scholar]
- 5.Bentivoglio M, Kuypers HGJM, Catsman-Berrevoets CE, Loewe H, Dann O. NeuroscL Lett. 1980;18:25–30. doi: 10.1016/0304-3940(80)90208-6. [DOI] [PubMed] [Google Scholar]
- 6.Katz LC, Burkhalter A, Dreyer WJ. Nature. 1984;310:498–500. doi: 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- 7.Vaney DI, Peichl L, Wässle H, Illing R-B. Brain Res. 1981;212:447–453. doi: 10.1016/0006-8993(81)90476-5. [DOI] [PubMed] [Google Scholar]
- 8.Brecha NC, Oyster CW, Takahashi ES. Invest. Ophthalmol. Vis. Sci. 1984;25:66–70. [PubMed] [Google Scholar]
- 9.Pamavelas JG, Kelly W, Franke E, Eckenstein F. J. Neurocytol. 1986;15:329–336. doi: 10.1007/BF01611435. [DOI] [PubMed] [Google Scholar]
- 10.Cuello AC, Galfré G, Milstein C. Proc. natn Acad. Sci. U.S.A. 1979;76:3532–3536. doi: 10.1073/pnas.76.7.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harmar AJ. Trends Neurosci. 1984;7:57–60. [Google Scholar]
- 12.Famiglietti EV, Brecha NC, Karten HJ. Neurosci. Abstr. 1980;6:212. [Google Scholar]
- 13.Cajal S. R. y. La Cellule. 1893;9:17–257. [Google Scholar]
- 14.Hughes A, Vaney DI. J. comp. Neurol. 1980;189:169–189. doi: 10.1002/cne.901890110. [DOI] [PubMed] [Google Scholar]
- 15.Vaney DI, Peichl L, Boycott BB. J. comp. Neurol. 1981;199:373–391. doi: 10.1002/cne.901990305. [DOI] [PubMed] [Google Scholar]
- 16.Vaney DI. J. comp. Neurol. 1980;189:215–233. doi: 10.1002/cne.901890202. [DOI] [PubMed] [Google Scholar]
- 17.Oyster CW, Takahashi ES, Hurst DC. J. Neurosci. 1981;1:1331–1346. doi: 10.1523/JNEUROSCI.01-12-01331.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masland RH, Mills JW, Hayden SA. Proc. R. Soc. B. 1984;223:79–100. doi: 10.1098/rspb.1984.0084. [DOI] [PubMed] [Google Scholar]
- 19.Holcombe V, Guillery RW. J. comp. Neurol. 1984;225:469–491. doi: 10.1002/cne.902250402. [DOI] [PubMed] [Google Scholar]
- 20.Jones EG. The Thalamus. Plenum; New York: 1985. [Google Scholar]
- 21.Kuljis RO, Karten HJ. J. comp. Neurol. 1985;240:1–15. doi: 10.1002/cne.902400102. [DOI] [PubMed] [Google Scholar]
- 22.Unger WG, Butler JM, Cole DF, Bloom SR, McGregor GP. Expl Eye Res. 1981;32:797–801. doi: 10.1016/0014-4835(81)90030-0. [DOI] [PubMed] [Google Scholar]
- 23.Amthor FR, Oyster CW, Takahashi E. Proc. R. Soc. B. 1983;217:341–355. doi: 10.1098/rspb.1983.0014. [DOI] [PubMed] [Google Scholar]
- 24.Vaney DI, Levick WR, Thibos LN. Expl Brain Res. 1981;44:27–33. doi: 10.1007/BF00238746. [DOI] [PubMed] [Google Scholar]
- 25.Bloomfield SA, Miller RF. J. Neurosci. 1986;6:1–13. doi: 10.1523/JNEUROSCI.06-01-00001.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holstege G, Collewijn H. J. comp. Neurol. 1982;209:139–175. doi: 10.1002/cne.902090204. [DOI] [PubMed] [Google Scholar]
- 27.Torrealba F, Partlow GD, Guillery RW. Neuroscience. 1981;6:1341–1360. doi: 10.1016/0306-4522(81)90192-5. [DOI] [PubMed] [Google Scholar]