Abstract
Compelling evidence demonstrates that intestinal commensal microbiota modulate conventional and regulatory T cell (Treg) responses that are required for effective host defence against pathogens and avoidance of autoimmunity and other immunopathologic conditions. Here, we investigated the contribution of the commensal microbiota and Toll-like receptor (TLR) signaling to homeostasis of Foxp3− conventional CD4+ T cells and Foxp3+ Tregs. Upon long-term antibiotics treatment, we observed a significant reduction of conventional CD4+ T cell proliferation in a systemic manner, whereas Foxp3+ Treg proliferation was locally impaired in gut-draining mesenteric lymph nodes and Peyer’s patches. The proliferative response to microbial components was not mediated by TLRs as MyD88- and various TLR-deficient mice displayed normal or even increased conventional T cell and Foxp3+ Treg proliferation. Thus, commensal microbiota-derived stimuli support cycling of both conventional CD4+ T cells and Foxp3+ Tregs with TLR-mediated recognition of bacterial components not being the major mechanism controlling microbiota-driven T cell homeostasis.
Keywords: T cell homeostasis, commensal microbiota, antibiotics, Foxp3−CD4+ T cells, Foxp3+CD4+ Tregs, MyD88-deficient mice, TLR-deficient mice
Introduction
Colonization with commensal microbiota starts immediately after birth and continues through childhood until stable microbiota are established [1, 2]. The presence of intestinal bacteria is an important stimulus for the development and maturation of the mucosal and systemic immune system [1–4]. In germ-free mice, Peyer’s Patches (PP) are hypoplastic, numbers of IgA-secreting plasma cells and lamina propria CD4+ T cells are greatly reduced and structural defects also affect spleen and peripheral LN (pLN). Colonization of germ-free mice with commensal microbiota from specific-pathogen-free (SPF) mice reversed these abnormalities [5].
The gut-associated immune system is capable of mounting an efficient host defense against pathogenic bacteria, but has to maintain at the same time regulatory control mechanisms to protect the organism from hyperresponsiveness toward antigens from the normal intestinal flora and nutrients, which could result in chronic inflammation [6]. In vivo break of tolerance toward intestinal microbiota is believed to be a major cause of inflammatory bowel disease (IBD) [6–8]. Moreover, the intestinal microbiota play a pivotal role in the development of T cells both within and outside the intestine by selectively expanding and activating different T cell subsets under normal and/or pathological conditions [9]. While it was demonstrated that total intestinal T cells do not proliferate in response to enterobacterial antigens in vitro [10–12], depletion of regulatory T cells (Tregs) [12] or exposure to heterologous bacterial antigens [10] reversed unresponsiveness in these cultures. Thus, antigens of gut commensals are not simply ignored, but rather trigger an active immunosuppressive process to maintain intestinal homeostasis.
Numerous experimental studies demonstrated a pivotal role for Foxp3-expressing Tregs in the maintenance of intestinal homeostasis [6]. Although initially being characterized as an anergic CD4+ T cell subset [13], Tregs show a high turnover in vivo [14]. Steady-state proliferation of Foxp3+ Tregs is commonly attributed to recognition of self-antigens [15], but recognition of microbial antigens by Foxp3+ Tregs has been reported as well [16, 17]. Interestingly, Tregs from germ-free mice display lower suppressive activity in vitro compared to Tregs from SPF-housed mice [18] and only Tregs previously exposed to commensal microbiota are capable of ameliorating intestinal inflammation in a transfer colitis model [19]. Collectively, these results indicate a crucial role of commensal microbiota for generation, expansion, and/or differentiation of Tregs in a healthy individual.
Recognition of pathogens and commensal bacteria by Toll-like receptors (TLRs) is of major importance for maintenance of intestinal homeostasis [20]. Accordingly, absence of TLR signaling impairs normal gut epithelial homeostasis [21] and increased allergic responses to food antigens [22]. Furthermore, numerous TLR polymorphisms have been linked to IBD susceptibility in humans, yet the functional outcome is still unclear [23, 24]. Importantly, conventional CD4+ T cells and Tregs also express TLRs, and their functional modulation by TLR agonists has been reported [25–32]. We therefore analyzed in the present study the influence of commensal microbiota and TLR signaling on the homeostatic proliferation of conventional Foxp3−CD4+ T cells and Foxp3+ Tregs. Our data demonstrate that microbial stimuli support cycling of both conventional CD4+ T cells and Foxp3+ Tregs in a TLR-signaling-independent manner.
Materials and methods
Mouse strains
C57BL/6 mice were purchased from Harlan Laboratories, Charles River or Janvier. MyD88−/− mice [56], backcrossed at least twelve generations onto the C57BL/6 background, and corresponding wt controls were obtained from the BfR (Berlin, Germany). TLR2−/−, TLR4−/−, TLR2/4−/− and TLR9−/− mice as well as corresponding wt controls were provided by the Max-Planck-Institut for Immunobiology (Freiburg, Germany). TLR2−/−, TLR4−/− and TLR2/4−/− mice were backcrossed at least seven generations onto the C57BL/10ScSn background as described elsewhere [57, 58]. TLR9−/− mice were backcrossed at least three times onto the C57BL/6 background as previously described [59]. TLR5−/− mice [60] and corresponding C57BL/6 wt controls were kindly provided by Fiona Powrie (Sir William Dunn School of Pathology, Oxford, UK). All mice were maintained in the animal facilities of the FEM (Charité, Berlin, Germany). In all experiments, gender- and age-matched mice were used. The experiments were performed in accordance with institutional, state and federal guidelines.
Antibiotics treatment
Mice formerly housed under SPF conditions were treated with an antibiotic regimen according to a standard protocol with minor modifications [21]. Groups of mice were given a cocktail of antibiotics (ampicillin, 1 g/L, Ratiopharm; vancomycin, 500 mg/L, Cell Pharm; ciprofloxacin, 200 mg/L, Bayer Vital; imipenem, 250 mg/L, MSD; metronidazole, 1 g/L, Fresenius) added to the drinking water ad libitum for a period of eight weeks. Mice were housed in sterile cages, which were changed on daily basis for the first two weeks. Efficient bacterial reduction was monitored by weekly bacteriologic evaluation of faeces from individual mice. Lack of any cultivable bacteria (aerobic and anaerobic cultures of Gram-negative rods, Gram-positive rods and Gram-positive cocci) for at least two consecutive weeks was taken as indication for successful removal of detectable commensal microbiota as previously described [34].
Antibodies and flow cytometry
Fluorochrom-conjugated anti-CD4 (RM4-5) and anti-Foxp3 (FJK-16s) were purchased from eBioscience. Fluorochrom-conjugated anti-BrdU (3D4) was purchased from BD™ Pharmingen. Intracellular BrdU and Foxp3 staining was performed according to the manufacturer’s instructions. Flow cytometry was performed using an LSRII (BD Biosciences) flow cytometer with Diva software and data were analyzed with FlowJo™ software (TreeStar).
Lymphocyte isolation from lymphoid organs
Single-cell suspensions were prepared from secondary lymphoid organs and classified as pLN (mandibular, superficial parotid, proper axillary, accessory axillary, subiliac and popliteal LNs), mesenteric LN (mLN; jejunal and colic LNs), spleen and PP (all macroscopically visible PP from small intestine). Single-cell suspensions were generated and erythrocytes were removed from spleen samples by hypotonic lysis in hypo-osmotic buffer. All samples were resuspended in defined volumes of PBS/0.2% BSA and subjected to subsequent processing.
Enumeration of lymphocytes
For high throughput cell counting, an aliquot from a defined volume of single cell suspension was added to a defined volume of suspension with a known number of Fluoresbrite™ beads supplemented with propidium iodide (0.1 µg/ml; Sigma) for exclusion of dead cells. Analysis was performed on a FACSCalibur™ operated with CellQuestPro™ software (BD™). Lymphocyte numbers were calculated according to acquired live cells in a lymphocyte gate relative to simultaneously detected numbers of beads. Absolute cell numbers were then calculated in consideration of the known amount of fluorescent beads in the sample and the size of the cell suspension aliquot in relation to total sample volume.
Double immunoenzymatic labeling
For immunostaining, 2–3 µm thick sections were cut, deparaffinized, and subjected to a heat-induced epitope retrieval step before incubation with antibodies. Sections were immersed in sodium citrate buffer solutions at pH 6.0 and heated in a high-pressure cooker. The slides were rinsed in cool running water, washed in Tris-buffered saline (pH 7.4), and incubated for 30 min with the antibody against Foxp3 (clone FJK-16s, eBioscience, dilution 1 : 100), followed by Alexa Fluor 488-conjugated secondary antibody (Invitrogen, dilution 1 : 100). Subsequently, slides were washed three times in PBS and incubated with anti Ki-67 antibody (TEC-3, Dako, dilution 1 : 50), followed by Alexa Fluor 555-conjugated secondary antibody (Invitrogen, dilution 1 : 100). Nuclei were counterstained with DAPI (Roche, dilution 1 : 1500) and slides were mounted in Fluoromount-G (Southern Biotech). Images were acquired using a fluorescence microscope (AxioImager Z1) equipped with a CCD camera (AxioCam MRm) and processed with Axiovision software (Carl Zeiss MicroImaging, Inc.). Negative controls were performed by omitting the primary antibodies.
Evaluation of in vivo proliferation via BrdU incorporation
For assessment of in vivo proliferative activity, mice were given 1 mg/ml 5-bromo-2′-deoxyuridine (BrdU; Sigma) ad libitum via the drinking water. After six days of BrdU feeding, mice were sacrificed, cell suspensions were prepared from different lymphoid organs and intracellulary stained with anti-BrdU-antibody according to the manufacturer’s instructions with the addition of anti-Foxp3-antibody.
Statistical analysis
Prism™ software (GraphPad) was utilized for statistical analysis and graphing. If not stated otherwise, statistical significance was assessed via ‘unpaired t-test’. All tests were performed with the assumption of a two-tailed probability and differences were considered statistically significant when p < 0.05 (*), and highly significant when p < 0.01 (**), p < 0.001 (***) or p < 0.0001 (****).
Results
Reduction of commensal microbiota affects size and composition of the CD4+ T cell compartment in mLN and PP
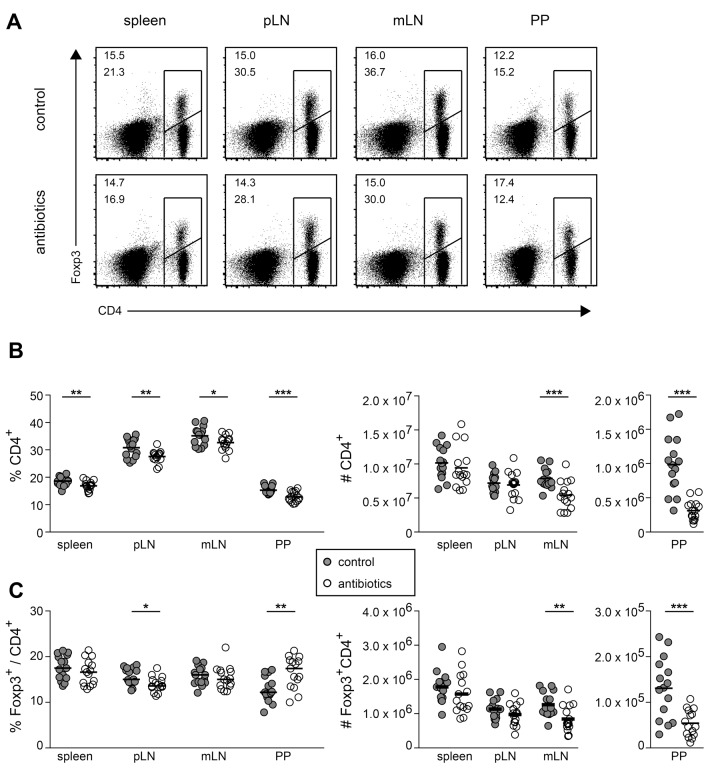
Germ-free mice display several developmental and cellular abnormalities within the mucosal and peripheral immune system [33], and thus represent a rather suboptimal model system to investigate the impact of commensal microbiota on homeostatic proliferation of conventional Foxp3–CD4+ T cells and Foxp3+CD4+ Tregs. To circumvent these limitations, we here treated adult SPF-housed mice, in which gut-draining lymphoid organs have developed normally, with a cocktail of antibiotics. Successful eradication of the gut microbiota was judged by regular microbacteriological investigations of feces samples [34]. In these mice, we first analyzed the total CD4+ T cell compartment compared to untreated controls (Fig. 1A). Antibiotics treatment resulted in a mild, but significant reduction of CD4+ T cell frequencies in all examined lymphoid organs, including spleen, pLN, mLN and PP, while total CD4+ T cell numbers were only reduced in mLN and PP (Fig. 1B). Frequencies of Foxp3+ Tregs within the CD4+ T cell compartment were largely unaffected in spleen, pLN and mLN, but PP showed a significantly increased Treg frequency when antibiotics-treated mice were compared to untreated controls (Fig. 1C). Absolute numbers of Foxp3+CD4+ Tregs were significantly reduced only in mLN and PP of antibiotics-treated mice compared to untreated controls (Fig. 1C). Together, these data demonstrate that the reduction of commensal microbiota by antibiotics treatment affects size and composition of the lymphocyte compartment particularly in gut-draining lymphoid organs.
Fig. 1.
Reduction of commensal microbiota affects size and composition of lymphocyte compartment in mLN and PP. Adult SPF-housed C57BL/6 mice were treated with antibiotics for a period of approximately eight weeks. Subsequently, lymphocytes from spleen, pLN, mLN and PP of antibiotics-treated and untreated control mice were analyzed for CD4 and Foxp3 expression by flow cytometry. (A) Representative dot plots show CD4 vs Foxp3 expression for indicated lymphoid organs of control mice (upper row) and antibiotics-treated mice (lower row). Frequencies are depicted according to the indicated gates for total CD4+ cells (lower numbers) and Foxp3+ among CD4+ cells (upper numbers). (B and C) Graphs summarize frequencies (left) and cell numbers (right) of conventional Foxp3–CD4+ T cells (B) and Foxp3+CD4+ Tregs for indicated lymphoid organs (C) in controls (gray filled circles) and antibiotics-treated mice (open circles). Pooled data from three independent experiments are depicted. Circles represent values from individual mice (n = 15) and lines indicate mean
Reduction of commensal microbiota affects in vivo proliferation of conventional CD4+ T cells and Foxp3+ Tregs
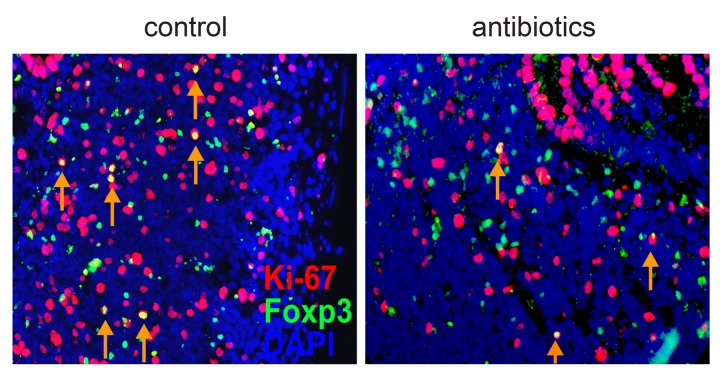
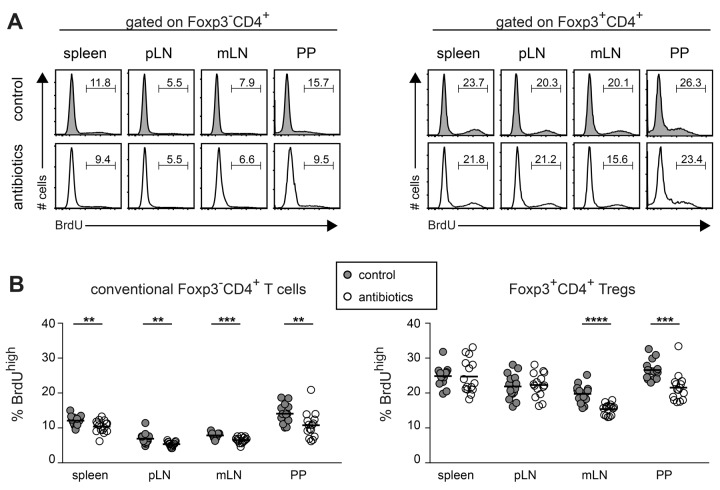
The initial characterization of Tregs as being anergic [13] was revised by several studies showing high turnover and proliferation of Tregs in vivo both under steady-state and inflammatory conditions [35–38], and steady-state proliferation of Foxp3+ Tregs has been commonly attributed to recognition of self-antigens [15]. Our observation that antibiotics treatment led to a reduction in the number of Foxp3+CD4+ Tregs in mLN and PP (Fig. 1C) suggested that at least part of the Tregs at these sites might proliferate in response to microbiota-derived antigens. Indeed, a large fraction of Foxp3-expressing cells in PP of SPF-housed, untreated mice also stained positive for the proliferation marker Ki-67 while the number of double positive cells was clearly diminished in antibiotic-treated mice (Fig. 2). We next quantified the proliferative activity of conventional CD4+ T cells and Foxp3+ Tregs in various compartments by in vivo labeling with BrdU. For this purpose, antibiotics-treated mice and untreated controls received BrdU via the drinking water. After six days of continuous feeding, mice were sacrificed and in vivo proliferating conventional Foxp3−CD4+ T cells and Foxp3+CD4+ Tregs were identified by staining of BrdU (Fig. 3A). In all lymphoid organs analyzed, conventional Foxp3–CD4+ T cells showed significantly reduced frequencies of BrdU+ cells when antibiotics-treated mice were compared with untreated controls (Fig. 3B). In contrast, antibiotics treatment affected proliferation of Foxp3+CD4+ Tregs only in mLN and PP, but not in spleen and pLN. In line with previously published data [35–38], Foxp3+ Tregs showed a higher overall proliferation when compared to conventional CD4+ T cells, and highest frequencies of cycling T cell subsets were observed in PP (Fig. 3A and B). Taken together, these data indicate that microbial stimuli affect proliferation of Foxp3+ Tregs only locally within mLN and PP, whereas conventional Foxp3−CD4+ T cells were influenced in a systemic manner.
Fig. 2.
Proliferating Ki-67-positive Foxp3+ cells can be found in the PP. Sections from PP of untreated control (left) and antibiotics-treated (right) mice were stained for DAPI (blue), Foxp3 (green) and Ki-67 (red). Arrows indicate Foxp3+Ki-67+ cells (yellow in overlay). A representative photomicrograph is shown (magnification ×400)
Fig. 3.
Reduction of commensal microbiota affects in vivo proliferation of conventional CD4+ T cells and Foxp3+ Tregs. Adult SPF-housed C57BL/6 mice were treated with antibiotics for a period of approximately eight weeks. Two days after the treatment was stopped, mice were fed with BrdU for another period of six days. Subsequently, lymphocytes from indicated organs of antibiotics-treated and untreated control mice were analyzed for BrdU, CD4 and Foxp3 expression by flow cytometry. (A) Representative histograms show BrdU incorporation in Foxp3–CD4+ T cells (left) and Foxp3+CD4+ Tregs (right) in indicated lymphoid organs from untreated controls (gray shaded) and antibiotics-treated mice (open). Numbers indicate frequencies of BrdUhigh cells among respective CD4+ T cell subset. (B) Graphs summarize frequencies of BrdUhigh cells among conventional Foxp3–CD4+ T cells (left) and Foxp3+CD4+ Tregs (right) from untreated controls (gray filled circles) and antibiotics-treated mice (open circles). Pooled data from three independent experiments are depicted. Circles represent values from individual mice and lines indicate mean (n = 15)
MyD88−/− mice display normal in vivo proliferation of conventional CD4+ T cells and Foxp3+ Tregs
Recognition of commensal bacteria by TLRs plays a crucial role for the maintenance of intestinal homeostasis [20, 21]. TLR-mediated signaling was reported to modulate the suppressive activity of Tregs in an indirect [39, 40] and direct manner [25–27, 29–31]. Here, we analyzed the contribution of TLR-mediated signaling to the in vivo proliferation of conventional CD4+ T cells and Foxp3+ Tregs and questioned whether lack of those signals might mimic the effects observed in antibiotics-treated mice. Thereto, BrdU labeling experiments were performed in mice being deficient for the common TLR-signaling adaptor molecule MyD88. No differences in frequencies of BrdU+ cells among conventional CD4+ T cells (Fig. 4A) and Foxp3+ Tregs (Fig. 4B) were observed when MyD88−/− mice were compared with wild type (wt) controls. In line with previously published data [41], MyD88−/− mice showed largely unaffected frequencies of Foxp3+ Tregs in all lymphoid organs analyzed (data not shown). Thus, lack of TLR-mediated signaling does not mimic the reduced in vivo proliferation of conventional CD4+ T cells and Foxp3+ Tregs observed in antibiotics-treated mice.
Fig. 4.
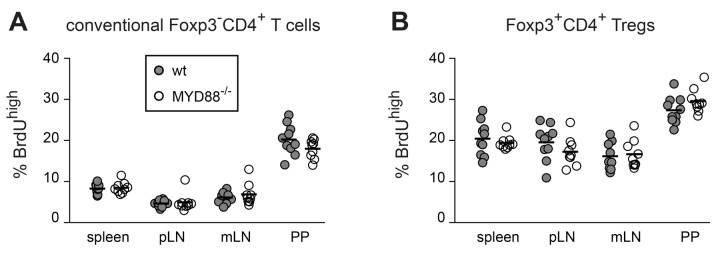
In vivo proliferation of Foxp3+ Tregs and Foxp3–CD4+ T cells occurs independently of MyD88 signaling. MyD88-deficient and wt control mice were fed with BrdU for a period of six days. Subsequently, lymphocytes from indicated organs of MYD88-deficient and control mice were analyzed for BrdU, CD4 and Foxp3 expression by flow cytometry. Graphs summarize frequencies of BrdUhigh cells among conventional Foxp3–CD4+ T cells (A) and Foxp3+CD4+ Tregs (B) from wt (gray filled circles) and MyD88−/− mice (open circles). Pooled data from two independent experiments are depicted. Circles represent values from individual mice and lines indicate mean (n = 9–10)
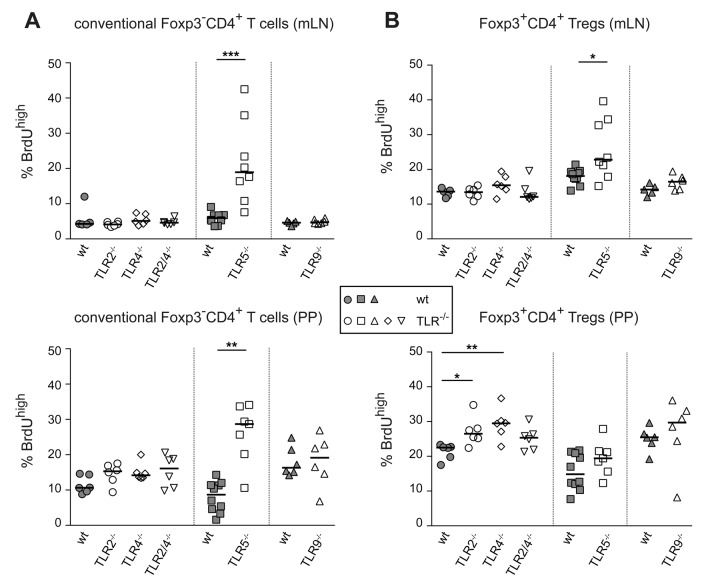
In vivo proliferation of conventional CD4+ T cells and Foxp3+ Tregs is not reduced in TLR-deficient mice
The unobtrusive phenotype observed in MyD88−/− mice might be explained by opposing effects of individual MyD88-dependent TLRs. Therefore, we here investigated the proliferative capacity of conventional CD4+ T cells and Foxp3+ Tregs in mice being deficient for individual TLRs known to recognize bacterial microbe-associated molecular patterns (MAMPs). BrdU labeling experiments were performed in TLR2−/−, TLR4−/−, TLR2/4−/−, TLR5−/− and TLR9−/− mice. Intriguingly, none of these TLR-deficiencies resulted in impaired in vivo proliferation of T cell subsets by showing reduced frequencies of BrdU+ cells among conventional CD4+ T cells (Fig. 5A) or Foxp3+ Tregs (Fig. 5B) when compared to corresponding wt controls. On the contrary, increased frequencies of cycling conventional CD4+ T cells were observed in both mLN and PP of TLR5−/− mice, and Foxp3+ Tregs from TLR5−/−, TLR2−/− and TLR4−/− mice displayed significantly enhanced BrdU-incorporation in mLN and PP (Fig. 5A and B). Together, these data indicate that lack of individual MAMP-sensing receptors was neither affecting homeostasis of conventional CD4+ T cells nor Foxp3+ Tregs in a manner mimicking the effects observed in antibiotics-treated mice.
Fig. 5.
Lack of bacterial MAMP-sensing TLRs does not resemble the effects observed in antibiotics-treated mice. TLR2-, TLR4-, TLR2/4-, TLR5- and TLR9-deficient mice as well as corresponding wt controls were fed with BrdU for a period of six days. Subsequently, lymphocytes from mLN and PP of TLR-deficient and control mice were analyzed for BrdU, CD4 and Foxp3 expression by flow cytometry. Graphs summarize frequencies of BrdUhigh cells among conventional Foxp3–CD4+ T cells (A) and Foxp3+CD4+ Tregs (B) in mLN (upper graphs) and PP (lower graphs) from wt controls (gray filled symbols) and indicated knockout mice (open symbols). Pooled data from three independent experiments are depicted. Symbols represent values from individual mice and lines indicate median (n = 5–9). Statistical analysis was performed via ‘Wilcoxon ranked sum test’
Discussion
The intestinal immune system is able to mount inflammatory immune responses against intestinal pathogens and, at the same time, to tolerate both indigenous commensal microbiota and food-derived antigens. Breakdown of intestinal tolerance can lead to overwhelming inflammatory immune responses directed against resident commensal microbiota which might result in chronic inflammatory conditions such as IBD [42]. Adoptive transfer experiments in germ-free and gnotobiotic mice demonstrated a critical importance of commensal microbiota for development of colitis [43, 44], and antibiotics treatment has been successfully applied for improvement of disease severity in human IBD patients [45]. On the other side, commensal microbiota have been shown to be crucial players for maintenance of intestinal homeostasis and modulation of Foxp3+ Treg responses [1, 6, 8]. Although some reports suggest that Foxp3+ Tregs isolated from germ-free mice display a normal phenotype with normal proliferative capacity [12, 46] others have demonstrated a reduced suppressive activity in vitro and in vivo [18, 19]. However, it is not entirely clear whether the observed effects are a direct consequence of the absence of microbial stimuli or if they are of indirect nature based on developmental abnormalities of the gut-associated immune system that are documented for germ-free mice [33].
In the present study, we investigated the contribution of commensal microbiota to the homeostatic proliferation of conventional CD4+ T cells and Foxp3+ Tregs. To exclude the aforementioned developmental abnormalities due to the complete absence of microbial stimuli during ontogeny, adult SPF-housed mice, which display a normally developed mucosal immune system, were treated with a cocktail of antibiotics to reduce their commensal microbiota [34]. Although being less pronounced, this regimen also led to minor changes of the gut-associated immune system. Notably, a reduced number of macroscopically visible PP with proximity to the caecum was observed. As this group of PP contained a twofold smaller Treg frequency (data not shown), this might explain the increased frequency of Foxp3+ Tregs in PP isolated from antibiotics-treated mice compared to untreated controls.
Analysis of the proliferative activity of conventional CD4+ T cells and Foxp3+ Tregs revealed a shaping of the whole CD4+ T cell compartment by commensal microbiota since both CD4+ T cell subsets were substantially regulated by the presence or absence of microbial stimuli. Interestingly, site-specific differences between conventional CD4+ T cells and Foxp3+ Tregs were observed. Whereas conventional CD4+ T cells from antibiotics-treated mice displayed reduced frequencies of BrdU+ cells in gut-draining mLN and PP as well as skin-draining LN and spleen, proliferation of Foxp3+ Tregs was affected only in gut-draining mLN and PP when antibiotics-treated mice were compared to untreated controls. These data fit to the observation that conventional CD4+ T cells display a largely recirculating phenotype showing similar T cell receptor (TCR) repertoires in all secondary lymphoid organs, while Foxp3+ Tregs seem to be more sessile and present unique TCR repertoires in individual lymphoid tissues [47]. Thus, it can be assumed that both conventional CD4+ T cells and Foxp3+ Tregs being specific for microbiota exist within gut-draining LN and PP, and that their survival depends on the continuous presence of commensal microbiota.
Foxp3+ Tregs recognizing microbial antigens have been recently reported and were mainly found within the subset of peripherally induced Tregs [16, 17, 48]. However, in the present study, we did not observe any significant changes in the TCR Vβ repertoire usage among Foxp3+ Tregs being isolated from mLN and PP of antibiotics-treated compared to untreated control mice (data not shown). Only minor changes were found for conventional CD4+ T cells derived from PP, but the aforementioned reduced number of macroscopically visible PP with proximity to the caecum in antibiotics-treated mice could explain these changes. An unchanged TCR repertoire upon treatment with antibiotics for eight weeks would be expected if the T cells would have a much longer half-life or when the proportion of conventional and Foxp3+ CD4+ T cells being reactive toward microbial antigens within gut-draining mLN and PP would be small. These questions still need to be clarified.
Both, commensals as well as pathogens, express MAMPs that are sensed by the host’s pattern recognition receptors (PRRs). To date, several classes of PRRs, such as Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), NOD-like receptors (NLRs), and DNA receptors (cytosolic sensors for DNA), have been discovered and characterized [49]. Key components of the immune system sensing the presence of bacteria are TLRs. A role of TLR-mediated signaling in response to commensal bacteria was not only identified with respect to intestinal homeostasis [21] but also for regulation of the suppressive activity of Tregs [32, 39, 40]. All TLRs except TLR3 recruit MyD88 and activate MyD88-dependent signaling pathways [49]. MyD88 deficiency in conventional CD4+ T cells results in impaired effector T cell differentiation both in vitro and in vivo [32], and MyD88−/− mice were reported to contain decreased numbers of Tregs [29] with reduced suppressive capacity [32]. Together, these data suggest a direct role of TLR signaling for T cell differentiation and function. Results of the present study, however, indicate MyD88-independent in vivo proliferation of conventional CD4+ T cells and Foxp3+ Tregs. Accordingly and in line with previously published data [41], MyD88−/− mice displayed a normal Treg compartment, suggesting that MyD88-dependent signaling is not controlling Treg homeostasis.
A growing number of reports demonstrated a direct influence of TLR agonists on induction, expansion and function of conventional CD4+ T cells and Foxp3+ Tregs [25–32, 50–52], and particularly TLR2 ligation has been demonstrated to enhance their proliferation in in vitro assays [29, 30, 51]. In the present study, however, neither TLR2−/−, TLR4−/−, TLR2/4−/−, TLR5−/− nor TLR9−/− mice showed reduced proliferation of conventional CD4+ T cells or Foxp3+ Tregs, further supporting our assumption that TLR-mediated signals are not essential for the microbiota-dependent part of proliferative activity. We rather observed an inhibitory effect of TLR2-, TLR4- and TLR5-dependent signaling on T cell proliferation within gut-draining mLN and PP, resembling the previously published Treg phenotype in TLR9−/− mice [53]. Although our data are supported by a recent report on a protective role for TLR5 deficiency for colitis development in a T cell transfer model [54], another study reported, in contrast, a potentiating effect of the TLR5 agonist flagellin on induction, expansion and suppressive activity of Foxp3+ Tregs [28, 52]. While differing compositions of commensal enteric communities between laboratories might explain these contrasting results, our data clearly show that microbiota-driven proliferation of conventional CD4+ T cells and Foxp3+ Tregs can occur in the absence of TLR-signaling. It is tempting to speculate that antigen-specific stimulation causes T cell expansion; however, other, TLR-independent MAMP-sensing mechanisms [49, 55] or metabolic influences might also be involved in the microbiota-dependent control of T cell proliferation.
In summary, our findings reveal an important role of microbial stimuli for maintenance of both conventional CD4+ T cells and Foxp3+ Tregs and suggest that TLR-mediated recognition of microbial components is not the critical signaling pathway for the control of microbiota-driven T cell homeostasis.
Acknowledgements
We thank Uta Lauer, Maria Ebel and Simone Spieckermann for expert technical assistance. TLR5−/− mice were kindly provided by Fiona Powrie (Sir William Dunn School of Pathology, Oxford, UK). We thank Marina Freudenberg (Max-Planck-Institut for Immunobiology, Freiburg, Germany) for providing TLR2−/−, TLR4−/−, TLR2/4−/− and TLR9−/− mice. This work was supported by the German Research Foundation (SFB621 to J.H. and SFB633 to A.H.).
Contributor Information
Sascha Cording, 1Experimental Immunology, Helmholtz Centre for Infection Research, 38124 Braunschweig, Germany.
Diana Fleissner, 1Experimental Immunology, Helmholtz Centre for Infection Research, 38124 Braunschweig, Germany.
Markus M. Heimesaat, 2Institute for Microbiology and Hygiene, Charité University Medicine Berlin, Campus Benjamin Franklin, 12200 Berlin, Germany.
Stefan Bereswill, 2Institute for Microbiology and Hygiene, Charité University Medicine Berlin, Campus Benjamin Franklin, 12200 Berlin, Germany.
Christoph Loddenkemper, 3Department of Pathology, Charité University Medicine Berlin, Campus Benjamin Franklin, 12200 Berlin, Germany.
Satoshi Uematsu, 4Division of Innate Immune Regulation, International Research and Development Center for Mucosal Vaccine, The Institute of Medical Science, University of Tokyo, 108-8639, Tokyo, Japan.
Shizuo Akira, 5Laboratory of Host Defense, WPI Immunology Frontier Research Center, Osaka University, 3-1 Yamada-oka, Suita, Osaka, 565-0871, Japan.
Alf Hamann, 6Experimental Rheumatology, Charité University Medicine Berlin, Campus Mitte, 10117 Berlin, Germany.
Jochen Huehn, 1Experimental Immunology, Helmholtz Centre for Infection Research, Inhoffenstr. 7, D-38124 Braunschweig, Phone: +49-531-6181-3310, FAX: +49-531-6181-3399, Email: jochen.huehn@helmholtz-hzi.de, Germany.
References
- 1.Tannock GW. What immunologists should know about bacterial communities of the human bowel. Seminars in immunology. 2007 Apr 1;19(2) doi: 10.1016/j.smim.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nature reviews. Immunology. 2011 Dec 9;12(1) doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- 3.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nature reviews. Immunology. 2004 Jun 1;4(6) doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 4.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Seminars in immunology. 2007 Apr 1;19(2) doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Macpherson AJ, Uhr T. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Annals of the New York Academy of Sciences. 2004 Dec 1;1029 doi: 10.1196/annals.1309.005. [DOI] [PubMed] [Google Scholar]
- 6.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011 Jun 15;474(7351) doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 7.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunological reviews. 2005 Aug 1;206 doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 8.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunological reviews. 2006 Aug 1;212 doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 9.Duan J, Kasper DL. Regulation of T cells by gut commensal microbiota. Current opinion in rheumatology. 2011 Jul 1;23(4) doi: 10.1097/BOR.0b013e3283476d3e. [DOI] [PubMed] [Google Scholar]
- 10.Duchmann R, Schmitt E, Knolle P, Meyer KH, Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. European journal of immunology. 1996 Apr 1;26(4) doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 11.Khoo UY, Proctor IE, Macpherson AJ. CD4+ T cell down-regulation in human intestinal mucosa: evidence for intestinal tolerance to luminal bacterial antigens. Journal of immunology (Baltimore, Md. : 1950) 1997 Apr 15;158(8) [PubMed] [Google Scholar]
- 12.Gad M, Pedersen AE, Kristensen NN, Claesson MH. Demonstration of strong enterobacterial reactivity of CD4+CD25- T cells from conventional and germ-free mice which is counter-regulated by CD4+CD25+ T cells. European journal of immunology. 2004 Mar 1;34(3) doi: 10.1002/eji.200324394. [DOI] [PubMed] [Google Scholar]
- 13.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. The Journal of experimental medicine. 1998 Jul 20;188(2) doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Boehmer H. Dynamics of suppressor T cells: in vivo veritas. The Journal of experimental medicine. 2003 Sep 15;198(6) doi: 10.1084/jem.20031358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein L, Jovanovic K. Regulatory T cell lineage commitment in the thymus. Seminars in immunology. 2011 Dec 1;23(6) doi: 10.1016/j.smim.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. The Journal of experimental medicine. 2006 Mar 20;203(3) doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011 Sep 21;478(7368) doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. European journal of immunology. 2006 Sep 1;36(9) doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- 19.Strauch UG, Obermeier F, Grunwald N, Gürster S, Dunger N, Schultz M, Griese DP, Mähler M, Schölmerich J, Rath HC. Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut. 2005 Nov 1;54(11) doi: 10.1136/gut.2004.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medzhitov R, Janeway C., Jr The Toll receptor family and microbial recognition. Trends in microbiology. 2000 Oct 1;8(10) doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- 21.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004 Jul 23;118(2) doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. Journal of immunology (Baltimore, Md. : 1950) 2004 Jun 1;172(11) doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 23.Török HP, Glas J, Tonenchi L, Bruennler G, Folwaczny M, Folwaczny C. Crohn's disease is associated with a toll-like receptor-9 polymorphism. Gastroenterology. 2004 Jul 1;127(1) doi: 10.1053/j.gastro.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 24.Michelsen KS, Arditi M. Toll-like receptors and innate immunity in gut homeostasis and pathology. Current opinion in hematology. 2007 Jan 1;14(1) doi: 10.1097/00062752-200701000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. The Journal of experimental medicine. 2003 Feb 17;197(4) doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, van Krieken JH, Hartung T, Adema G, Kullberg BJ. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. Journal of immunology (Baltimore, Md. : 1950) 2004 Mar 15;172(6) doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 27.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science (New York, N.Y.) 2005 Aug 26;309(5739) doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 28.Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. Journal of immunology (Baltimore, Md. : 1950) 2005 Dec 15;175(12) doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- 29.Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. The Journal of clinical investigation. 2006 Feb 1;116(2) doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2006 May 2;103(18) doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Current opinion in immunology. 2007 Feb 1;19(1) doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Fukata M, Breglio K, Chen A, Vamadevan AS, Goo T, Hsu D, Conduah D, Xu R, Abreu MT. The myeloid differentiation factor 88 (MyD88) is required for CD4+ T cell effector function in a murine model of inflammatory bowel disease. Journal of immunology (Baltimore, Md. : 1950) 2008 Feb 1;180(3) doi: 10.4049/jimmunol.180.3.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umesaki Y, Setoyama H. Structure of the intestinal flora responsible for development of the gut immune system in a rodent model. Microbes and infection / Institut Pasteur. 2000 Sep 1;2(11) doi: 10.1016/s1286-4579(00)01288-0. [DOI] [PubMed] [Google Scholar]
- 34.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, G UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. Journal of immunology (Baltimore, Md. : 1950) 2006 Dec 15;177(12) doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 35.Fisson S, Darrasse-Jèze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. The Journal of experimental medicine. 2003 Sep 1;198(5) doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2003 Jul 22;100(15) doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. The Journal of experimental medicine. 2003 Jul 21;198(2) doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siewert C, Lauer U, Cording S, Bopp T, Schmitt E, Hamann A, Huehn J. Experience-driven development: effector/memory-like alphaE+Foxp3+ regulatory T cells originate from both naive T cells and naturally occurring naive-like regulatory T cells. Journal of immunology (Baltimore, Md. : 1950) 2008 Jan 1;180(1) doi: 10.4049/jimmunol.180.1.146. [DOI] [PubMed] [Google Scholar]
- 39.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science (New York, N.Y.) 2003 Feb 14;299(5609) doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 40.Kubo T, Hatton RD, Oliver J, Liu X, Elson CO, Weaver CT. Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. Journal of immunology (Baltimore, Md. : 1950) 2004 Dec 15;173(12) doi: 10.4049/jimmunol.173.12.7249. [DOI] [PubMed] [Google Scholar]
- 41.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science (New York, N.Y.) 2011 Jan 21;331(6015) doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nature reviews. Immunology. 2003 Jul 1;3(7) doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 43.Cahill RJ, Foltz CJ, Fox JG, Dangler CA, Powrie F, Schauer DB. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infection and immunity. 1997 Aug 1;65(8) doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aranda R, Sydora BC, McAllister PL, Binder SW, Yang HY, Targan SR, Kronenberg M. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. Journal of immunology (Baltimore, Md. : 1950) 1997 Apr 1;158(7) [PubMed] [Google Scholar]
- 45.Prantera C, Zannoni F, Scribano ML, Berto E, Andreoli A, Kohn A, Luzi C. An antibiotic regimen for the treatment of active Crohn's disease: a randomized, controlled clinical trial of metronidazole plus ciprofloxacin. The American journal of gastroenterology. 1996 Feb 1;91(2) [PubMed] [Google Scholar]
- 46.Min B, Thornton A, Caucheteux SM, Younes SA, Oh K, Hu-Li J, Paul WE. Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. European journal of immunology. 2007 Jul 1;37(7) doi: 10.1002/eji.200737236. [DOI] [PubMed] [Google Scholar]
- 47.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. The Journal of experimental medicine. 2008 Dec 22;205(13) doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011 May 27;34(5) doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 49.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. International reviews of immunology. 2011 Feb 1;30(1) doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 50.Mills KH. TLR9 turns the tide on Treg cells. Immunity. 2008 Oct 17;29(4) doi: 10.1016/j.immuni.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Chen Q, Davidson TS, Huter EN, Shevach EM. Engagement of TLR2 does not reverse the suppressor function of mouse regulatory T cells, but promotes their survival. Journal of immunology (Baltimore, Md. : 1950) 2009 Oct 1;183(7) doi: 10.4049/jimmunol.0901465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores-Langarica A, Marshall JL, Hitchcock J, Cook C, Jobanputra J, Bobat S, Ross EA, Coughlan RE, Henderson IR, Uematsu S, Akira S, Cunningham AF. Systemic flagellin immunization stimulates mucosal CD103+ dendritic cells and drives Foxp3+ regulatory T cell and IgA responses in the mesenteric lymph node. Journal of immunology (Baltimore, Md. : 1950) 2012 Dec 15;189(12) doi: 10.4049/jimmunol.1202283. [DOI] [PubMed] [Google Scholar]
- 53.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008 Oct 17;29(4) doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardenberg G, Yao Y, Piccirillo CA, Levings MK, Steiner TS. Toll-like receptor 5 deficiency protects from wasting disease in a T cell transfer colitis model in T cell receptor-β-deficient mice. Inflamm Bowel Dis. 2012 Jan;18(1):85–93. doi: 10.1002/ibd.21738. [DOI] [PubMed] [Google Scholar]
- 55.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010 Mar 19;140(6) doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 56.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998 Jul 1;9(1) doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 57.Heimesaat MM, Fischer A, Siegmund B, Kupz A, Niebergall J, Fuchs D, Jahn HK, Freudenberg M, Loddenkemper C, Batra A, Lehr HA, Liesenfeld O, Blaut M, Göbel UB, Schumann RR, Bereswill S. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PloS one. 2007 Jul 25;2(7): doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heimesaat MM, Fischer A, Jahn HK, Niebergall J, Freudenberg M, Blaut M, Liesenfeld O, Schumann RR, Göbel UB, Bereswill S. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007 Jul 1;56(7) doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalis C, Gumenscheimer M, Freudenberg N, Tchaptchet S, Fejer G, Heit A, Akira S, Galanos C, Freudenberg MA. Requirement for TLR9 in the immunomodulatory activity of Propionibacterium acnes. Journal of immunology (Baltimore, Md. : 1950) 2005 Apr 1;174(7) doi: 10.4049/jimmunol.174.7.4295. [DOI] [PubMed] [Google Scholar]
- 60.Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, Hemmi H, Coban C, Kawai T, Ishii KJ, Takeuchi O, Miyasaka M, Takeda K, Akira S. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on. Nature immunology. 2006 Aug 1;7(8) doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]