Abstract
The occurrence of tetracycline resistance determinants in 203 Escherichia coli isolates recovered from clinical samples at three different hospitals in Nigeria between June 2009 and May 2010 was investigated. The isolates were subjected to standard procedures. Antibiotic susceptibility to a panel of eight antibiotics was also performed, and resistance genes were detected with the polymerase chain reaction (PCR) technique. One hundred and six E. coli isolates (52.2%) were obtained at LAUTECH Teaching Hospital Osogbo, 85 (41.9%) from OAUTHC Ile Ife and 12 (5.9%) from Osun State Hospital Asubiaro Osogbo. Result of the disk diffusion antibiotic susceptibility test showed 96.1% isolates to be resistant to ampicillin, 77.8% to tetracycline, 37.9% to cotrimoxazole, 38.4% to nalidixic acid, 20.7% to ofloxacin, 17.7% to ceftriaxone, 11.8% to gentamycin, and 2% to nitrofurantoin. One hundred and sixty two (79.9%) isolates had minimum inhibitory concentration (MIC) of tetracycline ≥ 128 μg/ml. The polymerase chain reaction (PCR) detected tetA gene in 89 (43.8%) isolates, tetB gene in 65 (32.0%), and both tetA and tetB genes in 9 (4.4%) isolates. The study demonstrated a relatively high level of gene mediated antibiotic resistance to tetracycline and other antibiotics in E. coli clinical isolates in Southwest region of Nigeria.
Keywords: resistance, tetracycline, tet gene, Escherichia coli
Introduction
In recent years, there have been increasing reports of bacteria resistant to multiple antimicrobial agents involved in clinical infections. This situation is threatening the effectiveness of even the most reliable antibiotics used to treat bacterial infections [1]. The problems of resistant bacteria are particularly more serious in hospitals and nursing homes where patients are treated for acute or chronic infective conditions [2]. In developing countries, on the other hand, antibiotic-resistant bacteria transmission often occurs in communities by person-to-person transfer, through contaminated food, unsafe drinking water, or by insects. Resistance can mean that people infected with such bacteria do not respond to conventional drugs and, if no other treatment options are available, must depend on their immune system to overcome the disease [1, 3].
Tetracyclines are antibiotics which inhibit bacterial growth by interfering with protein synthesis. The emergence of bacterial resistance to these antibiotics has nowadays limited their use. Three different mechanisms of tetracycline resistance have been identified so far: tetracycline efflux, ribosome protection, and tetracycline modification [4, 5]. Tetracycline efflux is achieved by an export protein from the major facilitator super family (MFS) which contains 12 transmembrane fragments (TMS) in Gram-negative bacteria and 14 in Gram-positive bacteria. Ribosome protection is mediated by a soluble protein which shares homology with the GTPases participating in protein synthesis by elongation factor-Tu (EF-Tu) and EF-G. The third mechanism involves a cytoplasmic protein that chemically modifies tetracycline, a reaction that takes place only in the presence of oxygen and NADPH and does not function in the natural host [4–6].
The most common resistance mechanism in Gram-negative bacteria is the energy-dependent efflux pump system which is encoded by the genes tetA, tetB, tetC, tetD, and tetG, with tetA and tetB genes being the most frequently described. The objective of this study is to determine the prevalence of tetracycline resistance genes (tetA and tetB) carriage in Escherichia coli, a prototype Gram-negative bacterium that has been reported to be commonly involved in clinical infections in our environment [1, 7–10].
Materials and methods
Study area
This study was conducted in three hospitals located in two major towns in Osun State: Ladoke Akintola University of Technology (LAUTECH) Teaching Hospital Osogbo, Obafemi Awolowo University Teaching Hospital Complex (OAUTHC) Ile-Ife, and Osun State Hospital Asubiaro Osogbo between June 2009 and May 2010. Osun State is located in the Southwestern part of Nigeria with Osogbo as the capital city.
Study design
This research is a clinico-laboratory investigation of tetracycline resistant determinants in E. coli isolates in Osun State, Nigeria.
Subjects
The subjects include all categories of patients with clinical illness on hospital admission or outpatient treatment. Informed consent was obtained from each subject participant, and ethical committee approval from the hospitals was obtained before the conduct of the study.
Specimen collection
Various clinical specimens (urine, high vaginal swab, stool, wound, and blood) were collected from the subjects. All samples were collected at regular intervals into sterile sampling bottles and transported in an insulated sampling case to the laboratory for examination. Demographic and clinical data of each patient were obtained from the request forms and laboratory register.
Isolation and identification of E. coli
The isolation of E. coli from clinical specimens was carried out according to standard microbiological methods [11]. Specimens were first grown in Buffered Peptone Water (Oxoid, UK) at 37 °C for 24 h and then cultured on TBX agar and incubated aerobically at 44 °C for 24 h. Suspected colonies of E. coli from the culture plates were confirmed by testing for oxidase (OXItest, Pliva-Lachema, CZ) and indole production (COLItest, Pliva-Lachema, CZ). Confirmed colonies were purified and used for susceptibility test and PCR.
Disk diffusion antimicrobial susceptibility testing
E. coli isolates were tested for their susceptibility against antimicrobial agents by the disk diffusion method according to Clinical and Laboratory Standards Institute (CLSI) guideline [12]. The antimicrobial disks (Oxoid, UK) used include: ampicillin (10 μg), gentamycin (10 μg), nalidixic acid (30 μg), nitrofurantoin (30 μg), ofloxacin (30 μg), ceftriaxone (30 μg), tetracycline (30 μg), and cotrimoxazole (25 μg). E. coli NCTC 1515 was used as the control strain. Isolate susceptibility was evaluated based on the size of the zones of inhibition and classified as susceptible (S), intermediate resistant (I), or resistant (R) according to the CLSI criteria [12].
Dilution test for determination of minimum inhibitory concentration (MIC) of E. coli
Double dilutions of antimicrobial agents were prepared in Mueller Hinton broth (Oxoid, UK) in test tubes according to standard procedures [12]. A standardized inoculum (5 · 105 CFU/ml) of the test organism was added to each of the prepared antibiotic dilutions in the tubes. The same was performed for the control strain. Incubation was done aerobically at 35–37 °C for 18–24 h, and tubes were observed for growth. The tube with lowest concentration (highest dilution) of antibiotics to show no growth (no turbidity) is the MIC. The tetracycline MIC breakpoints and interpretative criteria for each antibiotic to categorize isolate into susceptible (S), intermediate resistant (I), or resistant (R) were as set in the CLSI guideline [12]; if the MIC was <5 μg/ml, the isolate was considered sensitive; if it was 10–70 μg/ml, the isolate was considered intermediate resistant; and if MIC was >90 μg/ml, the isolate was considered highly resistant.
Detection of tetracycline resistance genes by PCR
All E. coli isolates resistant to tetracycline (n = 203) were tested for carriage of tetracycline resistant genes: tetA and tetB using polymerase chain reaction (PCR). First, bacterial DNA was isolated from a 24-hour culture of E. coli on nutrient agar by lysis of bacterial cell suspension at 95.5 °C for 10 min with the addition of 20% Chelex 100 (Bio-Rad, France) followed by centrifugation. The supernatant was used as template DNA. For the detection of the tet gene, primers used were as shown on Table 1, dissolved in 25 ml of reaction mixture containing Taq-Purple DNA polymerase and MgCl2 (Top-Bio, CZ). The PCR program consisted of an initial denaturation step at 94 °C for 30 s, followed by 30 cycles of DNA denaturation at 94 °C for 30 s, primer annealing for tetA at 62 °C and primer extension at 72 °C for 30 s while the annealing temperature of tetB was 58 °C and primer extension at 72 °C for 1 min. After the last cycle, a final extension step at 72 °C for 5 min was added. PCR products were analyzed by gel electrophoresis in 1.5% agarose (Serva, Germany) followed by visualization in a transilluminator after staining in ethidium bromide [13].
Table 1.
Primers used to amplify genes encoding tetracycline resistance in Escherichia coli
| Gene | Forward sequence Primer (5´ to 3´) | Reverse sequence Primer (5´ to 3´) | Annealing Temp (°C) | Product Size (bp) |
|---|---|---|---|---|
|
| ||||
| tetA | GGCGGTCTTCTTCTTCATCATGC | CGGCAGGCAGAGCAAGTAGA | 62 | 501 |
| tetB | CATTAATAGGCCCATCGCTG | TGAAGGTCATCGATAGCAGG | 58 | 929 |
|
| ||||
Data entry and analysis
Clinical and laboratory data were entered into a Windows 7 laptop computer with the Statistical Package for Social Sciences (SPSS 15.0) software. Frequency tables were generated and data analyzed using appropriate statistical methods.
Results
A total of 203 E. coli isolates were obtained from the three health institutions between June 2009 and May 2010; 106 isolates were obtained from LAUTECH Teaching Hospital, 85 from OAUTHC Ile Ife, and 12 from the State Hospital Asubiaro. The distribution of the 203 isolates in relation to gender of patients from whom they were recovered shows 92 (45.3%) males and 111 (54.7%) females, and in relation to age group distribution, age ≤10 years had 19.7% (40), 11–20 years 11.8% (24); 21–30 years 23.6% (48), 31–40 years 16.3% (33), 41–50 years 10.3% (21), 51–60 years 6.4% (13), 61–70 years 9.9% (20) and 71–80 years 2% (4) (Table 2).
Table 2.
Distribution of Escherichia coli isolates in relation to age group of patients
| Age group (years) | No. of isolates | Percentage |
|---|---|---|
|
| ||
| ≤10 | 40 | 19.7 |
| 11–20 | 24 | 11.8 |
| 21–30 | 48 | 23.6 |
| 31–40 | 33 | 16.3 |
| 41–50 | 21 | 10.3 |
| 51–60 | 13 | 6.4 |
| 61–70 | 20 | 9.9 |
| 71–80 | 4 | 2.0 |
|
| ||
| Total | 203 | 100.0 |
|
| ||
Table 3 shows the distribution of the 203. E. coli isolates in relation to clinical specimens from which they were recovered; 99 (48.7%) isolates were obtained from urine, 17 (8.4%) from high vaginal swab, 67 (33.0%) from stool, 16 (7.9%) from wound, and 4 (2.0%) from blood.
Table 3.
Distribution of Escherichia coli isolates from various clinical specimens
| Specimen types | No. of isolates | Percentages |
|---|---|---|
|
| ||
| Urine | 99 | 48.7 |
| High vaginal swab (HVS) | 17 | 8.4 |
| Wound | 16 | 7.9 |
| Stool | 67 | 33.0 |
| Blood | 4 | 2.0 |
|
| ||
| Total | 203 | 100.0 |
|
| ||
Table 4 shows the antibiotic disk susceptibility pattern of the isolates to eight antimicrobial agents used; 158 isolates (77.8%) were resistant to tetracycline, 195 (96.1%) to ampicillin, and 77 (37.9%) to cotrimoxazole. The susceptibility pattern to other antibiotics is shown in the table.
Table 4.
Antibiotic susceptibility of Escherichia coli isolates to antibiotics
| Antibiotics | No. of sensitive isolates (%) | No. of intermediately resistant isolates (%) | No. of resistant isolates (%) |
|---|---|---|---|
|
| |||
| Amp | 8 (3.9) | 0 (0) | 195 (96.1) |
| Gen | 127 (62.6) | 52 (25.6) | 24 (11.8) |
| Nit | 155 (76.4) | 44 (21.7) | 4 (2.0) |
| Nal | 76 (37.4) | 49 (24.1) | 78 (38.4) |
| Cot | 82 (40.4) | 44 (21.7) | 77 (37.9) |
| Cef | 135 (66.5) | 32 (15.8) | 36 (17.7) |
| Ofl | 120 (59.1) | 41 (20.2) | 42 (20.7) |
| Tet | 45 (22.2) | 0 (0) | 158 (77.8) |
|
| |||
| Key: Amp – ampicillin, Gen – gentamycin, Nit – nitrofurantoin, Nal – nalidixic acid, Cot – cotrimoxazole, Cef – ceftriaxone, Ofl – ofloxacin, Tet – tetracycline | |||
Table 5 shows the MIC values of tetracycline for the isolates with all the 203 isolates categorized as resistant (MIC >5 μg/ml); 13 isolates (6.4%) had MIC value of 8 μg/ml, 20 (9.9%) had MIC value of 16 μg/ml, 4 (2.0%) MIC value of 32 μg/ml, 4 (2.0%) had MIC value of 64 μg/ml, 20 (9.9%) had MIC value of 128 μg/ml, 114 (56.2%) had MIC value of 256 μg/ml, and 28 (13.8%) had MIC value of >256 μg/ml.
Table 5.
Minimum inhibitory concentration (MIC) of tetracycline for Escherichia coli isolates
| MIC value (µg/ml) (“S”, “I”, or “R”) | No. of isolates | Percentage |
|---|---|---|
|
| ||
| 8 (I) | 13 | 6.4 |
| 16(I) | 20 | 9.9 |
| 32 (I) | 4 | 2.0 |
| 64 (I) | 4 | 2.0 |
| 128 (R) | 20 | 9.9 |
| 2569 (R) | 114 | 56.2 |
| >256 (R) | 28 | 13.8 |
|
| ||
| Total | 203 | 100.0 |
|
| ||
| Key: “S”, sensitive; “I”, intermediate; “R”, resistant | ||
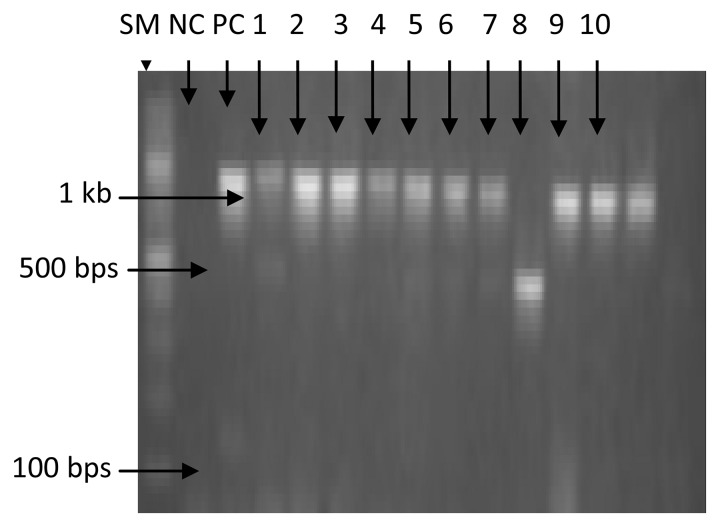
Of the 203 isolates analyzed by PCR, 43.8% (89 of 203) carry tetA gene, 32.0% (65 of 203) carry tetB gene, while 4.4% (9 of 203) carry both genes. Figures 1 and 2 show the gel electrophoretic band pattern of the amplified products of representative isolates with tetA band at approximately 500 bp and tetB band at approximately 1000 bp.
Fig. 1.
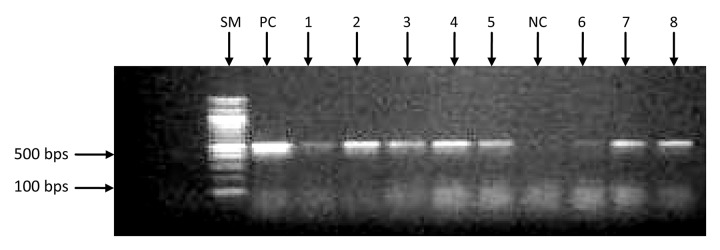
Agarose gel electrophoresis of amplified tetA gene products (~500 bp) from representative Escherichia coli isolates. SM: size marker; PC: positive control; NC: negative control; Lanes 1, 2, 3, 4, 5, 7, and 8 show the presence of tetA gene; Lane 6 shows that tetA gene is not present.
Fig. 2.
Agarose gel electrophoresis of amplified tetB gene products (~1000 bp) from representative Escherichia coli isolates. SM: size marker; PC: positive control; NC: negative control; Lanes 1, 2, 3, 4, 5, 6, 7, 9, and 10 indicate the presence of tetB gene; Lane 8 indicates the absence of tetB gene.
Discussion
E. coli strains have been widely implicated in various clinical infections as hospital and community acquired infections [14]. Pathogenic strains of E. coli have relatively high potentials for developing resistance [15]. High resistance of E. coli to antimicrobial agents tested was observed in this study, and this correlated well with the results from the study of antimicrobial resistant pattern of E. coli from human clinical samples in Osogbo, Southwestern Nigeria [1, 10] where high resistance rates (>85%) were reported against tetracycline, sulphonamides, and cotrimoxazole. Likewise, the prevalence of resistance in this study showed that resistance profile of E. coli is ≤40% for all drugs except nitrofurantoin, gentamycin, and nalidixic acid. This is in harmony with what was observed in a previous study [16]. The high resistance rate is an indication of poor antibiotic policy with indiscriminate use of antibiotics in this region along with poor hygiene and infection control practices which aid spread of resistance.
The four antibiotics for which a considerable rise in resistance rate was seen in this study were tetracycline, ampicillin, cotrimoxazole, and nalidixic acid, which are extensively used in Nigeria [1, 17]. Such multi-drug resistance has serious implications for the empiric therapy of infections caused by E. coli and for possible co-selection of antimicrobial resistance mediated by multi-drug resistance plasmids [18]. Ingestion of antibiotics is known to provide selective pressure ultimately leading to a higher prevalence of resistant bacteria. This study also shows the age distribution of the subjects used; age groups 21–30, ≤10, and 31–40 have the highest occurrence with 23.6%, 19.7%, and 16.3%, respectively. In contrast, age range 71–80 has the least occurrence with 2%. This implies that infection is common among children and middle aged group, which correlated well with what was observed in a study carried out by previous workers [19]. In this study, E. coli recovery was found to be very high in urine with 48.7% and stool with 33%, which also correlated with previous report [20]. This is because E. coli is responsible for over 70% urinary tract infections and also responsible for most cases of gastroenteritis.
From molecular identification of tet genes in E. coli in this study like previous works [21–23], it shows a rise in resistance pattern confirming the alarming spread of resistance genes in this region. The MIC values also correlated with the level of resistance displayed by isolates carrying tetA gene (43.8%) and tetB gene (32%). Several earlier reports on tetracycline resistance genes in both humans and animals have been reported [5, 24, 25], even from farm products, especially from cow milk. In prior clinical surveys, the tetB gene was the most prevalent tetracycline resistance determinant identified; evidence shows that it has wide host range due to the ability to reside on highly mobile genetic elements that effectively transfer them among bacterial genera. Other workers too have reported the ability of tetA gene to freely spread in farm animals than tetB, and that this determinant can be concluded or summarized that tetA gene can be spread more easily in the environment than tetB gene [5, 25]. According to some other workers, tetracycline resistance in relation to plasmid incompatibility has also been reported, moreover just like other determinants, tetC and tetD genes are located on conjugative plasmids of different compatibility groups with even tetA [21], whereas the tetE determinant is located on the chromosome in some isolates and also associated with large, non-conjugative, non-mobile plasmids [6], hence may not easily spread as others. Nine (4.4%) isolates were positive for both genes in this study, which as reported earlier by other workers, there is tendency for E. coli to harbor more than one gene [22, 25].
Although this may be the first report on molecular detection of tetracycline resistance in E. coli isolate in this region, the data obtained show that there is variation in the distribution pattern of tetA and tetB genes from the different centers. Both of these genes encode an energy-dependent efflux pump system, one of the most frequently used mechanisms of tetracycline resistance in bacteria of the family Enterobacteriaceae [13, 20, 23, 25].
Conclusion
Our study showed high antibiotic resistance in E. coli isolates to tetracycline and other commonly used antibiotics in this environment. The high carriage rate of tetA and tetB genes among the E. coli isolates suggests that these genes are the major determinant of resistance to tetracycline in this environment.
Contributor Information
O. A. Olowe, 1Department of Medical Microbiology and Parasitology, College of Health Sciences, Ladoke Akintola University of Technology, PMB 4400, Osogbo, Osun State, Email: olowekunle@yahoo.com, Nigeria.
O. J. Idris, 1Department of Medical Microbiology and Parasitology, College of Health Sciences, Ladoke Akintola University of Technology, PMB 4400, Osogbo, Osun State, Nigeria.
S. S. Taiwo, 1Department of Medical Microbiology and Parasitology, College of Health Sciences, Ladoke Akintola University of Technology, PMB 4400, Osogbo, Osun State, Nigeria.
References
- 1.Olowe OA, Okanlawon BM, Olowe RA, Olayemi AB. Antimicrobial resistant pattern of Escherichia coli from human clinical samples in Osogbo, Southwestern Nigeria. Afr J Microbiol Res. 2008;2:8–11. [Google Scholar]
- 2.Fish DN, Ohlinger MJ. Antimicrobial resistance: factors and outcomes. Critical care clinics. 2006 Apr 1;22(2) doi: 10.1016/j.ccc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Olowe OA. Mechanisms of antimicrobial resistance in bacteria; general approach. Int J Pharma Med Biol Sci. 2012;1(2):166–187. [Google Scholar]
- 4.Roberts MC. Tetracycline resistance determinants: mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS microbiology reviews. 1996 Oct 1;19(1) doi: 10.1111/j.1574-6976.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 5.Koo HJ, Woo GJ. Distribution and transferability of tetracycline resistance determinants in Escherichia coli isolated from meat and meat products. International journal of food microbiology. 2011 Feb 28;145(2-3) doi: 10.1016/j.ijfoodmicro.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Roberts MC. Epidemiology of tetracycline-resistance determinants. Trends in microbiology. 1994 Oct 1;2(10) doi: 10.1016/0966-842x(94)90610-6. [DOI] [PubMed] [Google Scholar]
- 7.Tobih JE, Taiwo SS, Olowe OA, Olaosun OA, Adejumao SD. Clinical and microbiological profiles of ear infections in Osogbo, Nigeria. Tropical doctor. 2006 Jul 1;36(3) doi: 10.1258/004947506777978109. [DOI] [PubMed] [Google Scholar]
- 8.Olowe OA, Grobbel M, Büchter B, Lübke-Becker A, Fruth A, Wieler LH. Detection of bla(CTX-M-15) extended-spectrum beta-lactamase genes in Escherichia coli from hospital patients in Nigeria. International journal of antimicrobial agents. 2010 Feb 1;35(2) doi: 10.1016/j.ijantimicag.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Olowe OA, Aboderin BW. Detection of extended spectrum beta-lactamase producing strains of Escherichia coli and Klebsiella spp. in a tertiary health centre in Ogun State. Int J Trop Med. 2010;3(3):62–64. [Google Scholar]
- 10.Taiwo SS, Fadiora SO, Fayemiwo SA. High antimicrobial resistance among bacterial isolates of blood stream infections (BSI) in a Nigerian University Teaching Hospital. World J Microbiol Biotechnol. 2008;24(2):231–236. [Google Scholar]
- 11.Cheesbrough M. Medical Laboratory Manual for Tropical Countries: Microbiology. Cambridge: University Press; 2000. [Google Scholar]
- 12.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Sixteenth Informational Supplement. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2006. document M100-S16; p. 183. [Google Scholar]
- 13.Fluit AC, Visser MR, Schmitz FJ. Molecular detection of antimicrobial resistance. Clinical microbiology reviews. 2001 Oct 1;14(4) doi: 10.1128/CMR.14.4.836-871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah AA, Hassan F, Hameed A. Study on the prevalence of enterobacteriaceae in hospital acquired and community acquired infections. Pak J Med Res. 2002;41:1. [Google Scholar]
- 15.Karlowsky JA, Jones ME, Draghi DC, Thornsberry C, Sahm DF, Volturo GA. Prevalence and antimicrobial susceptibilities of bacteria isolated from blood cultures of hospitalized patients in the United States in 2002. Annals of clinical microbiology and antimicrobials. 2004 May 10;3 doi: 10.1186/1476-0711-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwalokun BA, Gbenle GO, Smith SI, Ogunledun A, Akinsinde KA, Omonigbehin EA. Epidemiology of shigellosis in Lagos, Nigeria: trends in antimicrobial resistance. Journal of health, population, and nutrition. 2001 Sep 1;19(3) [PubMed] [Google Scholar]
- 17.Okeke IN, Lamikanra A, Edelman R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerging infectious diseases. 1999 Jan-Feb;5(1) doi: 10.3201/eid0501.990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherley M, Gordon DM, Collignon PJ. Evolution of multi-resistance plasmids in Australian clinical isolates of Escherichia coli. Microbiology (Reading, England) 2004 May 1;150(Pt 5) doi: 10.1099/mic.0.26773-0. [DOI] [PubMed] [Google Scholar]
- 19.Hawkey PM. The growing burden of antimicrobial resistance. The Journal of antimicrobial chemotherapy. 2008 Sep 1;62 Suppl 1 doi: 10.1093/jac/dkn241. [DOI] [PubMed] [Google Scholar]
- 20.Lewis K, Salyers AA, Taber HW, Wax RG. In: Bacterial Resistance to Antimicrobials. Lewis K, Salyers AA, Taber HW, Wax RG, editors, editor. New York: Marcel Dekker, Inc.; 2002. p. 495. [Google Scholar]
- 21.Jones CS, Osborne DJ, Stanley J. Enterobacterial tetracycline resistance in relation to plasmid incompatibility. Molecular and cellular probes. 1992 Aug 1;6(4) doi: 10.1016/0890-8508(92)90007-k. [DOI] [PubMed] [Google Scholar]
- 22.Ozgumus OB, Celik-Sevim E, Alpay-Karaoglu S, Sandalli C, Sevim A. Molecular characterization of antibiotic resistant Escherichia coli strains isolated from tap and spring waters in a coastal region in Turkey. Journal of microbiology (Seoul, Korea) 2007 Oct 1;45(5) [PubMed] [Google Scholar]
- 23.Tuckman M, Petersen PJ, Howe AY, Orlowski M, Mullen S, Chan K, Bradford PA, Jones CH. Occurrence of tetracycline resistance genes among Escherichia coli isolates from the phase 3 clinical trials for tigecycline. Antimicrobial agents and chemotherapy. 2007 Sep 1;51(9) doi: 10.1128/AAC.00625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryan A, Shapir N, Sadowsky MJ. Frequency and distribution of tetracycline resistance genes in genetically diverse, nonselected, and nonclinical Escherichia coli strains isolated from diverse human and animal sources. Applied and environmental microbiology. 2004 Apr 1;70(4) doi: 10.1128/AEM.70.4.2503-2507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skocková A, Cupáková Š, Karpíšková R, Janštová B. Detection of tetracycline resistance genes in Escherichia coli from raw cow’s milk. J Microbiol, Biotechnol Food Sci. 2012;1:777–784. [Google Scholar]