Abstract
Non-pathogenic Escherichia coli (Ec) strains K12 (EcK12) and Nissle 1917 (EcN) are used for gene technology and probiotic treatment of intestinal inflammation, respectively. We investigated intestinal colonization and potential pro-inflammatory properties of EcK12, EcN, and commensal E. coli (EcCo) strains in Toxoplasma (T.) gondii-induced acute ileitis. Whereas gnotobiotic animals generated by quintuple antibiotic treatment were protected from ileitis, mice replenished with conventional microbiota suffered from small intestinal necrosis 7 days post-T. gondii infection (p.i.). Irrespective of the Ec strain, recolonized mice revealed mild to moderate histopathological changes in their ileal mucosa. Upon stable recolonization with EcK12, EcN, or EcCo, development of inflammation was accompanied by pro-inflammatory responses at day 7 p.i., including increased ileal T lymphocyte and apoptotic cell numbers compared to T. gondii-infected gnotobiotic controls. Strikingly, either Ec strain was capable to translocate to extra-intestinal locations, such as MLN, spleen, and liver. Taken together, Ec strains used in gene technology and probiotic treatment are able to exert inflammatory responses in a murine model of small intestinal inflammation. In conclusion, the therapeutic use of Ec strains in patients with broad-spectrum antibiotic treatment and/or intestinal inflammation should be considered with caution.
Keywords: gnotobiotic mice, Escherichia coli, E. coli Nissle 1917, E. coli K12, security strains, probiotic, colonization resistance, Toxoplasma gondii, ileitis, acute intestinal inflammation, Th1-type immunopathology, T lymphocytes, bacterial translocation, mesenteric lymph nodes, liver, spleen, apoptosis, pro-inflammatory potential
Introduction
Over the past decades, Escherichia (E.) coli K12 (EcK12), originally detected in 1922, was extensively used in molecular biological laboratories for genetic cloning, such as recombination by conjugation and transformation [1, 2, 3]. EcK12 is considered an apathogenic “security” strain given that inherent genetic deficits prevent EcK12 from adhesion to intestinal epithelial cells and thus from colonization of the vertebrate intestinal tract [4–6]. In contrast to EcK12, E. coli Nissle 1917 (EcN) strain is capable to colonize the intestinal tract of vertebrates [7–9]. In 1917, Dr. Alfred Nissle isolated EcN from a fecal sample of a soldier during the first world war staying unaffected during a severe outbreak of shigellosis [10]. The well-characterized strain belongs to the heterogeneous serogroup O6 including pathogenic variants [11], is considered safe, and is recommended as a probiotic treatment option in acute enteritis in children [12] and inflammatory bowel diseases (IBD), such as ulcerative colitis [13] and Crohn’s disease [14]. We have recently shown that following oral high dose infection with Toxoplasma (T.) gondii, susceptible C57BL/6 mice harboring a conventional gut microbiota develop acute terminal ileitis within 7 days post-infection (p.i.) [15–17]. This immunopathology is mediated by a strong Th1-type immune response with a subsequent storm of pro-inflammatory cytokines such as IL-12, IL-18, IFN-γ, TNF-α, and nitric oxide (NO), but also involves IL-23 and IL-22 [15, 18–23]. Furthermore, acute ileitis is accompanied by characteristic shifts in ileal microbiota composition with a loss in species diversity and a luminal overgrowth of the inflamed ileum with Gram-negative commensals such as E. coli [17, 22, 24, 25]. Taken together, the Th1-type immunopathology together with the characteristic intestinal microbiota changes in the T. gondii-mediated ileitis model resemble immunological key features of acute episodes in human Crohn’s disease (ileitis terminalis).
Given that overgrowing intestinal commensal E. coli were shown to induce and further perpetuate this inflammatory scenario via TLR-4-dependent lipopolysaccharide (LPS) signaling [24, 25], we were hypothesizing that LPS from E. coli strains considered safe and probiotic might also exert pro-inflammatory actions in murine T. gondii-induced ileitis. We here for the first time investigated the pro-inflammatory potential of EcK12 and EcN strains in an acute ileitis model following selective reassociation of gnotobiotic mice with the respective E. coli strain before ileitis induction by oral T. gondii infection.
Materials and methods
Ethics statement
All animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin; Reg. no.: G0170/04). Animal welfare was monitored twice daily by assessment of clinical conditions and weight loss of mice.
Generation of gnotobiotic mice
Female C57BL/6j (wildtype) mice were bred and housed under specific pathogen-free (SPF) conditions in the Forschungsinstitut für Experimentelle Medizin (Charité, Berlin, Germany). To eradicate the commensal gut microbiota, conventionally colonized mice were transferred to sterile cages and treated by adding ampicillin (1 g/L; Ratiopharm), vancomycin (500 mg/L; Cell Pharm), ciprofloxacin (200 mg/L; Bayer Vital), imipenem (250 mg/L; MSD), and metronidazole (1 g/L; Fresenius) to the drinking water ad libitum for 8–10 weeks as described earlier [17] starting at 8 weeks of age.
E. coli strains
E. coli K12 (EcK12) MG1655 and E. coli Nissle 1917 (EcN) strains were kindly provided by Dr. Sya Ukena (Helmholtz Center for Infection Research, Braunschweig, Germany). Furthermore, a commensal E. coli control strain (EcCo) was isolated from naïve, healthy 3-month-old C57BL/6 mice by culture on MacConkey media as described previously (characterized and named “EcM” strain [17, 24, 26]). PCR-based detection revealed that this strain does not contain known virulence factors of pathogenic E. coli, such as stx 1 and 2, catA, hlyA, cspA, katP, and astA [17, 24, 26].
Recolonization of gnotobiotic mice
Recolonization experiments of gnotobiotic mice were performed as described earlier [17]. In brief, 2 days prior to recolonization experiments, the antibiotic cocktail was withheld and substituted by sterilized tap water. E. coli strains were grown in supplemented brain heart infusion broth to a turbidity equivalent of 6 McFarland units (a bacterial load of approximately 109–1010 per milliliter), harvested by centrifugation, washed once, resuspended in 5 mL PBS, and administered by gavage in a final volume of 0.3 mL for 3 consecutive days. Bacterial loads of oral suspensions were determined by cultivation of serial dilutions on solid media (Columbia and MacConkey Agar; Oxoid). To assure stable establishment of the respective E. coli strain within the entire gastrointestinal (GI) tract, recolonized animals were kept for 4 days before acute ileitis induction.
Parasites and ileitis induction
Four days following the final of three consecutive oral E. coli recolonizations, mice were infected perorally with 100 T. gondii cysts (ME49 strain) from homogenized brains of intraperitoneally infected NMRI mice in a volume of 0.3 mL phosphate-buffered saline (PBS, pH 7.4) by gavage in order to induce acute ileitis, as described previously [17].
Sampling procedures
In ileitis experiments, mice were sacrificed by isoflurane (Abbott) 7 days following T. gondii infection. Mesenteric lymph nodes, spleen and liver, as well as luminal samples taken from the terminal ileum of each mouse, were removed under sterile conditions for culture. In parallel, ileal tissue samples were collected in parallel for histopathological and immunohistochemical analyses.
Histological scores and determination of parasite load
Histological scores of ileitis and parasite loads were determined in tissue samples from terminal ileum immediately fixed in 5% formalin and embedded in paraffin as described earlier [17, 24]. Sections (5 µm) were stained with hematoxylin and eosin (HE) and examined by light microscopy. Our standardized “acute ileitis” score ranging from 0 to 6 was used for blinded duplicate evaluation (by M.M.H. and A.A.K.): 0, normal; 1, minimal focal inflammation, edematous blubbing, intact epithelium; 2, mild inflammation of mucosa and submucosa, cell-free exudate into the lumen, but intact epithelium; 3, moderate inflammation of mucosa and submucosa, erosions and/or ulcerations, cryptitis or crypt abscesses, cellular shedding into the lumen; 4, severe inflammation of mucosa and submucosa, ulcerations, fibrosis, distortion of villous architecture, beginning epithelial disintegration; 5, severe inflammation, mucosal destruction < 50% of small intestine length; 6, severe inflammation, complete destruction > 50% of small intestine length, severe necroses [17]. Numbers of parasitophorous vacuoles containing tachyzoites or tachyzoite antigens were determined by immunohistology using a rabbit anti-T. gondii-IgG antiserum and counted in the terminal ileum, as previously described [21]. In addition, T. gondii DNA in ileal biopsies was quantified as described earlier [22].
Immunohistochemistry
In situ immunohistochemical analysis of ileal paraffin sections was performed as described previously [27–30]. Primary antibodies against CD3 (#N1580, Dako, Denmark, dilution 1:10) and cleaved caspase-3 (Asp175; Cell Signaling, USA, 1:200) were used. For each animal, the average number of positive stained cells within at least six independent high power fields (HPF, 400× magnification) were determined microscopically by two independent investigators (M.M.H. and A.A.K.) and subjected to statistical analysis as indicated.
Cultural analysis of the intestinal microbiota and bacterial translocation
Cultural analyses of feces and luminal ileal contents were performed in serial dilutions on solid media such as Columbia and MacConkey (Oxoid) under aerobic conditions (2 days incubation at 37 °C), and the bacterial species identified morphologically and biochemically as described earlier [17]. For assessment of bacterial translocation, homogenates of MLN, spleen, and liver (in sterile PBS) were plated onto respective solid media and cultured as described above.
Statistical analysis
Mean values, medians, standard deviations (SD), and levels of significance were determined using appropriate tests as indicated (Mann–Whitney U-test or Student’s t-test). Two-sided probability (p) values ≤0.05 were considered significant. All experiments were repeated at least twice.
Results
E. coli Nissle 1917 and E. coli K12 stably colonize conventional mice following antibiotic treatment
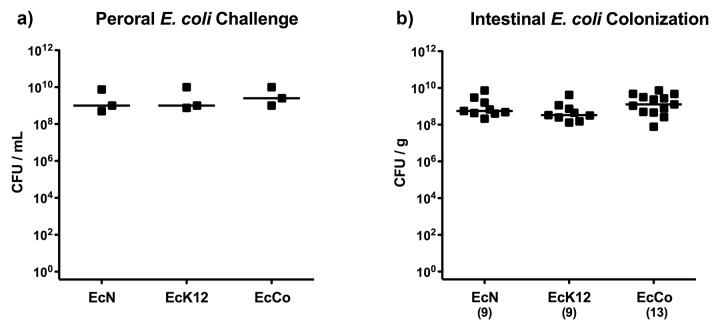
We were first investigating whether the E. coli strains Nissle 1917 and K12 (MG1655), the latter supposed to be incapable of colonizing the vertebrate GI tract [4–6], were able to establish within the murine intestines. We therefore generated gnotobiotic mice lacking any GI bacteria following an 8- to 10-week course of quintuple antibiotic therapy thereby abrogating physiological colonization resistance and thus facilitating colonization of the respective strains. Gnotobiotic mice were perorally challenged on 3 consecutive days with approximately 109 CFU EcK12, EcN, or EcCo, a commensal E. coli control strain previously isolated from our healthy conventionally colonized C57BL/6 wildtype mice (Fig. 1a). Four days thereafter (and right before ileitis induction), E. coli strains had stably colonized the intestinal tract as indicated by comparably high fecal loads of approximately 109 CFU per gram feces, irrespective of the E. coli strain used (Fig. 1b), indicating that even the EcK12 strain was able to establish within gnotobiotic animals.
Fig. 1.
Intestinal E. coli loads following oral recolonization of gnotobiotic mice. (A) Gnotobiotic mice generated by quintuple antibiotic treatment were perorally recolonized with E. coli Nissle 1917 (EcN), E. coli K12 (EcK12) or a commensal E. coli (EcCo) strain on 3 consecutive days as described in methods. Indicated are individual bacterial concentrations (CFU, colony forming units per mL; black squares) in suspensions used for gavage and medians (black bars). Data shown are representative for three independent experiments. (B) Four days following the latest of three consecutive oral recolonization of gnotobiotic mice (and thus right before ileitis induction), E. coli loads were determined in fecal samples by culture (CFU, colony forming units; black squares). Numbers of recolonized mice are given in parentheses and medians (black bars) indicated. Data shown were pooled from three independent experiments.
E. coli Nissle 1917 and E. coli K12 stably establish in mono-associated gnotobiotic mice following induction of acute ileitis
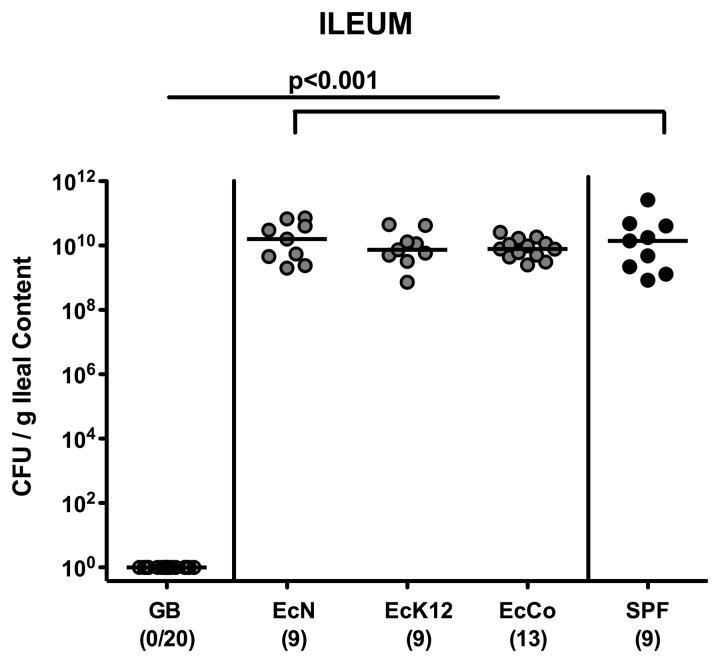
We then investigated whether (i) E. coli Nissle 1917 and E. coli K12 stably establish during acute intestinal inflammation, (ii) translocate to extra-intestinal tissues, or (iii) are involved in intestinal immunopathology despite their secure and probiotic properties. Seven days following ileitis induction using T. gondii, the terminal ileal lumen of E. coli mono-associated gnotobiotic mice harbored approximately 1010 CFU EcN, EcK12, or EcCo (Fig. 2). Interestingly, these bacterial loads were similar to commensal E. coli loads in control mice replenished with a conventional (SPF) microbiota, whereas gnotobiotic mice were still lacking any bacteria at day 7 p.i. (Fig. 2). Thus, under conditions of acute small intestinal inflammation, the respective E. coli strains are able to stably colonize the small intestines at high loads.
Fig. 2.
Ileal E. coli loads in recolonized gnotobiotic mice following ileitis induction. Gnotobiotic mice generated by quintuple antibiotic treatment were perorally recolonized with E. coli Nissle 1917 (EcN), E. coli K12 (EcK12), or a commensal E. coli (EcCo) strain as described in methods. Four days thereafter, E. coli recolonized mice (grey circles) were perorally infected with 100 cysts of Toxoplasma gondii in order to induce acute ileitis. With conventional microbiota recolonized (SPF; black circles) and gnotobiotic (GB; white circles) animals served as positive and negative controls, respectively. Indicated are E. coli loads within the ileal lumen at day 7 following ileitis induction determined by culture (CFU, colony forming units). Numbers of animals harboring E. coli out of the total number of analyzed animals are given in parentheses. Medians (black bars) and significance levels (p values) determined by the Mann–Whitney U test are indicated. Data shown were pooled from three independent experiments.
E. coli Nissle 1917 and E. coli K12 translocate to extra-intestinal tissue sites following acute ileitis induction
We next assessed whether the respective E. coli strains were capable of translocating to extra-intestinal tissue sites during induced acute ileitis. At day 7 following T. gondii infection, live EcN could be detected in 33.3% of mesenteric lymph nodes (MLN) of recolonized mice, whereas E. coli translocation rates in mice harboring EcK12, EcCo, or a replenished complex microbiota were up to 2.5 times higher (77.8%, 84.6% and 77.8%, respectively; Fig. 3a). In 44.4% of spleens derived from control mice harboring a complex microbiota, commensal E. coli could be cultured, whereas translocation rates were approximately 50% lower in EcK12 and EcCo and another 50% lower in EcN recolonized mice at day 7 p.i. (Fig. 3b). Interestingly, translocation into livers occurred at comparable frequencies in EcN and EcK12 (11.1% each) recolonized mice and four times more frequently in EcCo and SPF controls (46.2% and 44.4%, respectively; Fig. 3c). Taken together, EcK12 – supposed to be a non-colonizer – was not only capable of stably establishing within the intestinal tract of gnotobiotic mice but also translocated to extra-intestinal locations upon ileitis induction. Furthermore, extra-intestinal translocation frequencies into MLN, spleen, and liver were lowest in EcN-colonized mice and highest in control animals replenished with a complex microbiota at day 7 p.i.
Fig. 3.
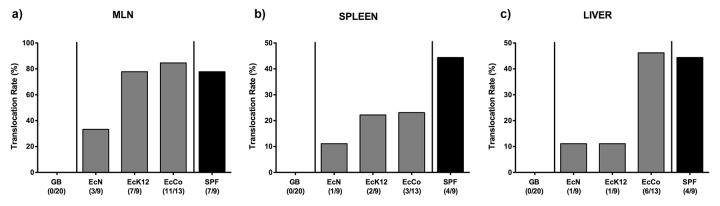
Translocation of E. coli in recolonized gnotobiotic mice following ileitis induction. Gnotobiotic mice generated by quintuple antibiotic treatment were perorally recolonized with E. coli Nissle 1917 (EcN), E. coli K12 (EcK12) or a commensal E. coli (EcCo) strain as described in methods. Four days thereafter, E. coli recolonized mice (grey bars) were perorally infected with 100 cysts of Toxoplasma gondii in order to induce acute ileitis. With conventional microbiota recolonized (SPF; black bars) and gnotobiotic (GB; white bars) animals served as positive and negative controls, respectively. Indicated are E. coli translocation rates into (a) mesenteric lymphnodes (MLN), (b) spleen, and (c) liver at day 7 following ileitis induction determined by culture of organ homogenates. Relative percentages of translocation frequencies are indicated, and absolute numbers of animals harboring the respective E. coli strain out of the total number of analyzed animals are given in parentheses. Data shown were pooled from three independent experiments.
E. coli K12 and E. coli Nissle 1917 induce intestinal inflammatory responses in mono-associated gnotobiotic mice following T. gondii infection
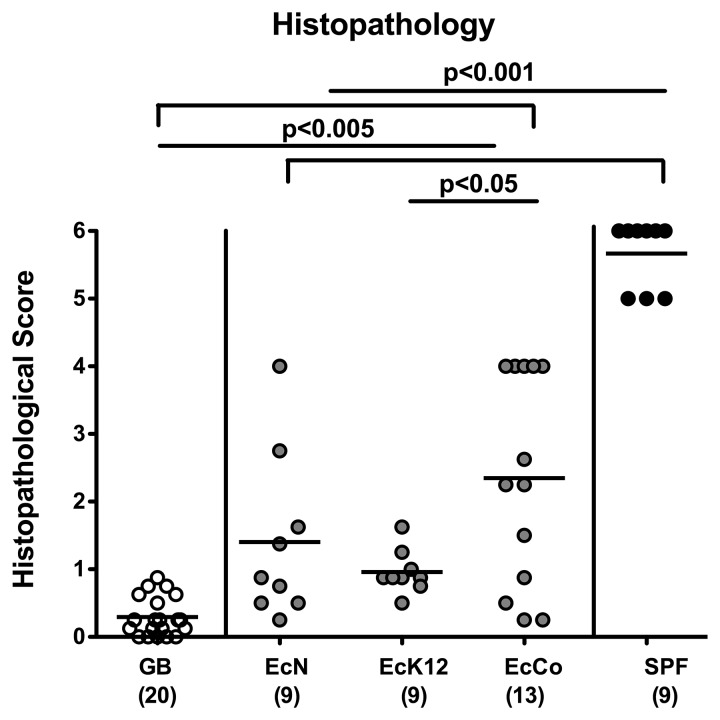
Given that commensal E. coli exacerbate T. gondii-induced acute ileitis in mice [17, 24], we investigated the pro-inflammatory potential of E. coli “security” strains used in this study. As expected, control mice harboring a complex microbiota suffered from acute ileitis with severe transmural necroses (as indicated by maximal histopathological scores of 5.7 ± 0.5; p < 0.001 vs. all other groups; Fig. 4) and succumbed to death, whereas gnotobiotic mice lacking any bacteria were clinically uncompromised and displayed an intact epithelial layer with corresponding histopathological scores of less than 1 (0.3 ± 0.3; Fig. 4). Interestingly as compared to gnotobiotic controls, mice recolonized with EcN, EcK12, or EcCo displayed significantly higher histopathological score (1.4 ± 1.2; 1.0 ± 0.3; 2.4 ± 1.5, respectively; p < 0.001; Fig. 4). Within the EcK12 group, mild to moderate inflammatory foci in the ileal mucosa and submucosa with intact epithelium were detected (range of histopathological scores between 0.5 and 1.5; relatively low SD) with significantly lower histopathological score as compared to EcCo mono-associated mice (p < 0.05; Fig. 4). A substantial variability of histopathological aspects, however, could be observed in EcN and EcCo mono-associated mice at day 7 following ileitis induction. Whereas single mice displayed an almost unaffected mucosa, others exhibited mild to moderate inflammatory changes with an intact epithelial layer, villous edema, and cell-free exudate into the lumen. In some mice, however, more severe histopathological changes such as erosions or ulceration, cryptitis or crypt abscesses, fibrosis, cellular shedding into the lumen or even epithelial disintegration and distortion of the villous architecture could be observed (Fig. 4). Furthermore, in ileal biopsies taken at day 7 p.i., T. gondii DNA and numbers of parasitophorous vacuoles containing tachyzoites or tachyzoite antigens were comparable in either group (data not shown). Thus, EcN, EcK12, and EcCo considered as “security,” probiotic, or apathogenic commensal strains, respectively, contribute to mild to moderate small intestinal inflammation upon T. gondii infection.
Fig. 4.
Ileal histopathology in recolonized gnotobiotic mice following ileitis induction. Gnotobiotic mice generated by quintuple antibiotic treatment were perorally recolonized with E. coli Nissle 1917 (EcN), E. coli K12 (EcK12) or a commensal E. coli (EcCo) strain as described in methods. Four days thereafter, E. coli recolonized mice (gray circles) were perorally infected with 100 cysts of Toxoplasma gondii in order to induce acute ileitis. With conventional microbiota recolonized (SPF; black circles) and gnotobiotic (GB; white circles) animals served as positive and negative controls, respectively. Histopathological scores of the terminal ileum were assessed at day 7 following ileitis induction. Numbers of analyzed animals are given in parentheses. Mean values and significance levels as determined by the Student’s t-test are indicated. Data were pooled from three independent experiments.
Pro-inflammatory immune responses in E. coli Nissle 1917 and K12 mono-associated gnotobiotic mice following ileitis induction
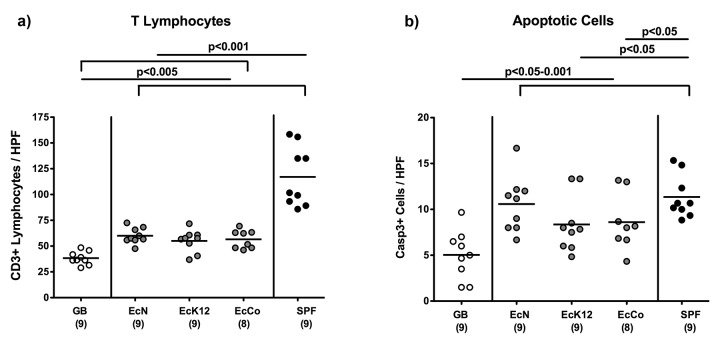
Given that the underlying immunopathogenesis of T. gondii-induced acute ileitis is due to an exaggerated T cell response, we next quantitatively assessed T lymphocyte numbers within the ileal mucosa and lamina propria applying in situ immunohistochemistry. Irrespective of the E. coli strain, mono-colonized mice displayed significantly higher numbers of ileal CD3+ T lymphocytes at day 7 following T. gondii infection (p < 0.005) as compared to gnotobiotic control animals lacking any bacteria (Fig. 5a). These increased T cell numbers were by far exceeded in control animals replenished with complex microbiota (p < 0.001), indicative for an even more pronounced T cell infiltration of the ileal mucosa and lamina propria during acute ileitis (Fig. 5a). Given that apoptosis is a commonly used diagnostic marker in the histopathological evaluation and grading of intestinal disease [31], we quantitatively assessed apoptotic cells within the ileal mucosa. The more pronounced T cell influx was accompanied by higher numbers of caspase-3+ cells in the ileal mucosa (Fig. 5b; p < 0.05–0.001), indicative for more distinct apoptosis in the ileal mucosa and thus further underlining results derived from histopathological grading of mucosal changes (Fig. 4).
Fig. 5.
Ileal pro-inflammatory immune responses in recolonized gnotobiotic mice following ileitis induction. Gnotobiotic mice generated by quintuple antibiotic treatment were perorally recolonized with E. coli Nissle 1917 (EcN), E. coli K12 (EcK12) or a commensal E. coli (EcCo) strain as described in Materials and methods. Four days thereafter, E. coli recolonized mice (grey circles) were perorally infected with 100 cysts of Toxoplasma gondii in order to induce acute ileitis. With conventional microbiota recolonized (SPF; black circles) and gnotobiotic (GB; white circles) animals served as positive and negative controls, respectively. The average number of cells positive for (a) CD3 (T Lymphocytes), (b) caspase-3 (Casp3, Apoptotic Cells) per animal were determined microscopically (HPF, 400× magnification) in immunostained ileum sections of mice at day 7 post ileitis induction. Numbers of analyzed animals are given in parentheses. Mean values and significance levels as determined by the Student’s t-test are indicated. Data are pooled from three independent experiments.
Taken together, the presented data clearly show that EcN and EcK12 security as well as commensal intestinal E. coli strains bear a pro-inflammatory potential in acute ileitis model following mono-association of gnotobiotic mice.
Discussion
One week following high-dose oral T. gondii infection, susceptible mice harboring a conventional intestinal microbiota develop acute ileitis accompanied by a tremendous overgrowth of the ileal microbiota with commensal E. coli [17, 22, 25]. In the present study, we observed that gnotobiotic mice mono-associated with safety and probiotic E. coli strains displayed ileal immunopathology, whereas gnotobiotic mice generated by broad-spectrum antibiotic treatment were protected from disease upon ileitis induction as shown previously [17]. Furthermore, we demonstrate that even E. coli strains considered as security or beneficial/therapeutic probiotic strains (i.e., EcK12 and EcN, respectively) bear harmful potential in themselves by exerting pro-inflammatory actions in mono-associated gnotobiotic mice upon ileitis induction using T. gondii.
Our results are of surprise for several reasons. First, E. coli K12 (MG1655) strain is considered incapable of colonizing vertebrates due to specific genetic defects preventing from adhesion to intestinal epithelial cells [4–6] and therefore considered an apathogenic security strain commonly used in laboratories for cloning purposes. Following mono-association, however, EcK12 not only stably established within the intestinal tract of gnotobiotic mice generated by broad-spectrum antibiotic treatment but could also be cultured from the inflamed small intestines at high loads following ileitis induction. This was also true for E. coli Nissle 1917, a strain exerting beneficial effects when administered as a probiotic drug in inflammatory bowel disease [13, 14, 32]. Remarkably, whereas gnotobiotic mice lacking any bacteria were protected from immunopathology, both EcK12 and EcN induced significant mucosal and submucosal histopathological changes in the presented murine acute ileitis model. This finding was further supported by higher numbers of apoptotic cells and increased influx of pro-inflammatory immune cell such as T lymphocytes, the “master driving force” in T. gondii-induced ileitis [15], infiltrating the ileal lamina propria of EcK12 or EcN mono-associated mice. Interestingly, in the EcN group, the intestinal histopathological outcome was rather heterogenous ranging from almost uncompromised epithelium to mild, moderate, and severe changes seen in the ileal mucosa and submucosa after ileitis induction. For development of the full-blown inflammatory scenario, however, the complex microbiota was necessary as demonstrated here and earlier [17]. Strikingly, either strain was not only able to stably colonize the GI tract and induce small intestinal inflammation but also translocated to extra-intestinal tissue sites such as MLN, spleens, and livers. Translocation of live E. coli from the intestinal lumen to the lamina propria and adjacent lymphoid tissues (i.e., the ileum draining MLN) is facilitated by a disrupted epithelial cell barrier. Bacteria subsequently come in contact with immune cells thereby exacerbating the vicious cycle within the inflammatory scenario. When translocating, bacteria reach the systemic blood stream, dissemination to extra-intestinal organs, such as spleen and liver, occur and finally peritonitis, sepsis, and subsequent multiple organ failure might cause fatality [17, 24]. Interestingly, live EcK12 translocated more frequently to MLN and spleen as compared to EcN despite comparable abundance in the intestinal lumen and similar ileal histopathology.
Beyond the non-questionable plethora of beneficial characteristics and effects exerted by EcN, such as modulation of intestinal microbiota, tightening epithelial barrier function by interference with tight junction protein expression, upregulation of anti-inflammatory cytokines, and chemokines, among many others [33–35], another murine study revealed adverse, inflammatory actions exerted by EcN in the intestinal tract and extra-intestinal locations [36]. Depending on the genetic background, isolator-raised germ-free mice became severely sick following EcN, but not EcK12 (MG1655) strain infection, and displayed intestinal as well as extra-intestinal pyogranulomatous changes [36]. The authors concluded that the potentially harmful side of EcN might become overt under distinct genetic and environmental conditions. In support of this, Ukena et al. demonstrated that genes upregulated following in vitro host cell–EcN interaction were encoding for pro-inflammatory cytokines or molecules and mediators involved in inflammatory pathways [37]. Interestingly, genomic comparisons of EcN and uropathogenic E. coli strain CFT073 [38] revealed similarities regarding expression of pathogenic virulence factors associated with fitness and adaptative properties [39, 40].
We showed previously that TLR-4-dependent signaling of LPS derived from commensal E. coli overgrowing the intestinal lumen during ileitis development aggravated immunopathology [24, 25]. EcN has been shown to possess an avirulent semirough chemotype of LPS with a shortened 06 side chain responsible for serum resistance [39]. Whereas beneficial in vivo effects exerted by EcN have been shown to be TLR-4 mediated [41], it is tempting to speculate whether the pro-inflammatory action of EcN in this acute ileitis model was – at least in part – due to TLR-4 signaling. We are aware that the gnotobiotic mouse model – as any experimental model – does not exactly mirror the (patho-) physiological situation in humans and one should be generally very careful to deduce experimental data from mice to men. However, potential unwanted immunopathological effects exerted by TLR-4-dependent signaling of truncated EcN-LPS in humans are even more worrisome given that humans are up to 1000 times more sensitive to TLR-4 ligands than rodents [42]. Previously, a case of severe sepsis in a preterm infant following EcN treatment has been reported [43]. Even though EcN is known for almost a century and many beneficial effects were elucidated during the past 20 years, many facets of EcN–host interaction await further unraveling. In this context, a dichotomy of effects exerted by the same strain (to use the metaphor, like “the double-edged sword”) needs to be taken into consideration.
In conclusion, results of the present study reveal that EcN may have adverse in addition to a plethora of beneficial effects. Those potentially harmful effects should be taken into account before considering use of EcN as a treatment option in patients, particularly in light of broad-spectrum antibiotic treatment and/or compromised mucosal barrier functions due to acute intestinal inflammation.
Further studies need to be undertaken to establish appropriate risk assessment criteria for use of E. coli strains in health and disease.
Acknowledgements
E. coli Nissle 1917 and E. coli K12 MG1655 strains were kindly provided by Dr. Sya Ukena (Helmholtz Center for Infection Research, Braunschweig, Germany). This work was supported by grants from the German Research Foundation (DFG) to UBG, SB and AF (SFB633, TP A7), MMH (SFB633, TP B6), AAK (SFB633, TP Z1), and from the German Federal Ministry of Education and Research (BMBF) to SB (“Lab in a hanky” projects TP 1.1 and TP 8.2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Michaela Wattrodt, Ursula Rüschendorf, Alexandra Bittroff-Leben, Gernot Reifenberger, Simone Spieckermann, and the staff of the animal research facility for excellent technical assistance and breeding of mice, respectively.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
Contributor Information
S. Bereswill, 1Department of Microbiology and Hygiene, Charité, University Medicine Berlin, Berlin, Germany.
A. Fischer, 1Department of Microbiology and Hygiene, Charité, University Medicine Berlin, Berlin, Germany.
I. R. Dunay, 1Department of Microbiology and Hygiene, Charité, University Medicine Berlin, Berlin, Germany; 2Department of Microbiology and Hygiene, University of Magdeburg, Magdeburg, Germany.
A. A. Kühl, 3Department of Internal Medicine, Rheumatology and Clinical Immunology/Research Center Immunosciences (RCIS), Charité, University Medicine Berlin, Berlin, Germany.
U. B. Göbel, 1Department of Microbiology and Hygiene, Charité, University Medicine Berlin, Berlin, Germany.
O. Liesenfeld, 1Department of Microbiology and Hygiene, Charité, University Medicine Berlin, Berlin, Germany.
M. M. Heimesaat, 1Department of Microbiology and Hygiene, Charité, University Medicine Berlin, Berlin, Germany.
References
- 1.Smith HW. Is it safe to use Escherichia coli K12 in recombinant DNA experiments? The Journal of infectious diseases. 1978 May 1;137(5) doi: 10.1093/infdis/137.5.655. [DOI] [PubMed] [Google Scholar]
- 2.Curtiss 3rd R. Biological containment and cloning vector transmissibility. The Journal of infectious diseases. 1978 May 1;137(5) doi: 10.1093/infdis/137.5.668. [DOI] [PubMed] [Google Scholar]
- 3.Lederberg J, Tatum EL. Gene recombination in Escherichia coli. Nature. 1946 Oct 19;158(4016) doi: 10.1038/158558a0. [DOI] [PubMed] [Google Scholar]
- 4.Smith HW. Survival of orally administered E. coli K 12 in alimentary tract of man. Nature. 1975 Jun 5;255(5508) doi: 10.1038/255500a0. [DOI] [PubMed] [Google Scholar]
- 5.Møller AK, Leatham MP, Conway T, Nuijten PJ, de Haan LA, Krogfelt KA, Cohen PS. An Escherichia coli MG1655 lipopolysaccharide deep-rough core mutant grows and survives in mouse cecal mucus but fails to colonize the mouse large intestine. Infection and immunity. 2003 Apr 1;71(4) doi: 10.1128/IAI.71.4.2142-2152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi K, Morooka N, Yamamoto Y, Fujita K, Isono K, Choi S, Ohtsubo E, Baba T, Wanner BL, Mori H, Horiuchi T. Highly accurate genome sequences of Escherichia coli K-12 strains MG1655 and W3110. Molecular systems biology. 2006 Feb 21;2 doi: 10.1038/msb4100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodinová-Zádniková R, Sonnenborn U. Effect of preventive administration of a nonpathogenic Escherichia coli strain on the colonization of the intestine with microbial pathogens in newborn infants. Biology of the neonate. 71(4) doi: 10.1159/000244421. [DOI] [PubMed] [Google Scholar]
- 8.Shanahan F. Probiotics and inflammatory bowel disease: is there a scientific rationale? Inflammatory bowel diseases. 2000 May 1;6(2) doi: 10.1097/00054725-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Shanahan F. Probiotics in inflamatory bowel disease. Gut. 2001 May 1;48(5) doi: 10.1136/gut.48.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nissle A. Die antagonistische Behandlung chronischer Darmstörungen mit Colibekaterien. Med Klin. 1918;2:29–30. in German. [Google Scholar]
- 11.Blum G, Marre R, Hacker J. Properties of Escherichia coli strains of serotype O6. Infection. 1995 Jul-Aug;23(4) doi: 10.1007/BF01781204. [DOI] [PubMed] [Google Scholar]
- 12.Henker J, Laass M, Blokhin BM, Bolbot YK, Maydannik VG, Elze M, Wolff C, Schulze J. The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. European journal of pediatrics. 2007 Apr 1;166(4) doi: 10.1007/s00431-007-0419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M, Wolff C, Schulze J. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004 Nov 1;53(11) doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimori S, Tatsuguchi A, Gudis K, Kishida T, Mitsui K, Ehara A, Kobayashi T, Sekita Y, Seo T, Sakamoto C. High dose probiotic and prebiotic cotherapy for remission induction of active Crohn's disease. Journal of gastroenterology and hepatology. 2007 Aug 1;22(8) doi: 10.1111/j.1440-1746.2006.04535.x. [DOI] [PubMed] [Google Scholar]
- 15.Liesenfeld O, Kosek J, Remington JS, Suzuki Y. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. The Journal of experimental medicine. 1996 Aug 1;184(2) doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? The Journal of infectious diseases. 2002 Feb 15;185 Suppl 1 doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- 17.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Göbel UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. Journal of immunology (Baltimore, Md. : 1950) 2006 Dec 15;177(12) doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 18.Khan IA, Schwartzman JD, Matsuura T, Kasper LH. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proceedings of the National Academy of Sciences of the United States of America. 1997 Dec 9;94(25) doi: 10.1073/pnas.94.25.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mennechet FJ, Kasper LH, Rachinel N, Li W, Vandewalle A, Buzoni-Gatel D. Lamina propria CD4+ T lymphocytes synergize with murine intestinal epithelial cells to enhance proinflammatory response against an intracellular pathogen. Journal of immunology (Baltimore, Md. : 1950) 2002 Mar 15;168(6) doi: 10.4049/jimmunol.168.6.2988. [DOI] [PubMed] [Google Scholar]
- 20.Liesenfeld O, Kang H, Park D, Nguyen TA, Parkhe CV, Watanabe H, Abo T, Sher A, Remington JS, Suzuki Y. TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite immunology. 1999 Jul 1;21(7) doi: 10.1046/j.1365-3024.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- 21.Vossenkämper A, Struck D, Alvarado-Esquivel C, Went T, Takeda K, Akira S, Pfeffer K, Alber G, Lochner M, Förster I, Liesenfeld O. Both IL-12 and IL-18 contribute to small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii, but IL-12 is dominant over IL-18 in parasite control. European journal of immunology. 2004 Nov 1;34(11) doi: 10.1002/eji.200424993. [DOI] [PubMed] [Google Scholar]
- 22.Muñoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Hölscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. The Journal of experimental medicine. 2009 Dec 21;206(13) doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Struck D, Frank I, Enders S, Steinhoff U, Schmidt C, Stallmach A, Liesenfeld O, Heimesaat MM. Treatment with Interleukin-18 binding protein ameliorates Toxoplasma gondii-induced small intestinal pathology that is induced by bone marrow cell-derived Interleukin-18. Eur J Microbiol Immunol. 2012;2:249–257. doi: 10.1556/EuJMI.2.2012.3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heimesaat MM, Fischer A, Jahn HK, Niebergall J, Freudenberg M, Blaut M, Liesenfeld O, Schumann RR, Göbel UB, Bereswill S. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007 Jul 1;56(7) doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erridge C, Duncan SH, Bereswill S, Heimesaat MM. The induction of colitis and ileitis in mice is associated with marked increases in intestinal concentrations of stimulants of TLRs 2, 4, and 5. PloS one. 2010 Feb 9;5(2) doi: 10.1371/journal.pone.0009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haag LM, Fischer A, Otto B, Plickert R, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM. Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PloS one. 2012 May 1;7(5) doi: 10.1371/journal.pone.0035988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heimesaat MM, Fischer A, Siegmund B, Kupz A, Niebergall J, Fuchs D, Jahn HK, Freudenberg M, Loddenkemper C, Batra A, Lehr HA, Liesenfeld O, Blaut M, Göbel UB, Schumann RR, Bereswill S. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PloS one. 2007 Jul 25;2(7) doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, Steinhoff U, Tchaptchet S, Thiel E, Freudenberg MA, Göbel UB, Uharek L. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010 Aug 1;59(8) doi: 10.1136/gut.2009.197434. [DOI] [PubMed] [Google Scholar]
- 29.Bereswill S, Muñoz M, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Loddenkemper C, Göbel UB, Heimesaat MM. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PloS one. 2010 Dec 3;5(12) doi: 10.1371/journal.pone.0015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haag LM, Fischer A, Otto B, Grundmann U, Kühl AA, et al. Campylobacter jejuni infection of infant mice: acute enterocolitis is followed by asymptomatic intestinal and extra-intestinal immune response. Eur J Microbiol Immunol. 2012;2:2–11. doi: 10.1556/EuJMI.2.2012.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Dasti JI, Dashti JI, Zautner AE, Muñoz M, Loddenkemper C, Gross U, Göbel UB, Heimesaat MM. Novel murine infection models provide deep insights into the "m nage trois" of Campylobacter jejuni, microbiota and host innate immunity. PloS one. 2011 Jun 15;6(6) doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zigra PI, Maipa VE, Alamanos YP. Probiotics and remission of ulcerative colitis: a systematic review. The Netherlands journal of medicine. 2007 Dec 1;65(11) [PubMed] [Google Scholar]
- 33.Senok AC, Ismaeel AY, Botta GA. Probiotics: facts and myths. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2005 Dec 1;11(12) doi: 10.1111/j.1469-0691.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 34.Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nature reviews. Microbiology. 2010 Mar 1;8(3) doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 35.Trebichavsky I, Splichal I, Rada V, Splichalova A. Modulation of natural immunity in the gut by Escherichia coli strain Nissle 1917. Nutrition reviews. 2010 Aug 1;68(8) doi: 10.1111/j.1753-4887.2010.00305.x. [DOI] [PubMed] [Google Scholar]
- 36.Bleich A, Sundberg JP, Smoczek A, von Wasielewski R, de Buhr MF, Janus LM, Julga G, Ukena SN, Hedrich HJ, Gunzer F. Sensitivity to Escherichia coli Nissle 1917 in mice is dependent on environment and genetic background. International journal of experimental pathology. 2008 Feb 1;89(1) doi: 10.1111/j.1365-2613.2007.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ukena SN, Westendorf AM, Hansen W, Rohde M, Geffers R, Coldewey S, Suerbaum S, Buer J, Gunzer F. The host response to the probiotic Escherichia coli strain Nissle 1917: specific up-regulation of the proinflammatory chemokine MCP-1. BMC medical genetics. 2005 Dec 13;6 doi: 10.1186/1471-2350-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welch RA, Burland V, Plunkett 3rd G, Redford P, Roesch P, Rasko D, Buckles EL, Liou SR, Boutin A, Hackett J, Stroud D, Mayhew GF, Rose DJ, Zhou S, Schwartz DC, Perna NT, Mobley HL, Donnenberg MS, Blattner FR. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 2002 Dec 24;99(26) doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grozdanov L, Raasch C, Schulze J, Sonnenborn U, Gottschalk G, Hacker J, Dobrindt U. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. Journal of bacteriology. 2004 Aug 1;186(16) doi: 10.1128/JB.186.16.5432-5441.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun J, Gunzer F, Westendorf AM, Buer J, Scharfe M, Jarek M, Gössling F, Blöcker H, Zeng AP. Genomic peculiarity of coding sequences and metabolic potential of probiotic Escherichia coli strain Nissle 1917 inferred from raw genome data. Journal of biotechnology. 2005 May 4;117(2) doi: 10.1016/j.jbiotec.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Adam E, Delbrassine L, Bouillot C, Reynders V, Mailleux AC, Muraille E, Jacquet A. Probiotic Escherichia coli Nissle 1917 activates DC and prevents house dust mite allergy through a TLR4-dependent pathway. European journal of immunology. 2010 Jul 1;40(7) doi: 10.1002/eji.200939913. [DOI] [PubMed] [Google Scholar]
- 42.Warren HS, Fitting C, Hoff E, Adib-Conquy M, Beasley-Topliffe L, Tesini B, Liang X, Valentine C, Hellman J, Hayden D, Cavaillon JM. Resilience to bacterial infection: difference between species could be due to proteins in serum. The Journal of infectious diseases. 2010 Jan 15;201(2) doi: 10.1086/649557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guenther K, Straube E, Pfister W, Guenther A, Huebler A. Sever sepsis after probiotic treatment with Escherichia coli NISSLE 1917. The Pediatric infectious disease journal. 2010 Feb 1;29(2) doi: 10.1097/INF.0b013e3181c36eb9. [DOI] [PubMed] [Google Scholar]