Abstract
Tim-3 has opposing roles in innate and adaptive immunities. It not only dampens CD4+ and CD8+ T cells responses but also enhances the ability of macrophages to eliminate intracellular pathogens. After peroral infection with 100 cysts of Toxoplasma gondii genetically susceptible C57BL/6 mice develop an unchecked Th1 response associated with the development of small intestinal immunopathology. Here we report that upon infection with T. gondii, both susceptible C57BL/6 and resistant BALB/c mice exhibit increased frequencies of Tim-3+ cells in spleens and mesenteric lymph nodes. The number of Tim-3+ cells was significantly higher in C57BL/6 than in BALB/c mice. Tim-3 was expressed by macrophages, dendritic, natural killer, as well as CD4+ and CD8+ T cells. Highest frequencies of Tim-3+ cells were observed at the peak of Th1 responses (day 7 post infection) concurrent with the development of ileal immunopathology. Infected Tim-3-deficient BALB/c mice did not develop ileal immunopathology nor did their parasite loads differ from those in wildtype BALB/c mice. Thus, although Tim-3 is markedly upregulated upon infection and differentially regulated in susceptible and resistant mice upon infection with T. gondii, the absence of Tim-3 is not sufficient to overcome the genetic resistance of BALB/c mice to the development of Th1-driven small intestinal immunopathology.
Keywords: acute ileitis, immune cell response, oral infection, T cells, Th1 type immunopathology, Tim-3, Toxoplasma gondii
Introduction
The T cell immunoglobulin Tim-3 protein (Tim-3) is expressed on terminally differentiated murine Th1 but not on Th2 cells [1]. Tim-3 is also expressed on non-T cells including dendritic cells (DCs) [1], monocytes, macrophages [2], natural killer (NK) and NK-T cells, and at lower levels in Th17 cells [3–5]. Galectin-9, discussed as the ligand for Tim-3 [6, 7], is expressed on antigen-presenting cells dependent on interferon γ (IFN-γ) production by Th1 cells [8]. Ligation of Tim-3 with galactin-9 induced by IFN-γ results in deletion of Th1 cells and thereby prevents protracted inflammation in target organs, e.g. in cerebrospinal fluid T cells of patients with multiple sclerosis [9]. The expression of Tim-3 on human T cells directly regulates proliferation and secretion of IFN-γ, as observed in ex vivo experiments with CD4+ T cells of multiple sclerosis patients since blocking of Tim-3 by small interfering RNA enhanced proliferation and IFN-γ secretion [9] suggesting a role for Tim-3 dysregulation in autoimmune diseases. Evidence also points at a regulation of antigen-presenting cells by Tim-3 since the protein is constitutively expressed on these cells and can synergize with Toll-like receptors to promote pro-inflammatory responses [10].
The role of Tim-3 in infections is less well understood. Recent studies observed that in chronic viral diseases, such as infection with human immunodeficiency virus (HIV), hepatitis B, and hepatitis C, Tim-3 characterizes a population of exhausted CD8+ T cells lacking the ability to proliferate, become cytotoxic, and produce cytokines [11, 12], indicating that there is a functional connection between Tim-3 expression and T cell dysfunction. However, Leitner et al. [7] did not find a functional role of Tim-3 in human T cell activation. An antimicrobial role of Tim-3 has been described in chronic infection with Mycobacterium tuberculosis since binding of Tim-3 to galectin-9 on infected macrophages contributed to restriction of intracellular replication of mycobacteria [13]. Thus, the current view is that Tim-3 has opposing roles in innate and adaptive immunity by enhancing the ability of macrophages to eliminate intracellular pathogens and dampening the CD4 and CD8 T cell responses [9, 10, 12, 13].
Infection with Toxoplasma gondii (T. gondii) elicits a strong type 1 immune response [14]. IFN-γ is recognized as the major mediator of resistance against T. gondii by controlling parasite replication. T lymphocytes and NK cells are critical sources of IFN-γ. Macrophages (MØ) and dendritic cells (DC) process parasite antigens and produce pro-inflammatory cytokines, including IL-12 and IL-18, thereby inducing IFN-γ production [14]. On the other hand, IFN-γ-dependent pro-inflammatory responses can be highly detrimental to the host [15]. After high-dose oral infection with T. gondii, genetically susceptible C57BL/6 mice develop lethal immunopathology of the small intestine [15]. Interestingly, the immunopathogenesis of T. gondii-induced ileal pathology in mice resembles that of Crohn’s disease in humans [16]. While IL-23 and IL-22 have been identified as essential pro-inflammatory mediators of small intestinal pathology, IL-17 does not play a key role and was actually down-regulated during infection [17]. IL-10 and TGF-b have been described as counter-regulatory molecules in this model [15, 18].
In the present study, we investigated the role of Tim-3 in the regulation of immune responses against oral infection with T. gondii. We initially determined the frequency of Tim-3+ immune cell populations in naive and infected BALB/c and C57BL/6 mice. Finally, Tim-3 knockout mice on the BALB/c background were used to investigate the role of Tim-3 in resistance against development of small intestinal pathology. Taken together, our results indicate that peroral high-dose T. gondii infection results in increased frequencies of Tim-3+ cells and that the expression of Tim-3 is under genetic control; however, the absence of Tim-3 is not sufficient to overturn genetic resistance of BALB/c mice against development of Th1-mediated small intestinal immunopathology.
Materials and methods
Mice and infection with T. gondii
Female wildtype and Tim-3-deficient BALB/c mice [1], and C57BL/6 mice were 6 to 8 weeks old and bred and maintained in the animal facility of the Charité-University Medicine Berlin, Campus Benjamin Franklin under specific pathogen-free (SPF) conditions. Clinical conditions and body weight were monitored daily; all experiments were conducted according to the German animal protection laws. Cysts of the T. gondii ME49 strain were obtained from homogenized brains of NMRI mice that had been infected intraperitoneally with 10 cysts 2–3 months earlier. For peroral infection, mice were infected with 100 cysts in a volume of 0.3 mL of PBS (pH 7.4) by gavage. There were three to five mice in each experimental group, and each experiment was performed at least three times.
Histopathology
Mice were sacrificed by cervical dislocation following anesthesia with isofluran (Abbott, Wiesbaden, Germany) at 3 and 7 days after peroral infection with T. gondii. Their liver, spleen, and small intestine were removed and immediately fixed in a solution containing 10% formalin, 70% ethanol, and 5% acetic acid. Two to four 5-μm-thin sections (50 or 100 μm distance between sections) of each organ from each mouse were stained with hematoxylin and eosin and evaluated for inflammatory changes. A standardized histological inflammation score ranging from 0 to 6 (0, normal; 1, edematous blubbing; 2, cell-free exudates into the lumen but intact epithelium; 3, cellular shedding into the lumen; 4, beginning epithelial disintegration; 5, mucosal destruction <50% of small intestine length; 6, complete destruction >50% of small intestine length, severe necrosis) as described previously [19] was used for blinded duplicate evaluation. Histological changes were consistent between individual mice in the same group and between sections from the same organ of each mouse.
Flow cytometry
Spleen and mesenteric lymph nodes were removed, put on ice with 10 mL RPMI medium (Invitrogen Life Technologies, Darmstadt, Germany) + 5% FCS (PAA Laboratories, Pasching, Austria) and 0.5% penicillin and streptomycin (Biochrom, Cambridge, UK), cut into pieces, grinded and then homogenized through a nylon mesh sieve (70 μm) followed by centrifugation at 1200 rpm for 10 min at 4°C. Supernatant was discarded and the pellet resuspended in 5 mL medium: distilled water (1:2) and centrifugated at 1200 rpm for 10 min at 4°C. The cell suspension was washed with RPMI medium + 10% FCS and counted. Cells were treated with rat serum to avoid non–Ag-specific binding of Abs and stained with mouse phycoerythrin (PE) Tim-3 MAb (Clone 215008 R & D Systems, Wiesbaden-Nordenstadt Germany), fluorescein isothiocyanate (FITC) anti-CD69 and anti-CD86, peridinin chlorophyll (PerCP) anti-CD11b, anti-CD4, and anti-NK1.1, and allophycocyanin (APC) anti-CD8 and anti-CD11c mAbs (all BD Biosciences, Heidelberg, Germany). Fluorescence of stained cells was measured using a FACScan (BD Biosciences), and data were analyzed using CellQuest software (BD Biosciences).
Cytokine concentrations
Biopsies from small intestine, spleen, and liver were cultured in 12-well plates containing 1 mL of RPMI medium 1640 supplemented with penicillin and streptomycin. After 24 h at 37°C supernatants were harvested and stored at −80°C. Blood was collected by cardiac puncture; the serum was separated and stored at −80°C. Cytokine concentrations were determined using commercial ELISA kits for IFN-γ, TNF-α, IL-10 (all BD Biosciences), IL-17, and IL-22 (both R&D).
T. gondii DNA
Samples of liver, spleen, mesenteric lymph nodes, and small intestine were homogenized in a rotor-stator in 500 µL lysis buffer containing 100 mM Tris–HCl, pH 8.0, 200 mM NaCl, 5 mM EDTA, 0.2% SDS, and 200 µg/mL proteinase K (Sigma-Aldrich, Munich, Germany). DNA was precipitated by centrifugation after adding isopropanol to the supernatant. DNA concentrations were measured with an Eppendorf BioPhotometer 6131. All PCR assays were performed using the LightCycler (Roche Diagnostics GmbH, Mannheim, Germany) as described previously [19]. The amplification mixture consisted of 2 µL of 10× reaction mix (LightCycler Fast Start Master Hybridization Probes, Roche), 2 mM MgCl2, 1 µM of each oligonucleotide primer (TOX-9: 5´-AggAgAgATATCAggACTgTAg-3´; TOX-10 as: 5´-gCgTCgTCTCgTCTAgATCg-3´), 0.2 µM of each oligonucleotide probe (TOX-HP-1: 5´-gAgTCggAgAgggAgAAgATgTT-FAM-3´; TOX-HP-2: 5´-RED-640-CCggCTTggCTgCTTTTCCTg-PH-3´), and 500 ng of template DNA in a final volume of 20 µL. A standard curve was performed using 500–5 pg T. gondii DNA of GFP-tachyzoites. Fluorescence was analyzed by Light Cycler Data Analysis software 3.5 (Roche).
Statistical analysis
All data were analyzed with GraphPad Prism version 5.00 for Windows, GraphPad Software (San Diego California, USA). Normally distributed variables were expressed as the mean ± SD, not normally distributed variables as median ± interquartile range, and statistical differences between two groups were analyzed using two-sided Student’s t test or Mann–Whitney U test, respectively. One-way ANOVA with post hoc test or Kruskall–Wallis and Mann–Whitney U test with Bonferroni adjustment were used for normally and not normally distributed variables, respectively, when more than two groups were compared. Correlation between variables was assessed with Spearman test. Values of P < 0.05 were considered significant.
Results
Genetically susceptible C57BL/6 mice display significantly higher frequencies of Tim-3 expressing immune cells than genetically resistant BALB/c mice upon T. gondii infection
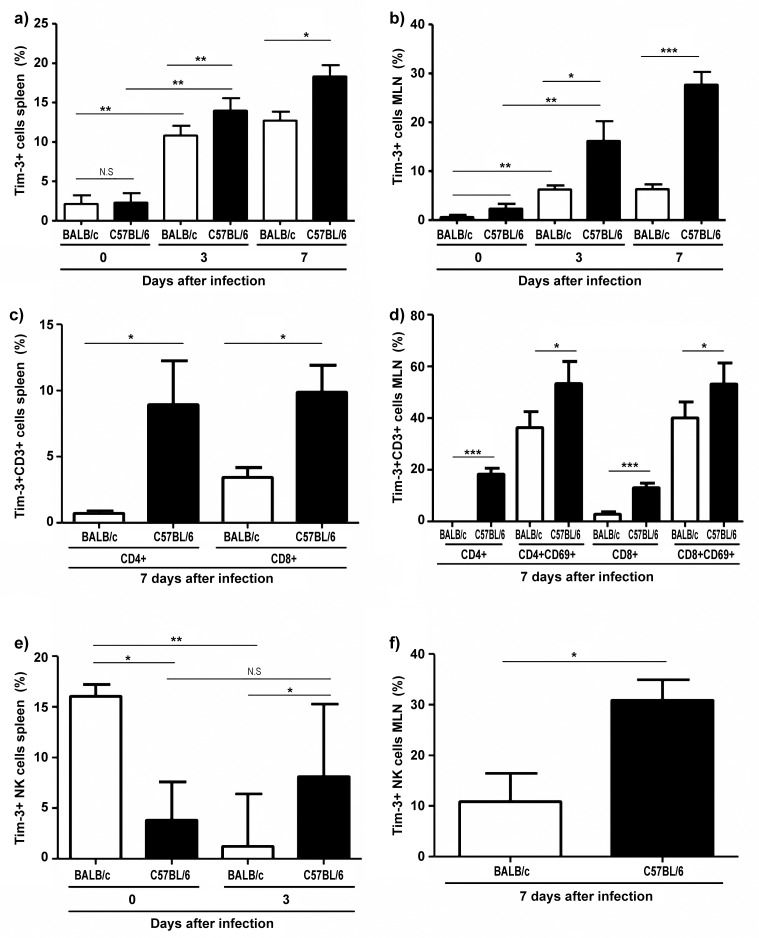
To investigate the effects of Tim-3 on different immune cell populations, we infected susceptible C57BL/6 and resistant BALB/c mice with 100 cysts of T. gondii orally. At day 3 post infection (p.i.), there were significantly more cells expressing Tim-3 in the spleen of both susceptible C57BL/6 and resistant BALB/c mice compared to naive control mice; the mean percentage of cells expressing Tim-3 was 2.1% in naive versus 10.8% in infected BALB/c mice, and 2.3% in naive C57BL/6 versus 14.0% in infected C57BL/6 mice (P = 0.006 and P < 0.0001, respectively; Fig. 1a). Similarly, the percentage of cells expressing Tim-3 was significantly higher in the mesenteric lymph nodes of infected (day 3 p.i.) versus naive mice of both genetic backgrounds (mean 0.6% in naive versus 6.2% in infected BALB/c mice and 2.4% in naive versus 16.2% in infected C57BL/6 mice, P = 0.004 and P = 0.008, respectively; Fig. 1b). Similar results were obtained when determining the percentage of Tim-3-expressing cells on day 7 after infection (Fig. 1a,b). These results indicate that oral infection with T. gondii results in the induction of Tim-3 expression on mononuclear cells of the spleen and mesenteric lymph nodes in both genetically susceptible and resistant mice.
Fig. 1.
Frequency of Tim-3+ cells in the spleen and mesenteric lymph nodes of BALB/c and C57BL/6 mice before (day 0) and at days 3 and 7 following oral infection with 100 cysts of T. gondii ME49 strain as determined by flow cytometry. (a) Total Tim-3+ cells in spleen (mean ± SD); (b) total Tim-3+ cells in mesenteric lymph nodes (mean ± SD); (c) Tim-3+, CD4+, and CD8+ T cells (CD3+) in spleen (median ± interquartile range); (d) Tim-3+, CD4+, and CD8+ T cells in mesenteric lymph nodes (mean ± SD); (e) Tim-3+ NK cells in spleen (median ± interquartile range); (f) Tim-3+ NK cells in mesenteric lymph nodes (mean ± SD). *P < 0.05, **P < 0.01, ***P < 0.001, NS: not significant. Data are representative of at least three independent experiments (n = 12 for each group/time point)
Comparing infected mice at days 3 and 7 post infection, we observed a significantly higher frequency of Tim-3+ cells in the spleen of susceptible C57BL/6 compared to resistant BALB/c mice at 3 and 7 days post infection (mean percentage of cells expressing Tim-3 on day 3: 10.0% in BALB/c versus 14.0% in C57BL/6 mice, and 12.7% in BALB/c versus 18.3% in C57BL/6 mice on day 7, P = 0.001 and P = 0.03, respectively; Fig. 1a). Similarly, the frequency of Tim-3+ in the mesenteric lymph nodes was significantly increased in susceptible C57BL/6 compared to resistant BALB/c mice. Mean percentages on day 3 were 6.2% in BALB/c versus 16.2% in C57BL/6 mice (P = 0.02; Fig. 1b), and 6.2% in BALB/c versus 27.7% in C57BL/6 mice on day 7 (P ≤ 0.0001; Fig. 1b).
Thus, Tim-3 expression on mononuclear cells of spleen and mesenteric lymph nodes is under genetic control, and susceptible mice display significantly increased frequencies of Tim-3+ cells compared to resistant mice.
To further investigate which cell subsets contributed to the marked differences in the frequency of Tim-3 expression, we determined the frequency of CD4+, CD8+ T cells, natural killer cells, macrophages, and dendritic cells expressing Tim-3.
A significant difference in the frequency of CD4+Tim-3+ and CD8+Tim-3+ cells was found in the spleen of BALB/c versus C57BL/6 mice at day 7 post infection (median percentage of CD4+Tim-3+ cells: 0.7% in BALB/c versus 8.9% in C57BL/6 mice, P = 0.021; median percentage of CD8+Tim-3+ cells 3.4% in BALB/c versus 9.9% in C57BL/6 mice, P = 0.007; Fig. 1c). This difference was more pronounced in mesenteric lymph node CD4+ (mean 0% in BALB/c versus 18.2% in C57BL/c mice, P = 0.0001) and CD8+ T cells (mean 1.0% in BALB/c versus 53.3% in C57BL/6, P = 0.024; Fig. 1d). Thus, susceptible C57BL/6 mice harbor higher frequencies and higher absolute numbers (data not shown) of CD4+ and CD8+ T cells expressing Tim-3 upon infection.
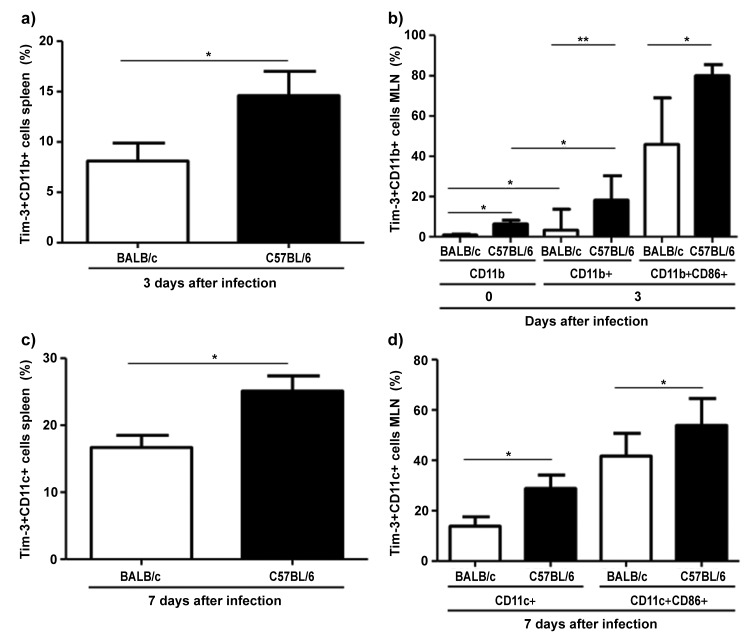
Interestingly, uninfected BALB/c mice displayed higher frequencies of splenic Tim-3-expressing NK1.1+ cells than C57BL/6 mice (median 16.0% in BALB/c versus 3.8% in C57BL/6, P = 0.021; Fig. 1e). This ratio was reversed after infection when median percentages of splenic NK1.1+ cells in BALB/c versus C57BL/6 mice were 2.6% versus 10%, P = 0.01; Fig. 1e), and mean percentages of mesenteric lymph nodes NK1.1+ cells were 10.8% in BALB/c versus 30.9% in C57BL/6 mice, P = 0.012: Fig. 1f). C57BL/6 mice also harbored higher absolute numbers of NK1.1 + cells than BALB/c mice (data not shown). In addition, C57BL/6 mice had significantly higher frequencies of splenic Tim-3+CD11b+ cells compared to BALB/c mice at day 3 post infection (mean 8.1% in BALB/c versus 14.6% in C57BL/6, P = 0.044; Fig. 2a). Similarly, frequencies of Tim-3-expressing CD11b+ cells in susceptible mice were significantly higher in uninfected mice and at day 3 post infection in the mesenteric lymph nodes compared to resistant mice (mean 0.85% in BALB/c versus 6.4% in C57BL/6; mean 7.0% in BALB/c versus 24.2% in C57BL/6, P = 0.02, P = 0.008, respectively; Fig. 2b). Furthermore, there were more splenic Tim-3-expressing CD11c+ cells in C57BL/6 mice at day 7 post infection (mean 16.7% in BALB/c versus 25.1% in C57BL/6, P = 0.008; Fig. 2c), although both mice strains had equal absolute numbers of CD11c+ cells (data not shown). Similar observations were made in mesenteric lymph nodes of these mice (mean 13.8% in BALB/c versus 28.9% in C57BL/6 and 41.7% in BALB/c versus 54% in C57BL/6 mice, P = 0.024 and P = 0.046, respectively; Fig. 2d) in which the C57BL/6 mice not only had more relative frequencies of Tim-3+ cells but also more absolute numbers of CD11c+ cells (data not shown). Similar results were observed in activated CD11b+ and CD11c+ cells double positive for CD86 (Fig. 2b and d). Thus, natural killer cells and antigen-presenting cells in C57BL/6 mice express more Tim-3 after T. gondii infection than that in BALB/c mice.
Fig. 2.
Frequency of Tim-3+ cells in the spleen and mesenteric lymph nodes of C57BL/6 and BALB/c mice before (day 0) and at days 3 and 7 following oral infection with 100 cysts of T. gondii ME49 strain as determined by flow cytometry. (a) Tim-3+ CD11b+ cells in spleen (mean ± SD); (b) Tim-3+ CD11b+ cells in mesenteric lymph nodes (mean ± SD); (c) Tim-3+ CD11c+ cells in spleen (mean ± SD); (d) Tim-3+ CD11c+ cells in mesenteric lymph nodes (mean ± SD). *P < 0.05, **P < 0.01. Data are representative of at least three independent experiments (n = 12 for each group/time point)
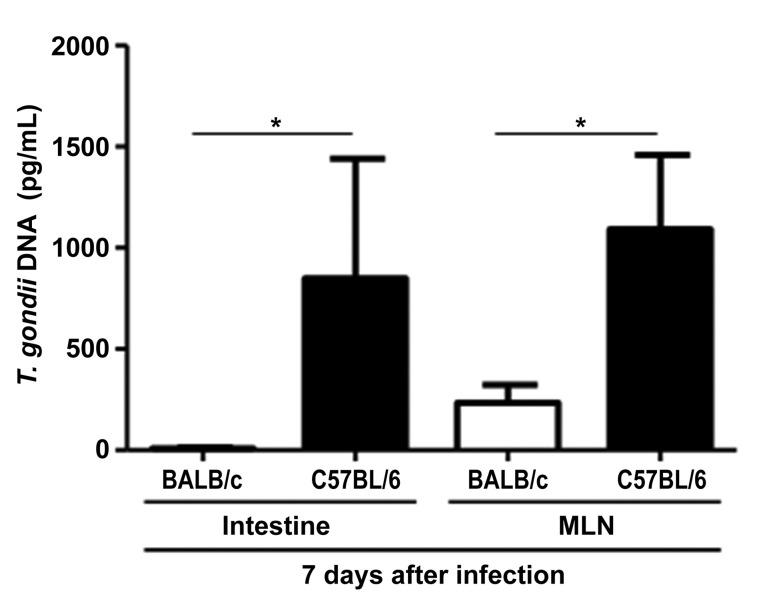
C57BL/6 display higher parasite loads than BALB/c mice and their parasite load correlates with the frequency of Tim-3+ cells
To investigate whether increased frequencies of Tim-3+ immune cells are associated with the parasite load in mice, T. gondii DNA concentrations were determined in the small intestine and mesenteric lymph nodes. As expected, C57BL/6 mice showed a significantly higher parasite load than BALB/c mice in their intestine and mesenteric lymph nodes at 7 days after infection (P = 0.022 and P = 0.021, respectively; Fig. 3). Interestingly, in mesenteric lymph nodes in C57BL/6 mice, the parasite load was positively correlated with the frequency of all Tim-3+ cells and of Tim-3+/CD86– dendritic cells at day 3 post infection (correlation coefficients 0.76, P = 0.003 and 0.60, P = 0.03, respectively; Table 1). In contrast, in BALB/c mice the splenic parasite load was predominantly negatively correlated with the frequencies of Tim-3+/CD69+ CD4+ and CD8+ T cells, and CD86+/CD11c+ dendritic cells (P = 0.002, P = 0,001, P = 0,001, respectively; Table 1) at day 7 post infection. However, in BALB/c mice the correlation with the CD86/CD11c+ dendritic cell population was positive (P = 0.004, Table 1). Thus, in both mouse strains the parasite load appears to be predominantly positively correlated with the frequency of Tim-3+ resting cells whereas the association of parasite load and Tim-3+ activated cells is predominantly negative.
Fig. 3.
Concentrations of T. gondii DNA in the small intestine and mesenteric lymph nodes of C57BL/6 and BALB/c mice 7 days following oral infection with 100 cysts of T. gondii ME49 strain as determined by RT-PCR. n = 12 for each group/time point. (median ± interquartile range) *P < 0.05. Data are representative of at least three independent experiments (n = 12 for each group/time point)
Table 1.
Correlation between T. gondii parasite and frequency of Tim-3+ cells in C57BL/6 and BALB/c mice 3 and 7 days following oral infection with 100 cysts of T. gondii ME49 strain
| C57BL/6, 3 days after infection MLN | Correlation coefficient R2 | 95% C.I | P value |
|---|---|---|---|
| T. gondii DNA/Tim-3+ cells | 0.769 | 0.3–0.9 | 0.003 |
| T. gondii DNA/Tim-3+CD11c+CD86– cells | 0.60 | 0.02–0.8 | 0.03 |
| BALB/c, 7 days after infection spleen | |||
| T. gondii DNA/Tim-3+CD4+CD69+ cells | −0.783 | −0.93 to −0.38 | 0.002 |
| T. gondii DNA/Tim-3+CD8+CD69+ cells | −0.80 | −0.94 to −0.43 | 0.001 |
| T. gondii DNA/Tim-3+CD11c+ cells | 0.76 | 0.36–0.92 | 0.004 |
| T. gondii DNA/Tim-3+CD11c+CD86+ cells | −0.81 | −0.94 to −0.45 | 0.001 |
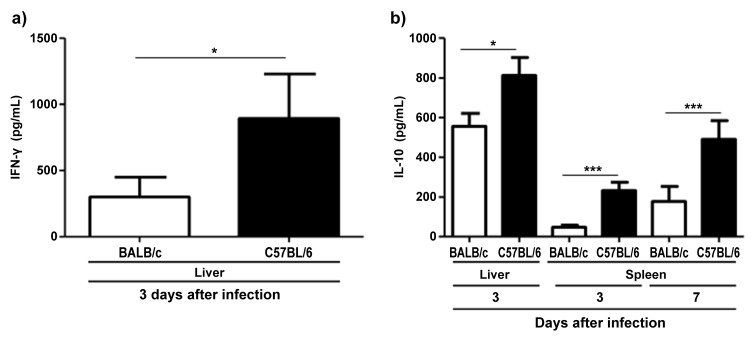
Levels of IFN-γ, IL-17 and IL-10 but not IL-22 are increased in C57BL/6 compared to BALB/c mice following infection with T. gondii
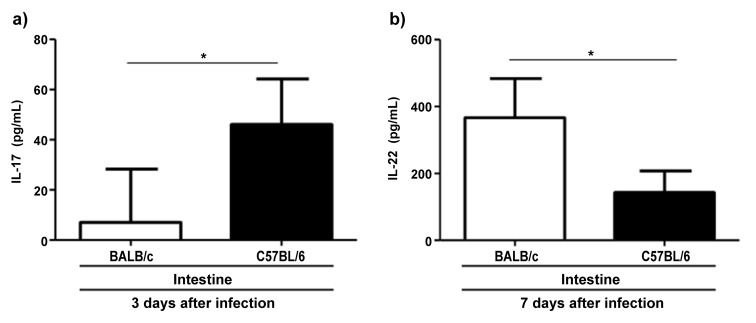
To assess differences in cytokine production between both mouse strains after infection with T. gondii, we determined IFN-γ, IL-17, IL-10, and IL-22 concentrations in different organs by ELISA. C57BL/6 mice displayed higher concentrations of IFN-γ than BALB/c mice at 3 days post infection in the liver but not in the spleen, intestine, or mesenteric lymph nodes (P = 0.024; Fig. 4a). IL-10 in the liver was also increased in C57BL/6 compared to BALB/c mice at day 3 after infection (P = 0.018; Fig. 4b). Similarly, IL-10 in the spleen was elevated at days 3 and 7 post infection (P < 0.0001 and P = 0.001, respectively; Fig. 4b). IL-17 concentration in the small intestine was significantly higher in C57BL/6 compared to BALB/c mice at day 3 but not at day 7 after infection (P = 0.022; Fig. 5a). In contrast, BALB/c mice exhibited higher concentrations of IL-22 in the small intestine at day 7 post infection (P = 0.033; Fig. 5b).
Fig. 4.
Concentrations of IFN-γ in liver (a) and of IL-10 in liver and spleen (b) derived from C57BL/6 and BALB/c mice 3 and 7 days following oral infection with 100 cysts of T. gondii ME49 strain as determined by ELISA (mean ± SD) *P < 0.05, ***P < 0.001. Data are representative of three independent experiments (n = 12 for each group/time point)
Fig. 5.
Concentrations of IL-17 (a) and IL-22 (b) in small intestines derived from C57BL/6 and BALB/c mice 3 or 7 days following oral infection with 100 cysts of T. gondii ME49 strain as indicated. Cytokine concentrations were determined by ELISA (mean ± SD) *P < 0.05. Data are representative of three independent experiments (n = 12 for each group/time point)
Genetic resistance to development of intestinal pathology in BALB/c mice is independent of Tim-3
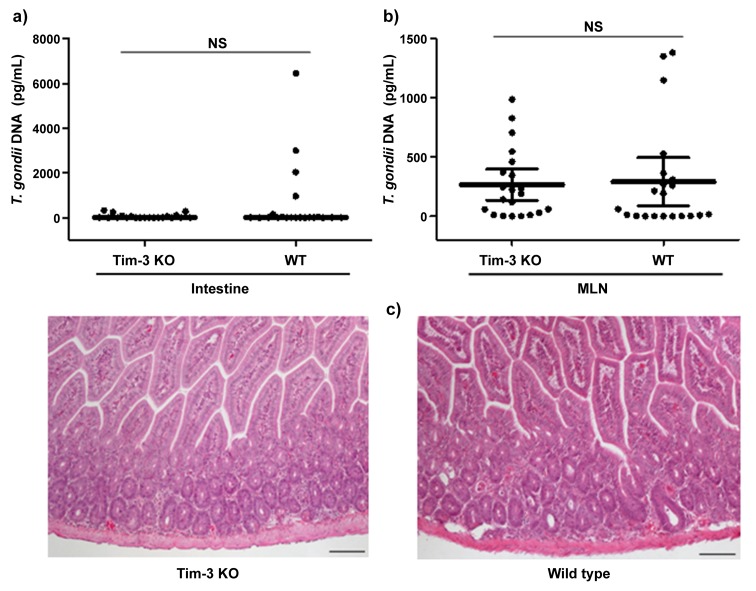
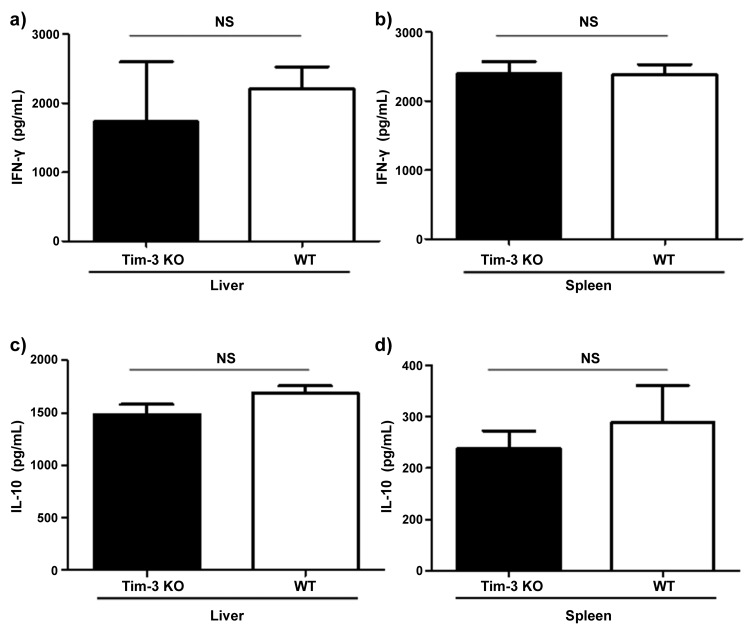
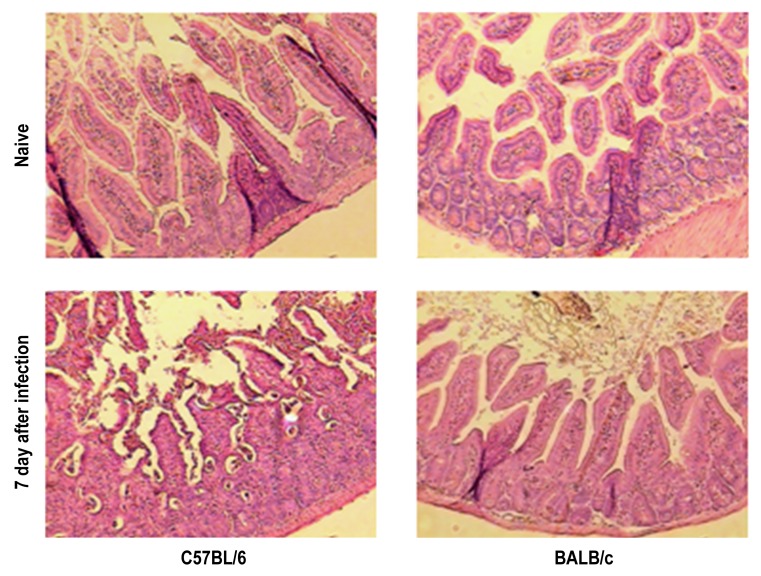
To study the role of Tim-3 in the resistance of BALB/c mice against oral infection with T. gondii, we infected wildtype and Tim-3-deficient BALB/c mice. We did not observe significant differences in parasite loads in the small intestine or mesenteric lymph nodes in BALB/c wildtype compared to Tim-3-deficient BALB/c mice. In addition, neither wildtype nor Tim-3-deficient BALB/c mice developed signs of immunopathology in their ilea 7 days post infection (Fig. 6a–c). Concentrations of IFN-γ and IL-10 did not differ in serum, small intestine, liver, or spleen of wildtype compared to Tim-3-deficient BALB/c mice (Fig. 7a–d; data not shown). In contrast, C57BL/6 mice developed intestinal pathology characterized by severe ileal necrosis as described previously [18] (Fig. 8). Thus, parasite loads and development of small intestinal changes did not differ between wildtype and Tim-3-deficient mice suggesting that Tim-3 does not play an essential role in the genetic resistance of BALB/c mice against the development of Th1-induced small intestinal immunopathology.
Fig. 6.
Concentrations of T. gondii DNA in small intestines (a) and mesenteric lymph nodes (b), and HE-stained ileal paraffin sections (100× magnification); (c) derived from Tim-3-deficient (KO) and wild type (WT) mice 7 days following oral infection with 100 cysts of T. gondii ME49 strain. T. gondii DNA was determined by RT-PCR. (median ± interquartile range). NS: not significant. Data and photomicrographs shown are representative of three independent experiments (n = 12 for each group/time point)
Fig. 7.
Concentrations of IFN-γ in liver (a) and spleen (b), and IL-10 in liver (c) and spleen (d) of Tim-3-deficient (KO) and BALB/c wild type (WT) mice 7 days following oral infection with 100 cysts of T. gondii ME49 strain. Cytokine concentrations were determined by ELISA. NS: not significant. Data are representative of three independent experiments (n = 12 for each group/time point)
Fig. 8.
Histopathological changes in ilea derived from C57BL/6 and BALB/c mice before (Naive) and 7 days following oral infection with 100 cysts of T. gondii ME49 strain. Shown photomicrographs of H&E stained ileal paraffin sections (100× magnification) are representative of three independent experiments (n = 12 for each group/time point)
Discussion
Tim-3 was initially described as a negative regulator of T cell responses based on observations in patients with multiple sclerosis [9] and chronic viral infections such as human immunodeficiency virus (HIV) and hepatitis C virus infection [11, 20]. However, recent evidence indicates that Tim-3 can also promote proinflammatory cytokine production and enhance the ability of macrophages to eliminate intracellular pathogens [10, 13]. Therefore, Tim-3 has been proposed to play a dual role in inflammatory responses in the host. This dichotomous function of Tim-3 has been partially explained by its different tyrosine phosphorylation patterns found in T cells compared to myeloid cells [10].
T. gondii is an intracellular parasite that triggers a strong Th1 immune response in the infected host [15]. In genetically susceptible C57BL/6 mice, peroral infection with 100 cysts of T. gondii induces elevated levels of IFN-γ and other proinflammatory mediators that lead to the development of small intestinal immunopathology resembling that of Crohn’s disease in humans [16]. However, BALB/c-infected mice do not develop immunopathology. Although genetic regulation of susceptibility to this infection is not fully understood, it is plausible that differentially expressed molecules in susceptible versus resistant mice are key to the development of immunopathology [15, 16, 18]. Pro-inflammatory cytokines such as IFN-γ might be part of these factors. Indeed, we here observed significantly higher IFN-γ levels in the liver of C57BL/6 compared to BALB/c mice after T. gondii infection. In addition, we found significantly higher frequencies of Tim-3+-expressing CD4+ and CD8+ T cells in infected C57BL/6 compared to infected BALB/c mice, particularly at the peak of the Th1 response (i.e., day 7 p.i.). This suggests that the intensity of Tim-3 induction on T cells might depend on the development of strong Th1 responses and the amount of IFN-γ. Tim-3 has been shown to be specifically upregulated only on T cells that have differentiated to produce IFN-γ, CD4+ T helper 1, and CD8+ T cytotoxic 1 (Tc1) cells [21]. Interestingly, exposure of the proposed Tim-3 ligand, Galectin-9, to T cells triggers cell death and therefore downregulates Th1 immune responses. This suggests that Tim-3 induction might be a modulatory mechanism initiated by IFN-γ-producing cells in order to prevent the development of immunopathology. However, the hyperinflammatory response induced by T. gondii in susceptible mice might override the effects of a Tim-3-mediated autoregulatory loop. A recent study with another intracellular pathogen, Mycobacterium tuberculosis, showed that Tim-3 was expressed by only 1–2% of lung T cells in naive mice; however, after low-dose aerosol Mycobacterium tuberculosis infection, the percentage of pulmonary CD4+ and CD8+ T cells that express Tim-3 gradually increased [13].
Moreover, we observed significantly higher frequencies of CD11b+ and CD11c+ Tim-3+ cells in C57BL/6 compared to BALB/c mice after infection, suggesting that Tim-3 is not only induced on T cells during strong Th1 responses but also on myeloid cells. Similarly, overexpression of Tim-3 on T cells has been shown to increase the frequencies of CD11b+Gr1+ cells that can in turn suppress inflammatory immune responses [22].
In HIV-infected individuals, Tim-3 expression was positively correlated with HIV viral load [11]. Similarly, we found a positive correlation between parasite loads and resting Tim-3+ cells whereas this correlation was mostly negative in activated TIM-3+ cells. Peroral infection with 100 cysts induces an inflammatory cytokine storm in C57BL/6 mice, which do not survive beyond 12 days after infection. Therefore, we investigated whether Tim-3 absence could impact IFN-γ production in BALB/c mice after T. gondii infection and might be involved in the resistant phenotype displayed by BALB/c mice. However, we did not find any differences in IFN-γ and IL-10 levels in the liver and the spleen between wildtype and Tim-3-deficient BALB/c mice after T. gondii infection. Moreover, no signs of immunopathology were observed in Tim-3 deficient BALB/c mice compared to wildtype BALB/c mice upon infection. These results suggest that Tim-3 is not a key player in the resistance of BALB/c mice against development of Th1-driven immunopathology. This is in strong contrast to the role of IL-10. While wildtype BALB/c mice are resistant to development of small intestinal immunopathology following high-dose oral infection, we previously reported that BALB/c mice when rendered deficient in IL-10 develop small intestinal immunopathology upon infection with an inoculum as low as 20 cysts [23] demonstrating the key role of IL-10 in the resistance against development of immunopathology. Our results suggest that the role of Tim-3 is less important in the downregulation of Th1 responses than IL-10. However, due to the unavailability of Tim-3-deficient C57BL/6 mice, we were unable to demonstrate whether Tim-3 plays a role in the induction of immunopathology in C57BL/6 mice after T. gondii infection. Further experiments using Tim-3-deficient C57BL/6 mice or blocking antibodies against Tim-3 might help answer this question.
In conclusion, the finding that C57BL/6 mice harbor more Tim-3+ cells than BALB/c mice after T. gondii infection demonstrates that Tim-3 expression is genetically regulated in mice. However, Tim-3 deficiency is not sufficient to overturn the genetically determined resistance of BALB/c mice against development of Th1-driven small intestinal immunopathology.
Acknowledgments
This work was supported by grants from the German Research Foundation (DFG) to MM, MMH, and OL (SFB633, TP B6), AAK (SFB633, TP Z1). LCBA is recipient of a scholarship from the DFG (grant IRTG1673). We thank Michaela Wattrodt and the staff of the animal facility (FEM, Charite) for excellent technical assistance and animal breeding, respectively.
Contributor Information
L. C. Berrocal Almanza, 1Department of Microbiology and Hygiene, Charité, University Medicine Berlin, Berlin, Germany.
M. Muñoz, 1Department of Microbiology and Hygiene, Charité, University Medicine Berlin, Berlin, Germany; 2Experimental Immunology, Department of Rheumatology and Clinical Immunology, Charité, University Medicine Berlin, Berlin, Germany; 3German Rheumatism Research Center (DRFZ), A Leibniz Institute, Berlin, Germany.
A. A. Kühl, 4Department of Gastroenterology, Infectology and Rheumatology / Research Center ImmunoSciences (RCIS), Charité, University Medicine Berlin, Berlin, Germany.
T. Kamradt, 5Institute for Immunology, Jena University Hospital, Jena, Germany.
M. M. Heimesaat, 1Department of Microbiology and Hygiene, Charité, University Medicine Berlin, Berlin, Germany.
O. Liesenfeld, 1Department of Microbiology and Hygiene, Charité, University Medicine Berlin, Berlin, Germany.
References
- 1.Sánchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, Gutiérrez-Ramos JC, Coyle AJ, Strom TB. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003 Nov;4(11):1093–10101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 2.Frisancho-Kiss S, Nyland JF, Davis SE, Barrett MA, Gatewood SJ, Njoku DB, Cihakova D, Silbergeld EK, Rose NR, Fairweather D. Cutting edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J Immunol. 2006 Jun 1;176(11):6411–6415. doi: 10.4049/jimmunol.176.11.6411. [DOI] [PubMed] [Google Scholar]
- 3.Oikawa T, Kamimura Y, Akiba H, Yagita H, Okumura K, Takahashi H, Zeniya M, Tajiri H, Azuma M. Preferential involvement of Tim-3 in the regulation of hepatic CD8+ T cells in murine acute graft-versus-host disease. J Immunol. 2006 Oct 1;177(7):4281–4287. doi: 10.4049/jimmunol.177.7.4281. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006 May;116(5):1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khademi M, Illés Z, Gielen AW, Marta M, Takazawa N, Baecher-Allan C, Brundin L, Hannerz J, Martin C, Harris RA, Hafler DA, Kuchroo VK, Olsson T, Piehl F, Wallström E. T Cell Ig- and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear cells in multiple sclerosis. J Immunol. 2004 Jun 1;172(11):7169–7176. doi: 10.4049/jimmunol.172.11.7169. [DOI] [PubMed] [Google Scholar]
- 6.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005 Dec;6(12):1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 7.Leitner J, Rieger A, Pickl WF, Zlabinger G, Grabmeier-Pfistershammer K, Steinberger P. TIM-3 does not act as a receptor for galectin-9. PLoS Pathog. 2013 Mar;9(3):e1003253. doi: 10.1371/journal.ppat.1003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuchroo VK, Meyers JH, Umetsu DT, DeKruyff RH. TIM family of genes in immunity and tolerance. Adv Immunol. 2006;91:227–249. doi: 10.1016/S0065-2776(06)91006-2. [DOI] [PubMed] [Google Scholar]
- 9.Koguchi K, Anderson DE, Yang L, O'Connor KC, Kuchroo VK, Hafler DA. Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp Med. 2006 Jun 12;203(6):1413–1418. doi: 10.1084/jem.20060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, Bruce JN, Kane LP, Kuchroo VK, Hafler DA. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007 Nov 16;318(5853):1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 11.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008 Nov 24;205(12):2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011 Aug;32(8):345–349. doi: 10.1016/j.it.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayaraman P, Sada-Ovalle I, Beladi S, Anderson AC, Dardalhon V, Hotta C, Kuchroo VK, Behar SM. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J Exp Med. 2010 Oct 25;207(11):2343–2354. doi: 10.1084/jem.20100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denkers EY. From cells to signaling cascades: manipulation of innate immunity by Toxoplasma gondii. FEMS Immunol Med Microbiol. 2003 Dec 5;39(3):193–203. doi: 10.1016/S0928-8244(03)00279-7. [DOI] [PubMed] [Google Scholar]
- 15.Liesenfeld O, Kosek J, Remington JS, Suzuki Y. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996 Aug 1;184(2):597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J Infect Dis. 2002 Feb 15;185(Suppl 1):S96–S101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Hölscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009 Dec 21;206(13):3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buzoni-Gatel D, Debbabi H, Mennechet FJ, Martin V, Lepage AC, Schwartzman JD, Kasper LH. Murine ileitis after intracellular parasite infection is controlled by TGF-beta-producing intraepithelial lymphocytes. Gastroenterology. 2001 Mar;120(4):914–924. doi: 10.1053/gast.2001.22432a. [DOI] [PubMed] [Google Scholar]
- 19.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Göbel UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006 Dec 15;177(12):8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 20.Moorman JP, Wang JM, Zhang Y, Ji XJ, Ma CJ, Wu XY, Jia ZS, Wang KS, Yao ZQ. Tim-3 pathway controls regulatory and effector T cell balance during hepatitis C virus infection. J Immunol. 2012 Jul 15;189(2):755–766. doi: 10.4049/jimmunol.1200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002 Jan 31;415(6871):536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 22.Dardalhon V, Anderson AC, Karman J, Apetoh L, Chandwaskar R, Lee DH, Cornejo M, Nishi N, Yamauchi A, Quintana FJ, Sobel RA, Hirashima M, Kuchroo VK. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J Immunol. 2010 Aug 1;185(3):1383–1392. doi: 10.4049/jimmunol.0903275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki Y, Sher A, Yap G, Park D, Neyer LE, Liesenfeld O, Fort M, Kang H, Gufwoli E. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol. 2000 May 15;164(10):5375–5382. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]