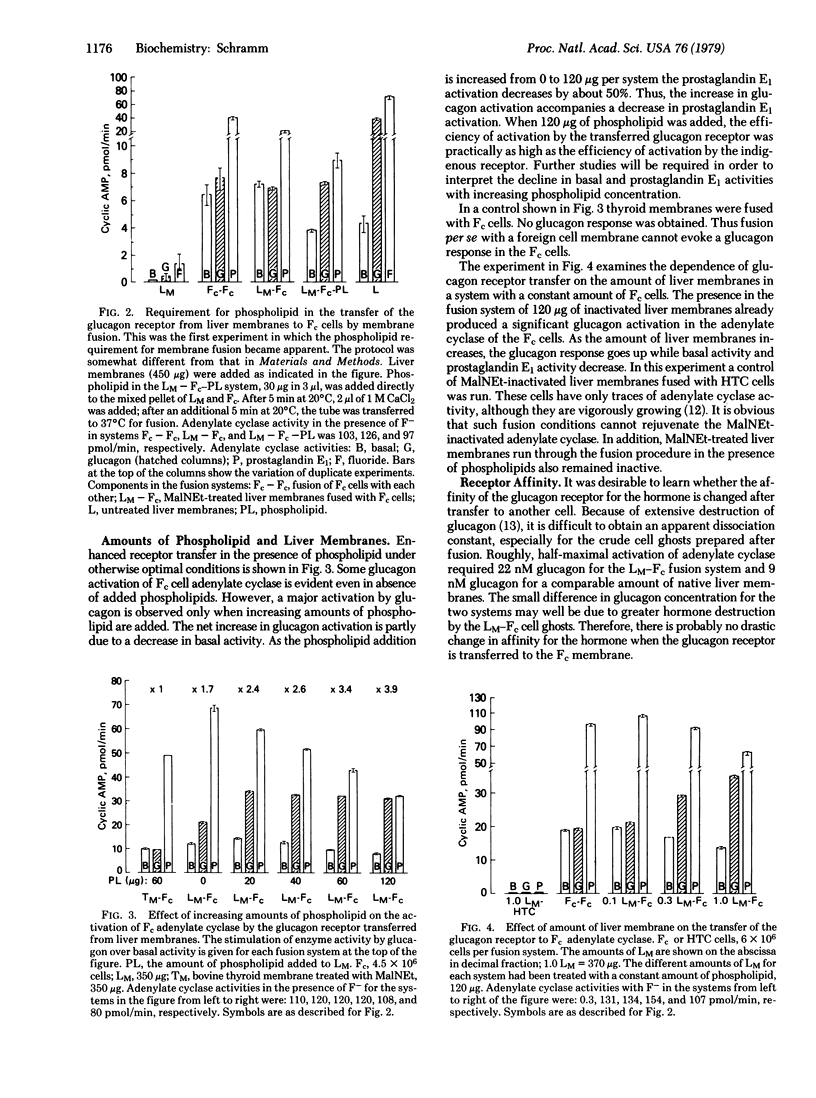
Abstract
Previous work had demonstrated the coupling of a β-adrenergic receptor on an erythrocyte with the adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] of a tissue culture cell when the two cells were fused by Sendai virus. The validity of this finding for animal tissues in general, for membrane preparations, and for peptide hormone receptors could hitherto not be assessed. Available fusion procedures worked efficiently only with certain intact cells from tissue culture and with erythrocytes. In the present work a membrane fusion method was developed that causes the transfer of the glucagon receptor from purified rat liver membranes to Friend erythroleukemia cells; even direct transfer to a membrane fraction prepared from Friend cells became feasible. It can therefore be concluded that a peptide hormone receptor in a normal tissue membrane has properties similar to those demonstrated for a β-adrenergic receptor in an erythrocyte: it exists in the membrane as a dissociable independent unit that can readily couple with the adenylate cyclase of a foreign cell. The efficiency of the membrane fusion procedure is due to the combined action of polyethylene glycol, phospholipids, stearylamine, and ATP in a salt medium. The method promises to be applicable to membranes of various cells and tissues, and it can probably be used to analyze hormone receptors and adenylate cyclase systems in states of malfunction by transfer to their respective counterpart in a normal cell membrane. Studies in biochemical hybridization of membrane components need not be limited to hormone activation of adenylate cyclase. With the aid of the membrane fusion method, this approach could be applied to any dissociable multicomponent system in biological membranes.
Keywords: hormone receptors, membrane hybridization, cell fusion, β-adrenergic receptor, phospholipids
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Fisher D., Tampion W., Lucy J. A. Mechanisms of cell fusion. Nature. 1975 Jan 17;253(5488):194–195. doi: 10.1038/253194a0. [DOI] [PubMed] [Google Scholar]
- Chi E. Y., Lagunoff D., Koehler J. K. Freeze-fracture study of mast cell secretion. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2823–2827. doi: 10.1073/pnas.73.8.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., Gerald P. S. Improved techniques for the induction of mammalian cell hybridization by polyethylene glycol. Somatic Cell Genet. 1976 Mar;2(2):165–176. doi: 10.1007/BF01542629. [DOI] [PubMed] [Google Scholar]
- Granner D., Chase L. R., Aurbach G. D., Tomkins G. M. Tyrosine aminotransferase: enzyme induction independent of adenosine 3', 5'-monophosphate. Science. 1968 Nov 29;162(3857):1018–1020. doi: 10.1126/science.162.3857.1018. [DOI] [PubMed] [Google Scholar]
- Hales A. A procedure for the fusion of cells in suspension by means of polyethylene glycol. Somatic Cell Genet. 1977 Mar;3(2):227–230. doi: 10.1007/BF01551817. [DOI] [PubMed] [Google Scholar]
- Heppel L. A., Makan N. Methods for rapidly altering the permeability of mammalian cells. J Supramol Struct. 1977;6(3):399–409. doi: 10.1002/jss.400060313. [DOI] [PubMed] [Google Scholar]
- Lawson D., Raff M. C., Gomperts B., Fewtrell C., Gilula N. B. Molecular events during membrane fusion. A study of exocytosis in rat peritoneal mast cells. J Cell Biol. 1977 Feb;72(2):242–259. doi: 10.1083/jcb.72.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Ahkong Q. F., Lucy J. A. Poly(ethylene glycol), surface potential and cell fusion. Biochem J. 1976 Sep 15;158(3):647–650. doi: 10.1042/bj1580647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. J., MacDonald R. C. Lipid vesicle-cell interactions. I. Hemagglutination and hemolysis. J Cell Biol. 1976 Sep;70(3):494–505. doi: 10.1083/jcb.70.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson A., Jr Crystallization of proteins from polyethylene glycol. J Biol Chem. 1976 Oct 25;251(20):6300–6303. [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Orly J., Schramm M. Coupling of catecholamine receptor from one cell with adenylate cyclase from another cell by cell fusion. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4410–4414. doi: 10.1073/pnas.73.12.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orly J., Schramm M. Fatty acids as modulators of membrane functions: catecholamine-activated adenylate cyclase of the turkey erythrocyte. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3433–3437. doi: 10.1073/pnas.72.9.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLSON A., POTGIETER G. M., LARGIER J. F., MEARS G. E., JOUBERT F. J. THE FRACTIONATION OF PROTEIN MIXTURES BY LINEAR POLYMERS OF HIGH MOLECULAR WEIGHT. Biochim Biophys Acta. 1964 Mar 16;82:463–475. doi: 10.1016/0304-4165(64)90438-6. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Poste G., Schaeffer B. E. Fusion of mammalian cells by unilamellar lipid vesicles: inflluence of lipid surface charge, fluidity and cholesterol. Biochim Biophys Acta. 1973 Sep 27;323(1):23–42. doi: 10.1016/0005-2736(73)90429-x. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L. Adenyl cyclase in fat cells. 3. Stimulation by secretin and the effects of trypsin on the receptors for lipolytic hormones. J Biol Chem. 1970 Feb 25;245(4):718–722. [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schramm M., Orly J., Eimerl S., Korner M. Coupling of hormone receptors to adenylate cyclase of different cells by cell fusion. Nature. 1977 Jul 28;268(5618):310–313. doi: 10.1038/268310a0. [DOI] [PubMed] [Google Scholar]
- Schulster D., Orly J., Seidel G., Schramm M. Intracellular cyclic AMP production enhanced by a hormone receptor transferred from a different cell. beta-adrenergic responses in cultured cells conferred by fusion with turkey erythrocytes. J Biol Chem. 1978 Feb 25;253(4):1201–1206. [PubMed] [Google Scholar]
- Schwarzmeier J. D., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase activity: interaction of components following cell-cell and membrane-cell fusion. J Cyclic Nucleotide Res. 1977 Aug;3(4):227–238. [PubMed] [Google Scholar]
- Zakai N., Kulka R. G., Loyter A. Fusion of human erythrocyte ghosts promoted by the combined action of calcium and phosphate ions. Nature. 1976 Oct 21;263(5579):696–699. doi: 10.1038/263696a0. [DOI] [PubMed] [Google Scholar]