SUMMARY
Obesity is associated with a chronic, low-grade, systemic inflammation that may contribute to the development of insulin resistance and type 2 diabetes. Resveratrol, a natural compound with anti-inflammatory properties, is shown to improve glucose tolerance and insulin sensitivity in obese mice and humans. Here we tested the effect of a 2-year resveratrol administration on pro-inflammatory profile and insulin resistance caused by a high-fat, high-sugar (HFS) diet in white adipose tissue (WAT) from rhesus monkeys. Eighty mg/day of resveratrol for 12-month followed by 480 mg/day for the second year decreased adipocyte size, increased sirtuin 1 expression, decreased NF-κB activation and improved insulin sensitivity in visceral but not subcutaneous WAT from HFS-fed animals. These effects were reproduced in 3T3-L1 adipocytes cultured in media supplemented with serum from monkeys fed HFS +/− resveratrol diets. In conclusion, chronic administration of resveratrol exerts beneficial metabolic and inflammatory adaptations in visceral WAT from diet-induced obese monkeys.
INTRODUCTION
Over two-thirds of United States adults are overweight or obese, a condition that is assumed to be produced by excess nutrient intake and possibly by lack of exercise. Obesity is associated with insulin resistance and an increased risk for type 2 diabetes (Olefsky and Glass, 2010). Moreover, it is now well established that excess white adipose tissue (WAT) is related with a state of chronic, low-grade, systemic inflammation, which induces and sustains insulin resistance (Xu et al., 2003). Activation of the NF-κB pathway has been shown to directly increase pro-inflammatory cytokine and chemokine gene expression (Suganami et al., 2007). NF-κB is a major pro-inflammatory nuclear transcription factor that is sequestered in the cytoplasm in its inactive state in a complex with members of the IκB family of inhibitor proteins. Following cell activation, IκB kinase β (IKK-β) phosphorylates IκB, which causes its ubiquitination and proteolytic degradation. This, in turn, frees NF-κB and enables its translocation to the nucleus where it activates the transcription of target genes. Hence, pharmacological inhibition of the NF-κB signaling pathway in adipose tissue may reduce chronic, low-grade, systemic inflammation and confer protection against insulin resistance and the development and progression of type 2 diabetes.
Resveratrol, a product of red grapes and nuts, has been shown to inhibit NF-κB activation and expression of inflammatory genes (Heynekamp et al., 2006), thereby improving glucose tolerance and insulin sensitivity in mice (Baur et al., 2006). The biological effects of resveratrol in mammals are largely attributed to its ability to activate sirtuin 1 (SIRT1) (Howitz et al., 2003), a NAD+-dependent deacetylase that regulates a wide range of biological processes such as gene silencing, aging, cellular differentiation, and metabolism (Blander and Guarente, 2004). However, it was recently shown that resveratrol might not activate SIRT1 directly, but rather exert its effects on SIRT1 through inhibition of cAMP-degrading phosphodiesterases (Park et al., 2012), although direct SIRT1 activation is not completely excluded (Dai et al., 2010). Regardless of the mode of activation, SIRT1-mediated deacetylation of p65 subunit (RelA) of NF-κB, at lysine 310, contributes to the down-regulation of NF-κB transcriptional activity and leads to decreased inflammatory response (Yeung et al., 2004) and improved insulin sensitivity in adipocytes and macrophages (Yoshizaki et al., 2009; Yoshizaki et al., 2010).
A number of recent studies have determined that short-term resveratrol supplementation (4-6 weeks) decreases inflammation (Ghanim et al., 2010; Timmers et al., 2011) and improves insulin sensitivity and metabolic function in adults who are obese, have type 2 diabetes, or older individuals with impaired glucose tolerance (Timmers et al., 2011; Crandall et al., 2012; Brasnyó et al., 2011). In contrast, no improvement in metabolic profile was noted after a 12-week resveratrol supplementation in nonobese women with normal glucose tolerance (Yoshino et al., 2012) while promising effects on glucose tolerance has been reported in primates fed chronically a standard diet supplemented with resveratrol (Marchal et al., 2012). Data from our laboratory have also shown beneficial effects of long-term resveratrol administration in non-human primates fed a high-fat, high-sugar (HFS) diet, a diet formulated to encourage obesity as well as chronic low grade inflammation and insulin resistance. Specifically, resveratrol prevented β-cell dedifferentiation in pancreas (Fiori et al., 2013) and conferred protection against inflammatory profile of aortic wall from rhesus monkeys fed HFS diet (Mattison et al., unpublished data). Despite these advances, there are no studies showing the effectiveness of chronic administration of resveratrol for improving insulin sensitivity, and metabolic and inflammatory profile altered by HFS diet in WAT from a non-human primate model. Then, the aim of the present study was to establish the effect of a 2-year resveratrol supplementation on the pro-inflammatory profile and insulin resistance caused by HFS diet in subcutaneous and visceral WAT from rhesus monkeys, a species close to human.
RESULTS
Rhesus monkey characteristics
Table S1 shows the characteristics pre-intervention (baseline) of 24 rhesus monkeys included in this study. The monkeys assigned to diets did not differ in age, weight, waist, hip, lipids and glucose metabolism parameters. Post-intervention, previous data (Fiori et al., 2013) have proven no metabolic dysregulation at 24 months of dietary interventions, based on fasting insulin and glucose levels. However, an increase in insulin area under the curve (AUC) from intravenous glucose tolerance test (IVGTT) and a decrease in insulin sensitivity index (ISI) was shown with the HFS +/− resveratrol (Resv) diets compared to baseline, consistent with a weight gain and insulin resistance (Fiori et al., 2013). In the present study, an additional analysis was carried out in order to determine the resveratrol effects at systemic level after 24 months of dietary intervention. Since the animals at pre-intervention state were fed with a standard diet (SD) and non-significant differences were observed between monkeys fed with SD at baseline (SDb) and post-intervention, we considered the baseline measurements for the experimental animals to represent the control state (Table 1). Post-intervention, HFS +/− Resv diets increased the waist and hip measurements (P < 0.05) when compared with SDb control group. An increase in LDL plasma levels, a characteristic associated to insulin resistance, was observed in monkeys fed HFS diet (P = 0.011) respect to animals in SDb control group.
Table 1.
| SDb | HFS diet | HFS + Resv diet | P value | |
|---|---|---|---|---|
| N | 24 | 10 | 10 | |
| Waist, cm | 41.06±2.03* | 55.95±5.93 | 53.05±3.28 | <0.001 |
| Hips, cm | 39.07±1.31* | 50.16±3.20 | 48.60±1.83 | <0.001 |
| Total Cholesterol, mg/dL | 141.87±6.01 | 162.40±19.48 | 159.30±12.18 | 0.103 |
| LDL-C, mg/dL | 57.54±12.55# | 84.20±13.84 | 73.00±6.59 | 0.008 |
| HDL-C, mg/dL | 71.67±4.31 | 58.60±7.80 | 63.90±7.57 | 0.037 |
| NEFA, mEq/L | 0.83±0.08 | 0.64±0.06 | 0.54±0.06 | 0.068 |
Values are presented as mean ± SEM. The data were analyzed using using a linear mixed-effects model Hips: n=24 (SDb); n=9 (HFS diet); n=10 (HFS + Resv diet). SDb: Standard diet baseline; HFS: High-fat, high-sugar; Resv: Resveratrol.
P ± 0.05 SDb vs. HFS ± Resv diet
P = 0.011 SDb vs. HFS diet.
Blood samples were obtained from fasting animals.
See also Table S1 and Figure S2.
Changes in gene expression of subcutaneous and visceral WAT by resveratrol
To examine possible effects of Resv in adipose tissue, we performed a genome-wide microarray analysis on subcutaneous WAT from rhesus monkeys at the start of the experiment (SDb) and after a two-year treatment with HFS +/− Resv diet (Figure 1). Analysis of the transcript profile revealed that a significant proportion of the genes whose expression was modified by HFS were shifted in the opposite direction in response to Resv supplementation (Figure 1A). Further analysis showed a highly related gene signature when comparing the Z-score of a given gene from Resv to either HFS (Resv_HFS) or SDb (Resv_SDb) (Figure 1A). Parametric analysis of gene-set enrichment (PAGE) was then used to highlight functional pathways most affected by the various dietary regimens. Focusing in gene categories related to immune responses and inflammation (i.e., inflammatory response, tumor necrosis factor receptor activity, chemokine activity, and others), the Z-score of a given GO term showed striking differences when comparing Resv to HFS (Resv_HFS) vs. HFS to SDb (HFS_SDb) (Figure 1B). Ingenuity pathway analysis revealed diet-induced alterations in inflammation-related biological functions in subcutaneous WAT (Table S2).
Figure 1. see also Table S2 and Table S3. Resveratrol supplementation induces changes in gene expression in two fat depots.
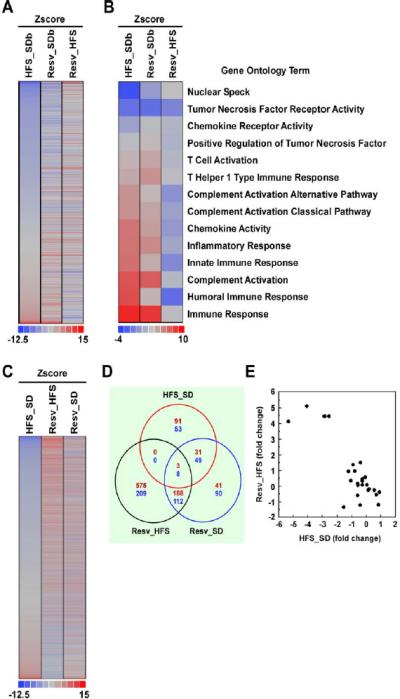
(A) Gene expression profile from subcutaneous fat of rhesus monkeys fed for 2 years with a high-fat, high-sugar diet (HFS) without or with resveratrol (Resv) supplementation compared to standard diet-fed animals at baseline (SDb). (B) Heatmap of immune-related GO Terms in subcutaneous fat depot. The Z-score of a given GO Term showed striking difference when comparing Resv to HFS (Resv_HFS) vs. HFS to SDb (HFS_SDb). (C) Gene expression profile from visceral fat of rhesus monkeys fed for 2 years with HFS diet +/− Resv supplementation compared to SD-fed animals. (D) Venn diagram of overlapping genes significantly changed in the comparison HFS_SD, Resv_HFS, and Resv_SD. (E) Scatter plot of gene expression values in Resv_HFS vs. HSF_SD within the ‘stresspathway’ gene set. (A to E) SDb, n=2; SD, n=2; HFS diet, n=4 and HFS + Resv diet, n=4.
The fact that our animal research protocol did not allow for the collection of visceral WAT at the onset of the experiment has prevented us to compare the effect of HFS +/− Resv diet vs. SDb in this fat depot. Nevertheless, visceral WAT from 2-year SD group was collected and used for subsequent comparisons. Consistent with the results obtained in subcutaneous fat, Resv supplementation elicited a genome-wide expression profiling that was opposite to that observed with HFS feeding (Figure 1C). A clear overlap between the genes whose expression was significantly altered by Resv supplementation compared to either SD (Resv_SD) or HFS (Resv_HFS) is shown in the Venn diagram (Figure 1D). Using PAGE analysis, we focused on select genesets known for their susceptibility to Resv (e.g., Electron_Transport_Chain, Proteasome, Aged_Rhesus_Up, TollPathway, and others), and found a number of genesets that were regulated in opposite directions by HFS_SD vs. Resv_HFS in visceral WAT (Table S3). Indeed, scatter plot of gene expression values in HSF_SD within the ‘stresspathway’ geneset showed strikingly opposite changes as compared to Resv_HFS (Figure 1E). Moreover, the transcriptional effect of HFS feeding appears to have intrinsic fat depot-specific difference in the regulation of some genesets, including Proteasome and Aged_Rhesus_Up (Table S3). These data indicate that HFS feeding +/− Resv supplementation elicits a set of transcriptional changes that is likely to shape the distinct depot-specific responses in rhesus monkeys.
Resveratrol increases the number of small adipocytes in visceral WAT
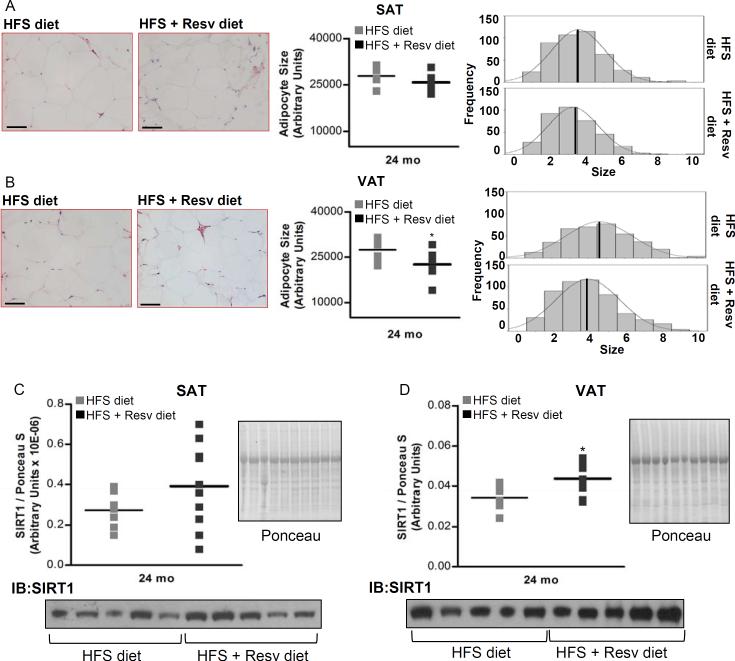
A H&E staining of WAT showed non-significant differences in the mean size of adipocytes and adipocyte frequency distribution when subcutaneous WAT from monkeys assigned to HFS diet was compared to that of HFS + Resv diet group (Figure 2A). However, the analysis of adipocyte frequency distribution in visceral WAT showed a distribution change toward smaller adipocytes in the HFS + Resv diet-fed monkeys compared with HFS diet-fed group. As a result, a significant difference in the mean size of adipocytes between both groups was observed (P = 0.032) (Figure 2B).
Figure 2. see also Figure S3. Resveratrol decreases mean adipocyte size and increases SIRT1 protein expression in visceral WAT of rhesus monkeys maintained on HFS diet for 2 years.
(A) Morphologic characteristics of subcutaneous WAT. (B) Morphologic characteristics of visceral WAT. (C) SIRT1 protein levels in subcutaneous WAT. (D) SIRT1 protein levels in visceral WAT. (A and B) H&E-stained sections of WAT from monkeys fed HFS and HFS + Resv diet are shown. Images were captured at 20× magnification. Scale bar: 200 μm. Mean adipocyte size and adipocyte frequency distribution show cell surface areas in both fat depots after 24-mo of dietary intervention. (A to D) Results are expressed in a dot plot format, which represents the individual data and the mean. (A) n=7 (HFS diet); n=8 (HFS + Resv diet). (B) n=8 for each group. (C and D) n=10 for each group. The data were analyzed using Independent-Samples t test to analyze statistical significance between HFS vs. HFS+Resv diet at 24-mo of dietary intervention. *, P < 0.05 (HFS vs. HFS + Resv diet). HFS: high-fat, high-sugar; Resv: resveratrol; VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue.
Increased SIRT1 protein levels in visceral WAT by resveratrol supplementation
SIRT1 protein expression was measured in visceral and subcutaneous WAT after dietary intervention (Figure 2). No change in SIRT1 protein levels was found in subcutaneous fat between the groups (Figure 2C). However, HFS + Resv diet induced a significant increase of SIRT1 protein levels in visceral fat when compared to HFS diet (P = 0.010) (Figure 2D).
Resveratrol inhibits NF-κB activation and expression of target genes in visceral WAT
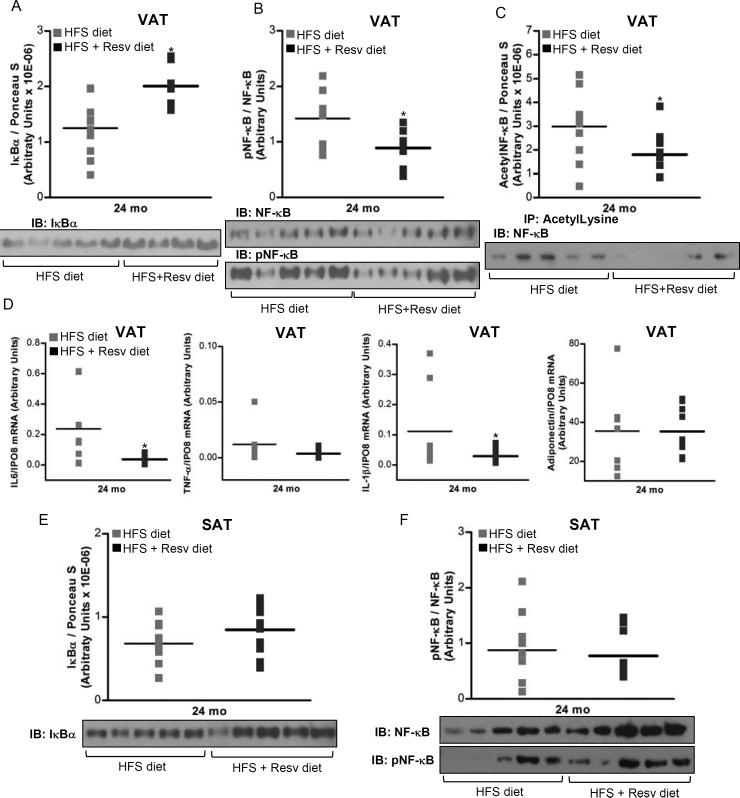
A decrease in NF-κB activation, as evidenced by a substantial increase of IκBα protein levels (P = 0.003) (Figure 3A), and reduction of NF-κB phosphorylation (P = 0.02) (Figure 3B) and acetylation (P = 0.05) (Figure 3C) was found in visceral fat of rhesus monkeys fed with HFS + Resv diet as compared with HFS diet. Inhibition of the NF-κB signaling by Resv was accompanied by a decrease of NF-κB target gene expression (IL-6, P = 0.05; IL-1β, P = 0.048) (Figure 3D). However, regulation of NF-κB in the subcutaneous adipose tissue was refractory to Resv supplementation (Figure 3E-F).
Figure 3. see also Table S4 and Table S5. Resveratrol decreases inflammatory response in visceral WAT of rhesus monkeys fed a HFS diet for 2 years.
(A) IκBα protein levels in visceral WAT. (B) phosphorylated NF-κB/NF-κB ratio in visceral WAT. (C) Acetylated NF-κB protein content in visceral WAT. IP with a control IgG did not result in NF-κB detection (data not shown). (D) mRNA expression for IL-6, TNF-α, IL-1β and adiponectin in visceral WAT. (E) IκBα protein levels in subcutaneous WAT. (F) Phosphorylated NF-κB/NF-κB ratio in subcutaneous WAT. (A to F) Results are expressed in a dot plot format, which represents the individual data and the mean. (A and E) n=10 for each group. (B) n=9 (HFS diet); n=8 (HFS + Resv diet). (C) n=10 (HFS diet); n=9 (HFS + Resv diet). (D) IL-6 and IL-1β: n=8 (HFS diet); n=10 (HFS + Resv diet). TNF-α and adiponectin: n=7 (HFS diet); n=10 (HFS + Resv diet). (F) n=9 (HFS diet); n=10 (HFS + Resv diet). (A to F) The data were analyzed using Independent-Samples t test to analyze statistical significance between HFS vs. HFS + Resv diet at 24-mo of dietary intervention. IL-1β gene expression was log-transformed before statistical analysis. *, P < 0.05 (HFS vs. HFS + Resv diet). HFS: high-fat, high-sugar; Resv: resveratrol; VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue; pNF-κB: phosphorylated NF-κB.
To extend the results obtained in visceral fat, we next quantified the circulating levels of several inflammatory pathway components and found the concentration of pro-inflammatory IL-1β to be significantly higher after consumption of a HFS ± Resv diets compared to SDb control (P < 0.05) (Table S4). Non-significant differences between groups were observed in the other variables analyzed (IL-6, C-reactive protein (CRP), TNF-α and Adiponectin) (Table S4).
Resveratrol modulates the expression of insulin-signaling protein markers in visceral WAT
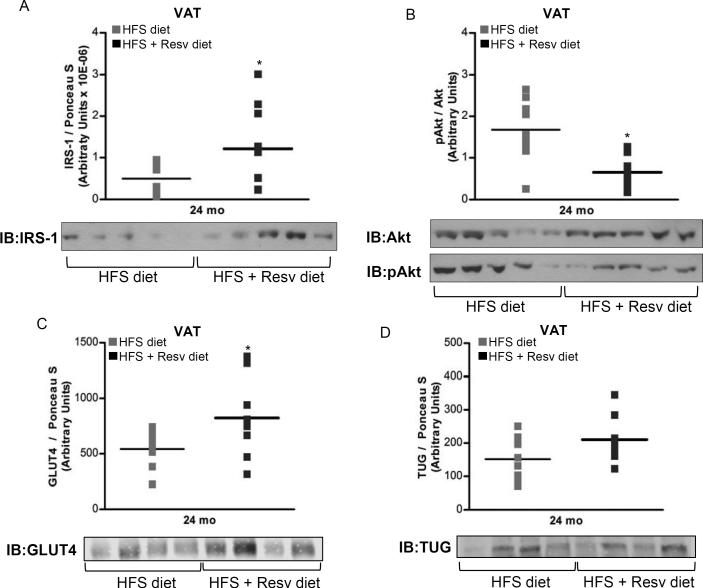
An increase of IRS-1 protein levels (P = 0.05) (Figure 4A), a decrease of Akt serine 473 phosphorylation (P = 0.003) (Figure 4B) and an increase in the content of insulin-responsive GLUT4 glucose transporter (P = 0.033) (Figure 4C) were observed in visceral fat depot from fasted monkeys on HFS + Resv group as compared to HFS diet controls. It is important to note that chronic stimulation of Akt in adipocytes and transgenic mice overexpressing constitutively active Akt elicit negative feedback on insulin signaling (Ozes et al., 2001; Nagoshi et al., 2005). Therefore, reduction in phosphoactive Akt in adipocytes of fasted monkeys maintained on HFS + Resv diet vs. HFS supports the notion of an improved insulin sensitivity in visceral WAT from the HFS + Resv diet-fed animals.
Figure 4. see also Figure S1. Resveratrol improves insulin sensitivity in visceral WAT of rhesus monkeys fed a HFS diet for 2 years.
(A) IRS-1 protein expression. (B) Phosphorylated Akt/Akt ratio. (C) GLUT4 protein levels. (D) TUG protein content. (A to D) Results are expressed in a dot plot format, which represents the individual data and the mean. (A) n=8 (HFS diet); n=10 (HFS + Resv diet). (B) n=9 (HFS diet); n=10 (HFS + Resv diet). (C and D) n=10 (HFS diet); n=9 (HFS + Resv diet). The data were analyzed using Independent-Samples t test to analyze statistical significance between HFS vs. HFS + Resv diet at 24-mo of dietary intervention. *, P < 0.05 (HFS vs. HFS + Resv diet). HFS: high-fat, high-sugar; Resv: resveratrol; VAT: visceral adipose tissue; pAkt: phosphorylated Akt.
In unstimulated fat and muscle cells, GLUT4 is sequestered intracellularly in part by binding the Tether, containing a UBX domain, for GLUT4 (TUG) protein (Bogan et al., 2003). Insulin stimulates TUG endoproteolytic cleavage to mobilize intracellular GLUT4 storage vesicles to the plasma membrane, an action that allows glucose to enter the cells by facilitative transport (Bogan et al., 2012). Here, TUG protein levels tended to be higher in HFS + Resv group compared to HFS group, although these differences did not reach statistical significance (Figure 4D).
Refractoriness of visceral WAT mitochondrial to resveratrol treatment
To test if the Resv-mediated expression of classical markers of insulin signaling pathway was associated to an effect of this polyphenol in visceral WAT mitochondria, we analyzed mitochondrial content (citrate synthase activity, mRNA expression for cytochrome b (CytB), NADH dehydrogenase 1 (ND1), ND2, ND5 and ND6, and NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 9 (NDUFA 9) protein levels), mitochondrial biogenesis (mRNA expression for PPARγ co-activator 1 (PGC-1α) and dimethyladenosine transferase 2 mitochondrial (TFB2M)) and oxidative stress (H2O2 and lysine 4-hydroxynonenal (HNE) levels) in this fat depot from rhesus monkeys after dietary intervention. Non-significant changes between HFS and HFS + Resv diet were observed in these parameters (Figure S1).
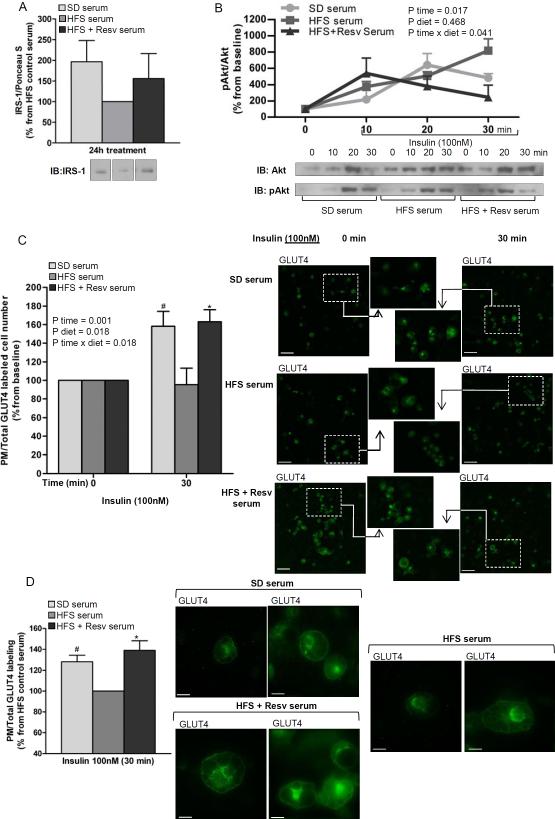
Serum from resveratrol-treated monkeys elicits anti-inflammatory effects and improves insulin signaling in 3T3-L1 adipocytes
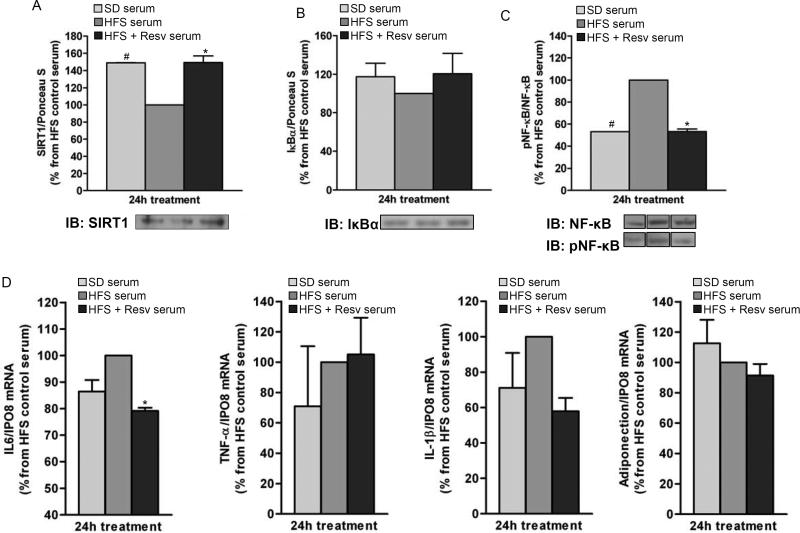
The inflammatory profile of fully-differentiated 3T3-L1 adipocytes was assessed after a 24-h incubation with media containing serum from monkeys fed with SD, HFS and HFS + Resv diets for 24 months. The results indicated an increase in SIRT1 protein level (P < 0.05) (Figure 5A) and decrease in NF-κB phosphorylation (P < 0.05) (Figure 5C) after treatment of 3T3-L1 adipocytes with serum from animals fed SD or HFS + Resv diet as compared to HFS serum. Moreover, a decrease in NF-κB target gene expression (IL-6, P = 0.016) was also observed when 3T3-L1 adipocytes were incubated in media containing HFS + Resv serum vs. HFS serum (Figure 5D). A trend toward lower IL-6 mRNA was found with SD serum vs. HFS serum (P = 0.056).
Figure 5. Serum from resveratrol-treated monkeys on HFS diet exerts anti-inflammatory effects in 3T3-L1 adipocytes.
Fully-differentiated 3T3-L1 adipocytes were incubated for 24-h with media containing serum from SD and HFS +/− Resv diet-fed monkeys for 2 years. (A) SIRT1 protein levels. (B) IκBα protein levels. (C) Phosphorylated NF-κB/NF-κB ratio. Lanes were run on the same gel but were noncontiguous. (D) mRNA expression for IL-6, TNF-α, IL-1β and adiponectin. (A to D) The graphs show the mean ± SEM from 3 independent experiments, and the results are expressed as percent increase over the values observed in adipocytes treated with HFS serum. The data were analyzed using One-Way ANOVA. *, P < 0.05 (HFS vs. HFS + Resv serum); #, P < 0.05 (HFS vs. SD serum). SD: standard diet; HFS: high-fat, high-sugar; Resv: resveratrol; pNF-κB: phosphorylated NF-κB.
This reduction of the pro-inflammatory profile by the HFS + Resv serum may correlate with an improvement in insulin sensitivity. Control experiments showed no difference in IRS-1 protein levels upon treatment of 3T3-L1 adipocytes with the three serums at baseline (Figure 6A). However, a significant effect of time (P = 0.017) and the interaction -diet and time- (P = 0.041) in Akt activation was found when 3T3-L1 adipocytes that were pretreated with monkey serum for 24 h were stimulated with insulin for 30 min. Specifically, the duration of insulin-induced Akt phosphorylation was markedly longer when 3T3-L1 adipocytes were pretreated with serum from HFS diet group as compared to that of SD and HFS + Resv diet (Figure 6B). Moreover, a significant increase in the number of cells expressing GLUT4 at the plasma membrane (P < 0.05) (Figure 6C) and greater insulin-induced cell surface GLUT4 labeling (P < 0.05) (Figure 6D) were observed when 3T3-L1 adipocytes were incubated with SD or HFS + Resv serum as compared with HFS serum.
Figure 6. Serum from resveratrol-treated monkeys on HFS diet improves insulin signaling in 3T3-L1 adipocytes.
(A) IRS-1 protein levels in 3T3-L1 adipocytes incubated for 24-h with media containing serum from SD and HFS +/− Resv diet-fed monkeys for 2 years (n=3). Lanes were run on the same gel but were noncontiguous. (B) Phosphorylated Akt/Akt ratio in 3T3-L1 adipocytes pretreated for 24-h with media containing serum from SD and HFS +/− Resv diet-fed monkeys for 2 years and stimulated with insulin (100 nM) for 10, 20 and 30 min (n=3). (C) Number of cells expressing GLUT4 at the plasma membrane. 3T3-L1 adipocytes were incubated for 24-h with media containing serum from SD and HFS +/− Resv diet-fed monkeys for 2 years and then treated in the absence (0 min) or presence of 100 nM insulin (30 min). GLUT4-labeled sections of 3T3-L1 adipocytes are shown. Images were captured at 10× magnification. Scale bar: 90 μm. The number of cells that were stained for GLUT4 at the plasma membrane over total cell number both in control and insulin-treated cells were counted (n=5). (D) Insulin-induced cell surface GLUT4 labeling. 3T3-L1 adipocytes were pretreated for 24-h with media containing serum from SD and HFS +/− Resv diet-fed monkeys for 2 years and then stimulated with insulin (100 nM) for 30 min. GLUT4-labeled sections of 3T3-L1 adipocytes are shown. Images were captured at 60× magnification. Scale bar: 15 μm. The amount of GLUT4 labeling at the plasma membrane of insulin-treated cells was normalized by the total GLUT4 staining in the same cells (n=5). (A to D) The graphs show the mean ± SEM, and the results are expressed as percent increase over the values observed in adipocytes treated with HFS serum (A and D) or as percent increase over the values observed in adipocytes at baseline (B and C). The data were analyzed using One-Way ANOVA and RM-ANOVA was used to calculate the time effect (P time), the diet effect (P diet) and the diet × time interaction (P diet × time). *, P < 0.05 (HFS vs. HFS + Resv serum); #, P < 0.05 (HFS vs. SD serum). SD: standard diet; HFS: high-fat, high-sugar; Resv: resveratrol; pAkt: phosphorylated Akt; PM: plasma membrane.
DISCUSSION
Obesity is recognized as a chronic, low-grade, systemic inflammation, which contributes to the development of insulin resistance (Gustafson et al., 2007). Approaches aimed to reduce adipose tissue inflammation may be effective at improving insulin resistance. In this sense the polyphenol Resv has been shown to improve glucose tolerance and insulin sensitivity in diet-induced obese mice (Baur et al., 2006; Lagouge et al., 2006) and in obese people (Timmers et al., 2011). Here, gene expression studies and pathway analyses revealed that Resv supplementation partly protect adipose tissue from transcriptional changes that lead to activation of pro-inflammatory and stress responses in rhesus monkeys upon HFS feeding. Some of the transcriptional changes induced by a 2-year HFS +/− Resv supplementation were fat depot-specific, which may be the cause of the observed alterations in visceral vs. subcutaneous WAT after dietary intervention. To our knowledge, we show for the first time that long-term Resv supplementation reduced adipocyte size, increased SIRT1 protein levels, and decreased both NF-κB activation and several inflammatory markers in visceral WAT of diet-induced obese rhesus monkeys. Moreover, chronic administration of Resv elicited an appropriate expression of classical markers of insulin signaling pathway in visceral WAT. The fact that these effects were partially reproduced in 3T3-L1 adipocytes incubated with media supplemented with serum from monkeys fed HFS +/− Resv indicated that adipocytes may contribute to the development of these metabolic and inflammatory adaptations.
Resveratrol has received considerable attention for its ability to activate SIRT1, an enzyme that deacetylates a range of substrates including PGC-1α, uncoupling protein (UCP)-2, NF-κB and FoxO1, which, in turn, results in a profound effect on glucose homeostasis and insulin secretion in rodents (Rodgers et al., 2005, Kitamura et al., 2005, Moynihan et al., 2005). In adipose tissue, SIRT1 represses adipocyte differentiation and expression of genes controlled by PPARγ (Picard et al., 2004), while increasing FoxO1-mediated adiponectin expression in adipocytes (Qiao and Shao, 2006) and PCG-1α–mediated adaptative thermogenesis in brown adipose tissues (Puigserver et al., 1998). Moreover, SIRT1 has been implicated in the negative regulation of the NF-κB inflammatory response in 3T3-L1 adipocytes (Yoshizaki et al., 2009). Because of the growing evidence that links chronic low-grade inflammation to the development of insulin resistance (Cai et al., 2005) and the ability of SIRT1 at reducing inflammation and improving glucose tolerance in mice (Ramsey et al., 2008), it was imperative to extend these findings to a species close to human. Toward this end, we studied the effect of chronic administration of Resv on the HFS diet-induced pro-inflammatory state and insulin resistance in rhesus monkey WAT and found that significant changes in adipocyte size, SIRT1 expression and NF-κB activity were observed in visceral WAT but not subcutaneous WAT, indicative of functional differences among fat depots (Coon et al., 1992; Gastaldelli et al., 2002). Consequently, the ability of Resv to regulate NF-κB target gene expression was examined in visceral WAT. A decrease in IL-6 and IL-1β mRNA levels was observed in HFS + Resv group when compared to HFS-fed animals, consistent with earlier observations showing that Resv reduces pro-inflammatory cytokine expression in cultured mouse and human adipocytes (Gonzales and Orlando, 2008; Kennedy et al., 2009) and in rodent WAT (Rivera et al., 2009). These findings in our study were associated with a reduction in the inflammatory profile of aortic wall (Mattison et al., unpublished data) from monkeys assigned to HFS + Resv diet, without changes in circulating pro-inflammatory cytokines levels. This latter result could be due to a variety of reasons as duration of dietary intervention and/or the effect of Resv in other contributors of systemic inflammation (e.g. liver and muscle).
Adipose tissue is composed of mature adipocytes and the stromavascular fraction that contains preadipocytes, blood cells, and macrophages. IL-1β and IL-6 are produced by adipocytes (Trayhurn and Wood, 2005) and the increase in inflammatory response following SIRT1 depletion in 3T3-L1 adipocytes is reversed upon cell treatment with SIRT1 activators (Yoshizaki et al., 2009). In support of the latter study, we observed that 3T3-L1 adipocytes treated with media supplemented with serum from monkeys fed with HFS + Resv diet had higher SIRT1 protein level but lower NF-κB phosphorylation and IL-6 mRNA expression when compared to the HFS-fed animals. Taken together, these data indicate that adipocytes contribute, at least partly, to the beneficial effects of Resv in visceral WAT.
Studies conducted in healthy obese men (Timmers et al., 2011) and patients with type 2 diabetes (Brasnyó et al., 2011) demonstrate that 4-week Resv administration exerts beneficial effects on systemic insulin sensitivity. However, others performed in subjects with impaired glucose tolerance (Crandall et al., 2012) and normal weight healthy individuals (Ghanim et a, 2010) have shown non-significant changes in fasting glucose or insulin levels after acute resveratrol supplementation (4-6 weeks). These contradictory results warrant additional studies on the effect of long-term resveratrol supplementation. Long-term treatment of adipocytes with IL-6 or IL-1β inhibits IRS-1 and GLUT4 expression (Rotter et al., 2003; Jager et al., 2007). Likewise, lower IRS-1 and GLUT4 content is observed in adipocytes from patients with type 2 diabetes (Carvalho et al., 1999; Garvey et al., 1991) and in insulin-resistant subjects (Carvalho et al., 1999; Shepherd and Kahn, 1999). Moreover, there is emerging evidence showing that prolonged activation of Akt leads to negative feedback of insulin signaling (Ozes et al., 2001; Nagoshi et al., 2005), possibly through p65/RelA-mediated increase in the phosphorylation and expression of Akt (Meng and D'Mello, 2003). We report that long-term Resv supplementation improved expression of classical markers of insulin signaling cascade in visceral adipose tissue of HFS-fed rhesus monkeys through increased IRS-1 protein levels, lower chronic activation of Akt and higher GLUT4 protein content. This effect in visceral WAT was asssociated with a preservation of pancreas morphology without improvement of insulin sensitivity at the systemic levels (Fiori et al., 2013). This latter finding may be partly explained by the fact that 2-year resveratrol supplementation may not be sufficient to observe systemic changes in glucose homeostasis in rhesus monkeys. In this sense, Marchal et al. (Marchal et al., 2012) did not observe differences in insulin sensitivity and glycemia markers between grey mouse lemurs fed standard diet and those fed with standard diet supplemented with resveratrol at 21 months of dietary intervention. However, after 33 months, resveratrol decreased glycemia after the oral glucose loading and HOMA-IR index compared with standard diet in these non-human primates. Finally, although Resv potently increases mitochondrial function and biogenesis through PGC-1α in muscle and brown adipose tissue and confers protection against diet-induced obesity and insulin resistance in mice (Lagouge et al., 2006), we did not observe an improvement of mitochondrial content in visceral WAT of HFS + Resv diet-fed rhesus monkeys, indicating a possible tissue-specific effect of Resv. In agreement with us, Lagouge et al. (Lagouge et al., 2006) did not observed changes in mitochondrial biogenesis in the heart, despite the coexpression of PGC-1α and SIRT1. Likewise, Chabi et al. (Chabi et al., 2009) found that liver, and to a lesser extent adipose tissue, from rats shows high levels of SIRT1 activity, despite having low PGC-1α protein content, which are in accordance with the reported role of SIRT1 in the activation of lipolysis and gluconeogenesis (Picard et al., 2004; Rodgers et al., 2005), but do not indicate that the SIRT1 expression pattern is related to the oxidative capacity of a given tissue. Alternatively, long-lived species, such as primates and humans, may have different bioenergetics response to Resv compared to short-lived species (e.g. mice and rats).
To demonstrate that Resv effectively improves insulin sensitivity in WAT, we studied the effects of this compound in 3T3-L1 adipocytes and found that the serum from rhesus monkeys fed with HFS + Resv diet normalized insulin-induced Akt activation, increased the number of cells expressing GLUT4 and the quantity of this glucose transporter at the plasma membrane in response to an acute insulin challenge. However, Resv was also found to increase the insulin-stimulated phosphorylation of Akt in 3T3-L1 adipocytes (Kang et al., 2012). Difference in experimental conditions (e.g., insulin concentration; monkey serum vs. inflammatory conditioned media from lipopolysaccharide-stimulated RAW264.7 cells; Resv administered to monkeys for 2 years vs. Resv addition to the inflammatory conditioned media) could ultimately account for the opposite results between the two studies. It is interesting that Resv reduces serine phosphorylation of IRS-1 and a subsequent degradation of the protein through inhibition of Akt and JNK activity in insulin-resistant muscle cells (Frojdo et al., 2011).
In conclusion, our data suggest that before evidence of dysglycemia is uncovered, the chronic administration of Resv produces beneficial metabolic and inflammatory adaptations in visceral WAT of a diet-induced obese non-human primate model. In addition, we demonstrate that adipocytes are partly responsible for the favorable adaptations produced by Resv in visceral WAT. Although lifestyle changes and pharmacotherapy are the primary intervention to improve insulin resistance, they are difficult to follow and maintain and may be too late as changes predisposing to diabetes could have already happened in pancreas and peripheral tissues. Since Resv was well tolerated at the tested concentrations, long-term Resv supplementation may provide a safe approach to reduce the chronic inflammatory properties associated with obesity while restoring insulin responsiveness in visceral WAT.
EXPERIMENTAL PROCEDURES
Animals
Twenty-four adults (7-13 years old) male rhesus monkeys (Macaca mulatta) were housed at the NIH Animal Center, Poolesville, MD, a center fully accredited by the American Association for Accreditation of Laboratory Animal Care. All procedures were approved by the Animal Care and Use Committee of the NIA Intramural Program. Details of animal care are provided in Supplemental Material.
After baseline assessment, the animals were subjected to a dose escalation study of 2-year of duration. Monkeys were quasi-randomized into one of three groups: HFS diet + Resv (n=10); HFS diet + placebo (n=10); Standard diet (SD) (n=4). The three diets were isoenergetics (~52 kcal / Kg body weight per day). The SD was a commercially available closed formula monkey chow (TestDiet® #5038, Purina Mills, Richmond, IN); 13% of kcal in fat and 2.24% sucrose by weight. The main sources of macronutrients in the SD were as follows: Protein— soy meal, corn; Fat— porcine fat, corn; Carbohydrate— corn, wheatmiddlings. The HFS diet was a specially formulated purified ingredient diet with 42% of kcal in fat and approximately 27% sucrose by weight (Custom formula #07802, Harlan, Teklad, Madison, WI). The main sources of macronutrients in the HFS diet were as follows: Protein —casein, lactalbumin; Fat —milk fat, soybean oil; Carbohydrate —sucrose and maltodextrin. Macronutrient composition in both diets was: 1) SD diet: 18.2% of kcal in protein, 13.1% of kcal in fat, 68.7% of kcal in carbohydrate, 2.24% sucrose; 2) HFS diet: 15.8% of kcal in protein, 42.3% of kcal in fat, 41.9% of kcal in carbohydrate, 27% sucrose. The monkeys were gradually switched to HFS diet over a 3-week period. Both groups received 2 meals per day in allotments that represented ad libitum feeding. Resv (resVida®) was supplied by DSM Nutritional Products (Parsippany, NJ). Each monkey in the HFS + Resv group received a total daily dose of 40 mg twice a day. Details of calculation of the dose are provided in Supplemental Material. No adverse effects to Resv were evident. Monkeys in non-Resv group received a placebo treat. After 1-year, the Resv dose was increased to 240 mg twice a day to document a dose effect. Again, no adverse effects were evident. An increase of serum trans-Resv and its O-sulfated metabolite concentration (P < 0.05) was observed with the dosage increments (40 vs. 240 mg twice daily) in HFS + Resv group (Figure S2).
Blood and adipose tissue sample collection
In monkeys anesthetized with ketamine (7-10mg/kg, intramuscular), fasting blood samples were obtained by venipuncture of the femoral vein at 0, 9 and 24 months of dietary intervention and subcutaneous adipose tissue was collected via an open incision at the ventral midline of the abdomen at 0 month of dietary intervention. Adipose tissue was obtained at 24 months of dietary intervention after the fasting monkeys were anesthetized with a lethal dose of sodium pentobarbital (50 mg/kg, intraperitoneal). Once deeply sedated, the monkeys were perfused with cold lactated Ringer's solution until death. A section of subcutaneous adipose tissue was collected via an open incision at the ventral midline of the abdomen. The peritoneal cavity was then exposed and visceral adipose tissue was collected from the omentum. Samples were stored in cryovials, flash frozen in liquid nitrogen, and stored at −80 °C until assayed.
Biochemical determinations
Total cholesterol, LDL-C, HDL-C were obtained by a VAP test by Atherotech Diagnostics Lab (Birmingham, AL). Serum fasting insulin was determined by ELISA using a commercially available kit (Mercodia, Inc., Uppsala, Sweden). Fasting glucose was determined from whole blood with an Ascensia Breeze 2 Blood Glucose Meter (Bayer Healthcare LLC, Mishawaka, IN). Serum trans-Resv and trans-Resv-3-O-sulfate concentration was measured by a chromatography and mass spectrometry method as described in Supplemental Material.
3T3-L1 cell culture and in vitro experimental setups
Mouse 3T3-L1 fibroblasts (American Type Culture Collection, Manassas, VA) were cultured and differentiated toward adipocytes as previously described (Student et al., 1980) with modifications. See Supplemental Material for further details.
For treatments, 3T3-L1 adipocytes were cultured with high glucose DMEM containing 4 mM glutamine and 1.5 g/L NaH2CO3 and supplemented with 10% pooled monkey serum from either the SD, HFS or HFS + Resv group. After a 24-h incubation period, cells were treated with 100 nM insulin for 30 min. 3T3-L1 adipocytes were harvested for RNA and protein determination and examined also for GLUT4 translocation by immunocytochemistry at the time points indicated in Figures.
Protein extraction
50-100 mg of adipose tissue were thawed by adding 250 μL of cold urea/thiourea buffer (7 M urea (Sigma-Aldrich), 2 M thiourea (Sigma-Aldrich), 4% CHAPS (USB Affymetrix, Santa Clara, CA), 45 mM Tris pH 7.4 (Sigma-Aldrich), 60 mM dithiothreitol (Sigma-Aldrich) and protease and phosphatase inhibitors (Sigma-Aldrich)). Cells were mechanically disrupted and briefly sonicated. Samples were incubated for 15 min at 35 °C and cooled on ice for 10 min. The homogenate was then centrifuged (15 min, 10 000 × g, 4 °C) and the aqueous phase between the upper lipid p hase and lower cellular debris phase was collected. Extensive delipidation was accomplished by tri-n-butylphosphate-acetone-methanol (Sigma-Aldrich) precipitation (Gesta et al., 2006). Precipitated proteins were resuspended in 80 μL of urea/thiourea buffer, and the Lowry assay method (Bio-Rad, Hercules, CA) was used to determine protein concentration.
For immunoprecipitation and determination of GLUT4 and TUG protein content, visceral adipose tissue was mechanically lysed and briefly sonicated in RIPA buffer supplemented with EDTA and EGTA (Boston BioProducts, Ashland, MA), protease and phosphatase inhibitors (Sigma-Aldrich) and 0.1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich). 3T3-L1 adipocytes were also lysed using the same buffer. The homogenate was then centrifuged and the aqueous phase was collected between the upper lipid phase and lower cellular debris phase. The Bradford assay method (Bio-Rad) was used to determine protein concentration.
Western blot and Immunoprecipitation
Protein expression by western blot and acetylated NF-κB quantification by immunoprecipitation were performed according to standard procedures as described in Supplemental Material. Ponceau S staining was selected as a loading control (Romero-Calvo et al., 2010), because the values of integrated densitometric peak areas correlated with NF-κB protein levels when serial dilutions of a visceral WAT protein sample were analyzed (r2 = 0.99; Figure S3).
Gene expression
Microarray and real-time PCR techniques were carried out according to standard procedures to determine the effects of Resv on gene expression of 3T3-L1 adipocytes and WAT from rhesus monkeys. Full methodological details are provided described in Supplemental Material. The primer sequences used in real-time PCR are summarized in Table S5.
Histology
H&E staining of paraffin-embedded tissue sections was performed by Histoserv, Inc. (Germantown, MD). Samples were visualized under an optical microscope (DAS Mikroskop Leitz DMR; Leica, Wetzlar, Germany) and image stacks were analyzed with ImageJ software. To evaluate mean size and adipocyte frequency distribution, the surface area of a minimum of 370 cells per group was analyzed.
Immunocytochemistry
3T3-L1 adipocytes were fixed in 4% paraformaldehyde (15 min), incubated with PBS containing 0.3% Triton-X-100 and 1% BSA (1 h at RT), and then exposed to rabbit anti-GLUT4 antibody (1:100; Abcam, Cambridge, MA). After an overnight incubation at 4°C, an anti-rabbit Alexa488-conjugat ed secondary antibody (1:500; Invitrogen) was added. Samples were visualized under a Delta-Vision high-resolution microscope (Applied Precision, Inc., Issaquah, WA). Image stacks were deconvolved with Delta-Vision microscope software and analyzed with ImageJ software. To evaluate the number of cells expressing GLUT4 at the plasma membrane, a ratio of the number of cells that were stained for GLUT4 at the cell surface over the total number of cells was calculated. A minimum of 650 cells per group was counted. Moreover, a minimum of 90 cells positive for cell surface GLUT4 labeling per group was used to assess quantitatively the effect of insulin on the amount of GLUT4 present at the plasma membrane over total GLUT4 staining. Negative control samples without the primary antibody were included to assess non-specific staining.
Enzyme-linked immunosorbent assay
Plasma or serum concentration of IL-6, TNF-α, IL-1β, CRP, adiponectin and NEFA were determined in duplicate with commercially available ELISA kits: IL-6, TNF-α and IL-1β (Cell Sciences, Inc., Canton, MA); adiponectin (AdipoGen, Burlington, NC); CRP (USCN Life Science, Inc., Wuhan, China); NEFA (Wako diagnostic, Richmond, VA).
Citrate synthase activity and hydrogen peroxide determination
Visceral adipose tissue was homogenized in RIPA buffer supplemented with EDTA and EGTA (Boston BioProducts), protease and phosphatase inhibitors (Sigma-Aldrich) and 0.1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich). Citrate synthase was determined by spectrophotometric methods in 20 μg of protein lysates following the method described by Bernier et al. (Bernier et al., 2011). Hydrogen peroxide was determined using an Amplex Red hydrogen peroxide determination kit (Invitrogen).
Data analysis
In vitro data presented in the text, figures and tables are expressed as mean ± SEM. The AUC was determined using GraphPad Prism (La Jolla, CA). Boxplot graphs were generated using statistical software to detect outliers within each variable. The repeated measures data was analyzed using a linear mixed-effects model. Fisher's exact test right-tailed, Z-test, Independent-Samples t test, One-Way ANOVA and repeated measures ANOVA (RM-ANOVA) were also used, as appropriate. P < 0.05 was considered statistically significant. Full statistical analysis details are described in Supplemental Material.
Supplementary Material
HIGHLIGHTS.
Resveratrol elicits transcriptional changes in visceral and subcutaneous WAT
Resveratrol exerts anti-inflammatory effects in visceral WAT of HFS-fed monkeys
Resveratrol's anti-inflammatory effect coincides with improved insulin sensitivity
Adipocytes play a key role in the adaptations evoked by resveratrol
ACKNOWLEDGMENTS
This work was funded by the Intramural Research Program of the National Institute on Aging, NIH, the Office of Dietary Supplements, NIH, and by the NIH R01 DK075772 (to J.S.B.) and F30 DK093198 (to J.P.B.). Y.J.-G. was supported by a Sara Borrell fellowship of the Institute de Salud Carlos III (CD07/00208) and by a grant for actividades y estancias formativas of the Consejeria de Salud, Junta de Andalucia (EF-0122/2010), Spain. The authors would like to thank DSM Nutritional Products Ltd. for providing us with the resveratrol (resVida®). None of the authors has any conflict of interest that could affect the performance of the work or the interpretation of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Number. Raw microarray data sets have been submitted to NCBI GEO database (http://www.ncbi.nlm.nih.gov/geo/) (GSE50005)
REFERENCES
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier M, Paul RK, Martin-Montalvo A, Scheibye-Knudsen M, Song S, He HJ, Armour SM, Hubbard BP, Bohr VA, Wang L, et al. Negative regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein. J. Biol. Chem. 2011;286:19270–19279. doi: 10.1074/jbc.M110.200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Bogan JS, Hendon N, McKee AE, Tsao TS, Lodish HF. Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature. 2003;425:727–733. doi: 10.1038/nature01989. [DOI] [PubMed] [Google Scholar]
- Bogan JS, Rubin BR, Yu C, Löffler MG, Orme CM, Belman JP, McNally LJ, Hao M, Cresswell JA. Endoproteolytic cleavage of TUG protein regulates GLUT4 glucose transporter translocation. 2012. [DOI] [PMC free article] [PubMed]
- Brasnyó P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, Mikolas E, Szijarto IA, Merei A, Halmai R, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho E, Jansson PA, Axelsen M, Eriksson JW, Huang X, Groop L, Rondinone C, Sjostrom L, Smith U. Low cellular IRS 1 gene and protein expression predict insulin resistance and NIDDM. FASEB J. 1999;13:2173–2178. doi: 10.1096/fasebj.13.15.2173. [DOI] [PubMed] [Google Scholar]
- Coon PJ, Roqus EM, Drinkwater D, Muller DC, Goldberg AP. Role of body fat distribution in the decline in insulin sensitivity and glucose tolerance with age. J. Clin. Endocrinol. Metab. 1992;75:1125–1132. doi: 10.1210/jcem.75.4.1400882. [DOI] [PubMed] [Google Scholar]
- Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:1307–1312. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, Perni RB, Riera TV, Szczepankiewicz B, Vlasuk GP, et al. SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator. J. Biol. Chem. 2010;285:32695–32703. doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori JL, Shin YK, Kim W, Krzysik-Walker SM, Gonzalez-Mariscal I, Carlson OD, Sanghvi M, Moaddel R, Farhang K, Gadkaree SK, et al. Resveratrol prevents β-cell dedifferentiation in non-human primates given a high fat/high sugar diet. Diabetes. 2013 doi: 10.2337/db13-0266. in press. Published online July 24, 2013. 10.2337/db13-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frojdo S, Durand C, Molin L, Carey AL, Assam EO, Bronwyn AK, Febbraio MA, Solari F, Vidal H, Luciano P. Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by SIRT1. Mol. Cell. Endocrinol. 2011;335:166–176. doi: 10.1016/j.mce.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Garvey WT, Maianu L, Huecksteadt TP, Birnbaum MJ, Molina JM, Ciaraldi TP. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J. Clin. Invest. 1991;87:1072–1081. doi: 10.1172/JCI115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldelli A, Miyazaki Y, Pettiti M, Matsuda M, Mahankali S, Santini E, DeFronzo RA, Ferrannini E. Metabolic effects of visceral fat accumulation in type 2 diabetes. J. Clin. Endocrinol. Metab. 2002;87:5098–5103. doi: 10.1210/jc.2002-020696. [DOI] [PubMed] [Google Scholar]
- Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. USA. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanim H, Sia CL, Abuaysheh S, Korzeniewski K, Patnaik P, Marumganti A, Chaudhuri A, Dandona P. An antiiinflammtory and reactive oxygen species suppressive effects of an extract of polygonum cuspidatum containing resveratrol. J. Clin. Endocrinol. Metab. 2010;95:E1–E8. doi: 10.1210/jc.2010-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales AM, Orlando RA. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. (Lond.) 2008;5:17. doi: 10.1186/1743-7075-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:2276–2283. doi: 10.1161/ATVBAHA.107.147835. [DOI] [PubMed] [Google Scholar]
- Heynekamp J, Weber W, Hunsaker L, Gonzales A, Orlando R, Deck L, Jagt DV. Substituted trans-stilbenes, including analogs of the natural product resveratrol, inhibit the TNFα-induced activation of transcription factor NF-κB. J. Med. Chem. 2006;49:7182–7189. doi: 10.1021/jm060630x. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148:241–251. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W, Hong HJ, Guan J, Kin DG, Yang EJ, Koh G, Park D, Han CH, Lee YJ, Lee DH. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: in vitro and in vivo experiments in rodents. Metabolism. 2012;61:424–433. doi: 10.1016/j.metabol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Kennedy A, Overman A, Lapoint K, Hopkins R, West T, Chuang CC, Martinez K, Bell D, McIntosh M. Conjugated linolenic acid-mediated inflammation and insulin resistance in human adipocytes are attenuated by resveratrol. Lipid Res. 2009;50:225–232. doi: 10.1194/jlr.M800258-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Marchal J, Blanc S, Epelbaum J, Aujard F, Pifferi F. Effect of chronic calorie restriction or dietary resveratrol supplementation on insulin sensitivity markers in a primate, Microcebus murinus. PLoS One. 2012;7:e34289. doi: 10.1371/journal.pone.0034289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, D'Mello SR. NF-κB stimulates Akt phosphorylation and gene expression by distinct signaling mechanisms. Biochim. Biophys. Acta. 2003;1630:35–40. doi: 10.1016/j.bbaexp.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte R, Gwathmey JK, Grazette L, et al. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J. Clin. Invest. 2005;115:2128–2138. doi: 10.1172/JCI23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc. Natl. Acad. Sci. USA. 2001;98:4640–4645. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong R, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPARγ. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J. Biol. Chem. 2006;281:39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 2009;77:1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Matinez-Augustin O, de Medina FS. Resversible ponceau staining as a loading control alternative to actin in western blots. Anal. Biochem. 2010;401:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-α overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Kahn BB. Glucose transporters and insulin action-implications for insulin resistance and diabetes mellitus. N. Engl. J. Med. 1999;341:248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- Student AK, Hsu RY, Lane MD. Induction of fatty acide synthetase synthesis in differentiating 3T3-L1 preadipocytes. J. Biol. Chem. 1980;255:4745–4750. [PubMed] [Google Scholar]
- Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, et al. Role of the Toll-like Receptor 4/NF-κB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metabolism. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem. Soc. Trans. 2005;33:1078–1081. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo M. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW, et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. Sirt1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol. Cell Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T, Schenk S, Imamura T, Babendure JL, Sonoda N, Bae EJ, Oh D, Lu M, Milne JC, Westphal C, et al. Sirt1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010;298:E419–428. doi: 10.1152/ajpendo.00417.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.